Abstract
Background
Adult intracranial ependymoma is rare, and the role for adjuvant radiotherapy (RT) is not well defined.
Methods
We used the National Cancer Database (NCDB) to select adults (age ≥ 22 years) with grade 2 to 3 intracranial ependymoma status postresection between 2004 and 2015 and treated with adjuvant RT vs observation. Four cohorts were generated: (1) all patients, (2) grade 2 only, (3) grade 2 status post–subtotal resection only, (4) and grade 3 only. The association between adjuvant RT use and overall survival (OS) was assessed using multivariate Cox and propensity score matched analyses.
Results
A total of 1787 patients were included in cohort 1, of which 856 patients (48%) received adjuvant RT and 931 (52%) were observed. Approximately two-thirds of tumors were supratentorial and 80% were grade 2. Cohorts 2, 3, and 4 included 1471, 345, and 316 patients, respectively. There was no significant association between adjuvant RT use and OS in multivariate or propensity score matched analysis in any of the cohorts. Older age, male sex, urban location, higher comorbidity score, earlier year of diagnosis, and grade 3 were associated with increased risk of death.
Conclusions
This large NCDB study did not demonstrate a significant association between adjuvant RT use and OS for adults with intracranial ependymoma, including for patients with grade 2 ependymoma status post–subtotal resection. The conflicting results regarding the efficacy of adjuvant RT in this patient population highlight the need for high-quality studies to guide therapy recommendations in adult ependymoma.
Keywords: adult, brain, ependymoma, intracranial, radiotherapy
Ependymoma is a rare CNS tumor that accounts for approximately 2% to 3% of primary CNS tumors diagnosed in the United States each year.1 The World Health Organization (WHO) classifies ependymomas by histology, molecular findings, and grade.2 The classification of “ependymoma” is considered grade 2 and “anaplastic ependymoma” is considered grade 3.2 Recently, RELA fusion status has also been incorporated into the pathologic diagnosis and can be either grade 2 or 3.2 Although ependymoma occurs both in children and adults, the location, grade, and underlying biology of tumors have distinct patterns based on age. Childhood ependymoma has a high probability of being located intracranially (approximately 80% of cases) and being anaplastic (approximately 30% of cases).3,4 This is in contrast to adults, in whom approximately 50% to 60% of cases are located in the spinal cord and/or cauda equina and only about 5% of cases are anaplastic.
Much of the recent research into the molecular classification and biology-based prognosis of intracranial ependymoma has been derived from and applied to the pediatric population.5–7 There are demonstrated differences in the molecular subtype of ependymoma that correlates with tumor location and patient age, but the therapeutic implications of these differences are not yet well delineated.8 Additionally, virtually all the prospective studies published on ependymoma that guide therapy recommendations have included primarily pediatric patients.9
The literature regarding adult ependymoma is sparse in comparison and consists largely of single- or multi-institutional retrospective studies limited by small patient numbers or population-based studies that have been limited by data quality and/or methodologic issues.4,10–16
This dearth of evidence is reflected in recent European Association of Neuro-Oncology (EANO) consensus guidelines for ependymoma in which the controversy regarding the role of adjuvant radiotherapy (RT) for adults with intracranial grade 2 ependymoma is acknowledged and the recommendation to administer adjuvant RT for adults with subtotally resected grade 2 intracranial ependymoma is based on class III evidence with a C level recommendation.17
The goal of the present study was to evaluate the role of adjuvant RT in adults (age ≥ 22 years) with intracranial ependymoma by assessing the association of adjuvant RT with overall survival (OS) in a large national cohort of patients with particular attention to patients with grade 2 ependymoma.
Materials and Methods
Data Collection and Cohort Definition
We conducted the analysis using the National Cancer Database (NCDB). The NCDB is a national hospital-based cancer registry that includes nearly 70% of incident cancer cases in the United States.18 Deidentified information related to patient, tumor, and treatment parameters and OS outcomes are collected from more than 1500 Commission on Cancer–accredited cancer programs. Owing to the publicly available deidentified nature of the NCDB database, this study was institutional review board exempt and patient consents were not needed.
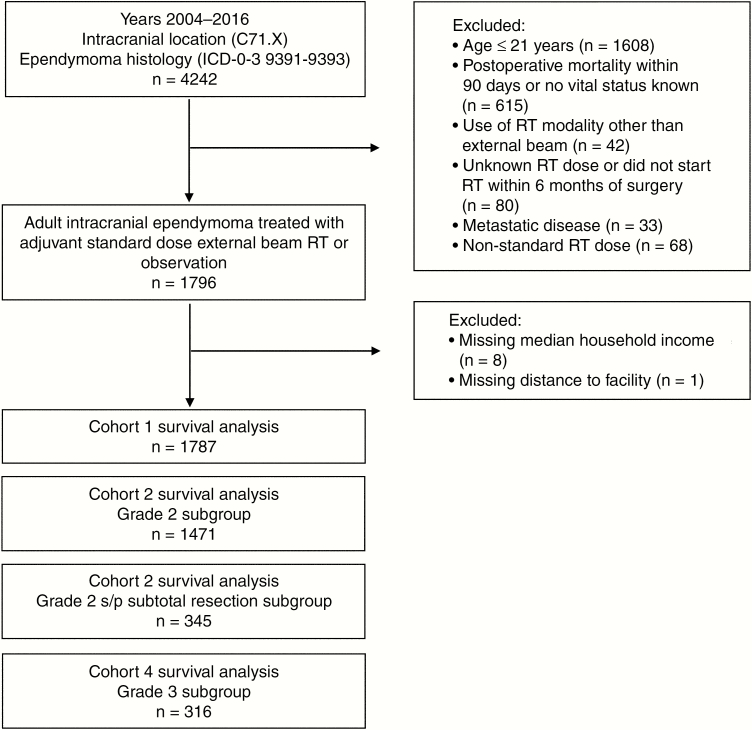
We defined “adult” as age 22 years or older based on the inclusion of patients aged 1 to 21 years on recent pediatric Children’s Oncology Group protocols including ACNS012119 and ACNS0831. We included adult patients (age ≥ 22 years) with grade 2 or 3 ependymoma (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histology codes 9391 (ependymoma–WHO grade II), 9392 (anaplastic ependymoma–WHO grade III), and 9393 (papillary ependymoma–WHO grade II). Tumor grade was defined based on ICD-0-3 histology code definitions. Tumor location was limited to intracranial as defined by ICD-0-3 topographical code C71.X. Other criteria were year of diagnosis 2004 to 2016 and no evidence of metastatic disease at diagnosis (CS_mets_at_dx value of 00, 10, or 99). To account for immortal time bias, we excluded patients who did not have known vital status or who died within 90 days of initial surgery.20 We additionally excluded patients who received adjuvant RT modalities other than external beam RT, who had unknown RT dose delivered, who received nonstandard RT dosing (standard defined as 45-66 Gy), or who did not start RT within 6 months of surgery. An additional 9 patients were excluded because of unknown median household income quartile based on zip code (n = 8) and unknown distance from home zip code to treatment facility (n = 1). This resulted in a cohort of 1787 patients (Fig. 1).
Fig. 1.
Patient selection flowchart. ICD indicates International Classification of Diseases; RT, radiotherapy; s/p, status post.
Predictor Variables
Variables included in the analysis included patient age, sex, race, insurance status, median household income based on zip code, proportion of residents with no high school diploma based on zip code, urban/rural category, distance from home zip code to facility, Charlson/Deyo comorbidity index, year of diagnosis, primary site (supratentorial vs infratentorial), tumor grade (2 vs 3), tumor size (≤ 4 cm vs >4 cm), extent of resection (subtotal resection [STR] vs gross total resection [GTR]), and facility type. Variables with missing data were categorized to include a variable level for unknown status in the analyses. Chemotherapy use was rare (5.6% of the overall cohort received chemotherapy), with very small patient numbers receiving chemotherapy in the various cohorts. As a result, we did not include chemotherapy use as a predictor variable.
Statistical Analyses
Patients were grouped according to receipt of RT (adjuvant RT vs observation). Continuous variables were reported as median with interquartile range [IQR]) and categorical variables were reported as number and percentage. Variables were compared using the chi-square test or Wilcoxon rank sum test, as appropriate.
The primary end point was to compare OS within cohorts based on receipt of adjuvant RT. The event for OS was death from any cause and the interval was from diagnoses to death or last known alive.
Given that grade and extent of resection are well-documented strong prognostic variables in intracranial ependymoma,21 we elected to stratify cohorts according to these variables to determine the effect of adjuvant RT within patient subsets. Four patient cohorts were generated in total: all patients (cohort 1), those with grade 2 ependymoma only (cohort 2), grade 2 ependymoma status post-STR only (cohort 3), and grade 3 ependymoma only (cohort 4, Fig. 1). We did not subdivide grade 3 by extent of resection because of resultant small numbers of patients who did not receive adjuvant RT and the consensus recommendation to administer adjuvant RT for grade 3 ependymoma regardless of extent of resection.17,22
Before matching, we performed a Cox proportional hazards model for OS including all predictor variables with backward selection (P = 0.1 to stay) within cohorts 1 and 4. We forced the variable of interest (adjuvant RT vs observation) into the model to obtain the adjusted hazard ratio (HR) of interest.
We used propensity score matching within each cohort to reduce imbalance in predictor variables between the patient cohort receiving adjuvant RT and those who were observed postoperatively. In cohort 1, propensity scores were estimated with a multivariate logistic regression model in which adjuvant therapy (adjuvant RT vs observation) was regressed on all variables outlined in the Predictor Variables section. The same process occurred in the other cohorts, except tumor grade was excluded for cohorts 2 and 4, and tumor grade and extent of resection were both excluded in cohort 3. Propensity score matching used the nearest-neighbor method with a caliper distance of 0.2 without replacement to perform a 1:1 match between adjuvant RT and observation.23
After matching, estimation of OS was performed using the Kaplan-Meier method. The association between receipt of RT and OS was evaluated using the Cox univariate proportional hazards model stratified on matched pair within each patient cohort.
Statistical analyses were performed using R version 3.2.5 and SPSS version 24 (IBM). All tests were 2-sided, and a P value < .05 was considered statistically significant.
Results
Patient Characteristics
A total of 1787 patients were included in cohort 1 (Fig. 1). Cohort 2 (grade 2), cohort 3 (grade 2 status post-STR), and cohort 4 (grade 3) included 1471, 345, and 316 patients, respectively. In cohort 1, a total of 856 patients (48%) received RT and 931 (52%) were observed postoperatively. Approximately two-thirds of tumors were supratentorial and 80% were grade 2. There were substantial missing data for tumor size (28% unknown) and extent of resection (49% unknown), which is common among NCDB studies of primary CNS tumors.24–26 There were significant imbalances in several variables between the adjuvant RT and observation groups in cohort 1 at baseline, including in Charlson/Deyo comorbidity score, primary site, grade, tumor size, age, distance from home zip code to facility, and median follow-up interval (Table 1). The median follow-up period for all patients was 53 months (IQR: 24.1-92). Similar imbalances between groups were found for cohorts 2, 3, and 4 at baseline. For patients who received adjuvant RT in cohort 1, the median RT dose was 54 Gy (IQR: 54-59.4 Gy).
Table 1.
Patient and tumor characteristics for unmatched and propensity score matched cohort 1
| Unmatched Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Variable | Observation No. (%) or Median (IQR) | Adjuvant RT No. (%) or Median (IQR) | P | Observation No. (%) or Median (IQR) | Adjuvant RT No. (%) or Median (IQR) | P |
| No. | 931 (52.1%) | 856 (47.9%) | 672 | 672 | ||
| Facility type | .08 | .99 | ||||
| Community | 20 (2.1%) | 17 (2%) | 15 (2.2%) | 13 (1.9%) | ||
| Comprehensive community | 199 (21.4%) | 162 (18.9%) | 133 (19.8%) | 138 (20.5%) | ||
| Academic/research | 346 (37.2%) | 283 (33.1%) | 222 (33%) | 221 (32.9%) | ||
| Integrated network | 74 (7.9%) | 83 (9.7%) | 63 (9.4%) | 65 (9.7%) | ||
| Unknown | 292 (31.4%) | 311 (36.3%) | 239 (35.6%) | 235 (35%) | ||
| Sex | .15 | .55 | ||||
| Male | 518 (55.6%) | 447 (52.2%) | 348 (51.8%) | 359 (53.4%) | ||
| Female | 413 (44.4%) | 409 (47.8%) | 324 (48.2%) | 313 (46.6%) | ||
| Race | .28 | .84 | ||||
| White | 801 (86%) | 718 (83.9%) | 566 (84.2%) | 564 (83.9%) | ||
| Black | 82 (8.8%) | 79 (9.2%) | 65 (9.7%) | 62 (9.2%) | ||
| Other | 48 (5.2%) | 59 (6.9%) | 41 (6.1%) | 46 (6.8%) | ||
| Insurance status | .07 | .83 | ||||
| No insurance | 45 (4.8%) | 53 (6.2%) | 41 (6.1%) | 41 (6.1%) | ||
| Private | 563 (60.5%) | 553 (64.6%) | 423 (62.9%) | 435 (64.7%) | ||
| Government | 304 (32.7%) | 238 (27.8%) | 199 (29.6%) | 185 (27.5%) | ||
| Unknown | 19 (2%) | 12 (1.4%) | 9 (1.3%) | 11 (1.6%) | ||
| Median income by zip code | .3 | .98 | ||||
| <$38 000 | 145 (15.6%) | 125 (14.6%) | 102 (15.2%) | 99 (14.7%) | ||
| $38 000-$47 999 | 217 (23.3%) | 201 (23.5%) | 166 (24.7%) | 162 (24.1%) | ||
| $48 000-$62 999 | 264 (28.4%) | 217 (25.4%) | 171 (25.4%) | 171 (25.4%) | ||
| ≥$63 000 | 305 (32.8%) | 313 (36.6%) | 233 (34.7%) | 240 (35.7%) | ||
| No high school diploma by zip code, % | .28 | .8 | ||||
| ≥ 21 | 161 (17.3%) | 147 (17.2%) | 120 (17.9%) | 120 (17.9%) | ||
| 13-20.9 | 234 (25.1%) | 197 (23%) | 156 (23.2%) | 156 (23.2%) | ||
| 7-12.9 | 301 (32.3%) | 262 (30.6%) | 215 (32%) | 201 (29.9%) | ||
| < 7 | 235 (25.2%) | 250 (29.2%) | 181 (26.9%) | 195 (29%) | ||
| Facility setting | .12 | .87 | ||||
| Metro | 741 (79.6%) | 699 (81.7%) | 543 (80.8%) | 545 (81.1%) | ||
| Urban | 132 (14.2%) | 125 (14.6%) | 102 (15.2%) | 100 (14.9%) | ||
| Rural | 21 (3.3%) | 18 (2.1%) | 17 (2.5%) | 14 (2.1%) | ||
| Unknown | 27 (2.9%) | 14 (1.6%) | 10 (1.5%) | 13 (1.9%) | ||
| Charlson/Deyo comorbidity | .001 | .83 | ||||
| 0 | 733 (78.7%) | 719 (84%) | 563 (83.8%) | 566 (84.2%) | ||
| 1 | 147 (15.8%) | 85 (9.9%) | 73 (10.9%) | 67 (10%) | ||
| ≥ 2 | 51 (5.5%) | 52 (6.1%) | 36 (5.4%) | 39 (5.8%) | ||
| Year of diagnosis | .29 | .89 | ||||
| 2004-2006 | 219 (23.5%) | 191 (22.3%) | 151 (22.5%) | 157 (23.4%) | ||
| 2007-2009 | 249 (26.7%) | 209 (24.4%) | 178 (26.5%) | 179 (26.6%) | ||
| 2010-2012 | 247 (26.5%) | 225 (26.3%) | 179 (26.6%) | 167 (24.9%) | ||
| 2013-2015 | 216 (23.2%) | 231 (27%) | 164 (24.4%) | 169 (25.1%) | ||
| Tumor location | .04 | .54 | ||||
| Supratentorial | 563 (60.5%) | 559 (65.3%) | 408 (60.7%) | 419 (62.4%) | ||
| Infratentorial | 368 (39.5%) | 297 (34.7%) | 264 (39.3%) | 253 (37.6%) | ||
| Tumor grade | < .001 | .23 | ||||
| 2 | 861 (92.5%) | 610 (71.3%) | 602 (89.6%) | 588 (87.5%) | ||
| 3 | 70 (7.5%) | 246 (28.7%) | 70 (10.4%) | 84 (12.5%) | ||
| Tumor size, cm | < .001 | .14 | ||||
| ≤ 4 | 434 (46.6%) | 351 (41%) | 321 (47.8%) | 299 (44.5%) | ||
| > 4 | 197 (21.2%) | 314 (36.7%) | 180 (26.8%) | 213 (31.7%) | ||
| Unknown | 300 (32.2%) | 191 (22.3%) | 171 (25.4%) | 160 (23.8%) | ||
| Extent of resection | .26 | .64 | ||||
| Subtotal | 213 (22.9%) | 221 (25.8%) | 168 (25%) | 173 (25.7%) | ||
| Gross total | 246 (26.4%) | 230 (26.9%) | 173 (25.7%) | 158 (23.5%) | ||
| Unknown | 472 (50.7%) | 405 (47.3%) | 331 (49.3%) | 341 (50.7%) | ||
| Age, y | 50 (37-62) | 45 (34-58) | < .001 | 47 (34-58) | 46 (34-58) | .77 |
| Distance to facility, miles | 20.4 (7.6-50.6) | 14.2 (6.1-33.6) | < .001 | 17.3 (7-41.9) | 14.3 (6.4-34.6) | .05 |
| Median follow-up, mo | 57.5 (26.2-94.6) | 50.5 (21.7-86.5) | .01 | 57 (26.5-93.2) | 55.6 (24.1-94.3) | .61 |
Abbreviations: IQ, interquartile range; RT, radiotherapy.
Survival Analysis in Cohort 1 (All Patients)
The multivariate model for OS in the unmatched cohort 1 demonstrated older age, male sex, urban vs metro location, higher Charlson/Deyo comorbidity score, earlier year of diagnosis, and grade 3 were significantly associated with increased risk of death (Table 2). Adjuvant RT was not associated with OS (HR: 1.19, P = .11).
Table 2.
Multivariate analysis of overall survival in unmatched cohort 1 (n = 1787)
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age, continuous | 1.04 | 1.03-1.04 | <.001 |
| Sex (female vs male) | 0.69 | 0.56-0.84 | <.001 |
| Insurance status | |||
| No insurance | Reference | ||
| Private | 0.7 | 0.44-1.13 | .14 |
| Government | 1.08 | 0.66-1.76 | .77 |
| Unknown | 1.56 | 0.76-3.2 | .22 |
| Facility setting: | |||
| Metro | Reference | ||
| Urban | 1.3 | 1.01-1.67 | .04 |
| Rural | 1.4 | 0.89-2.19 | .14 |
| Unknown | 1.48 | 0.84-2.61 | .17 |
| Charlson/Deyo comorbidity | |||
| 0 | Reference | ||
| 1 | 1.6 | 1.23-2.08 | <.001 |
| ≥ 2 | 1.72 | 1.24-2.37 | .001 |
| Year of diagnosis, continuous | 0.96 | 0.93-0.99 | .01 |
| Tumor grade (3 vs 2) | 4.4 | 3.52-5.5 | <.001 |
| Adjuvant therapy (adjuvant RT vs observation) | 1.19 | 0.96-1.47 | .11 |
Abbreviation: RT, radiotherapy.
Propensity score matching yielded 672 pairs (n = 1344) in the matched cohort 1. All variables were well balanced in the matched cohort 1 except for longer median distance from home zip code to facility in the observation group (median 17.3 vs 14.3 miles, P = .05, Table 1). The 3- and 5-year OS with adjuvant RT was 83.4% and 79.3% compared with 86.4% and 81.8% with observation. Adjuvant RT was not associated with OS in the matched cohort 1 (HR: 1.15, 95% CI: 0.86-1.53, P = .35).
Survival Analysis in Cohort 2 (Grade 2)
Propensity score matching yielded 569 pairs (n = 1138) in the matched cohort 2. All variables were well balanced except for longer median distance from home zip code to facility in the observation group (median 18 vs 14.2 miles, P = 0.01, Supplemental Table 1). For patients who received adjuvant RT in the matched cohort 2, the median RT dose was 54 Gy (IQR: 54-55.8 Gy). The 3- and 5-year OS with adjuvant RT was 86.3% and 82.4% compared with 90.6% and 88.4% with observation. Adjuvant RT was not associated with OS in the matched cohort 2 (HR: 1.3, 95% CI: 0.93-1.82, P = .13).
Survival Analysis in Cohort 3 (Grade 2 Status Post–Subtotal Resection)
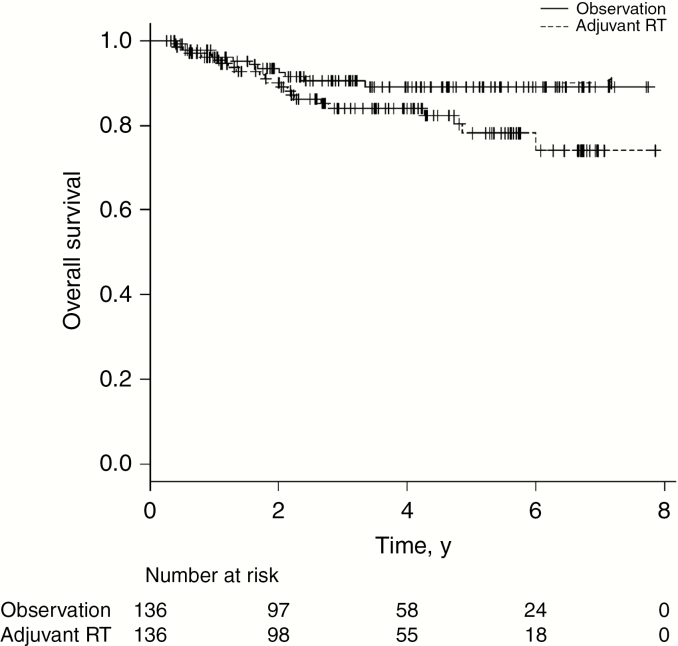
Propensity score matching yielded 136 pairs (n = 272) in the matched cohort 3. All variables were well balanced (Supplemental Table 2). For patients who received adjuvant RT in the matched cohort 3, the median RT dose was 54 Gy (IQR: 54-59.4 Gy). The 3- and 5-year OS with adjuvant RT was 84.1% and 78.1% compared with 90.4% and 89.1% with observation (Fig. 2). Adjuvant RT was not associated with OS in the matched cohort 3 (HR: 1.63, 95% CI: 0.67-3.92, P = .28).
Fig. 2.
Overall survival in propensity score matched cohort 3 (grade 2 ependymoma status post–subtotal resection) by receipt of adjuvant radiotherapy (RT)
As part of a sensitivity analysis, we also categorized adjuvant RT by RT dose (45-53.9 Gy, 54-59.3 Gy, and 59.4-66 Gy) in the propensity score matched cohorts 1 and 3 and there was no statistically significant association between receipt of adjuvant RT at any specific dose level and OS in these analyses (data not shown).
Survival Analysis in Cohort 4 (Grade 3)
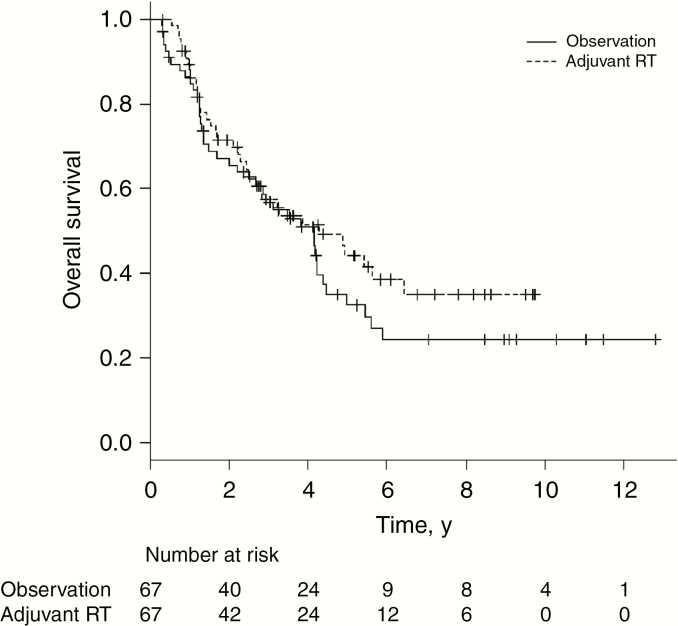
Adjuvant RT was administered to 246 of 316 patients (78%) in the unmatched cohort 4. Sample size was limited in this analysis because of the relatively small number of patients who were observed. We conducted a multivariate analysis in the unmatched cohort 4 in addition to propensity score matching to evaluate prognostic factors within this specific cohort and make full use of the data for the adjuvant RT analysis given the limited patient numbers. Multivariate analysis demonstrated significantly higher risk of death for older age, male sex, no insurance, supratentorial location, and STR. Receipt of adjuvant RT was not associated with OS (HR: 0.84, 95% CI: 0.58-1.22, P = .36, Supplemental Table 3). Propensity score matching yielded 67 pairs (n = 134) in the matched cohort 4. All variables were well balanced (Supplemental Table 4). For patients who received adjuvant RT in the matched cohort 4, the median RT dose was 59.4 Gy (IQR 59.2-60 Gy). The 3- and 5-year OS with adjuvant RT was 57.4% and 44.2% compared with 56.8% and 32.5% with observation (Fig. 3). Adjuvant RT was not associated with OS in the matched cohort 4 (HR: 0.79, 95% CI: 0.49-1.26, P = .32).
Fig. 3.
Overall survival in propensity score matched cohort 4 (grade 3 ependymoma) by receipt of adjuvant radiotherapy (RT)
Discussion
This NCDB retrospective cohort study included a large population of adult patients with intracranial ependymoma with the goal of determining the effect of adjuvant RT on OS. We were not able to demonstrate an association between adjuvant RT and OS in the overall cohort or in the restricted cohorts of grade 2 ependymoma, grade 2 ependymoma status post-STR, or grade 3 ependymoma. Multivariate analysis did demonstrate several predictor variables that were significantly associated with worse OS, including older age, male sex, earlier year of diagnosis, and grade 3 histology. Within the grade 3 cohort, supratentorial location and STR were also statistically significantly associated with increased risk of death.
Owing to the rarity of the disease, the literature regarding adjuvant therapy for adult intracranial ependymoma is limited to retrospective single- or multi-institutional studies and population-based studies that demonstrate variable results as to the efficacy of adjuvant RT. The consensus guidelines for adjuvant RT reflects this with conflicting recommendations between societies based on generally low-rated evidence. The EANO guidelines recommend observation for adults with grade 2 intracranial ependymoma status post-GTR and adjuvant RT for patients with grade 2 ependymoma status post-STR or grade 3 regardless of extent of resection.17 However, the National Comprehensive Cancer Network recommends adjuvant RT for grade 2 or grade 3 intracranial ependymoma regardless of extent of resection.22
There is mixed evidence regarding the role of adjuvant RT in this population. Metellus et al demonstrated no difference in OS or progression-free survival (PFS) based on receipt of adjuvant RT in 114 adults with grade 2 intracranial ependymoma both in univariate and multivariate analyses, but there was a significant benefit in both end points in univariate analysis within the STR subgroup.10 Dützmann and colleagues studied 33 adult patients with grade 2 intracranial ependymoma, and adjuvant RT was not associated with PFS or OS in the overall cohort or in the STR subgroup.12 The largest multi-institutional retrospective study (Vera-Bolanos et al) included 153 adults with intracranial ependymoma. They found that adjuvant RT was associated with statistically significantly improved PFS regardless of extent of resection primarily for patients with infratentorial tumor location adjusting for age and grade.13 OS was not reported in their study.
Our study shows no demonstrable OS benefit for adjuvant RT in grade 2 intracranial ependymoma and more specifically in grade 2 ependymoma status post-STR. We also did not demonstrate an OS benefit with adjuvant RT in the grade 3 ependymoma cohort, but that analysis was limited by the high proportion of patients with grade 3 tumors receiving adjuvant RT and resultant small patient numbers in the RT vs observation analysis. Nonetheless, the HR and OS point estimates did favor adjuvant RT in the grade 3 cohort.
A previous study on this subject using the NCDB was published by Nuño et al in 2016.14 That study did not find an OS benefit with adjuvant RT in multivariate analysis or in univariate analyses stratified by grade or extent of resection. Though the results of their study are similar to ours, the differences in patient selection and statistical methodology, and consequently the ability to make conclusions, are significant and worth discussing. First, the patient population used for the OS analysis in the Nuño study were diagnosed between 1998 and 2006.14 The NCDB no longer provides case data from before 2004 because of significant changes in data capture made in 2003 and 2004 that improved the quality of NCDB data, including comorbidity adjustment, required reporting of RT modality and treatment details, and inclusion of staging and site-specific details.18 Second, OS data were available for only approximately 60% of the included cohort (799 of 1318 patients). Patient, tumor, and treatment characteristics at baseline were presented for the larger cohort stratified by tumor grade, but predictor variable balance for the larger cohort or the OS cohort stratified by adjuvant RT vs observation (the association of interest) were not assessed or presented in the paper, which severely limits evaluation of residual confounding. Third, the patient cohort selection as reported by Nuño and colleagues14 did not exclude patients with early mortality, thereby retaining risk of immortal time bias, did not restrict the adjuvant RT group to known and standard RT dosing, thereby potentially including nonstandard or palliative courses in the RT group, which would bias toward the null, and did not report the extent of missing data (ie, tumor size and extent of resection) in the OS cohort and how that was accounted for. Lastly, the Nuño study14 performed only a multivariate analysis in the OS cohort and univariate analyses of adjuvant RT vs observation stratifying by grade and extent of resection. There were no additional efforts to reduce the influence of selection bias and confounding by treatment indication by using other methods such as propensity score matching, as was performed in the present study. For all these reasons, we feel that our study design, statistical analyses, and results are more internally and externally valid compared to the prior NCDB-based study.
The limitations of NCDB-based studies are well known and inherent to their retrospective nature, and include risk of misclassification and miscoding because of the database structure, risk of selection bias and confounding by treatment indication because of nonrandomized treatment assignment, lack of central pathology review, relatively high levels of missing data for certain variables including extent of resection and tumor size (not uncommon in NCDB studies of primary CNS tumors), and lack of recurrence or cancer-specific survival data. Additionally, more granular information on extent of resection including percentage extent of resection, specific definition of GTR used, and postoperative imaging modality used was not available. The high level of missing data for extent of resection is noteworthy and is specific to patients treated before 2010, which is the year the NCDB started regularly capturing extent of resection for patients with primary brain tumors. All patients in cohort 3 (grade 2 status post-STR) were treated on or after 2010 because of the requirement for known extent of resection in this cohort. We used rigorous patient selection criteria to generate a homogeneous and externally valid study population and employed multiple methods of adjustment, including multivariate analysis, propensity score matching, and stratification by grade and extent of resection to minimize risk of confounding. The propensity score matched cohorts 1, 2, and 4 had similar proportions for GTR, STR, and unknown extent of resection between arms. This minimizes bias as much as possible, but there is still residual potential confounding due to the undetermined true extent of resection for patients coded as unknown, which may not be equal between arms. However, all patients in cohort 3 had known extent of resection (all underwent STR) and there was no observed benefit for adjuvant RT in this cohort. An additional strength of this study is the large patient population, which allows for adjusting for multiple covariates and subgroup analyses, not possible with smaller patient cohorts.
Conclusions
This large NCDB observational study did not demonstrate a statistically significant association between adjuvant RT and OS for adults with intracranial ependymoma status postresection. This was also true more specifically for patients with grade 2 ependymoma status post-STR. Older age, male sex, urban vs metro location, higher Charlson/Deyo comorbidity score, earlier year of diagnosis, and grade 3 were statistically significantly associated with increased risk of death. The conflicting evidence in this space highlights the needs for high-quality studies with detailed pathology and treatment information to guide therapy recommendations in adult ependymoma. In the absence of these trials and based on the current literature, we recommend observation for adult patients with grade 2 intracranial ependymoma status post-GTR, consideration and discussion of adjuvant RT for patients with grade 2 tumors status post-STR based on risk factors and patient preference, and adjuvant RT for grade 3 disease regardless of extent of resection.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. None declared.
References
- 1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl 4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3. Villano JL, Parker CK, Dolecek TA. Descriptive epidemiology of ependymal tumours in the United States. Br J Cancer. 2013;108(11):2367–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a Surveillance, Epidemiology, and End Results study. J Neurosurg. 2009;110(4):725–729. [DOI] [PubMed] [Google Scholar]
- 5. Wu J, Armstrong TS, Gilbert MR. Biology and management of ependymomas. Neuro Oncol. 2016;18(7):902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parker M, Mohankumar KM, Punchihewa C, et al. C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature. 2014;506(7489):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J Clin Oncol. 2016;34(21):2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merchant TE. Current clinical challenges in childhood ependymoma: a focused review. J Clin Oncol. 2017;35(21):2364–2369. [DOI] [PubMed] [Google Scholar]
- 10. Metellus P, Guyotat J, Chinot O, et al. Adult intracranial WHO grade II ependymomas: long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro Oncol. 2010;12(9):976–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR.. Adult ependymal tumors: prognosis and the M.D. Anderson Cancer Center experience. Neuro Oncol. 2010;12(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dützmann S, Schatlo B, Lobrinus A, et al. A multi-center retrospective analysis of treatment effects and quality of life in adult patients with cranial ependymomas. J Neurooncol. 2013;114(3):319–327. [DOI] [PubMed] [Google Scholar]
- 13. Vera-Bolanos E, Aldape K, Yuan Y, et al. ; CERN Foundation Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015;17(3):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nuño M, Yu JJ, Varshneya K, et al. Treatment and survival of supratentorial and posterior fossa ependymomas in adults. J Clin Neurosci. 2016;28:24–30. [DOI] [PubMed] [Google Scholar]
- 15. Amirian ES, Armstrong TS, Gilbert MR, Scheurer ME.. Predictors of survival among older adults with ependymoma. J Neurooncol. 2012;107(1):183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amirian ES, Armstrong TS, Aldape KD, Gilbert MR, Scheurer ME. Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology. 2012;39(2):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rudà R, Reifenberger G, Frappaz D, et al. EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol. 2018;20(4):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 19. Merchant TE, Bendel AE, Sabin ND, et al. Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J Clin Oncol. 2019;37(12):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park HS, Gross CP, Makarov DV, Yu JB.. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(5):1365–1373. [DOI] [PubMed] [Google Scholar]
- 21. Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA.. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Comprehensive Cancer Network. Central nervous system cancers version 1.2019. 2019. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed May 31, 2019. [DOI] [PubMed] [Google Scholar]
- 23. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kann BH, Lester-Coll NH, Park HS, et al. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2017;19(2):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kann BH, Park HS, Lester-Coll NH, et al. Postoperative radiotherapy patterns of care and survival implications for medulloblastoma in young children. JAMA Oncol. 2016;2(12):1574–1581. [DOI] [PubMed] [Google Scholar]
- 26. Haque W, Verma V, Butler EB, Teh BS.. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol. 2017;133(2):369–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.