Abstract
Limbic-predominant age-related TDP-43 encephalopathy (LATE) is a recently established neurodegenerative disease entity. LATE neuropathological change (LATE-NC) is characterized by a TDP-43 proteinopathy that mainly involves the amygdala and medial temporal structures, with or without hippocampal sclerosis. LATE-NC is typically observed in individuals aged 80 years or older and manifests clinically as amnestic memory decline. Herein, we report a case of LATE diagnosed by brain autopsy in an 82-year-old male who had an 11-year history of memory impairment. Pathological examination revealed high Alzheimer disease neuropathological changes, as well as amygdala-predominant Lewy body pathology. In addition, immunohistochemistry for TDP-43 revealed neuronal and glial cytoplasmic inclusions in the dentate gyrus of the hippocampus, amygdala, and inferior temporal cortex. Increasing awareness of the newly defined entity LATE will enhance our understanding of the neurodegenerative processes that occur in the oldest individuals.
Keywords: TDP-43 proteinopathy, limbic system, Alzheimer disease, Lewy body disease, autopsy
INTRODUCTION
Transactive response DNA binding protein of 43 kDa (TDP-43) is a ubiquitinated protein associated with diseases, such as amyotrophic lateral sclerosis and certain variants of frontotemporal lobar degeneration.1,2 TDP-43 proteinopathy is frequently identified in the limbic area in people aged 80 years and older, and is associated with Alzheimer disease neuropathologic changes (ADNC) and/or hippocampal sclerosis.3,4
A new term, limbic-predominant age-related TDP-43 encephalopathy (LATE) has recently been introduced.5 LATE neuropathological change (LATE-NC) is defined pathologically as TDP-43 proteinopathy, in which phosphorylated TDP-43 accumulates in the cytoplasm or nuclei of neurons or in the cytoplasm of glial cells localized primarily to the limbic structures.
The clinical characteristics of LATE include amnestic cognitive decline progressing to advanced cognitive deficits. Individuals with both ADNC and LATE-NC manifest more rapid and severe cognitive impairment than the persons with ‘pure’ LATE-NC.5 Herein, we present the brain autopsy findings of a patient who presented with persistent cognitive decline and was found to have both high ADNC and LATE-NC.
CASE REPORT
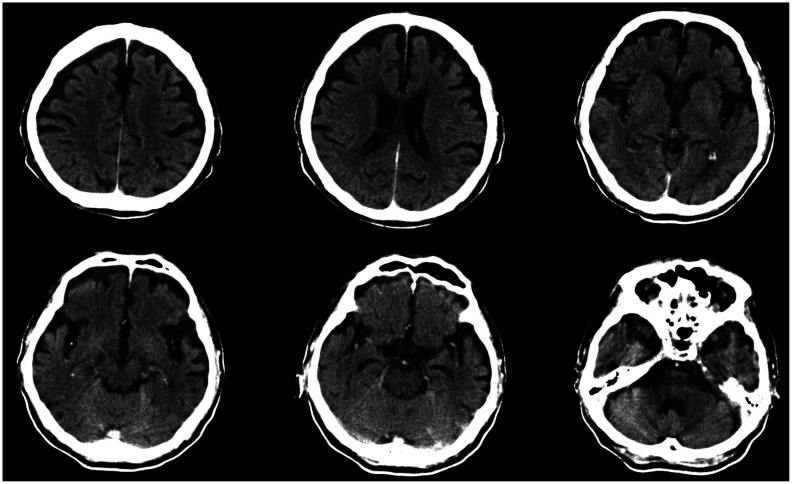
An 82-year-old male with a known history of dementia was referred to Chonnam National University Hospital brain bank (CNUHBB) for brain autopsy. His memory began to decline at the age of 71 years and further deteriorated thereafter. After the clinical diagnosis was determined, the patient was prescribed an acetylcholinesterase inhibitor. At the age of 73 years, he had trouble writing and finding his way home. He also had difficulty remembering words and names, and his orientation to time worsened. He further demonstrated impairment in daily living activities by failing to pay after eating at a restaurant and by experiencing difficulties with hygiene management. At this time, he recorded a Mini-Mental State Examination (MMSE) score of 20 (patient's score/maximum score): temporal orientation (4/5), spatial orientation (3/5), registration (3/3), attention and calculation (2/5), remote memory (1/3), language (7/8), and copy the diagram (0/1). No family history of dementia or other neurologic diseases was reported. Axial brain computed tomography images revealed mild atrophy in the bilateral medial temporal and frontal lobes, probably meeting the criteria for Alzheimer disease (AD) (Fig. 1).6 His cognition continued to deteriorate rapidly: MMSE scores of 17 at 74, 13 at 76, 5 at 77, 4 at 80, and 0 at 82 years of age. The patient died of aggravated pneumonia at the age of 82 years. Written informed consent was obtained from a legal surrogate of the patient.
Fig. 1. Brain computed tomography (CT) scan acquired 10 years before death. Axial brain CT image demonstrating cortical and mild hippocampal atrophy.
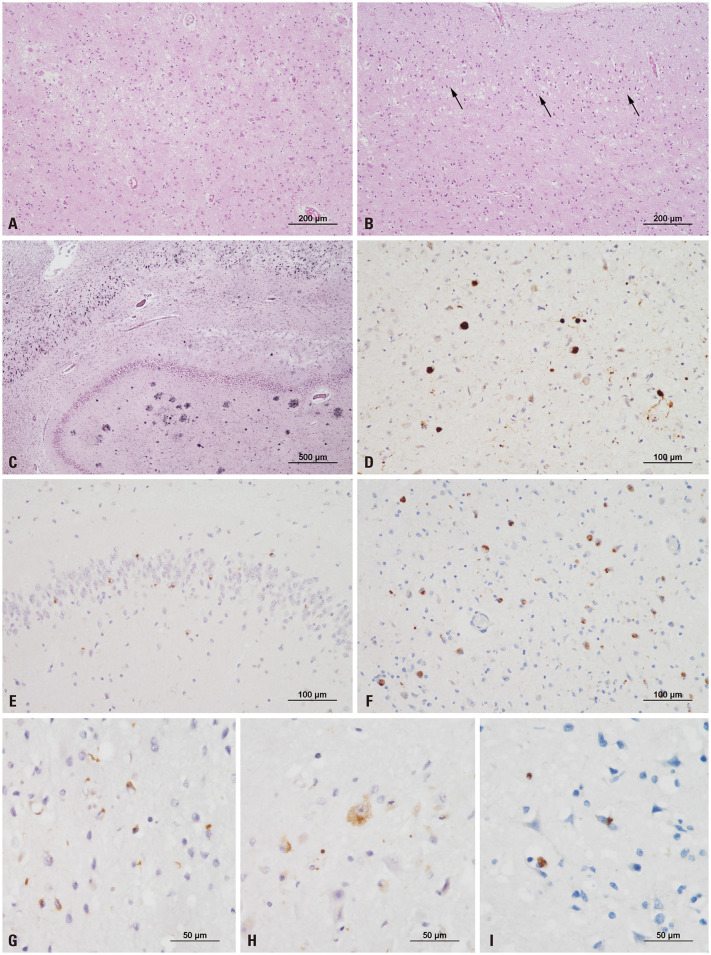
The major pathological findings of his autopsy were suggestive AD. Grossly, atrophy was severe in the neocortex and hippocampus (Fig. 2). Microscopically, neuronal loss and gliosis were severe in the hippocampus and moderate in the neocortex and amygdala (Fig. 3A). Superficial microvacuolation in cortical layer II was present in the inferior temporal area (Fig. 3B). Neurofibrillary tangles were frequent in the entorhinal cortex, hippocampus, and neocortex (Fig. 3C). Neuritic plaques were frequent in the neocortex. Amyloid deposition extended up to CA4 of the hippocampus. Based on scores of A3 (Thal amyloid phase 4), B3 (Braak neurofibrillary tangle stage VI), and C3 [Consortium to Establish a Registry for Alzheimer's Disease (CERAD) neuritic plaque score frequent], the pathological findings were compatible with high ADNC. α-Synuclein immunostaining revealed multiple Lewy bodies in the amygdala, but none in the midbrain or cingulate (Fig. 3D), consistent with amygdala-predominant Lewy body disease (LBD). Immunohistochemistry for TDP-43 revealed neuronal cytoplasmic inclusions (NCIs) in the dentate gyrus of the hippocampus (Fig. 3E). Abundant NCIs and some glial cytoplasmic inclusions were observed in the amygdala, entorhinal cortex, hippocampus, subiculum, and inferior temporal cortex (Fig. 3F, G, and H). NCIs were sparsely present in the insula (Fig. 3I). The frontal cortex, basal ganglia, and midbrain lacked TDP-43-positive inclusions. The TDP-43 pathology of the case indicated LATE-NC stage 2.5
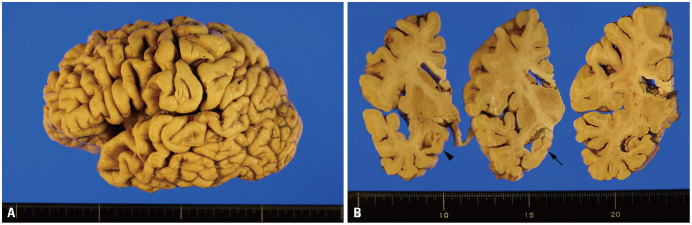
Fig. 2. Gross findings of a case with comorbid high Alzheimer disease neuropathologic changes, amygdala-predominant Lewy body disease, and limbicpredominant age-related TDP-43 encephalopathy (LATE) neuropathological changes. (A) The lateral view shows diffuse neocortical atrophy with remarkable involvement of the frontal cortex and temporal pole. (B) Coronal sections show thinning of the medial temporal lobe with dilatation of the temporal horn of the lateral ventricle (arrowhead) and severe atrophy of the hippocampus (tailed arrow).
Fig. 3. Histopathological features of a case with LATE-NC. (A) The amygdala shows remarkable neuronal loss and gliosis. (B) Superficial microvacuolation in cortical layer II (black arrows) is observed in the inferior temporal cortex (A and B, hematoxylin and eosin; original magnification, ×100). (C) The hippocampus shows an abundance of neurofibrillary tangles and neuritic plaques, consistent with high Alzheimer disease neuropathologic changes (Gallyas silver stain; original magnification, ×40). (D) Alpha-synuclein immunostaining highlights abundant Lewy bodies solely in the amygdala, consistent with amygdala-predominant Lewy body disease. (E) Immunohistochemistry for TDP-43 indicates neuronal cytoplasmic inclusions (NCIs) in the dentate gyrus of the hippocampus. (F–H) Abundant NCIs and a few dystrophic neurites are observed in the amygdala (F), entorhinal cortex (G), and hippocampus (H). (I) NCIs are sparsely present in the insula (D–F, original magnification, ×200; G–I, original magnification, ×400).
DISCUSSION
We report the autopsy findings of a patient with severe amnestic syndrome, who in fact had LATE-NC. The brain pathology exhibited high ADNC, amygdala-predominant LBD, and LATE-NC. Previous studies have indicated that cases with coexisting ADNC and LATE-NC are more likely to show hippocampal atrophy than subjects with ADNC only.7 Indeed, structural alteration of the amygdala reportedly indicates underlying LATE-NC and may be linked to cognitive decline.8 The current case showed severe hippocampal atrophy in association with both high ADNC and LATE-NC. The amygdala showed notable neuronal loss and gliosis in association with ADNC, LBD, and LATE-NC.
Although TDP-43 proteinopathy largely confined to medial temporal areas has been reported,4,9 a consensus definition of LATE has been reached only recently. The consensus staging scheme for LATE-NC is a three-tier system encompassing hierarchical spreading of TDP-43 proteinopathy from the amygdala (stage 1) to the hippocampus (stage 2) and middle frontal gyrus (stage 3),5 which represents a simpler system than the previously proposed six-stage scheme.9 To prevent under-recognition of LATE-NC during brain autopsy, it is critical to perform TDP-43 immunohistochemistry on three anatomical sections, as proposed previously.10 The prevalence of LATE-NC increases gradually with age, while that of severe ADNC decreases.11 Consequently, LATE plays a pivotal role in amnestic-type cognitive impairment among the rapidly growing oldest old population.5
For confirmation of LATE-NC, it is important to differentiate it from frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP). In comparison to those with LATE-NC, most patients with FTLD-TDP are diagnosed at a younger age and show severe symptoms, such as behavioral problems or language issues.12 LATE-NC typically involves the limbic regions, whereas FTLD-TDP affects the neocortices more widely.
There were several limitations to the current case report. First, the interval between brain imaging and brain autopsy was long, and brain imaging was not performed close to the death of the patient. Second, no other examinations, such as amyloid positron emission tomography or genetic analysis, were carried out to elucidate the etiology of LATE. Nevertheless, this case is noteworthy, because it is the first autopsy-proven case of LATE to be documented in South Korea and will assist other neurologists and pathologists in identifying LATE cases.
In conclusion, we suggest that LATE-NC can occur as a combined pathology or a single entity. When brain autopsy is performed in cases with amnestic memory decline, routine TDP-43 immunohistochemistry should also be performed in critical anatomic locations. Because there is no biofluid or neuroimaging biomarker for determining LATE status in vivo, acquisition of postmortem data would facilitate the diagnosis and treatment of cognitive impairment among the oldest individuals, which is important given the continual aging of society.
ACKNOWLEDGEMENTS
This study was supported by the KBRI Basic Research Program through the Korea Brain Research Institute funded by the Ministry of Science and ICT (19-BR-03-04 for BCK), a National Research Foundation of Korea grant funded by the Korean government (2019R1A2B5B01070598 for LKH), and the Chonnam National University Hospital Biomedical Research Institute (BCRI20012 for SHC). CNUHBB would like to acknowledge the generosity shown by the donor and donor families in donating brain tissue to the CNUHBB.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Byeong C. Kim and Kyung-Hwa Lee.
- Funding acquisition: Soo Hyun Cho, Byeong C. Kim, and Kyung-Hwa Lee.
- Investigation: Soo Hyun Cho and Seong-Min Choi.
- Methodology: Hyung-Seok Kim.
- Project administration: Won-Young Song.
- Supervision: Byeong C. Kim and Kyung-Hwa Lee.
- Validation: Seong-Min Choi and Hyung-Seok Kim.
- Visualization: Soo Hyun Cho and Kyung-Hwa Lee.
- Writing—original draft: Soo Hyun Cho.
- Writing—review & editing: Kyung-Hwa Lee.
- Approval of final manuscript: all authors.
References
- 1.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Bigio EH. TDP-43 variants of frontotemporal lobar degeneration. J Mol Neurosci. 2011;45:390–401. doi: 10.1007/s12031-011-9545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142:1503–1527. doi: 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. 2011;6:e26286. doi: 10.1371/journal.pone.0026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makkinejad N, Schneider JA, Yu J, Leurgans SE, Kotrotsou A, Evia AM, et al. Associations of amygdala volume and shape with transactive response DNA-binding protein 43 (TDP-43) pathology in a community cohort of older adults. Neurobiol Aging. 2019;77:104–111. doi: 10.1016/j.neurobiolaging.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, et al. Updated TDP-43 in Alzheimer's disease staging scheme. Acta Neuropathol. 2016;131:571–585. doi: 10.1007/s00401-016-1537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KH, Seo SW, Lim TS, Kim EJ, Kim BC, Kim Y, et al. Proposal guidelines for standardized operating procedures of brain autopsy: brain bank in South Korea. Yonsei Med J. 2017;58:1055–1060. doi: 10.3349/ymj.2017.58.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease. Acta Neuropathol Commun. 2018;6:33. doi: 10.1186/s40478-018-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider JA, Nelson PT. Reply: Limbic-predominant age-related TDP-43 encephalopathy (LATE) Brain. 2019;142:e43. doi: 10.1093/brain/awz186. [DOI] [PubMed] [Google Scholar]