Abstract
Purpose
Neonatal hypoxic ischemic encephalopathy (HIE) is an essential factor underlying neonatal death and disability. This study sought to explore the role of miR-146b-5p in regulating neonatal HIE.
Materials and Methods
In vitro and in vivo HIE models were established in PC12 cells and 10-day neonatal Sprague Dawley rats, respectively. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to assess miR-146b-5p expression and inflammatory factors [interleukin (IL)-6 and tumor necrosis factor (TNF)-α] in brain lesions and PC12 cells, while enzyme-linked immunosorbent assay was employed to detect the expression of oxidative stress factors (SOD and GSH-Px). Gain- and loss-assays of miR-146b-5p were conducted to verify its role in modulating the viability and apoptosis of PC12 cells under oxygen-glucose deprivation (OGD) treatment. Expression of TLR4, IRAK1, TRAF6, TAK1, and NF-κB were examined by qRT-PCR and/or Western blot. Dual luciferase activity assay was conducted to identify relationships between miR-146b-5p and IRAK1.
Results
In the HIE models, significant oxidative stress and inflammatory responses emerged upon upregulation of TLR4/IRAK1/TRAF6/TAK1/NF-κB signaling. Overexpression of miR-146b-5p greatly inhibited OGD-induced PC12 cell injury, inflammatory responses, and oxidative stress. Inhibiting miR-146b-5p, however, had the opposite effects. IRAK1 was found to be a target of miR-146b-5p, and miR-146b-5p overexpression suppressed the activation of IRAK1/TRAF6/TAK1/NF-κB signaling.
Conclusion
This study demonstrated that miR-146b-5p overexpression alleviates HIE-induced neuron injury by inhibiting the IRAK1/TRAF6/TAK1/NF-κB pathway.
Keywords: Neonatal hypoxic ischemic encephalopathy, miR-146b-5p, inflammation, oxidative stress
INTRODUCTION
Neonatal hypoxic ischemic encephalopathy (HIE) refers to a series of clinical encephalopathy manifestations resulting from fetus or neonatal hypoxic ischemic brain damage (HIBD). The damage, attributed to perinatal asphyxia, has been regarded as a common cause of neonatal death or motor dysfunction.1,2 Multiple factors are involved in mediating neuron death after hypoxic ischemic brain injury, including overexpression of apoptosis-related proteins and the activation of inflammatory cells and proteins.3 The inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α, in particular, act as crucial components in neuron apoptosis and death upon hypoxic ischemic insult.4,5 Accordingly, exploring the molecular mechanism underlying HIBD could help in the development of therapeutic targets for the treatment of hypoxic ischemic brain injury.
MicroRNAs (miRNAs) belong to a category of small non-coding RNAs that regulate many biological processes, including cell proliferation, apoptosis, and inflammation, by targeting a wide range of mRNAs.6,7 In addition, miRNAs are considered to be diagnostic biomarkers of human inflammation. They function by either promoting or inhibiting inflammation: for example, miR-1 aggravates endothelial induced inflammation.8 miR-101-3p is dysregulated and plays a role in synovial fibroblast-like cells in patients with rheumatoid arthritis.9 Interestingly, many miRNAs have been shown to have regulatory effects on HIE. For instance, miR-499-5p exhibits neuroprotective effects on HIE in neonatal rats by blocking C-reactive protein.10 miR-210 modulates microglial activation and regulates microglial-mediated neuroinflammation in HIE.11 miR-146b-5p has been found to exert actions in multiple diseases, including papillary thyroid cancer,12 atherosclerosis,13 and obesity.14 However, the role of miRNAs in HIBD remains unclear.
Interleukin-1 receptor-associated kinase 1 (IRAK1) is a serine/threonine protein kinase associated with IL-1R and toll-like receptor (TLR) signal transduction, exercising paramount influence in innate immunity and inflammatory diseases.15,16 Research has demonstrated that miRNAs are able to modulate a variety of inflammatory diseases by regulating the expression of IRAK1.17 For example, through targeting IRAK1 in MRC-5 cells, miRNA-206 promotes lipopolysaccharide-induced inflammatory injury.18 Additionally, miRNA-146a suppresses nuclear factor kappa B (NF-κB) activation and the production of proinflammatory cytokines by regulating IRAK1 expression in THP-1 cells.19 miRNAs also take part in HIE by regulating similar signaling pathways. For instance, miR-146b-5p prevents oligodendrocyte precursor cells from oxygen-glucose deprivation (OGD)-induced damage by targeting Brd4.20 Nevertheless, studies have yet to investigate whether miR-146b-5p regulates HIE inflammatory responses by targeting IRAK1.
Here, we report the down-regulated of miR-146b-5p and the up-regulation of IRAK1 in the brain lesions of HIBD rat pups and PC12 cells under OGD. Functionally, miR-146b-5p overexpression inhibited OGD-induced PC12 cell injury and attenuated inflammation and oxidative stress following OGD. Mechanism studies indicated that IRAK1 is an important target of miR-146b-5p and that miR-146b-5p overexpression conspicuously inhibits the activation of IRAK1, TNF receptor-associated factor 6 (TRAF6), transforming growth factor beta-activated kinase 1 (TAK1), and NF-κB. Collectively, this study demonstrates that miR-146b-5p overexpression ameliorates HIBD by suppressing IRAK1/TRAF6/TAK1/NF-κB signaling.
MATERIALS AND METHODS
Cell culture and OGD cell model
The neuronal cell line PC12 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The PC12 cells were cultured in DMEM high glucose medium containing 10% fetal bovine serum (FBS) and 10% horse serum at 5% CO2 and 37℃. Cells in the logarithmic growth phase were trypsinized using 0.25% trypsin (Beyotime Biotechnology, Shanghai, China). For OGD treatment, the cells were cultured in glucose free DMEM medium containing 10% FBS in an incubator for 12 hours at 2% O2 and 37℃.
Cell transfection
MiR-146b-5p mimics, inhibitors, and their negative controls (miR-NC or NC-in) were obtained from GenePharma (Shanghai, China). A total of 1×105 PC12 cells were seeded in 24-well plates. The cells were transfected with the above vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. After 24 hours of transfection, the medium was exchanged to normal culture medium.
HIE model with rat pups
A modified Rice-Vannucci hypoxia-ischemia model was developed using rat pups at the seventh day after birth (P7) as previously described.21 Briefly, the rat pups were anesthetized with inhalation of isoflurane (5% for induction and 2–3% for maintenance), and the right common carotid artery (CCA) was permanently occluded with 8/0 silk surgical suture. After surgery, pups recovered at 37℃ for 1 h and then placed in a hypoxic incubator containing humidified 8% oxygen balanced with 92% nitrogen at 37℃ for 2.5 h. At the end of hypoxia, the pups were returned to their dams. For the sham group, the right CCA was exposed but not ligated, and the pups were not exposed to hypoxia treatment. The animal research was approved by the Animal Ethics Committee of Shanxi Children's Hospital and performed in line with guidelines on the protection and use of experimental animals at Shanxi Children's Hospital. Our study was approved by the Ethics Review Board of Shanxi Medical University (IRB No. 2016LL083).
Immunohistochemistry
The brain tissues of the rats were fixed in 10% formaldehyde and embedded in paraffin. Next, citrate buffer (10 mM sodium citrate 0.05% tween, pH 6) was used to restore the brain sections for 20 min at 96℃. Afterwards, the sections were incubated with endogenous peroxidase blocking buffer [70% methanol and 3% hydrogen peroxide (H2O2)] for 15 min. Then, the slices were blocked in 0.5% bovine serum albumin with 0.05 M trisbuffered saline and 0.5% triton for at least 1 hour. IRAK1 antibody (1:200, Abcam, ab238, MA, USA) and Caspase-3 antibody (1:150, Abcam, ab13847, MA, USA) were incubated with the sections at 4℃ overnight. The following day, after being washed with PBS buffer, the sections were incubated first with secondary antibodies for 1 hour at room temperature and then with horseradish peroxidase (HRP)-coupled streptavidin. Finally, we sealed the sections for microscope observation. Five high power (×400) fields of view were randomly selected, and the exact number of positive cells in each section were counted.
ELISA
At 72 hours after HIE surgery, the brain tissues in the rats were collected, cut into pieces, and mixed in 0.9% physiological saline. The mixture was centrifuged at 6000 r/min (15 min, 4℃), and the supernatant was retrieved. The contents of IL-6, TNF-α, SOD, and GSH-Px in the supernatant were determined according to the manufacturer's instructions included with the ELISA kits (Nanjing Jiancheng Bioengineering Institute).
qRT-PCR
Total RNA was extracted from cells or brain tissues using TRIzol (Invitrogen). Total RNA was reversely transcribed into cDNA with the RNA First Strand cDNA Synthesis Kit (Sangon Biotech, Shanghai, China). PCR was conducted using SYBR Prime Script RT-PCR kits (Invitrogen). The primers were synthesized by Sangon Biotech Co., Ltd. GAPDH and U6 were used as the internal references for IRAK1 and miR-146b-5p, respectively. The primers sequences are as follows: miR-146b-5p, forward: 5′-ATGCGCGCTGAGAACTGAATT-3′, reverse: 5′-CAGTGCAGGGTCCGAGGT-3′; IRAK1, forward: 5′- CCTCCAGGTTCCACTCTCTG-3′, reverse: 5′-AACCACCCTCTCCAATCCTG-3′;GAPDH, forward: 5′-AACGGATTTGGTCGTATTG-3′;GAPDH, reverse: 5′-GGAAGATGGTGATGGGATT- 3′. U6, forward: 5′-GCTTCGGCACATATACTAAAAT-3′, reverse: 5′- CGCTTCACGAATTTGCGTGTCAT-3′.
Western blot
After cell treatments were finished, the cells were collected, and the total protein in the cells was isolated by RIPA lysate (Roche, Basel, Switzerland). Afterwards, 50 µg of total protein was loaded on a 12% sodium dodecyl sulfate-polyacrylamide gel and electrophoresed for 2 h at 100 V. Then, the protein was electrically transferred to polyvinylidene fluoride membranes. After being blocked with 5% skimmed milk powder for 1 h at room temperature, the membranes were washed three times with TBST (10 min each time) and incubated with primary antibodies against IRAK1 (Abcam, ab238, 1:1000), NF-κB (phospho S536) (Abcam, ab86299, 1:1000), NF-κB (Abcam, ab16502, 1:1000), TRAF6 (Abcam, ab33915, 1:1000), TAK1 (Abcam, ab109526, 1:1000), TLR4 (Abcam, ab22048, 1:1000), Caspase3 (Abcam, ab13847, 1:1000), and Bax (Abcam, ab32503, 1:1000) (concentration 1: 1000) at 4℃ overnight. Next, the membranes were again washed with TBST and incubated with HRP-labeled anti-rabbit secondary antibodies (concentration 1: 3000) at room temperature for 1 h. After another washing with TBST, the Novex™ ECL Chemiluminescent Substrate Reagent Kit (Invitrogen) was utilized for bands imaging, and the gray values of each protein were analyzed using Image J software.
CCK 8 assay
Cells in the logarithmic growth phase in each group were subjected to trypsinization, centrifugation, and counting. They were then inoculated into 96-well plates at a density of 2×104/mL each well. The culture solution was removed 24 hours later, and 10 µL of CCK-8 solution (Beyotime Biotechnology) was added to each well. Next, the cells were incubated at 37℃ for another 1 h. Finally, absorbance at 450 nm was examined on a microplate reader. Four parallel wells were set in each group, and the experiments were repeated three times.
Flow cytometry
Cells that did not undergo CCK-8 assay were trypsinized and collected by centrifugation (1500 r/min, 3 min). The cells were the examined in accordance with the instructions of the apoptosis detection kit (Shanghai Aladdin Biological Reagent Co., Ltd., Shanghai, China). Briefly, the cells were washed twice wash with PBS, after which 400 µL of pre-chilled PBS, 10 µL of Annexin V-FITC and 5 µL of propidium iodide were added to the cell samples. After incubation for 30 min in the dark at 4℃, cell apoptosis was immediately measured via flow cytometry. The percentage of apoptotic cells was acquired using computer algorithms.
Luciferase reporter assay
Amplified DNA sequences were cloned into pmirGLO dual luciferase vectors (Promega, Madison, WI, USA) to form wild-type (WT) IRAK1 and mutant (MT) IRAK1 reporter vectors. PC12 cells were seeded in 24-well plates and incubated overnight. Then, we transfected IRAK1-WT or IRAK1-MUT reporter vectors and miR-146b-5p mimics or negative control with Lipofectamine 2000 (Invitrogen). At 48 hours after transfection, the luciferase activity was measured using a dual luciferase reporter gene assay system (Promega).
Statistical analysis
SPSS software (version 20.0, IBM Corp., Armonk, NY, USA) was used for statistical analysis. All data are reported as a mean±standard deviation (x±s). Statistical analysis was carried out using Student's t-test. One-way analysis of variance was used to analyze normally distributed data among multiple groups. p values <0.05 were regarded as statistically significant.
RESULTS
miR-146b-5p is down-regulated in HIBD
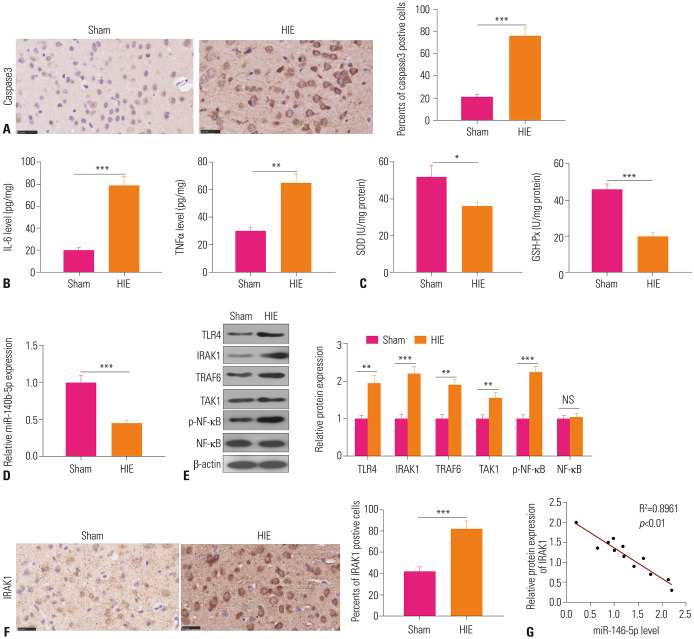
To explore the function of miR-146b-5p in HIBD, we established an HIE model in rat pups. First, histopathological changes in the HIE brain tissues were detected. Staining for cells expressing Caspase-3 revealed significant increases therein in HIE brain tissues, compared with tissues from the Sham group (Fig. 1A). ELISA assay was conducted to detect the expression of inflammatory and oxidative stress factors in the control and HIE groups. The results revealed up-regulated expression of inflammatory factors IL-6 and TNF-α (Fig. 1B) and down-regulated expression of oxidative stress factors SOD and GSH-Px in the HIE group (Fig. 1C). qRT-PCR significant downregulation of miR-146b-5p in the HIE group (Fig. 1D). The results of Western blot and immunohistochemistry showed higher expressions of TLR4, IRAK1, TRAF6, TAK1, and p-NF-κB in the HIE group than the control group (Fig. 1E and F). Linear regression analysis suggested that miR-146b-5p expression is negatively correlated with IRAK1 expression (Fig. 1G). Collectively, these results indicated that miR-146b-5p might be involved in regulating HIE-induced inflammation and oxidative stress.
Fig. 1. miR-146b-5p down-regulated in HIE model. (A) Brain tissue necrosis in HIE rats was evaluated via Caspase-3 immunohistochemistry staining. (B) The expressions of inflammatory cytokines IL-6 and TNF-α were measured in the control group and HIE group via ELISA. (C) The oxidative stress factors SOD and GSH-Px levels were estimated via ELISA. (D) qRT-PCR was used to detect miR-146b-5p expression in the brain lesions. (E) Relative expressions of TLR4, IRAK1, TRAF6, TAK1, and NF-κB were measured via Western blot. (F) Immunohistochemistry staining was used to detect IRAK1 expression in the brain tissues. (G) The correlation between IRAK1 and miR-146b-5p in rat brain tissues was analyzed by linear regression analysis. *p<0.05, **p<0.01, ***p<0.001. HIE, hypoxic ischemic encephalopathy; IL, interleukin; TNF-α, tumor necrosis factor.
miR-146b-5p overexpression attenuates OGD-induced PC12 cell damage
Allowing for the downregulation of miR-146b-5p in the HIE model, we developed an in vitro model of neuronal injury in PC12 cells by OGD. Similar to the in vivo experiments, expression of miR-146b-5p was lower in PC12 cells upon OGD insult (Fig. 2A). Next, we selectively regulated the levels of miR-146b-5p in PC12 cells using mimics and inhibitors (Fig. 2B). Additionally, CCK8 assay, flow cytometry, and Western blot were conducted to evaluate cell viability and apoptosis, respectively. The results demonstrated that OGD leads to marked decrease in cell viability and an increase in apoptosis (Fig. 2C–E). Overexpressing miR-146b-5p, however, reversed these features, while inhibiting miR-146b-5p decreased cell viability and promoted apoptosis (Fig. 2C–E). Overall, these data indicated that miR-146b-5p overexpression attenuates OGD-induced PC12 cell damage.
Fig. 2. Overexpression of miR-146b-5p attenuates OGD-induced PC12 cell damage. (A) PC12 cells were treated with OGD and the level of miR-146b-5p in the cells were detected by qRT-PCR. (B) Construction of a miR-146b-5p model in OGD-treated PC12 cells in vitro. Relative expression of miR-146b-5p in control group, OGD+NC group, OGD+miR-146b-5p group, OGD+NC-in group, and OGD+miR-146b-5p-in group via qRT-PCR. (C) Cell vitality was measured via CCK8 assay. (D) The apoptosis of PC12 cells was detected by flow cytometry. (E) The apoptotic markers of Caspase-3 and Bax in PC12 cells were detected by Western blot. *p<0.05, **p<0.01, ***p<0.001. OGD, oxygen-glucose deprivation; NC, negative control; FITC, fluorescin isothiocyanate; OD, optical density.
miR-146b-5p overexpression attenuates OGD-induced inflammatory responses and oxidative stress
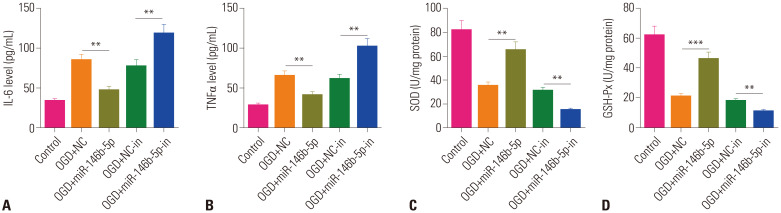
As the inflammatory response and oxidative stress were significant in the HIE model, we further assessed these reactions in PC12 cells under OGD. Therein, overexpression of miR-146b-5p inhibited IL-6 and TNF-α levels, but promoted antioxidative stress factors (SOD and GSH-Px) (Fig. 3). Interestingly, downregulating miR-146b-5p not only enhanced IL-6 and TNF-α expression, but also decreased SOD and GSH-Px. The above results suggested that miR-146b-5p overexpression attenuates OGD-induced inflammatory response and oxidative stress and that the inhibition of miR-146b-5p has the opposite effects.
Fig. 3. Overexpression of miR-146b-5p attenuates OGD-induced inflammatory response and oxidative stress. (A and B) Levels of inflammatory factors IL-6 and TNF-α in the cell supernatant were tested via ELISA. (C and D) Levels of oxidative stress factors SOD and GSH-Px were evaluated by ELISA. **p<0.01, ***p<0.001. IL, interleukin; TNF-α, tumor necrosis factor; OGD, oxygen-glucose deprivation; NC, negative control.
Overexpression of miR-146b-5p attenuates IRAK1/TRAF6/TAK1/NF-κB pathway activation
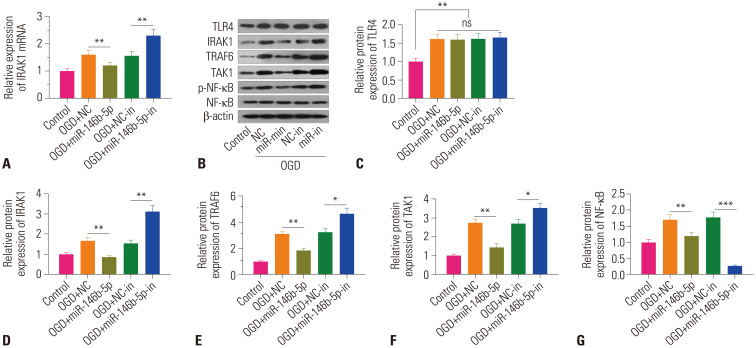
For the purpose of further investigation of the mechanism of miR-146b-5p on OGD-induced HIE, qRT-PCR and Western blot were performed to examine the relative expression of TLR4/IRAK1/TRAF6/TAK1/NF-κB in PC12 cells. The results revealed that IRAK1 mRNA levels in the OGD+miR-146b-5p group were considerably lower than those in the OGD+NC group (Fig. 4A). Similarly, the protein levels of IRAK1, TRAF6, TAK1, and p-NF-κB were also markedly downregulated. While the levels of TLR4, an upstream protein of IRAK1, were increased by OGD treatment, they were not significantly altered in the OGD+miR-146b-5p group, compared with the OGD+NC group (Fig. 4B–G). Also, in the OGD+miR-146b-5p-in group, IRAK1, TRAF6, TAK1, and p-NF-κB levels, but not TLR4, were notably higher than those in the OGD+NC-in group (Fig. 4). Accordingly, we deemed that miR-146b-5p overexpression suppresses IRAK1/TRAF6/TAK1/NF-κB pathway activation.
Fig. 4. MiR-146b-5p overexpression inhibits activation of the IRAK1/TRAF6/TAK1NF-κB pathway. (A) Relative expression of IRAK1 mRNA was determined via qRT-PCR. (B–G) Relative expressions of TLR4, IRAK1, TRAF6, TAK1, phosphorylated (p)-NF-κB, and NF-κB in PC12 cells were examined via Western blot. No significance (ns) p>0.05, *p<0.05, **p<0.01, ***p<0.001. OGD, oxygen-glucose deprivation; NC, negative control.
IRAK1 is a functional target of miR-146b-5p
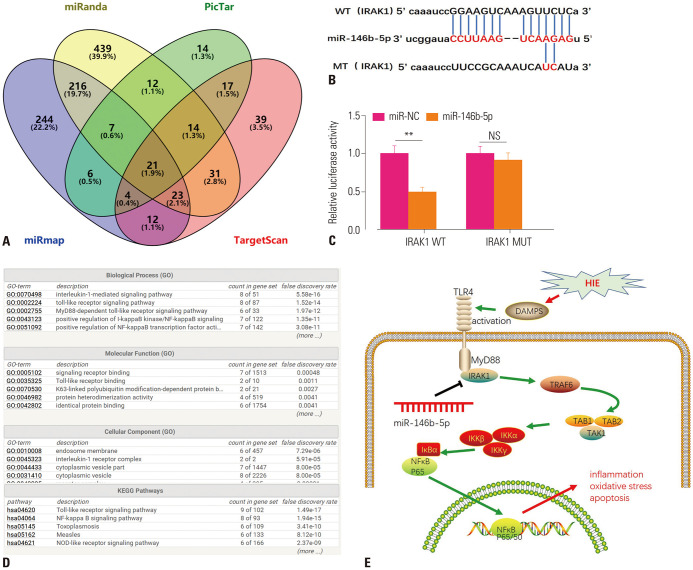
To further study the downstream molecular targets of miR-146b-5p, we analyzed miR-146b-5p using the miRanda, PicTar, miRmap, and TargetScan databases, and Venn Diagrams were drawn to analyze shared targets among the four databases. The results highlighted 21 candidate genes, including IRAK1 (Fig. 5A and B). Next, we verified targeted binding relationships between miR-146b-5p and IRAK1 via the dual luciferase gene reporter method. The results demonstrated that miR-146b-5p mimics significantly reduced the luciferase viability of IRAK1-WT, but not for IRAK1-MT (Fig. 5C). Moreover, we analyzed the biofunctions of IRAK1 through GO and KEGG pathway mapping using String (https://string-db.org/cgi/network). Interestingly, IRAK1 was found to be a vital protein involved in TLR4-MyD88-IRAK1-TRAF6-TAK1-NF-κB signaling (Fig. 5D and E). Overall, these results indicated that miR-146b-5p elicits anti-inflammatory and anti-oxidative stress responses by modulating IRAK1-TRAF6-TAK1-NF-κB signaling.
Fig. 5. MiR-146b-5p, a functional target of IRAK1. (A) Potential targets of miR-146b-5p were analyzed through miRanda, PicTar, miRmap, and TargetScan databases. (B) Binding sites between miR-146b-5p and IRAK1 are shown. (C) Luciferase viability was detected after co-transfection of wild-type IRAK1 or mutant IRAK1 with miR-146b-5p mimics or miR-NC into PC12 cells. (D) Go and KEGG mapping was conducted to predict the regulatory pathway of IRAK1 through String (https://string-db.org/cgi/network). (E) Sketch map of miR-146b-5p on TLR4/IRAK1/TRAF6/TAK1/NF-κB axis-mediated inflammation, oxidative stress, and apoptosis. No significance (ns) p>0.05, **p<0.01.
DISCUSSION
In the present study, we found that miR-146b-5p was downregulated in the brain lesions of HIE rat pups, which was correlated inflammatory and oxidative stress responses. Further exploration indicated that overexpression of miR-146b-5p attenuates OGD-induced PC12 cell damage via restraining the IRAK1-TRAF6-TAK1-NF-κB pathway.
Neonatal HIBD is a comorbid brain disorder caused by neonatal asphyxia, which threatens the life and health of newborns. Accordingly, the search of more effective methods against neonatal HIBD has become a greater focus in perinatal medicine.21,22 In the pathogenesis of HIE, the inflammatory reaction cascade is integral. The expressions of several inflammatory factors, including IL-6, IL-8, and TNF-α, increase greatly after HIE.23 Among these, IL-6 attracts neutrophils to the injured site, resulting in destruction of endothelial cell integrity.24 TNF-α interacts with NF-κB to aggravate the inflammatory response. Eventually, a series of damage, such as blood brain barrier destruction, cerebral edema, thrombosis and bleeding, may occur.25 Meanwhile, dysregulated oxidative stress elicits cytotoxic reactions. In HIE, the production of reactive oxygen species ruins antioxidant defenses, thereby permitting the modification or degeneration of cellular macromolecules, such as membranes, proteins, lipids, and DNA. Moreover, reactive oxygen species can lead to a cascading inflammatory response and protease secretion.26 Here, we constructed a HIE model in rat pups and found observed obvious inflammation and oxidative stress responses.
Increasing studies have demonstrated that miRNAs play a role in HIE. For instance, miR-17-5p was found to be downregulated in neonatal hypoxic-ischemic rat brains, and overexpression of miR-17-5p was shown to significantly relieve brain injury in rats by modulating IRE1α-mediated TXNIP/NLRP3 inflammasome activation.27 Additionally, over-expression of miR-128 has been found to attenuate brain edema and apoptosis of nerve cells in DEX-treated HIBD mice. Mechanistically, miR-128 exerts its neuroprotective effects by inhibiting WNT1.28 Previous studies have found that miR-146b regulates multiple inflammation processes by targeting multiple genes. For example, miR-146b overexpression inhibits the NF-κB activation signaling pathway by suppressing MYD88, thereby reducing the inflammatory factor production in the serum of patients with pneumonia.29 Additionally, miR-146b overexpression inhibits TRAF6, thus reducing inflammatory factor expression to alleviate hypercholesterolemia.30 In the present study, we found that miR-146b-5p was significantly downregulated both in HIE rat pups and OGD-treated PC12 cells. Overexpression of miR-146b-5p not only significantly inhibited OGD-induced PC12 damage, but also attenuated inflammatory and oxidative stress responses. Accordingly, we deemed that miR-146b-5p exerts neuroprotective effects in HIE.
Interestingly, accumulating evidence has proven that many miRNAs are dysregulated in patients with HIE. For example, a clinical study of 78 patients with ischemic stroke indicated that three novel miRNAs (miRNA-221-3p, miRNA-382-5p, and miRNA-4271) are significant biomarkers in the diagnosis of ischemic stroke.31 Another study revealed that miR-125a-5p, miR-125b-5p, and miR-143-3p in the circulatory system serve as promising early diagnostic markers for the patients suffering from acute ischemic stroke.32 Additionally, exosomal microRNA-126 from the venous blood of remote ischemic preconditioning was found to induce hypoxia tolerance of SH-SY5Y cells via targeting DNMT3B.33 Collectively, these studies suggest that miRNAs plays important roles in HIE. Notwithstanding, the clinical therapeutic effects of miRNAs on human diseases have rarely been reported, and the roles of miRNAs have primarily been elucidated using cells or animal models. Thus, there is a tremendous amount of works remaining to be done to clarify the biofunctions of miRNAs in human.
TLR-mediated NF-κB pathway signaling has been found to be activated after HIE.34 As a vital transcription factor, NF-κB is phosphorylated and then penetrates into the nucleus, thus promoting the expression of inflammatory factors. Moreover, multiple drugs have been found to ameliorate HIE by regulating TLR-mediated NF-κB signaling: For instance, tanshinone IIA relieves HIE through TLR-4-mediated NF-κB signaling.35 Similarly, ginkgolide B ameliorates hypoxic-ischemic brain injury in neonatal male rats by dampening NLRP3 inflammasome activation.36 Furthermore, resveratrol mitigates hypoxicischemic induced oxidative stress and brain injury in neonatal rats via Nrf2/HO-1 pathway.37 Presently, to investigate the underlying mechanism of the neuroprotective role of miR-146b-5p in HIE, we conducted bioinformatics analysis and found that IRAK1 was a promising candidate target of miR-146b-5p. Indeed, we found that IRAK1 was upregulated in HIE brain lesions and negatively correlated with the expression of miR-146b-5p. Moreover, we verified that miR-146b-5p targets the 3'UTR of IRAK1 mRNA and inhibits activation of the IRAK1-mediated TRAF6/TAK1NF-κB axis in OGD-treated PC12 cells. Interestingly, previous studies have found that IRAK-1/4 inhibition decreases the nuclear translocation of the NF-κB subunit p65 and protects against acute hypoxia/ischemia-induced neuronal injury in vivo and in vitro.38 Overall, we demonstrated that miR-146b-5p targets IRAK1 and inhibits IRAK1 expression, thereby reducing TRAF6/TAK1NF-κB pathway activation and the expression of inflammatory factors and oxidative stress.
In short, miR-146b-5p overexpression relieves HIE-induced neuronal damage by regulating the TRAF6/TAK1NF-κB signaling pathway by targeting IRAK1. Altogether, in this study, we identified a new regulatory axis of miR-146b-5p/IRAK1/TRAF6/TAK1/NF-κB in HIE, which could be a promising therapeutic target for neuronal HIE.
ACKNOWLEDGEMENTS
The authors appreciate the support from all lab members.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Guang Yang.
- Data curation: Guang Yang and Yuan Zhao.
- Formal analysis: Guang Yang.
- Funding acquisition: Guang Yang.
- Investigation: Guang Yang.
- Methodology: Guang Yang.
- Project administration: Guang Yang.
- Resources: Guang Yang and Yuan Zhao.
- Software: Yuan Zhao.
- Supervision: Guang Yang.
- Validation: Guang Yang.
- Visualization: Yuan Zhao.
- Writing—original draft: Guang Yang.
- Writing—review & editing: Guang Yang.
- Approval of final manuscript: Guang Yang and Yuan Zhao.
References
- 1.Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169:397–403. doi: 10.1001/jamapediatrics.2014.3269. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Concepcion K, Meng X, Zhang L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog Neurobiol. 2017;159:50–68. doi: 10.1016/j.pneurobio.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borjini N, Sivilia S, Giuliani A, Fernandez M, Giardino L, Facchinetti F, et al. Potential biomarkers for neuroinflammation and neurodegeneration at short and long term after neonatal hypoxicischemic insult in rat. J Neuroinflammation. 2019;16:194. doi: 10.1186/s12974-019-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou KQ, Green CR, Bennet L, Gunn AJ, Davidson JO. The role of connexin and pannexin channels in perinatal brain injury and inflammation. Front Physiol. 2019;10:141. doi: 10.3389/fphys.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driscoll DJO', Felice VD, Kenny LC, Boylan GB, O'Keeffe GW. Mild prenatal hypoxia-ischemia leads to social deficits and central and peripheral inflammation in exposed offspring. Brain Behav Immun. 2018;69:418–427. doi: 10.1016/j.bbi.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev. 2013;253:167–184. doi: 10.1111/imr.12050. [DOI] [PubMed] [Google Scholar]
- 7.Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72:156–166. doi: 10.1016/j.jhep.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Wei Q, Lv F, Zhang H, Wang X, Geng Q, Zhang X, et al. MicroRNA-101-3p inhibits fibroblast-like synoviocyte proliferation and inflammation in rheumatoid arthritis by targeting PTGS2. Biosci Rep. 2020;40:BSR20191136. doi: 10.1042/BSR20191136. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Jia H, Qu M, Fan G, Wu H, Wang L. miR-499-5p suppresses C-reactive protein and provides neuroprotection in hypoxic-ischemic encephalopathy in neonatal rat. Neurosci Res. 2019:S0168-0102(19)30370-0. doi: 10.1016/j.neures.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Dasgupta C, Huang L, Meng X, Zhang L. MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol Immunol. 2019 doi: 10.1038/s41423-019-0257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Zhang L, Luo D, Yan F, Liu J, Shao S, et al. MicroRNA-146b-3p promotes cell metastasis by directly targeting NF2 in human papillary thyroid cancer. Thyroid. 2018;28:1627–1641. doi: 10.1089/thy.2017.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin N, An Y. Blockade of 146b-5p promotes inflammation in atherosclerosis-associated foam cell formation by targeting TRAF6. Exp Ther Med. 2017;14:5087–5092. doi: 10.3892/etm.2017.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulsmans M, Van Dooren E, Mathieu C, Holvoet P. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS One. 2012;7:e32794. doi: 10.1371/journal.pone.0032794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degirmenci I, Ozbayer C, Kebapci MN, Kurt H, Colak E, Gunes HV. Common variants of genes encoding TLR4 and TLR4 pathway members TIRAP and IRAK1 are effective on MCP1, IL6, IL1β, and TNFα levels in type 2 diabetes and insulin resistance. Inflamm Res. 2019;68:801–814. doi: 10.1007/s00011-019-01263-7. [DOI] [PubMed] [Google Scholar]
- 16.Inoue M, Arikawa T, Chen YH, Moriwaki Y, Price M, Brown M, et al. T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. Proc Natl Acad Sci U S A. 2014;111:5295–5300. doi: 10.1073/pnas.1321427111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassin C, Hempel C, Stockinger S, Dupont A, Kübler JF, Wedemeyer J, et al. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 2012;4:1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu H, Qu X, Wang F, Chang J, Cheng R, Song X, et al. MicroRNA-206 promotes lipopolysaccharide-induced inflammation injury via regulation of IRAK1 in MRC-5 cells. Int Immunopharmacol. 2019;73:590–598. doi: 10.1016/j.intimp.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Zhao L, Wang K, Qi Q, Wang M, Yang L, et al. MicroRNA-146a inhibits NF-κB activation and pro-inflammatory cytokine production by regulating IRAK1 expression in THP-1 cells. Exp Ther Med. 2019;18:3078–3084. doi: 10.3892/etm.2019.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Zhang W, Xiao M, Wang F, Zhou P, Yang J, et al. MicroRNA-146b-5p protects oligodendrocyte precursor cells from oxygen/glucose deprivation-induced injury through regulating Keap1/Nrf2 signaling via targeting bromodomain-containing protein 4. Biochem Biophys Res Commun. 2019;513:875–882. doi: 10.1016/j.bbrc.2019.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE) Semin Perinatol. 2016;40:549–555. doi: 10.1053/j.semperi.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169:397–403. doi: 10.1001/jamapediatrics.2014.3269. [DOI] [PubMed] [Google Scholar]
- 23.Li SJ, Liu W, Wang JL, Zhang Y, Zhao DJ, Wang TJ, et al. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci. 2014;18:905–909. [PubMed] [Google Scholar]
- 24.Yun JH, Han MH, Jeong HS, Lee DH, Cho CH. Angiopoietin 1 attenuates interleukin-6-induced endothelial cell permeability through SHP-1. Biochem Biophys Res Commun. 2019;518:286–293. doi: 10.1016/j.bbrc.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhuri AD, Dastgheyb RM, Yoo SW, Trout A, Talbot CC, Jr, Hao H, et al. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018;9:363. doi: 10.1038/s41419-018-0369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M, Zhu P, Fujino M, Zhuang J, Guo H, Sheikh I, et al. Oxidative stress in hypoxic-ischemic encephalopathy: molecular mechanisms and therapeutic strategies. Int J Mol Sci. 2016;17:2078. doi: 10.3390/ijms17122078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Dixon BJ, Doycheva DM, Li B, Zhang Y, Hu Q, et al. IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J Neuroinflammation. 2018;15:32. doi: 10.1186/s12974-018-1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang H, Li HF, Yang M, Wang RR, Wang QY, Zheng PC, et al. microRNA-128 enhances neuroprotective effects of dexmedetomidine on neonatal mice with hypoxic-ischemic brain damage by targeting WNT1. Biomed Pharmacother. 2019;113:108671. doi: 10.1016/j.biopha.2019.108671. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Dong L, Tang Y, Li M, Zhang M. MiR-146b protects against the inflammation injury in pediatric pneumonia through MyD88/NF-κB signaling pathway. Infect Dis (Lond) 2020;52:23–32. doi: 10.1080/23744235.2019.1671987. [DOI] [PubMed] [Google Scholar]
- 30.Desjarlais M, Dussault S, Rivard F, Harel S, Sanchez V, Hussain SNA, et al. Forced expression of microRNA-146b reduces TRAF6-dependent inflammation and improves ischemia-induced neovascularization in hypercholesterolemic conditions. Atherosclerosis. 2019;289:73–84. doi: 10.1016/j.atherosclerosis.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Ma Z, Kan P, Zhang B. The diagnostic value of serum miRNA-221-3p, miRNA-382-5p, and miRNA-4271 in ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26:1055–1060. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 32.Tiedt S, Prestel M, Malik R, Schieferdecker N, Duering M, Kautzky V, et al. RNA-Seq identifies circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ Res. 2017;121:970–980. doi: 10.1161/CIRCRESAHA.117.311572. [DOI] [PubMed] [Google Scholar]
- 33.Cui J, Liu N, Chang Z, Gao Y, Bao M, Xie Y, et al. Exosomal microRNA-126 from RIPC serum is involved in hypoxia tolerance in SH-SY5Y cells by downregulating DNMT3B. Mol Ther Nucleic Acids. 2020;20:649–660. doi: 10.1016/j.omtn.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stridh L, Mottahedin A, Johansson ME, Valdez RC, Northington F, Wang X, et al. Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia-ischemia. J Neurosci. 2013;33:12041–12051. doi: 10.1523/JNEUROSCI.0673-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang C, Xie L, Liu C, Fu C, Ye W, Liu H, et al. Tanshinone IIA improves hypoxic ischemic encephalopathy through TLR 4 mediated NF κB signal pathway. Mol Med Rep. 2018;18:1899–1908. doi: 10.3892/mmr.2018.9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen A, Xu Y, Yuan J. Ginkgolide B ameliorates NLRP3 inflammasome activation after hypoxic-ischemic brain injury in the neonatal male rat. Int J Dev Neurosci. 2018;69:106–111. doi: 10.1016/j.ijdevneu.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Fu R, Wang J, Yang X, Wen L, Feng J. Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharm Biol. 2018;56:440–449. doi: 10.1080/13880209.2018.1502326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang YF, Chen Z, Hu SL, Hu J, Li B, Li JT, et al. Interleukin-1 receptor associated kinases-1/4 inhibition protects against acute hypoxia/ischemia-induced neuronal injury in vivo and in vitro. Neuroscience. 2011;196:25–34. doi: 10.1016/j.neuroscience.2011.08.059. [DOI] [PubMed] [Google Scholar]