Abstract
Purpose
Exposure to particulate matter (PM) is a well-known risk factor in the triggering and exacerbation of allergic airway disease. Indoor environments, where people spend most of their time, are of utmost importance. To assess the effects of air purifiers [equipped with high-efficiency particulate air (HEPA) filters] on allergic rhinitis (AR) in adult patients, we performed a multicenter, randomized, double-blind, and placebo-controlled study.
Materials and Methods
Patients with house dust mite (HDM)-induced AR were randomly assigned to either active or mockup (placebo) air-purification groups. Two air purifiers (placed in living room and bedroom) were operated for 6 weeks in each home environment. The primary study endpoint was to achieve improvement in AR symptoms and medication scores. Secondary endpoints were to achieve improvement in the quality of life (QoL) and visual analog scale (VAS) scores, as well as in the indoor (bedroom and living room) concentrations of PM2.5 and PM10.
Results
After 6 weeks of air purifier use, medication scores improved significantly in the active (vs. placebo) group, although subjective measures (symptoms, VAS, and QoL scores) did not differ. Bedroom PM2.5 concentrations initially exceeded living room or outdoor levels, but declined (by up to 51.8%) following active purifier operation. Concentrations of PM2.5 in living room and PM10 in bedroom and living room were also significantly reduced through active purification.
Conclusion
The use of air purifiers with HEPA filters significantly reduced medication requirements for patients with HDM-induced AR and significantly lowered indoor PM2.5 concentrations, regardless of room placement. Active intervention to reduce household air pollutants may help improve allergic airway disease (clinicaltrials.gov NCT03313453).
Keywords: Air purifier, allergic rhinitis, house dust mite, indoor pollution, particulate matter
INTRODUCTION
Indoor air pollution is a critical issue, with up to 90% of daily living currently taking place indoors.1 In this regard, children and elderly adults, often confined to their homes, are especially vulnerable.2 Indoor air in schools and workplaces can be a factor affecting respiratory health.3,4 Among known air pollutants, particulate matter (PM) is an acknowledged hazard with diverse effects on human health. Ambient PM exposure may adversely impact various cardiopulmonary conditions, including allergic airway disease.5,6
PM concentrations vary considerably by country and city, based on geopolitical location and socio-economic status. According to the World Health Organization air quality guidelines (WHO AQG), 24-hour concentrations of <50 µg/m3 for PM ≤10 µm (PM10) and <25 µg/m3 for PM ≤2.5 µm (PM2.5) are recommended (annual mean: PM10, <20 µg/m3; PM2.5, <10 µg/m3).7 However, the average annual PM10 level in the Seoul metropolitan area, which has climbed steadily since 2012 and now stands at 51.0 µg/m3, exceeds the WHO AQG limit.8
It has been shown that the use of an air purifier equipped with a high-efficiency particulate air (HEPA) filter to reduce indoor air pollution helps control allergic diseases. A number of reports have indicated that air purifiers are beneficial for patients with allergic rhinitis (AR),9,10,11 atopic dermatitis,12 and asthmatic children.13,14 In addition, air purifiers are credited with removing pollen, fungal spores,15 house dust-mite (HDM) allergens,10 and dog allergens.16
Previous studies evaluating the efficacy of air purifiers in relieving AR have focused solely on allergen removal, conducted research in lesser polluted countries, or had flawed study design (i.e., too few participants, single-center recruitment, or absence of a control/placebo group). This multicenter, randomized, double-blind, and placebo-controlled study of air purifiers in patients with AR was undertaken in an effort to overcome such limitations.
MATERIALS AND METHODS
Study design and ethical statement
By design, this was a 6-week multicenter, double-blind, and placebo-controlled study conducted at two centers in South Korea: Allergy and Asthma Center of Yonsei University in Seoul and Chonnam National University Medical School in Gwangju (clinicaltrials.gov NCT03313453). Our interventional protocol was approved by the Institutional Review Boards of Yonsei University Health System (Approval no. 4-2017-0588) and Chonnam National University Hospital (Approval no. CNUH-2017-184). The period of purifier operation lasted from late autumn to early winter in South Korea, out of pollen season without indoor heating (from October to November). The study was conducted in all of the enrolled individuals during the same 6-week periods.
Inclusion and exclusion criteria for enrollment
Inclusion criteria were as follows: 1) patients with AR, ages 18–65 years; 2) persistent moderate-to-severe AR sensitized to HDM, with retrospective rhinoconjunctivitis total symptom score (RRCTSS) ≥8; and 3) and written consent of voluntary participants. The severity of rhinitis was determined by RRCTSS during the month prior to the start of the study, based on six parameters: sneezing, rhinorrhea, nasal itchiness, nasal obstruction, ocular itchiness, and watery eyes. Each symptom was scored (0–3 points) as follows: 0, no symptom; 1, mild (mild symptoms/signs of rhinitis, well-controlled); 2, moderate (moderate symptoms/signs, difficult to control and interrupting daily activities or sleep); or 3, severe (severe symptoms/signs, difficult to control and interfering with daily activities or sleep).
The reasons for study exclusion were as follows: 1) seasonal allergies sensitized to tree, grass, or weed pollen; 2) rhinitis due to other causes (vasomotor, infectious, gustatory, or drug induced); 3) substantial and potentially obstructive nasal defects, such as deviated nasal septum; 4) chronic use of systemic corticosteroids (continuous for ≥3 months in the 12 months prior to study); 5) planned move or residential change within study period; 6) current air purifier usage; and 7) exposure to indoor tobacco smoke.
Sensitization profiles were determined by skin-prick test (SPT) and serum specific immunoglobulin E (IgE) test. Inhalant allergens (53 types), including tree, grass, weed pollen, HDMs, animal danders, molds, insects [Allergopharma (Hamburg, Germany) or Hollister-Stier (Spokane, WA, USA)], were tested; as well as controls [negative: normal saline with 0.3% phenol and 50% glycerol; positive: 0.1% histamine (Allergy Therapeutics, West Sussex, UK)]. SPTs were considered positive if wheal diameters at allergen sites were >3 mm on average. AdvanSure AlloScreen (LG Life Sciences, Seoul, South Korea) or ImmunoCAP (Thermo Fisher Scientific, Waltham, MA, USA) assays were peformed to detect specific IgE in serum. Values >0.35 kUA/L were interpreted as positive. Paranasal sinus series or Waters' view x-rays were obtained to delineate nasal defects, and blood eosinophil counts were measured.
A total of 44 patients were randomly assigned to either active purifier (AP, n=22) or mockup (placebo) purifier (MP, n=22) groups. Randomization was 1:1 via computer-generated schedule (in the order registered) and was carried out by a non-participating third party. Results were undisclosed until the end of study period. During the 6-week trial, patients had unrestricted access to medications previously used for rhinitis. Hospital visits were mandatory for all participants before, during, and after air purifier operation.
Air purifiers
Two air purifiers (LG Electronics, Seoul, South Korea) were provided per subject (Supplementary Fig. 1, only online). High-capacity purifier (capacity: 91 m2) was operated in the living room (AS281DAW, LG Electronics) (Supplementary Fig. 1A, only online), and low-capacity purifier (capacity: 58 m2) was operated in the bedroom (AS181DAW, LG Electronics) (Supplementary Fig. 1B, only online). The purifier instructor team guided the subjects to the optimal position and orientation to operate the air purifiers. Both machines ran continuously for 24 hours/day without interruption during 6 weeks of research, as confirmed by the LG Electronics study center via remote (Wi-Fi) monitoring. Purifiers of the AP group were equipped with HEPA filters (Supplementary Fig. 1C, only online, greencolored cylindrical apparatus), whereas units of MP members were operated without HEPA filters. Attached stickers secured the units, preventing secretive access to HEPA filters, and blinding was maintained until the end of the study. All of the participants were provided with a manual of the operation, precautions, and emergency contact number for error.
Indoor and outdoor air analyses
Indoor PM2.5, PM10 concentrations was measured by PPD4260B sensor (Shineyei, Kobe, Japan) equipped in the purifiers.17 Detection limits of the sensor were as follows: PM size, 1.0 µm; range of PM concentrations, 8–999 µg/m3. Capability of the sensor installed inside the purifiers was also well-correlated with that of Portable Aerosol Spectrometer Model 1.109 (GRIMM, Ainring, Germany, data not shown). For this study, we measured and collected 2.5 µm and 10 µm-sized PM concentrations using the optical particle measurement method.
The status of purifier operation and PM concentrations (living rooms and bedrooms) were sent to LG Electronics in real-time via Wi-Fi network machine mounted inside purifiers. All subjects agreed to wireless internet transmission of household PM concentrations and purifier operational updates to authorized centers. Display areas of all purifiers were blinded before study initiation. As a result, neither participant nor study personnel (medical staffs, clinical coordinator, LG researcher, and statistician) were not aware of the PM levels in their rooms until the end of the study.
Outdoor concentrations of PM2.5 and PM10 were recorded using the Air Korea monitoring system (https://www.airkorea.or.kr/eng/), a service of the Korean Ministry of Environment and Korea Environment Corporation that publicly disseminates real-time air quality data online. Measurements obtained at observatory stations nearest to the various households were used for analysis. Locations of observatories and home sites are shown in Supplementary Fig. 2 (only online).
Primary and secondary endpoints
Clinical manifestations of AR were monitored at 0, 3, and 6 weeks using questionnaires based on the averages of the prior week. Each patient received a diary outlining questions pertinent to their symptoms, medication use, etc., and was asked to record daily, starting from 1 week before the beginning of purifier operation. All of the subjects were required to bring diaries to each of their three hospital visits.
The primary endpoint of the study was to achieve improvement of AR symptoms and medication scores. Four nasal symptoms (itchy nose, sneezing, runny nose, and blocked nose) were scored using a 4-point scale ranging from 0 (no symptom) to 3 (severe symptoms, interfering with daily activities or sleeping). Daily average medication scores were also based on a 3-point-scale: 1 (oral or topical antihistamine use); 2 (intranasal corticosteroid use, with or without anti-histamine); and 3 (oral corticosteroid use, with or without intra-nasal corticosteroid or anti-histamine).18 Secondary endpoints were improvement in the quality of life (QoL) questionnaire scores (administered in Korean language);19 visual analog scale (VAS) scores (0 to 10, larger numbers indicating more severe symptoms); and indoor PM concentration (PM2.5 and PM10), PM10 signifying coarse particles (2.5–10 µm) and PM2.5 representing fine particulates (<2.5 µm).
Statistical analysis
Sample size was calculated using PASS v12 (NCSS LLC, Kaysville, UT, USA). A sample size of 22 for each of the two groups (1:1 allocation, 5% expected drop-out rate) was determined to achieve an 80% power, assuming a change in total nasal symptom score of 2.5 (as in a previous study),9 standard deviations of 2.3 (group 1) and 1.4 (group 2), a significance level (alpha) of 0.050, and the use of a two-sided two-sample unequal-variance t-test. For group comparisons, SPSS Statistics v23.0 for Windows (IBM Corp., Armonk, NY, USA) and SAS v9.4 (SAS Inc., Cary, NC, USA) were used. For continuous data, t-test and Mann-Whitney U-test were applied for variables following normal and non-normal distributions, respectively. Chi-squared test or Fisher's exact test was used for categorical data. Differing concentrations of PM were analyzed by place and group via linear mixed model. Outdoor and indoor PM levels were subjected to Pearson correlation analysis. Significance was set at p<0.05.
RESULTS
Population characteristics
Characteristics of the study population are shown in Table 1. No significant group-wise differences were observed. One MP group member dropped out of the study due to personal reasons, but the two groups did not differ in intention-to-treat or per-protocol analysis.
Table 1. Characteristics of Study Population by Assigned Group.
| Variables | Mockup purifier (n=22) | Active purifier (n=22) | p value |
|---|---|---|---|
| Age (yr) | 35.68±10.55 | 33.27±8.91 | 0.418 |
| Sex (male:female) | 6:16 | 9:13 | 0.340 |
| Diagnosis | |||
| Allergic rhinitis | 22 (100) | 22 (100) | - |
| Asthma | 11 (50) | 12 (54.6) | 0.763 |
| Allergic conjunctivitis | 7 (31.8) | 7 (31.8) | >0.999 |
| Atopic dermatitis | 5 (22.7) | 3 (13.7) | 0.698 |
| Rhinoconjunctivitis symptom score | 10 (9–12) | 9 (8–11) | 0.205 |
| Allergy testing | |||
| Total IgE (kU/L) | 162.75 (63.3–457) | 308 (157–524) | 0.242 |
| Blood eosinophils (cells/µL) | 205 (100–460) | 245 (170–400) | 0.981 |
| Wheal size to Dp (mm) | 8.91±4.73 | 8.77±4.39 | 0.922 |
| Specific IgE for Dp (kUA/L) | 7.93 (1.58–10.20) | 10.5 (3.83–24.50) | 0.153 |
| Indoor furry animals | |||
| Scoring of allergic rhinitis | |||
| Symptom score | 6.8±2.1 | 6.8±2.5 | >0.999 |
| Medication score | 1 (0.1–2.0) | 0.8 (0.0–1.0) | 0.223 |
| Visual analog scale | 5.5 (4.2–6.9) | 5.6 (4.5–6.8) | 0.672 |
| Quality of life | 70.8±13.5 | 69±17.7 | 0.711 |
IgE, immunoglobulin E; Dp, dermatophagoides pteronyssinus.
Continuous data are expressed as mean±standard deviation or median (interquartile range, 25 and 75 percentiles of data); categorical data are expressed as number (%). T-test was used for age and specific IgE for Dp; other continuous variables were compared via Mann-Whitney U-test. Chi-squared test was used for categorical data, except atopic dermatitis (Fisher's exact test).
Indoor (bedroom/living room) and outdoor environments
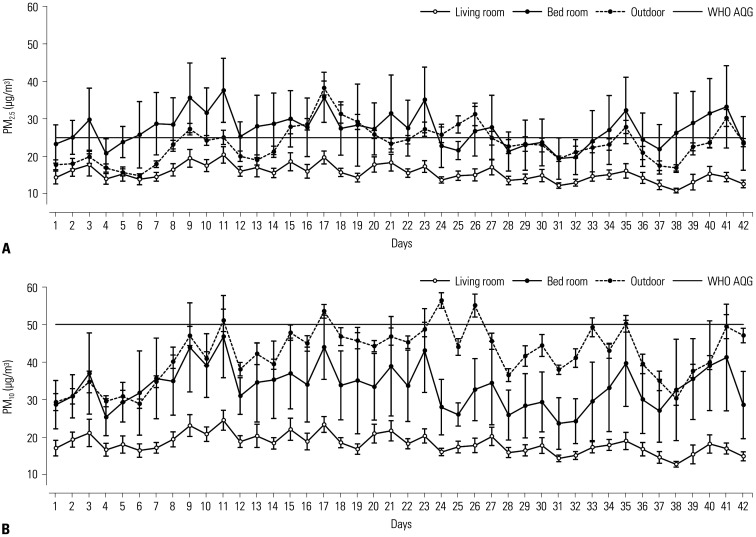
During the study period, the average bedroom PM2.5 concentration was 27.2±4.4 µg/m3 (mean±standard deviation), which exceeded the WHO AQG limit. In living rooms, PM2.5 concentration averaged 15.5±2.1 µg/m3 (Fig. 1A). Therefore, the bedroom PM2.5 level was 1.8 times higher than that of the living room (p<0.001), surpassing the outdoor average (23.5±4.8 µg/m3) as well. The mean bedroom PM10 concentration was 33.4±5.6 µg/m3, which was also higher than the living room level (18.1±2.5 µg/m3; p<0.001) (Fig. 1B). During this study, the outdoor PM10 concentration was 42.0±7.2 µg/m3 on average. The correlation between outdoor and indoor (bedroom and living room) levels is shown in Supplementary Table 1 (only online). Outdoor PM levels correlated better with determinations of the living room than with those of the bedroom.
Fig. 1. Outdoor and indoor concentrations of PM2.5 (A) and PM10 (B) during study period. PM, particulate matter; WHO AQG, World Health Organization air quality guideline.
Improvement in indoor PM concentration after air purifier operation
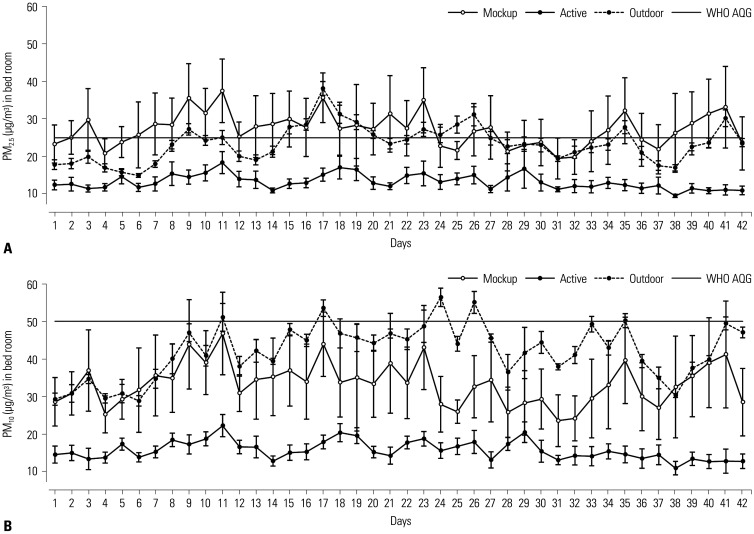
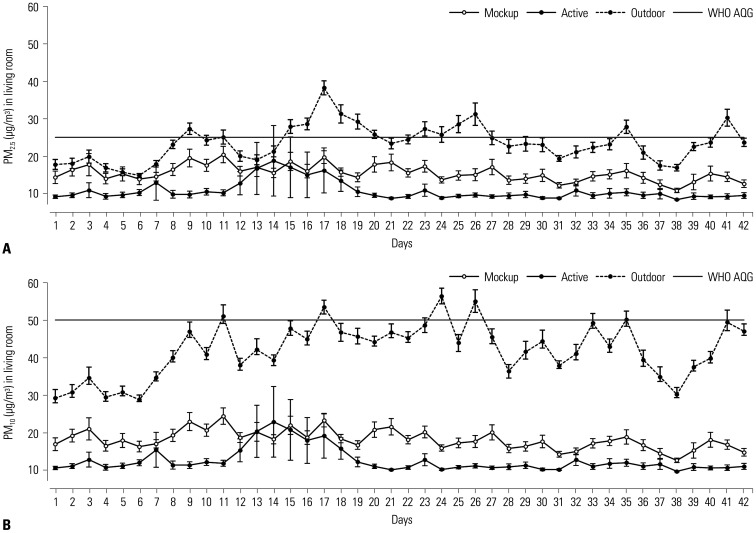
Amelioration of bedroom PM levels after air purifier operation is shown in Fig. 2. Average PM2.5 concentration was reduced by 51.8% (from 27.2±4.4 µg/m3 to 13.1±2.0 µg/m3; p=0.045) (Fig. 2A). PM10 concentration also declined by 53.2% on average (from 33.4±5.6 µg/m3 to15.6±2.5 µg/m3; p=0.048) (Fig. 2B). Living room PM concentrations determined after air purifier operation are shown in Fig. 3. The mean PM2.5 level fell by 30.5% (from 15.5±2.1 µg/m3 to 10.7±2.5 µg/m3; p=0.026) (Fig. 3A), and PM10 concentration showed 30.7% improvement (from 18.1±2.5 µg/m3 to 12.5±3.2 µg/m3; p=0.035) (Fig. 3B). The number of days at levels beyond the WHO AQG limits was significantly lower after air purifier operation, especially in terms of bedroom PM2.5 concentrations (Table 2). Since this study involved two South Korean cities that were 167.8 miles (270 km) apart, we also checked for city-wide differences in air quality before and after operating the air purifiers. However, we found no difference between the two cities.
Fig. 2. Changes in bedroom PM2.5 (A) and PM10 (B) concentrations after air purifier operation. PM, particulate matter; WHO AQG, World Health Organization air quality guideline.
Fig. 3. Changes in living room PM2.5 (A) and PM10 (B) concentrations after air purifier operation. PM, particulate matter; WHO AQG, World Health Organization air quality guideline.
Table 2. Comparison of the Number of Days Exceeding WHO AQG Standards by Group.
| Mockup purifier (total of 924 days*) | Active purifier (total of 924 days*) | p value† | |
|---|---|---|---|
| Bed room | |||
| PM2.5 (>25 μg/m3) | 239 (25.9) | 58 (6.3) | <0.001 |
| PM10 (>50 μg/m3) | 94 (10.2) | 18 (2.0) | <0.001 |
| Living room | |||
| PM2.5 (>25 μg/m3) | 72 (7.8) | 22 (2.4) | <0.001 |
| PM10 (>50 μg/m3) | 8 (0.9) | 14 (1.5) | 0.200 |
PM, particulate matter; WHO AQG, World Health Organization air quality guideline.
*Total days: 22 sites (per group)×42 days (air purifier operation period), †p-value was calculated by chi-square test.
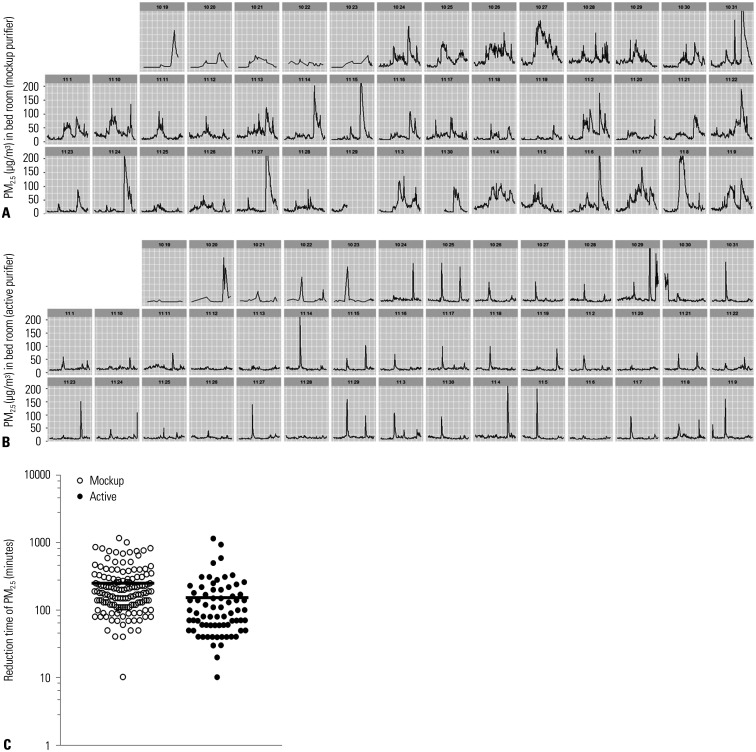
In addition to average PM concentrations, the exposure times to high concentrations of PM were significantly reduced after air purifier use. Due to various indoor activities, the concentration of fine dust was expected to surge and then diminish gradually (Fig. 4A, MP group depiction). However, PM levels declined rapidly under such circumstances in AP (vs. MP) households. (Fig. 4B, AP group depiction). When calculated, the time required for PM2.5 concentration in bedrooms to fall from >150 µg/m3 to <25 µg/m3 (WHO threshold) was reduced by 38.1% (from 249.3±209.0 min to 153.6±187.9 min; p<0.001) (Fig. 4C).
Fig. 4. Time taken to reduce PM2.5 concentration from 150 to 25 µg/m3. Representative PM levels in placebo group (A), active group (B), and comparison of reduction times between groups (C). PM, particulate matter.
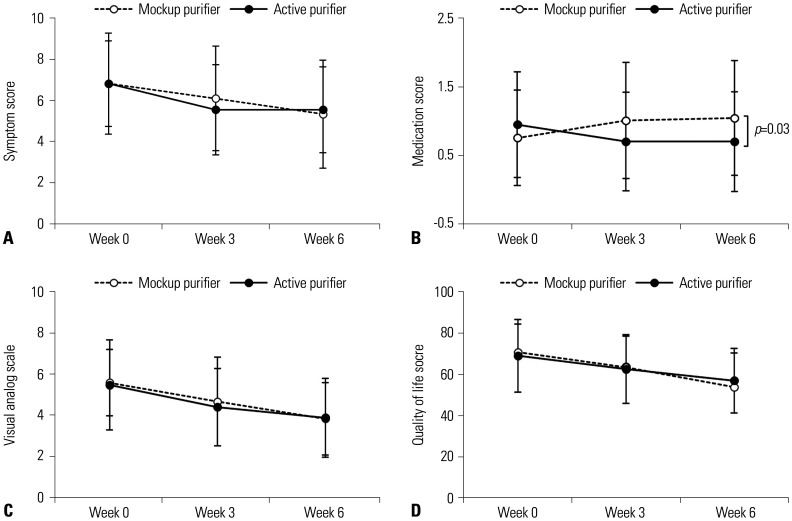
Improvement in allergic rhinitis after operation of air purifiers
Subjective measures of AR, such as symptoms, VAS, and QoL scores improved similarly in both AP and MP groups (Fig. 5A, C, and D), but objective medication scoring showed significantly greater improvement in the AP group at 6 weeks, compared with placebo (Fig. 5B). AP group members registered a 26.3% reduction in AR medication use at 6 weeks (p=0.033). These differences emerged in the third week of air purifier operation and persisted until the end of the study. In addition, AP group members were using 1.26 times the AR medications used by the MP group at study onset. After 6 weeks of use, this figure was reduced to 0.67 times (p=0.033).
Fig. 5. Changes in symptoms (A), medication use (B), visual analog scale (C), and quality of life scores (D) of allergic rhinitis after air purifier operation. Values are expressed as mean±standard deviation.
DISCUSSION
The results of this intricately designed study clearly demonstrate that air purifiers are beneficial for adults with persistent AR, underscoring the importance of indoor air pollution as a critical environmental issue. Despite apparent placebo effect reflected in subjective measures, we nevertheless confirmed objective gains in AR medication scores for users of active air purifiers, as opposed to mockup devices. Therefore, managing indoor pollutants on an individual basis may have merit in AR patients.
There are several explanations for the reduction of medication scores observed in this study. Air purifiers effectively reduce the levels of PM, a well-known risk factor in patients with allergic airway disease linked to indoor allergen exposure. Levels of household PM are attributable to outdoor PM, cooking fumes, cigarette smoking, microorganisms, and other sources such as HDM allergens.20 Patient's exposure times to high concentrations of PM are also significantly reduced by air purifier use; and PM2.5 concentrations, which may be especially noxious to patients with allergic airway disease, are effectively reduced by air purification. It has been established that smallcaliber PM poses a comparatively greater health hazard. In one previous study, an increased hospitalization rate due to respiratory diseases correlated more closely with PM2.5 than PM10 levels.2 Most of the studies heretofore in PM over airway allergy have been conducted in asthma,21,22 and the current study has the value to shed some light in the specific field of AR.
The difference we encountered in PM concentrations of bedroom and living room spaces was an unexpected finding, and may constitute a relative breakthrough in indoor air pollution management. Prior to this study, it was assumed that PM concentration in the living room (i.e., the gathering place and center of daily family activities) would exceed levels in more static bedroom environments. Moreover, the living room is closer to the kitchen, where cooking activity inordinately adds to the PM load. However, the opposite phenomenon was observed in this study. The higher levels of PM (both PM2.5 and PM10) in bedrooms compared to living room in this study may be explained by the effect of ventilation rate, which is typically lower than the standard setting.23 Although differing bedroom and living room ventilation rates were not fully investigated, a lower ventilation rate may be anticipated in the bedroom by comparison. An alarming fact is that similar conditions may exist in nursing homes, hospitals, or nurseries, rendering disadvantaged, marginalized, or inactive occupants more vulnerable to this type of indoor pollution. Further studies are needed to gather more related evidence. However, this premise is untenable if outdoor air is unexpectedly tainted by forest fires, dust storms, or seasonal pollen peaks.
Our data may also be applicable to other health conditions associated with PM, which presumably would benefit from the reductions achieved through air purification. PM is a reported risk factor in asthma hospitalizations,6 cardiovascular disease/mortality,24 pediatric atopic dermatitis,25 emergency room visits,26 and lung cancer.27 Long-term exposure to PM is reportedly associated with mortality rates.28,29 Likewise, various circulating inflammatory and thrombogenic indices,5 as well as stress hormone levels, have shown improvement in healthy patients after air purifier operation.30
Ultimately, this study had some limitations. First, the outdoor air of each home was not tested, and we relied instead on monitoring stations. However, the linear distance between observatory and residence locations (1.2 miles/1.9 kilometers on average) seemed acceptable for extrapolation. Another issue is that the lifestyles (i.e., in-home exposure times, proportionate bedroom/living room indoor occupancy, or cooking patterns and time spent in kitchens) of participants were not considered. Furthermore, we did not compare household indoor allergen levels. An attempt at monitoring HDM allergens using the Petri dish method31 was unproductive. Therefore, it was impossible to determine whether AR medication scores improved due to lower allergen concentrations, PM concentrations, or both. Although allergic biomarkers were not measured in our study, further research about either nasal or serum biomarker changes will help to support out findings. Finally, we could not check the efficacy of purifiers in reducing the PM of smokers, as exposure to indoor smokers disqualified study candidates.
In conclusion, the findings in this study confirm that the use of air purifiers with HEPA filters may mitigate medication requirements for patients with HDM-induced AR. We also determined that interventional air purification significantly lowered indoor PM2.5 and PM10 levels, regardless of room location, ensuring an overall healthier environment.
ACKNOWLEDGEMENTS
This research was supported by a grant from LG Electronics.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jung-Won Park and Kyung Hee Park.
- Data curation: Kyung Hee Park, Da Woon Sim, Sang Chul Lee, Sunyoung Moon, Eunju Choe, Sung Ryeol Kim, and Jae-Hyun Lee.
- Formal analysis: Kyung Hee Park and Hyejung Shin.
- Funding acquisition: Jung-Won Park, Hyung Ho Park, and Deok Huh.
- Investigation: Jung-Won Park and Kyung Hee Park.
- Methodology: Hyejung Shin.
- Project administration: Jung-Won Park and Kyung Hee Park.
- Resources: Jung-Won Park, Hyung Ho Park, and Deok Huh.
- Software: Hyejung Shin and Sunyoung Moon.
- Supervision: Jung-Won Park.
- Validation: Jung-Won Park.
- Visualization: Kyung Hee Park.
- Writing—original draft: Kyung Hee Park.
- Writing—review & editing: Jung-Won Park.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Air purifiers in living room (A) and bedroom (B); high-efficiency particulate air filters and airflow (C).
Distribution of subject home sites and outdoor air pollution observatory locations.
Correlation between Outdoor and Indoor (Bed-Room or Living Room) PM Levels
References
- 1.Habre R, Coull B, Moshier E, Godbold J, Grunin A, Nath A, et al. Sources of indoor air pollution in New York City residences of asthmatic children. J Expo Sci Environ Epidemiol. 2014;24:269–278. doi: 10.1038/jes.2013.74. [DOI] [PubMed] [Google Scholar]
- 2.Jo EJ, Lee WS, Jo HY, Kim CH, Eom JS, Mok JH, et al. Effects of particulate matter on respiratory disease and the impact of meteorological factors in Busan, Korea. Respir Med. 2017;124:79–87. doi: 10.1016/j.rmed.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Takaoka M, Suzuki K, Norbäck D. Current asthma, respiratory symptoms and airway infections among students in relation to the school and home environment in Japan. J Asthma. 2017;54:652–661. doi: 10.1080/02770903.2016.1255957. [DOI] [PubMed] [Google Scholar]
- 4.Hodas N, Loh M, Shin HM, Li D, Bennett D, McKone TE, et al. Indoor inhalation intake fractions of fine particulate matter: review of influencing factors. Indoor Air. 2016;26:836–856. doi: 10.1111/ina.12268. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65:2279–2287. doi: 10.1016/j.jacc.2015.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H, Kim H, Park YH, Lee JT. Assessment of temporal variation for the risk of particulate matters on asthma hospitalization. Environ Res. 2017;156:542–550. doi: 10.1016/j.envres.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Regional Office for Europe. Air quality guidelines: global update 2005. Copenhagen: World Health Organization; 2006. [Google Scholar]
- 8.Kim HC, Kim S, Kim BU, Jin CS, Hong S, Park R, et al. Recent increase of surface particulate matter concentrations in the Seoul Metropolitan Area, Korea. Sci Rep. 2017;7:4710. doi: 10.1038/s41598-017-05092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HK, Cheng KC, Tetteh AO, Hildemann LM, Nadeau KC. Effectiveness of air purifier on health outcomes and indoor particles in homes of children with allergic diseases in Fresno, California: a pilot study. J Asthma. 2017;54:341–346. doi: 10.1080/02770903.2016.1218011. [DOI] [PubMed] [Google Scholar]
- 10.Jia-Ying L, Zhao C, Jia-Jun G, Zi-Jun G, Xiao L, Bao-Qing S. Efficacy of air purifier therapy in allergic rhiniti. Asian Pac J Allergy Immunol. 2018;36:217–221. doi: 10.12932/AP-010717-0109. [DOI] [PubMed] [Google Scholar]
- 11.Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990;85:1050–1057. doi: 10.1016/0091-6749(90)90050-e. [DOI] [PubMed] [Google Scholar]
- 12.Park HC, Kim YH, Kim JE, Ko JY, Nam Goung SJ, Lee CM, et al. Effect of air purifier on indoor air quality and atopic dermatitis. Allergy Asthma Respir Dis. 2013;1:248–256. [Google Scholar]
- 13.Lee GH, Kim JH, Kim S, Lee S, Lim DH. Effects of indoor air purifiers on children with asthma. Yonsei Med J. 2020;61:310–316. doi: 10.3349/ymj.2020.61.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165:741–748. doi: 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng YS, Lu JC, Chen TR. Efficiency of a portable indoor air cleaner in removing pollens and fungal spores. Aerosol Sci Technol. 1998;29:92–101. [Google Scholar]
- 16.Green R, Simpson A, Custovic A, Faragher B, Chapman M, Woodcock A. The effect of air filtration on airborne dog allergen. Allergy. 1999;54:484–488. doi: 10.1034/j.1398-9995.1999.00029.x. [DOI] [PubMed] [Google Scholar]
- 17.Austin E, Novosselov I, Seto E, Yost MG. Laboratory evaluation of the Shinyei PPD42NS low-cost particulate matter sensor. PLoS One. 2015;10:e0137789. doi: 10.1371/journal.pone.0137789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI position paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 19.Jung MK, Hong SJ, Lee SH, Hong SJ, Son JW, Kang W, et al. Development and validation of a Korean allergic rhinitis-specific quality of life questionnaire (KARQLQ) Korean J Asthma Allergy Clin Immunol. 2008;28:113–120. [Google Scholar]
- 20.Wu W, Jin Y, Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol. 2018;141:833–844. doi: 10.1016/j.jaci.2017.12.981. [DOI] [PubMed] [Google Scholar]
- 21.Eeftens M, Hoek G, Gruzieva O, Mölter A, Agius R, Beelen R, et al. Elemental composition of particulate matter and the association with lung function. Epidemiology. 2014;25:648–657. doi: 10.1097/EDE.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 22.Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192:1052–1059. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitroulopoulou C. Ventilation in European dwellings: a review. Build Environ. 2012;47:109–125. [Google Scholar]
- 24.Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, et al. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc. 2017;6:e007170. doi: 10.1161/JAHA.117.007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EH, Kim S, Lee JH, Kim J, Han Y, Kim YM, et al. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PLoS One. 2015;10:e0119501. doi: 10.1371/journal.pone.0119501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang SH, Lee JY, Yi SM, Kim H. Associations of particulate matter and its components with emergency room visits for cardiovascular and respiratory diseases. PLoS One. 2017;12:e0183224. doi: 10.1371/journal.pone.0183224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamichhane DK, Kim HC, Choi CM, Shin MH, Shim YM, Leem JH, et al. Lung cancer risk and residential exposure to air pollution: a Korean population-based case-control study. Yonsei Med J. 2017;58:1111–1118. doi: 10.3349/ymj.2017.58.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SE, Bell ML, Hashizume M, Honda Y, Kan H, Kim H. Associations between mortality and prolonged exposure to elevated particulate matter concentrations in East Asia. Environ Int. 2018;110:88–94. doi: 10.1016/j.envint.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Kim OJ, Kim SY, Kim H. Association between long-term exposure to particulate matter air pollution and mortality in a South Korean National Cohort: comparison across different exposure assessment approaches. Int J Environ Res Public Health. 2017;14:1103. doi: 10.3390/ijerph14101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, et al. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. 2017;136:618–627. doi: 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
- 31.Tovey ER, Mitakakis TZ, Sercombe JK, Vanlaar CH, Marks GB. Four methods of sampling for dust mite allergen: differences in ‘dust’. Allergy. 2003;58:790–794. doi: 10.1034/j.1398-9995.2003.00228.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Air purifiers in living room (A) and bedroom (B); high-efficiency particulate air filters and airflow (C).
Distribution of subject home sites and outdoor air pollution observatory locations.
Correlation between Outdoor and Indoor (Bed-Room or Living Room) PM Levels