Abstract
Background
Primary CNS tumors constitute a heterogeneous group of neoplasms that share a considerable morbidity and mortality rate. To help control tumor growth and clinical outcomes (overall survival, progression-free survival, quality of life) symptoms, patients often resort to alternative therapies, including the use of cannabis. Despite rapidly growing popularity, cannabis and its impact on patients with primary malignant CNS tumors is understudied.
Methods
To shed light on the lack of scientific evidence in this field, in November 2018 we conducted a search and examination of cannabis in neuro-oncology in major journal databases and bibliographies of selected articles, and through abstracts of annual meetings using prespecified criteria in line with the Cochrane Collaboration guidelines.
Results
We identified 45 publications, of which 9 were selected. Five studies were included. Publication dates ranged from 2004 to 2018 and included varying histologies of primary brain tumors. The average survival at 1 year was 56.09% (95% CI: 48.28-63.9). There was no difference in risk ratio (RR) for death at 1 year between groups (RR: 1.069 [95% CI: 0.139-8.25]). We found strong evidence of heterogeneity (Q = 74.0%; P = .021). We found no statistical evidence of publication bias (P = .117; SD = 1.91).
Conclusions
There was limited moderate-quality evidence that supports the use of cannabinoids as adjuvant to the standard of care in the treatment of brain and CNS tumors. There was very low-quality evidence suggesting that cannabinoids were associated with adult-onset gliomas. Further prospective clinical trials are necessary to adequately evaluate the impact of cannabinoids on CNS tumors, specifically on survival and quality of life.
Keywords: adult glioma, cannabis, CNS tumors, clinical outcomes
Primary CNS tumors constitute a heterogeneous group of neoplasms that share a considerable morbidity and mortality. Glial tumors account for 85% to 90% of all primary CNS tumors.1 Estimated new cases of brain tumors and other CNS tumors in the United States in 2018 were estimated to be 23 880, and there were 16 830 deaths as a result of these neoplasms.2 Differences in survival are mainly due to the variation in histologic type and grade. However, there are demographic characteristics that have an impact on survival and prognosis such as age, sex, ethnicity, and geographical location.3,4
In many brain cancer patients, current treatment options are not curative, focusing instead on prolonging survival while maintaining or improving patients’ quality of life.5,6 As expected, patients with primary brain tumors face serious challenges to their quality of life. They often face symptoms secondary to neurological deterioration associated with the disease7 as well as symptoms related to different therapies. In studies conducted in populations with malignant glioma, patients scored significantly lower in all domains of functioning compared to healthy controls.8 Furthermore, health-related quality-of-life scores have been shown to be important prognostic factors in predicting survival.9,10
In the search to improve their symptoms and consequently their quality of life, patients often resort to unconventional therapies. Anecdotally, one of the most commonly used alternative therapies and one with significant media attention is cannabis, including edibles, oils, joints, vapors, etc.11,12 Despite rapidly growing popularity, the clinical literature on cannabis is still in its infancy. In fact, it was not until the early 1990s that the endocannabinoid derivatives were identified. The most notable cannabinoid isolated from Cannabis sativa is the phytocannabinoid tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis13; cannabidiol (CBD) is another major constituent of cannabis, although to date there are at least 113 different cannabinoids isolated from C sativa, exhibiting varied effects, making their separation difficult.14
Of course, it is well known that cannabinoids exert various palliative effects in cancer patients,15–18 including antiemetic, pain, seizure control, anxiety treatment, among others; however, there is a lack of prospective or systematic evidence for its use (reasons and efficacy) in the care of patients with primary brain tumors. Although there are ongoing efforts outside the United States to establish the safety and efficacy of CBD in the CNS tumor population, the legal status and social stigmatization leads to a gap in our current clinical knowledge,19 especially in the United States. In an effort to close this gap, the present systematic review aims to examine the effects of cannabinoids in adult patients with a diagnosis of malignant CNS neoplasms. Specifically, the present review will explore the differences in overall survival of cannabis users with primary malignant CNS tumors as a clinical end point and will describe the possible negative effects of these compounds.
Methods
This review followed guidance by the Cochrane Collaboration.20
Search Strategy
The systematic review and search strategy were performed by the authors according to a predetermined protocol to identify literature on the use of cannabis in adult patients with malignant CNS tumors of any grade. The terms searched included cannabis, cannabinoids, nabilone, marijuana, adult, CNS, cancer, and gliomas. The references were exported and managed using Mendeley Desktop. The search was conducted in November 2018 using Medline and Embase databases as well as the bibliographies of selected articles. Additionally, the search included a review of specialty journals such as Neuro-Oncology, Neuro-Oncology Practice, Journal of Neuro-Oncology, and Neuro-Surgical Frontiers. Furthermore, a search was performed of abstracts of scientific meetings of the Society for Neuro-Oncology, American Society of Clinical Oncology, European Association of Neuro-Oncology, American Association of Neurology, and the International Brain Tumor Alliance annual meeting. There were no restrictions by year or language, although the search was limited to human studies only, including clinical trials, systematic reviews, cohort studies, cross-sectional studies, observational studies, interventional studies, case series, and pilot studies. Preclinical studies were excluded from this review (cellular, molecular, and animal studies).
Study Selection
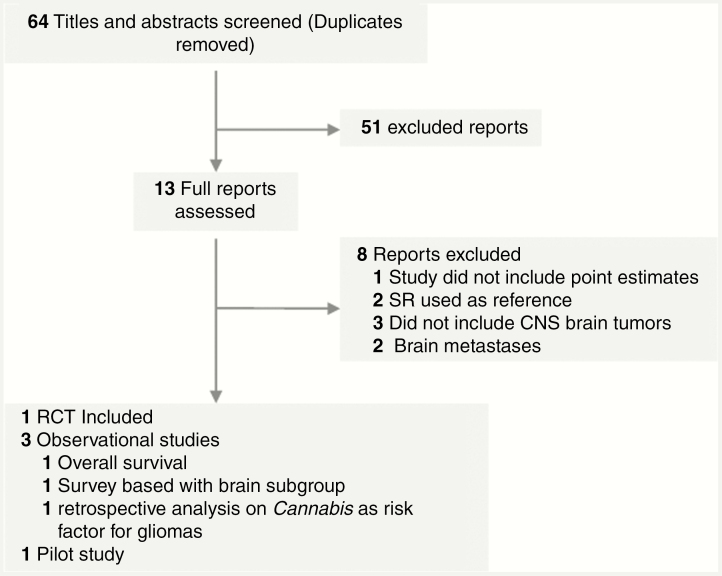
Titles and abstracts of all references were screened independently to identify literature that reported on the use of cannabis among adult human patients with malignant CNS tumors of any grade; those references with a different target population were excluded at this stage. The articles included must have met the following criteria: 1) population: adult patients with brain cancer. Studies that reported stratified outcomes, identifying a CNS group, were included; 2) intervention: report registry of cannabis or synthetic cannabinoid use in any form (edible, inhaled, infused, etc); 3) outcomes: all patient outcomes were considered (if available), namely overall survival, progression-free survival, quality of life scores, etc. Our study was primarily interested in overall survival. Secondary outcomes included KPS score, cognitive function, physical function, quality-of-life scores, symptom intensity, adverse outcomes, and side effects, as well as progression-free survival; 4) design: randomized controlled trials (RCTs), cohort studies, case-control studies, cross-sectional studies, and observational studies were included. Furthermore, longitudinal prospective and longitudinal retrospective studies were also included. Animal studies, molecular studies, and cellular studies were excluded (Figure 1).
Figure 1.
Flow of Studies Through the Review Process.
RCT indicates randomized controlled trial; SR: systematic review.
Data Extraction
Data were extracted from the identified studies using a structured data extraction form, and then entered into an electronic database to allow for analysis and writing of the final report. The data extracted included study design, year of publication, authors, type (histology and or World Health Organization grade) of tumor(s) included in the study (if available), type of adjuvant therapy (if any), use of cannabis or synthetic cannabinoids, total number of patients, patients in control and intervention groups (when applicable), mode of use, and outcomes (if available).
Data Quality
The quality of the studies and risk of bias were assessed following the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) scoring system recommended by the Cochrane Collaboration.20,21 The GRADE scoring system assesses the study design, statistical methods, publication bias, effect sizes, dose response, and residual confounding. This system results in an assessment of the quality of a body of evidence as high, moderate, low, or very low.
Data Synthesis and Analysis
The intervention being evaluated in this review is the use of cannabis and/or systemic cannabinoids and its impact on clinical outcomes (overall survival, progression-free survival) in the setting of patients with a diagnosis of CNS tumors. To assess for significant between-study heterogeneity, the Cochrane Q statistic was calculated along with a summary statistic. For 1-year survival, an average survival was calculated (Figure 2). Accordingly, a pooled risk ratio (RR) of death at 1 year was calculated (Figure 3). If significant heterogeneity was present (P < .05), a random-effects model was used. Publication bias was analyzed visually using funnel plots and subsequently the Begg test. The Egger test was performed in the event that the Begg test showed evidence for publication bias.
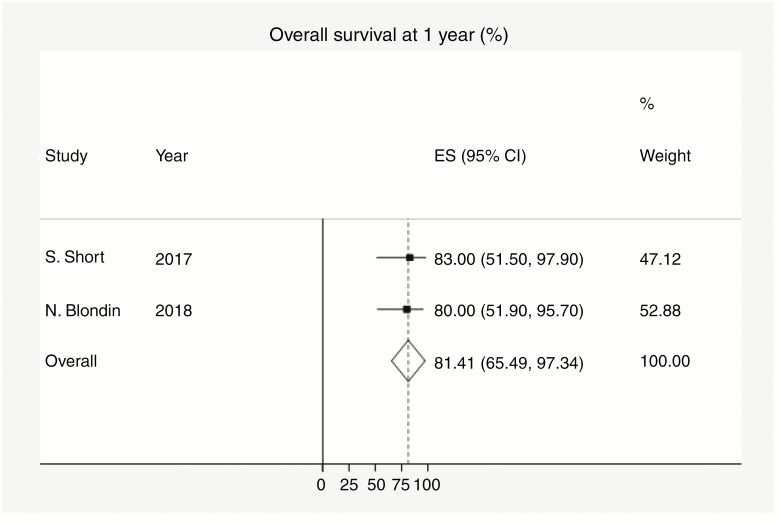
Figure 2.
Overall survival at 1 year. Dotted data markers indicate the percentage survival at 1 year among the population, and the statistical weight of the study using fixed-effects meta-analysis. Horizontal lines indicate 95% CI. Diamond data marker represents the overall percentage survival at 1 year (81.41%) and 95% CI (66.49-97.34). The vertical dashed line shows the summary effect estimate. The P value for Cochran Q test equals .85. Variation I 1-year percentage survival (%) attributable to heterogeneity equals 0.0%. RCT indicates randomized controlled trial; SR: systematic review.
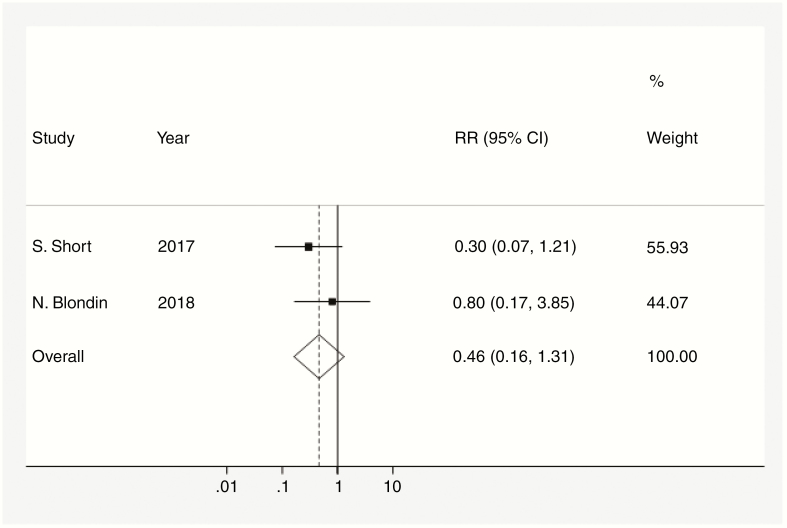
Figure 3.
Risk ratio (RR) of death among cannabis users. Dotted data markers indicate the RR for death compared to placebo or no intervention, with sizes reflecting the statistical weight of the study using fixed-effects meta-analysis. THhorizontal lines indicate 95% CI. Diamond data marker represents the overall RR (0.46) of death at 1 year and 95% CI (0.163-1.311). Vertical dashed line shows the summary effect estimate, and vertical solid line shows the line of no effect (RR = 1). The P value for the Cochran Q test equals .36. Variation in RR attributable to heterogeneity equals 0.0%.
For all tests, a P value of less than .05 was deemed to be significant. All statistical analyses were performed in STATA version 15 using the “metan” package.
Results
Identification and Description of Studies
The searches identified 45 results in PubMed, of which 9 were considered potentially relevant22–30 based on title and abstract screening. Similarly, 4 results were yielded from the search in the journal Neuro-Oncology, of which 230,31 were considered relevant based on title and abstract. Five studies were included23,26,27,30,31 (Table 1). Two studies were available only as abstracts30,31; the remaining 3 studies were reported in full-length journal articles.23,26,27 Publication dates ranged from 2004 to 2018. Studies were conducted in Spain, the United Kingdom, Israel, and the United States. A variety of unspecified cannabinoids were evaluated and compared with placebo or no treatment. From the pooled studies a total of 176 patients were examined with a mean age of 57.5 years (SD 1.6 years).
Table 1.
Characteristics of Included Studies
| Author | Year of publication | Study design | Primary outcome | Cannabinoidc | Type of cancer observed | Total No. of participants | Cannabinoid/Comparator (N)a | Population age, mean, y |
|---|---|---|---|---|---|---|---|---|
| Efird et al23 | 2004 | Observational retrospective | MPAGd | Unspecified | Glioma, NOS | 105005 | 60/9b | 51.9 |
| Blondin31 | 2018 | Observational prospective | Overall survival | Unspecified | Glioblastoma | 23 | 15/8b | 65 |
| Bar-Lev Schleider et al27 | 2018 | Observational prospective | Palliative effects | Unspecified | Multiple cancers | 2970 | 116/10b | 59.5e |
| Guzmán et al26 | 2006 | Pilot study | Survival | THC | Recurrent glioblastoma | 9 | 9/NA | 55.2 |
| Twelves et al30 | 2017 | Double-blind RCT | Safety and tolerance | CBD:THC | Recurrent glioblastoma | 21 | 12/9c | 58 |
Abbreviations: CBD, cannabidiol; MPAG, malignant primary adult-onset glioma; NA, not available; NOS, not otherwise specified; RCT, randomized controlled trial; THC, tetrahydrocannabinol.
aNumber of individuals with CNS tumors.
bComparator group received no intervention.
cComparator group received placebo.
dStudy evaluated risk of cannabis usage for developing MPAG.
eMean age of the overall population not brain/CNS specific.
The articles not selected were excluded for lack of detail on the number of participants in the CNS cancer cohort and/or did not report outcomes in this population group.
The 2017 study by Twelves et al30 was the only randomized, double-blind, placebo-controlled study. The study population was patients with recurrent glioblastoma multiforme (GBM). The mean age in this study was 58 years and the median baseline KPS was 90. This study stated that the intervention group was treated with temozolomide plus CBD:THC at a ratio of 1:1 (N = 12). The placebo group was treated with temozolomide plus placebo (N = 9). One-year survival was assessed in both groups (83% for the CBD:TCH group vs 44% for the placebo group). The main adverse events (AEs) were vomiting and dizziness.
The study by Blondin31 was an observational study in patients with GBM who consumed cannabis oil concentrate of at least 50 mg of cannabinoid per day for at least 1 month while also receiving standard-of-care treatments (N = 15), compared with patients who did not use cannabis oil concentrate (N = 8). No specific cannabinoids were assessed. The mean age was 53 years. Overall survival at 1 year was 80% in the cannabis group compared to 74% in the noncannabis user group. This group found no significant AEs.
The study by Guzmán and colleagues26 was a pilot study on the effects and efficacy of δ-9-tetrahydrocannabinol (THC) in patients with recurrent GBM. In this study 9 patients were enrolled, all of whom had failed standard therapy, which included surgery and external-beam radiotherapy, had clear evidence of tumor progression, and had a minimum KPS of 60. Following enrollment, patients underwent a surgical intervention aimed at resetting and creating a cavity in the recurrent tumor where an infusion catheter was placed. Patients received a daily infusion whose active component was THC. Patients then were followed. The median age was 55 years, and the mean KPS was 80. The median duration of administration was 10 days. The median survival from the surgical operation of tumor relapse was 24 weeks. Two patients survived 1 year.
The study by Bar-Lev Schleider et al27 was a prospective, observational study conducted on 2970 individuals who received a medical marijuana license, of whom 126 patients presented with a diagnosis of brain/CNS tumor (4.2%). Of these patients 116 individuals who did not stop treatment were considered cannabis users. Patients’ health status was assessed at baseline and was followed using a questionnaire. The average age was 59.5 years. The primary outcome was “success rate,” defined as at least moderate to significant improvement in the patient’s condition and no cessation of treatment or serious side effects. The success rate in the brain/CNS tumor subgroup was 67.8%. The survival rate at 6 months was 50.9%. The survival at 1 year was not reported for the brain/CNS tumor subgroup. The main side effects of cannabis treatment in the overall population were sleepiness, dry mouth, increased appetite, and psychoactive effect.
The study by Efird and colleagues23 was a retrospective observational study conducted on patients registered in a health network in Northern California who were enrolled between 1977 and 1985. The primary outcome was to determine the risk for malignant primary adult-onset glioma associated with cigarette smoking and other lifestyle behaviors in a large, multiethnic, managed-care cohort. Participants were at least age 25 years at enrollment with no history of benign or malignant brain tumors. Patients were followed until the occurrence of a primary malignant glioma or death. The occurrence of brain glioma was determined using the local tumor registry that reports to the Surveillance, Epidemiology, and End Results program. The mean age of the population was 62.2 years. There were 19 292 patients who had ever used cannabis who were identified. Of these individuals, 13 524 used cannabis at least once a month, and 5768 less than once a month. Nine patients who ever used cannabis presented with a primary glioma (RR = 1.9; 95% CI: 0.9-4.0; P = .1) and 60 in the nonuser group developed a primary brain glioma. The Efird study23 was excluded from all data analysis because the outcome (adult onset of primary glioma) and population (previously healthy individuals) were not comparable with other studies. However, because the goal of this review was to establish benefits as well as risks of cannabis use in the setting of brain and CNS tumors, it was deemed to be important to include this study as a means of including all possible risks of cannabis consumption (including onset of primary brain tumors).
Of the 5 included studies, 2 studies were judged to have a low risk of bias,26,30 1 had a high risk of bias,23 and 2 had an unclear risk of bias.27,31 The major potential source of bias in the trials was no randomization and incomplete data for patients with brain cancer. Two studies were survey based, being subject to recall bias and imprecision. Selective outcome reporting was a potential risk of bias in one study. Pooled results and GRADE ratings are presented in Table 2.
Table 2.
Outcomes and Grading of Recommendations Assessment, Development, and Evaluation Rating
| Study | Survival rate at 1 y cannabis/comparator, % | RRb (95% CI) | GRADE ratingc |
|---|---|---|---|
| Efird et al23,a | – | 1.33 | Very low |
| Guzmán et al26 | 22/NA | – | Low |
| Twelves et al30 | 83/44 | 0.3 (0.07-1.2)b | High |
| Blondin31 | 80/74 | 0.8 (0.17-3.85)b | Moderate |
| Bar-Lev Schleider et al27 | 50.9/100 | 10.812 (0.72-163.2)b | Low |
Abbreviations: NA, not available; RR, risk ratio.
aThe outcome for this study was the occurrence of adult-onset glioma with no survival data.
bRisk ratio of death among cannabis users.
cGRADE Working group grades of evidence: (1) high quality, further research is very unlikely to change the group’s confidence in the estimate of effect; (2) moderate quality, further research is likely to have an important impact on the group’s confidence in the estimate of effect and may change the estimate: (3) low quality, further research is very likely to have an important impact on the group’s confidence in the estimate of effect and is likely to change the estimate; (4) very low quality, the group is very uncertain about the estimate.
Overall Survival
Overall survival was directly assessed in 4 studies (179 participants).26,27,30,31 Two studies assessed CBD,30,31 1 study assessed THC,26 and 2 studies only report use of “marijuana” without specifying an active component.23,27 One study included a placebo control,30 3 studies included a control group,23,27,31 and 1 study did not include a comparison group.26 Of the 5 studies, risk of bias was high for 3,23,26,27 low risk for 1,30 and unclear for 1.31 Three studies suggested a great benefit of cannabinoids compared with placebo or no intervention, reaching statistical significance.27,30,31 One study showed an increased risk of developing brain cancer among marijuana users compared to nonusers.23 One study showed no benefit of the use of cannabis compounds (THC) compared to standard of care.26
The average survival at 1 year was 81.4% (95% CI: 65.5-97.3 [Figure 1]). Additionally, on average, there was no significant difference in the RR for death at 1 year in the cannabis-user group compared with the noncannabis-user group (RR: 0.46 [95% CI: 0.16-1.311][Figure 2]).
We found strong evidence of heterogeneity in the analysis when including studies for which there were enough data to be analyzed27,30,31 (Q = 74.0%; P = .021). Similarly, we found no evidence of heterogeneity when the Bar-Lev Schleider study was excluded (Q = 0.0%; P = .36), suggesting that it was not appropriate to include this study in the final analysis. Furthermore, we found no statistical evidence of publication bias supported by the adjusted rank correlation test (P = .317; SD = 1.0) and corroborated by the regression asymmetry test (bias score = 0.25).
Discussion
We conducted a systematic review of the benefits and risks associated with cannabis use in patients with CNS tumors. Ultimately, our review included only one RCT (21 patients) that evaluated overall tolerability of CBD:THC as well as survival at 1 year in patients with recurrent glioblastoma undergoing standard-of-care therapy with temozolomide.
Most studies suggested that cannabinoids were associated with improvement in overall survival, but this association did not reach statistical significance. Furthermore, one of the studies27 was powered to observe outcomes in cancer in general rather than brain tumors specifically. Based on the GRADE approach, there was low- to moderate-quality evidence to suggest cannabinoids may be beneficial as an adjuvant in the treatment of brain cancer. There was low-quality evidence suggesting cannabinoids were associated with a higher risk of death in the setting of brain cancer. There was very low-quality evidence suggesting cannabinoids were associated with malignant primary adult onset of glioma as well as low-quality evidence suggesting cannabinoids were associated with faster progression of recurrent glioblastoma. In contrast, there was low- to moderate-quality evidence suggesting cannabinoids (CBD/THC capsules and smoked marijuana) were associated with higher survival rates at 1 year in glioma patients.
Although the primary predictor in the Efird study23 was tobacco smoking as a risk factor for developing new-onset adult glioma, cannabis was also included and analyzed. This study yielded a positive association between cannabis consumption and new onset of adult glioma. However, this effect was not consistently observed when the cannabis-user group was divided by frequency in consumption. Furthermore, there was no explanation of whether cannabis users also were tobacco users or were considered a separate group. Regardless of these methodological issues, we considered it important to include this study to show the full spectrum of clinical outcomes that are being studied on cannabis in the CNS cancer setting.
Strengths and Weakness
This review followed the recommendations from the Cochrane Collaboration for rigorous systematic reviews. To identify as many relevant studies as possible and enhance the effort to avoid bias, a highly rigorous search strategy was used and an extensive range of resources were searched including electronic databases, abstracts from scientific meetings, and references from relevant articles. Published and unpublished trials were eligible for inclusion. There were no date or language restrictions. We used the GRADE approach21,32 to assess quality of evidence and risk of bias. This highlighted weaknesses in the included studies, including the observational nature of some research, mishandling of missing data, selective outcome reporting, lack of blinding and randomization, and lack of a comparison group. An additional limitation of some of the included studies was that brain cancer was not the main group, rather “cancer” data were captured and further reported as subgroups. However, no subgroup analysis based on type of cancer was conducted. Furthermore, the outcomes reported were heterogeneous, making the combination and further analysis of results difficult. Multiple different cannabinoids were evaluated in the included studies; however, only CBD and THC were specifically identified. Most of the studies specify only “marijuana” as the comparator group, preventing this analysis from stratifying by type of cannabinoid. Only one study presented a placebo group; the rest used “no intervention” as a comparator group. These differences in form, combined with the variety of outcomes, resulted in a very heterogeneous set of included studies.
A key strength of this review is that it highlights the lack of high-quality clinical evidence on cannabinoids and its effects on patients with brain or CNS tumors, despite its increasing use among this population.
Unanswered Questions and Future Research
Further large, robust, and rigorous RCTs are needed to confirm the effects of cannabinoids in patients with CNS tumors. Subsequent studies evaluating cannabis itself are also required because of the almost nonexistent evidence on the effects and AEs of cannabis in the specific setting of brain and CNS tumors. Future studies should assess patient-centered outcomes such as quality of life, physical functionality, progression-free survival, and AEs using validated and standardized outcome measures at prespecified time points to ensure improvement in the quality of the evidence for future meta-analyses.
Conclusions
The evidence presented here suggests cannabis does not increase risk of death in patients with malignant CNS tumors. Rather, although the overall effect of cannabis and cannabinoids in survival rates at 1 year was found not to be statistically significant, the direction of such an effect suggests an increase in survival rates at 1 year in CNS cancer populations. Owing to the lack of data, we were not able to perform an analysis of the direct impact of cannabis on quality-of-life scores of patients with CNS tumors. However, we found that the use of these compounds was safe and well tolerated, with the main side effects being dizziness, dry mouth, increased appetite, sleepiness, and psychoactive effects. Additionally, there was very low-quality evidence suggesting that cannabinoids were associated with adult-onset gliomas.
Although there is increasing preclinical evidence supporting the benefits of cannabis in CNS tumors,18,22,27,33,34 in this analysis we demonstrated that high-quality clinical evidence is still lacking and further clinical trials on the effects of cannabis and its derivatives in the CNS tumor population need to be conducted. As part of the effort to advance knowledge on the impact of cannabis on quality of life, our group is conducting an observational study exploring glioma patient behavior around the use of cannabis and cannabinoids as well as clinical outcomes in this population.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. None declared.
References
- 1. Brem SS, Bierman PJ, Brem H, et al. ; National Comprehensive Cancer Network Central nervous system cancers. J Natl Compr Canc Netw. 2011;9(4):352–400. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Brain and Other Nervous System Cancer.https://seer.cancer.gov/statfacts/html/brain.html#risk. Accessed September 20, 2018.
- 3. Howlader N, Noone A, Krapcho M, et al. . SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute; based on November 2016 SEER data submission, posted April 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed November 5, 2018. [Google Scholar]
- 4. Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl 5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minniti G, Scaringi C, Baldoni A, et al. . Health-related quality of life in elderly patients with newly diagnosed glioblastoma treated with short-course radiation therapy plus concomitant and adjuvant temozolomide. Int J Radiat Oncol Biol Phys. 2013;86(2):285–291. [DOI] [PubMed] [Google Scholar]
- 6. Taphoorn MJ, van den Bent MJ, Mauer ME, et al. ; European Organisation for Research and Treatment of Cancer Health-related quality of life in patients treated for anaplastic oligodendroglioma with adjuvant chemotherapy: results of a European Organisation for Research and Treatment of Cancer randomized clinical trial. J Clin Oncol. 2007;25(36):5723–5730. [DOI] [PubMed] [Google Scholar]
- 7. Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein M, Taphoorn MJ, Heimans JJ, et al. . Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19(20):4037–4047. [DOI] [PubMed] [Google Scholar]
- 9. de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubsts JA.. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37(3):332–339. [DOI] [PubMed] [Google Scholar]
- 10. Krex D, Klink B, Hartmann C, et al. ; German Glioma Network Long-term survival with glioblastoma multiforme. Brain. 2007;130(pt 10):2596–2606. [DOI] [PubMed] [Google Scholar]
- 11. Parmar JR, Forrest BD, Freeman RA. Medical marijuana patient counseling points for health care professionals based on trends in the medical uses, efficacy, and adverse effects of cannabis-based pharmaceutical drugs. Res Social Adm Pharm. 2016;12(4):638–654. [DOI] [PubMed] [Google Scholar]
- 12. Sexton M, Cuttler C, Finnell JS, Mischleyl LK.. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. [DOI] [PubMed] [Google Scholar]
- 14. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1646–1647. [Google Scholar]
- 15. Velasco G, Carracedo A, Blázquez C, et al. . Cannabinoids and gliomas. Mol Neurobiol. 2007;36(1):60–67. [DOI] [PubMed] [Google Scholar]
- 16. Bifulco M, Laezza C, Pisanti S, Gazzerro P.. Cannabinoids and cancer: pros and cons of an antitumour strategy. Br J Pharmacol. 2006;148(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abrams DI. Using medical cannabis in an oncology practice. Oncology (Williston Park). 2016;30(5):397–404. [PubMed] [Google Scholar]
- 18. Guzmán M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3(10):745–755. [DOI] [PubMed] [Google Scholar]
- 19. Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy. 2017;42:30–35. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Green S, eds; the Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; 2011. www.cochrane-handbook.org. [Google Scholar]
- 21. Balshem H, Helfand M, Schünemann HJ, et al. . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4): 401–406. [DOI] [PubMed] [Google Scholar]
- 22. Hall W, Christie M, Currow D. Cannabinoids and cancer: causation, remediation, and palliation. Lancet Oncol. 2005;6(1):35–42. [DOI] [PubMed] [Google Scholar]
- 23. Efird JT, Friedman GD, Sidney S, et al. . The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neurooncol. 2004;68(1):57–69. [DOI] [PubMed] [Google Scholar]
- 24. Colls BM. Cannabis and cancer chemotherapy. Lancet. 1980; 1(8179):1187–1188. [DOI] [PubMed] [Google Scholar]
- 25. Sallan SE, Zinberg NE, Frei E III. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med. 1975;293(16):795–797. [DOI] [PubMed] [Google Scholar]
- 26. Guzmán M, Duarte MJ, Blázquez C, et al. . A pilot clinical study of delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bar-Lev Schleider L, Mechoulam R, Lederman V, et al. . Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med. 2018;49:37–43. [DOI] [PubMed] [Google Scholar]
- 28. Cannabis-In-Cachexia-Study-Group; Strasser F, Luftner D, Possinger K, et al. . Comparison of orally administered cannabis extract and delta-9- tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24(21):3394–3400. [DOI] [PubMed] [Google Scholar]
- 29. Lichtman AH, Lux EA, McQuade R, et al. . Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manage. 2018;55(2):179–188.e1. [DOI] [PubMed] [Google Scholar]
- 30. Twelves C, Short S, Wright S. A two-part safety and exploratory efficacy randomized double-blind, placebo-controlled study of a 1:1 ratio of the cannabinoids cannabidiol and delta-9-tetrahydrocannabinol (CBD:THC) plus dose-intense temozolomide in patients with recurrent glioblastoma multiforme (GBM). J Clin Oncol. 2017;35(15 suppl):2046. [Google Scholar]
- 31. Blondin N. The evolving role of complementary cannabis therapy in glioblastoma treatment. Neuro Oncol. 2018;20(6):vi214–vi215. [Google Scholar]
- 32. Holger S, Jan B, Gordon G, Andrew O.. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; guidelinedevelopment.org/handbook. [Google Scholar]
- 33. Birdsall SM, Birdsall TC, Tims LA. The use of medical marijuana in cancer. Curr Oncol Rep. 2016;18(7):40. [DOI] [PubMed] [Google Scholar]
- 34. Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO.. Cannabidiol—recent advances. Chem Biodivers. 2007;4(8):1678–1692. [DOI] [PubMed] [Google Scholar]