Abstract
Maternal postpartum depression is a prominent risk factor for aberrant child socioemotional development, but there is little understanding about the neural phenotypes that underlie infant sensitivity to maternal depression. We examined whether newborn white matter fractional anisotropy (FA), a measure of white matter maturity, moderates the association between maternal postpartum depressive symptoms and infant negative reactivity at 6 months. Participants were 80 mother–infant dyads participating in a prospective population-based cohort, and included families whose newborns underwent a magnetic resonance/diffusion tensor imaging scan at 2–5 weeks of age and whose mothers reported their own depressive symptoms at 3 and 6 months postpartum and infant negative emotional reactivity at 6 months. The whole-brain FA moderated the association between maternal depressive symptoms and mother-reported infant negative reactivity at 6 months after adjusting for the covariates. Maternal depressive symptoms were positively related to infant negative reactivity among infants with high or average FA in the whole brain and in corpus callosum and cingulum, but not among those with low FA. The link between maternal depressive symptoms and infant negative reactivity was moderated by newborn FA. The variation in white matter microstructure might play a role in child susceptibility to parental distress.
Keywords: DTI, maternal depression, infancy, negative affect, susceptibility
Introduction
Because the first years of life are periods of dynamic central nervous system (CNS) development (Markant and Thomas, 2013), identifying who is susceptible to early stress exposure is central to the prevention of several psychiatric disorders (O’Donnell and Meaney, 2017). Importantly, stress exposures may cause adverse effects only in some individuals more susceptible to be affected by environmental adversities (i.e. diathesis-stress model) but these individuals may also benefit from good quality environments, as proposed by different theories reflecting environmental sensitivity (Belsky and Pluess, 2009; Boyce, 2016; Greven et al., 2019).
One important early life exposure for infants is the level of maternal postpartum depressive symptoms, the prevalence of which ranges from 10 to 20% in Western societies (Andersson et al., 2006). Maternal depressive symptoms of varying severity reportedly affect maternal caregiving (Field, 2010), child development (Luoma et al., 2001; Liu et al., 2017) and offspring brain development (Meaney, 2018). Negative reactivity, in turn, is a temperament trait linked to higher risk for psychopathology later in life (De Pauw and Mervielde, 2010; Kostyrka-Allchorne et al., 2019). Importantly, maternal depression is related to higher infant negative emotional reactivity (Ramchandani et al., 2011; Kingston et al., 2012). However, studies reporting heightened emotional reactivity after exposure to parental postpartum depression typically also reported modest effect sizes. The interindividual variation in child susceptibility (i.e. children being disproportionally affected by environmental exposures such as parental distress; Meaney, 2018) may explain part of this variability.
The mechanisms of susceptibility likely include genetic and behavioral factors (Belsky and Pluess, 2009; Boyce, 2016), but brain structure and function also appears as a viable mediator of such associations (Yap et al., 2008; Whittle et al., 2011; Schriber et al., 2017; Deane et al., 2019; Rifkin-Graboi et al., 2019). However, very little research has been conducted on the brain structural factors that may underlie such susceptibility in young children. White matter microstructure, more specifically its diffusion properties, is related to 5-HTTLPR and BDNF Val66met genotypes (Tost et al., 2013; Benedetti et al., 2015; Tatham et al., 2016), also linked to environmental susceptibility (e.g. Kim et al., 2007). White matter microstructure is also reportedly related to developmental and psychiatric disorders (Barnea-Goraly et al., 2005; Wolff et al., 2012; Vulser et al., 2018). Especially the white matter tracts that have a broad importance in connecting different areas of brain and the networks like default mode and salience network that have been indicated underlying variation in susceptibility (Greven et al., 2019) may be viable mediators of susceptibility to environment. Thus, variation in white matter microstructure can be considered one potential neural phenotype underlying interindividual differences in susceptibility. Further, exposure to maternal pre- and post-natal depressive symptoms have been related to offspring white matter diffusion properties in both neonates and older children (Lebel et al., 2016; Dean et al., 2018; El Marroun et al., 2018), suggesting an interplay between white matter microstructure, maternal perinatal depressive symptoms and child development.
The aim of the current study was to investigate whether the well-established association between maternal postpartum depressive symptoms and infant negative reactivity, a key trait reflecting higher risk for later psychopathology, is moderated by newborn white matter microstructure diffusion properties. We chose to focus broadly on mean fractional anisotropy (FA) in the whole brain as no previous studies with focus on white matter underlying the variation in susceptibility exist, and because in newborns, broad phenotypes including FA across the brain may have prominent implications for later development (Dubois et al., 2014; Gilmore et al., 2018; Girault et al., 2019). We tested the association separately at 3 and 6 months to examine whether timing of exposure has relevance in determining child outcomes in the context of FA as a marker of susceptibility, and additionally, we conducted an analysis differentiating continuously elevated, low and discontinuously elevated (elevated only at either 3 or 6 months postpartum) maternal symptoms.
Furthermore, we explored the moderation effect more locally by focusing on the FA of the corpus callosum (CC), cingulum bundle (CB) and uncinate fasciculus (UF) tracts. CC is a key tract responsible for interhemispheric brain connectivity (Horn et al., 2014; Roland et al., 2017) and can be reliably delineated in the developing brain (Gilmore et al., 2007; Jeurissen et al., 2013; Qiu et al., 2015). CC, CB and UF all also contribute to default mode and salience networks (Gordon et al., 2011; Von der Heide et al., 2013; Horn et al., 2014; Bubb et al., 2018), which are considered critical for the susceptibility to environment (Greven et al., 2019). Furthermore, structural alterations in these tracts have been linked with prenatal and early life stress exposures, including maternal symptomatology (Jackowski et al., 2008; Charil et al., 2010; Rifkin-Graboi et al., 2015; El Marroun et al., 2018), emotion regulation (Vulser et al., 2018) and a myriad of psychiatric disorders, including depression (Lacerda et al., 2005; Arnone et al., 2008; Walterfang et al., 2008; Barnea-Goraly et al., 2009; Bellani et al., 2009; Koch et al., 2014; Swartz et al., 2014; Jenkins et al., 2016; Tatham et al., 2016), which in turn is predicted by heightened negative reactivity in childhood. Finally, white matter microstructure alterations, namely lower FA of CC and CB, have been reported in adults with treatment-resistant depression (De Diego-Adeliño et al., 2014), further emphasizing the role of white matter microstructure in susceptibility to environmental influences. Although generally higher FA values reflect more advanced neural development (Gilmore et al., 2018) and lower FA values are reported in clinical populations, also increased FA values may represent a risk, especially in pediatric populations (Barnea-Goraly et al., 2005; Wolff et al., 2012; Koch et al., 2014; Bubb et al., 2018). With no previous studies on the topic, we did not set an a priori hypothesis about whether lower or higher FA would reflect susceptibility to maternal symptoms of depression.
Materials and methods
Participants
The data are part of the FinnBrain Birth Cohort Study (Karlsson et al., 2018), a prospective study starting from the prenatal period. A research nurse informed the families that underwent a first trimester ultrasound at gestational week 12 about the study, and of all the families informed, 66% enrolled in the study. A subset of families was invited to participate in the newborn brain magnetic resonance imaging (MRI) between 2 and 5 weeks of infant age. The families who participated in the newborn scan and whose parents also filled in the prenatal and 3- and 6-month postnatal questionnaires qualified for this study (N = 80 mother–infant dyads). The scanned infants were all born at gestational week 36 or later, weighed more than 2500 g, had Apgar scores >6 at 5 min after birth, did not have any diagnosed CNS anomaly or abnormal findings in the MRI scan, and had not undergone any invasive treatments after birth. Demographic characteristics of the sample are presented in Table 1. The sample of this study mainly resembled the main cohort (Karlsson et al., 2018) but the mothers who did not respond to the 6-month questionnaire including the main outcome were less educated and had more depressive symptoms in mid-pregnancy than the mothers who provided the full data (see the Supplementary Material).
Table 1.
The sample characteristics of mother–infant dyads (N = 80) in the study
| Mother–infant dyads (N = 80) | Mean or No. | % | SD | range |
|---|---|---|---|---|
| Maternal age at childbirth | 30.1 | 4.3 | 20–41 | |
| Duration of gestation | 39.8 | 1.3 | 36–42 | |
| Depressive symptoms (EPDS) | ||||
| During pregnancy (averaged) | 4.6 | 4.1 | 0–21 | |
| During postpartum (averaged) | 4.6 | 3.9 | 0–15.5 | |
| 3 months postpartum | 4.4 | 4.0 | 0–19 | |
| of which score ≥ 10 | 6 | 9.4 | ||
| 6 months postpartum | 4.9 | 4.4 | 0–19 | |
| of which score ≥ 10 | 9 | 14.1 | ||
| Race/ethnicity, White/Caucasian | 80 | 100 | ||
| Educational level | ||||
| High school/vocational | 18 | 23 | ||
| Polytechnics | 28 | 35 | ||
| University | 34 | 42 | ||
| Maternal weight status (BMI) at gwk 14 | 24.3 | 4.2 | 17.5–38.4 | |
| Self-reported use of alcohol | ||||
| during first trimester | 16 | 20 | ||
| during third trimester | 5 | 6 | ||
| Self-reported smoking | ||||
| during first trimester | 5 | 6 | ||
| during third trimester | 2 | 3 | ||
| Parity | ||||
| Primiparous | 49 | 61 | ||
| Multiparous | 31 | 39 | ||
| Infant sex | ||||
| Male | 40 | 50 | ||
| Female | 40 | 50 | ||
| Infant age at scan from birth, days | 24.3 | 8.0 | 11–54 | |
| Infant age at scan from conception (due date), days | 306.0 | 7.2 | 293–323 | |
| Infant birth weight, grams | 3540 | 461 | 2580–4700 | |
| APGAR score at 5 min after birth | 9.13 | 0.65 | 6–10 | |
| Whole brain mean FA | 0.25 | 0.02 | 0.22–0.30 | |
| Corpus callosum mean FA | 0.32 | 0.02 | 0.27–0.39 | |
| Cingulum mean FA | 0.43 | 0.05 | 0.19–0.50 | |
| Uncinate fasciculus mean FA | 0.26 | 0.02 | 0.19–0.33 | |
| Infant negative reactivity at 6 months | 2.98 | 0.80 | 1.48–4.89 | |
Ethical considerations
The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland and was performed according to the Declaration of Helsinki. Parents gave informed written consent on behalf of themselves and their children.
Measures
Maternal depressive symptoms during pregnancy and at postpartum
Maternal depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS; Cox et al., 1987) that is also validated for use in the prenatal period (Bergink et al., 2011). The questionnaire was filled in by mothers at gestational weeks 14, 24 and 34; at 3 months; and at 6 months (Cronbach’s α = 0.84–0.89). The EPDS consists of 10 items, each rated from 0 to 3, resulting in a maximum score of 30, higher scores indicating more depressive symptoms, and typically with 10 or greater indicating possible depression in community samples (e.g. Cox et al., 1987; Eberhard-Gran et al., 2001). Maternal depressive symptoms at 3 and 6 months postpartum were used as continuous variables in the main analysis. Further, an averaged sum of depressive symptoms throughout pregnancy was calculated to be used as a covariate in the analyses, as based on previous studies, prenatal distress could independently affect child brain (Scheinost et al., 2017; Pulli et al., 2018).
Continuity of maternal depressive symptoms
To examine the significance of exposure continuity, an additional analysis based on whether the mother had experienced depressive symptoms throughout the postnatal period or only at 3 or at 6 months was conducted. The classification was made based on the slightly lower EPDS score of 9 in comparison to the traditional threshold of 10 to maintain sufficient group sizes in the analyses and based on the literature showing that even subclinical symptoms are related to mother–infant interactions and infant outcomes (e.g. Campbell et al., 1995; West and Newman, 2003; Moehler et al., 2006; Giallo et al., 2015). The mothers with this score or higher at both postpartum time points were considered to have continuously elevated symptoms. The classification resulted in N = 58 mothers with low symptoms across postpartum, N = 13 mothers with high symptoms across postpartum (both at 3 and 6 months), and N = 8 mothers with high symptoms only at 6 months. Only one mother reported high symptoms only at 3 but not at 6 months, so the classes including mothers with high symptoms only in either of the time points (discontinuously elevated symptoms) were collapsed into one class.
The descriptive statistics for maternal depressive symptoms are reported in Table 1.
DTI-MRI data acquisition and processing
The scans were performed in the Medical Imaging Centre of Turku University Hospital in a family–friendly manner as previously described (Lehtola et al., 2019). MRI scans were conducted on a Siemens Magnetom Verio 3 T scanner (Siemens Medical Solutions, Erlangen, Germany) using a 12-element Head Matrix coil. A 96-direction DTI protocol was divided into three parts (each part with either 31, 32 or 33 individual diffusion encoding directions using b = 1000 s/mm2 in addition to three b = 0 s/mm2 images, per each part, and a duration of approximately 6 min). In each part, the spread of diffusion encoding directions was evenly distributed across the 3D space. The sequences were acquired using Spin Echo-Echo Planar Imaging sequence at 2 mm3 isotropic resolution (FOV 208 mm; 64 slices; TR 8500 ms; TE 90 ms). Images were screened for incidental findings, and they did not affect white matter (Kumpulainen et al., 2020).
DTI data analysis
First, we visually identified b0 volumes with acceptable quality (no motion artifacts in visual inspection) and moved average of them to the front of the 4D series. We then created a brain mask from the average b0 volume with FSL’s FMRIB Software Library v 5.0.9 (Jenkinson et al., 2012) Brain Extraction Tool (Smith, 2002). Second, the qualities of diffusion datasets were quantitatively evaluated using DTIprep software (Oguz et al., 2014). Datasets were then formed from the three quality-controlled parts into single image with an in-house script. The combined data now included a variable number of diffusion encoding directions, and those containing less than 40 diffusion encoding directions were excluded from later analyses. Finally, directions in excess to 40 were removed, while always maximizing the angular resolution (Merisaari et al., 2019), so that each participant now had exactly 40 diffusion encoding directions in their data. Finally, the 3D distribution of available diffusion encoding directions was assured to be even, as per planned coverage of the sequences and visual inspection of the distributions after preprocessing (Roalf et al., 2016). Prior work points out that 40 directions provide a robust prerequisite for tensor estimation (Ni et al., 2006; Giannelli et al., 2010).
Next, we corrected the data for eddy currents using FSL (Andersson and Sotiropoulos, 2016). Correlation to minor residual motion (after dtiprep) was assessed in our prior work (Merisaari et al., 2019) and they did not bias the estimates. Finally, we processed the 4D diffusion dataset with FSL’s dtifit, using the brain mask to limit the modeling to brain tissue only. All steps were followed by careful visual inspection of the data.
For further preprocessing, the tract-based spatial statistics (TBSS) pipeline of FSL (Smith et al., 2007) was employed, limiting the analysis only to the skeletons of white matter tracts, estimated from individual images that are projected to a common skeleton space. The ‘tbss_2_reg -n’ option was used to create a study-specific template for spatial transformations. A modified version of the ‘tbss_3_postreg -S’ step was then run to incorporate registrations to the study-specific template and up-sample the data to 1 mm3 resolution as per TBSS defaults. An FA threshold of 0.15 in the ‘tbss_4_prestats’ module was used to create an FA skeleton (Merisaari et al., 2019).
Finally, an automated, skeleton-based, ROI delineation was performed by masking the FA skeleton images with the JHU template (Oishi et al., 2011). The JHU labels were warped to the study-specific infant mean FA space (see the Supplementary Material), and the JHU atlas was used to mask and estimate mean FA from the anatomical areas within the skeleton (all the tracts, including the CC and UF). The co-registration was visually inspected to assure accurate coverage of the labels for each of the FA skeletons. The whole brain mean FA was calculated as a mean of FA values in all white matter areas (inside the skeleton), and the mean FA’s of CC, (cingular part of) CB and UF were used in post-hoc analyses separately. Thus, the ROI values for the whole brain FA and the anatomical JHU labels/regions have been defined from the thresholded individual FA skeleton.
Mother-reported infant negative reactivity
Maternal reports of the negative affectivity scale of the Infant Behavior Questionnaire Revised Short Form (IBQ-R; Putnam et al., 2014) were used to measure negative reactivity at the infant age of 6 months. The negative affectivity scale (Cronbach’s α = 0.90) of the IBQ-R includes 25 items, where the parent assesses infant behaviors and expressions of distress, sadness, and fear during the past 1 or 2 weeks on a scale from 1 to 7, with higher scores on each scale indicating higher levels of negative reactivity.
Statistical analyses
We evaluated the associations between relevant confounders, white matter FA in the whole brain, and mother-rated infant negative reactivity using Pearson correlations and pairwise T-tests, except for postnatal age and gestational age, which we tested using partial correlations controlling for each other. Because mother-reported infant negative reactivity was not found to deviate from normal distribution, we used linear regression models to analyze whether the interaction of newborn whole brain FA and maternal postnatal depressive symptoms at 3 and 6 months would predict infant negative reactivity at 6 months of age. The following covariates were included based on the previous literature on factors that may affect child brain and/or negative affect: infant sex (e.g. Else-Quest et al., 2006), parity (Fish and Stifter, 1993), maternal alcohol/tobacco use during pregnancy (see a review in Pulli et al., 2018) and maternal prenatal depressive symptoms (e.g. Dean et al., 2018) and post-conceptional age, resulting in the following models:
Negative reactivity = infant sex + post-conceptional age + maternal parity + maternal alcohol/tobacco use during pregnancy + maternal EPDSprenatal + whole brain FA + EPDSpostnatal + (EPDSpostnatal × whole brain FA), where EPDSpostnatal was either:
(a) EPDS at 3 months or (b) EPDS at 6 months.
We used logarithm-transformed and standardized maternal symptoms in the analyses. As a sensitivity analysis, we also conducted the model separately controlling for gestational age and postnatal age at scan instead of post-conceptional age to make sure that the age variable used did not affect the results (Rasmussen et al., 2017). Further, we tested whether the results remain after controlling for several prenatal exposures (see the Supplementary Material). The main analyses were run using SPSS V25.0.
Finally, as a post-hoc analysis, we ran the analyses for the preselected regions of interest; CC, CB and UF. The P-values of the foci of interest interaction terms in all models (i.e. EPDS3 months × FA and EPDS6 months × FA in the main models and the following three post-hoc models) were corrected using the Benjamini–Hochberg method (Benjamini and Hochberg, 1995) using false discovery rate P < 0.05 threshold. Next, we probed interactions (Roisman et al., 2012) for significant FA moderators using simple slopes using PROCESS Macro (Hayes, 2017) in SPSS. Thus, we tested whether maternal depressive symptoms and negative reactivity are associated at the low or high (± 1 SD) ends of the distribution of the FA mean (Aiken and West, 1991). Figures were made using the median split of whole brain FA and the package ggplot2 in R (R Core Team, 2018). Finally, as white matter microstructure only interacted with 6-month maternal depression, we ran an ANCOVA model using continuity groups of maternal depressive symptoms to distinguish whether infant exposure to continuous high maternal distress vs high maternal distress at 6 months only was important in terms of infant negative reactivity as a function of white matter microstructure.
Results
Potential confounders, newborn whole brain fractional anisotropy, and infant negative reactivity
The correlations between the variables are shown in Table 2. Newborn whole brain FA correlated positively with post-conceptional age. Mother-rated negative reactivity was positively related to length of gestation and maternal parity (T = −2.17, P = 0.033, Cohen’s d = 0.49), with multiparous mothers rating their children higher in negative reactivity (M = 3.22, SD = 0.88) than primiparous mothers (M = 2.83, SD = 0.72). Whole brain FA or mother-rated negative reactivity was not significantly associated with depressive symptoms during pregnancy, child sex, or maternal alcohol or tobacco use during pregnancy (P > 0.05).
Table 2.
Correlations between the whole brain mean FA, infant negative reactivity, maternal depressive symptoms at 3 and 6 months, and the covariates
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 Whole brain FA | |||||||
| 2 Negative reactivity | 0.10 | ||||||
| 3 EPDS at 3 months | −0.04 | 0.38** | |||||
| 4 EPDS at 6 months | −0.06 | 0.26* | 0.69** | ||||
| 5 Length of gestation | 0.37*a | 0.21 | 0.30** | 0.26* | |||
| 6 Postnatal age at scan | 0.39**a | −0.12 | −0.26* | −0.18 | −0.63** | ||
| 7 Age from conception | 0.42** | 0.11 | 0.05 | 0.05 | 0.49** | 0.33** | |
| 7 EPDS prenatal | 0.08 | 0.13 | 0.64** | 0.64** | 0.15 | -0.14 | 0.01 |
* P < 0.05, FA = fractional anisotropy, EPDS = Edinburgh postnatal depression scale.
** P < 0.01.
aA partial correlation adjusted for the other age variable (postnatal age for the length of gestation and length of gestation for postnatal age at scan).
Maternal postnatal depressive symptoms, newborn whole brain fractional anisotropy, and infant negative reactivity
Maternal depressive symptoms at 3 or 6 months postpartum were not related to newborn whole brain FA values. Maternal depressive symptoms at 3 (r = 0.38, P = 0.001) and 6 months (r = 0.26, P = 0.019) were positively related to mother-rated infant negative reactivity. Newborn whole brain FA was not related to mother-rated infant negative reactivity.
The interaction of maternal postpartum depressive symptoms and whole brain fractional anisotropy in predicting infant negative reactivity
The interaction of whole brain FA and maternal postpartum depressive symptoms at 6 months predicted infant negative reactivity at 6 months, so that infants with higher FA exhibited more negative reactivity when exposed to increased maternal symptoms and less negative reactivity when exposed to low maternal symptoms (Table 3 and Figure 1). The interaction between maternal depressive symptoms at 3 months and newborn FA did not predict infant negative reactivity (see the Figure S2 in the Supplementary Material). Results remained significant regardless of the infant age variable included in the model, as well as when controlled for several prenatal exposures (see the Supplementary Material).
Table 3.
The interaction of maternal postpartum depressive symptoms and newborn whole brain FA in predicting infant negative reactivity (N = 80)
| Model 1a 3-month EPDS | Model 1b 6-month EPDS | |||||
|---|---|---|---|---|---|---|
| B (SE) | P | B (SE) | P | P adj. | ∆R2 | |
| Step 1 | ||||||
| Infant sex | −0.04 (0.17) | 0.795 | −0.14 (0.18) | 0.437 | ||
| Parity | 0.36 (0.18) | 0.045 | 0.38*(0.19) | 0.047 | ||
| Alcohol/tobacco use | 0.04 (0.20) | 0.848 | 0.18 (0.21) | 0.386 | ||
| Maternal EPDS (pregnancy) | −0.04 (0.03) | 0.193 | −0.01 (0.03) | 0.702 | ||
| Age from conception | 0.00 (0.01) | 0.778 | 0.00 (0.1) | 0.840 | ||
| Maternal EPDS (postpartum) | 0.38 (0.12) | 0.002 | 0.20 (0.12) | 0.097 | ||
| Whole brain FA | 6.04 (5.48) | 0.274 | 5.96 (5.73) | 0.302 | ||
| Whole brain FA × EPDS (postpartum) | 4.13 (5.83) | 0.48 | 18.20** (6.27) | 0.005 | 0.040 | 0.09** |
** P < 0.01, *P < 0.05, all the beta coefficients and standard errors are unstandardized; the interaction terms in the main models and three sets of post-hoc models were corrected using the Benjamini–Hochberg method; ∆R2 refers to the significant interaction in Model 1b; the results are similar when postnatal age at scan and duration of gestation are controlled for separately.
Fig. 1.
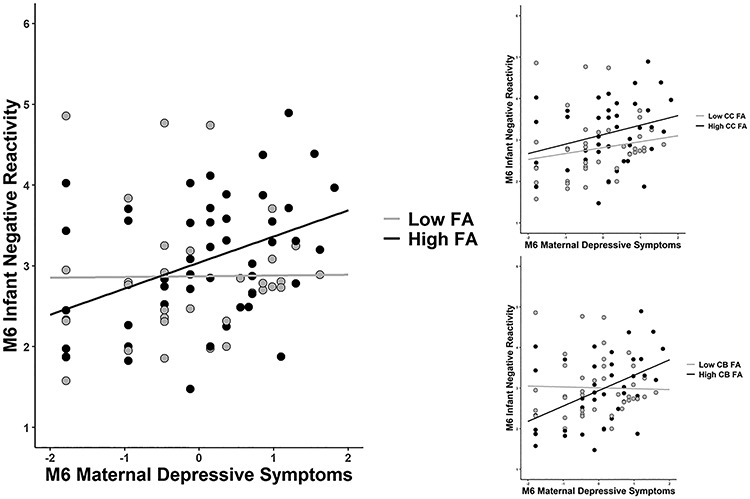
The association between maternal depressive symptoms at 6 months and infant negative reactivity at 6 months: moderation by low and high infant whole brain, CC and CB FA (groups based on median).
Post-hoc analyses: corpus callosum, cingulum bundle and uncinate fascicle
The interaction of maternal depressive symptoms at 6 months and CC FA (B = 10.55, P = 0.020) as well as CB FA (B = 5.21, P = 0.014) predicted infant negative reactivity in a similar manner to whole brain FA, while the interaction between the 3-month depressive symptoms and CC FA was not significant (P = 0.28–0.42). Maternal depressive symptoms in interaction with UF FA were not, however, found to predict infant negative reactivity (P > 0.05) (see detailed results in the Supplementary Material).
Post-hoc analyses: probing the interaction
The simple slope analysis indicated that maternal depressive symptoms at 6 months were associated with higher infant negative reactivity when whole brain FA was high (= 0.27, B = 0.58 the 95% confidence interval = [0.23, 0.92], P = 0.001) or average (= 0.25, B = 0.2579 [0.03, 0.48], P = 0.026), but not when newborn FA was low (= 0.23, B = −0.06 [−0.34, 0.22], P = 0.68). Similar results were replicated for CC FA and CB FA as moderators (see the Supplementary Material).
Post-hoc analyses: continuity of maternal symptoms
We ran the analyses for whole-brain FA and the significant ROIs (CC and CB FA) using a grouping of maternal depressive symptoms based on the continuity of symptoms. All the analyses replicated a similar pattern of interactions as in the models where only the 6-month EPDS was a significant predictor together with infant FA values (see Table 4). Only the infants exposed to continuously high maternal symptoms (high maternal symptoms both at 3 and 6 months) differed from infants exposed to low symptoms (Figure 2). The infants exposed to symptoms only at 6 or at 3 months did not differ from those exposed to low maternal symptoms (when whole brain or CC FA were used as moderator) or either of the other groups (when CB was used as a moderator) in terms of their negative reactivity.
Table 4.
The ANCOVA models testing interaction between white matter FA and maternal depressive symptom continuity across postpartum
| B (SE) | P | ∆ƞ2 | |
|---|---|---|---|
| Continuity of symptoms | 0.077 | ||
| Low vs continuous high | 9.49 (4.17) | 0.026 | |
| Temporary vs continuous high | 9.68 (5.41) | 0.078 | |
| Whole brain FA | 41.39 (15.15) | 0.046 | |
| Whole brain FA × continuity | 0.057 | ||
| Low x FA vs High x FA | −39.56 (16.41) | 0.019 | 0.08 * |
| Temporary x FA vs High x FA | −40.29 (21.55) | 0.066 | 0.05 |
| Low x FA vs Temporary x FA | 0.74 (16.60) | 0.965 | 0.00 |
| Continuity of symptoms | 0.035 | ||
| Low vs continuous high | 9.68 (3.67) | 0.010 | |
| Temporary vs continuous high | 9.36 (5.02) | 0.066 | |
| CC FA | 30.90 (10.59) | 0.066 | |
| CC FA x Continuity | 0.24 | ||
| Low x CC FA vs High x CC FA | −32.12 (11.48) | 0.007 | 0.10 ** |
| Temporary x CC FA vs High x CC FA | −31.056 | 0.053 | 0.05 |
| Low x CC FA vs Temporary x CC FA | −1.06 (12.41) | 0.932 | 0.00 |
| Continuity of symptoms | 0.054 | ||
| Low vs continuous high | 5.804 (2.08) | 0.064 | |
| Temporary vs continuous high | −0.463 (4.85) | 0.924 | |
| CB FA | 10.23 (6.80) | 0.137 | |
| CB FA x Continuity | 0.037 | ||
| Low x CB FA vs High x CB FA | −14.53 (6.95) | 0.040 | 0.06 ** |
| Temporary x CB FA vs High x CB FA | 0.337 (11.18) | 0.976 | 0.00 |
| Low x CB FA vs Temporary x CB FA | −14.86 (8.95) | 0.101 | 0.04 |
* P < 0.05, **P < 0.01. The significant interactions are bolded. All the beta coefficients and standard errors are unstandardized; all the models are controlled for parity, maternal depressive symptoms during pregnancy, child sex, post-conceptional age and tobacco/alcohol exposure during pregnancy.
Fig. 2.
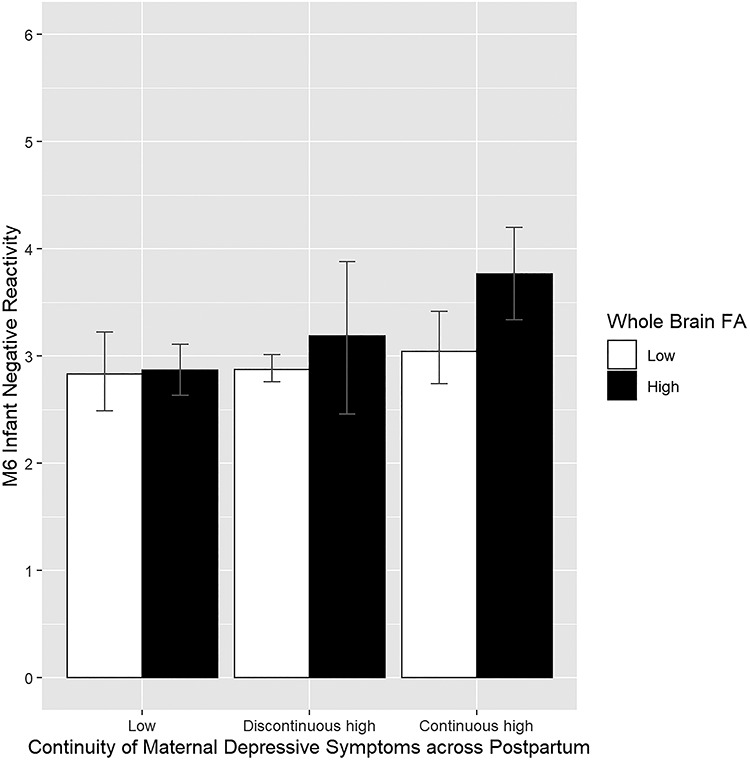
The continuity of maternal depressive symptoms across postpartum and infant negative reactivity within groups of low and high (based on median) whole brain FA. The error bars represent bootstrapped 95% confidence intervals of the group means.
Discussion
The aim of the current study was to investigate whether newborn white matter microstructure measured as FA reflects infant sensitivity to maternal depressive symptoms with an outcome of infant negative reactivity at 6 months. Although maternal depressive symptoms were expectedly found to be related to negative reactivity overall, when examining the moderating effect of newborn whole brain FA, the association was found only among infants with average or high white matter FA as newborns, but not among the infants with low FA. Although the association was detected specifically between 6-month maternal symptoms and infant negative reactivity among infants with higher FA, further analyses suggested that infants with higher FA that were exposed to continuously elevated maternal depressive symptoms differed from those exposed to low or discontinuously high maternal depressive symptoms, and were at specific risk of showing heightened negative reactivity. This suggests that white matter FA may play a role in linking longer term exposure to maternal distress and infant reactivity, an association reported in previous studies (Giallo et al., 2015; Prenoveau et al., 2017). Similar findings were detected when using CC and CB FA, but not UF FA, as a moderator.
Better understanding of the interindividual differences in susceptibility to maternal mental health and subsequent offspring psychopathology has been stressed in the literature (Meaney, 2018). Moreover, conceptually similar results have been reported in adolescent populations linking limbic volumes or reduced frontal cortical thinning to susceptibility to the effects of parenting (Yap et al., 2008; Whittle et al., 2011; Deane et al., 2019). Furthermore, a recent study reported larger newborn hippocampal volumes underlying infant susceptibility to maternal sensitivity in terms of later disorganized attachment style (Rifkin-Graboi et al., 2019). The current study is thus among the first to report conceptually similar associations in early infancy when postnatal factors have only minimally shaped the brain, and the first to reports such associations with a focus on white matter metrics as a moderator. The study is of specific importance because white matter metrics typically show a mixed pattern of associations with several psychiatric and developmental outcomes. Our findings provide preliminary evidence for overall white matter FA underlying postnatal plasticity, proposing that high white matter FA at birth may moderate the sensitivity of the infant to the influence of continuously elevated levels of postpartum maternal distress. However, due to the small sample and the novelty of the findings, more research is needed to replicate the findings and make conclusions about the type of susceptibility.
Our finding that high newborn whole brain FA values were relevant for infant susceptibily in terms of negative reactivity was rather surprising, because lower FA values are typically related to psychopathology, including mood disorders (Barnea-Goraly et al., 2005; Vulser et al., 2018), which in turn are predicted by childhood negative reactivity. However, higher FA values have also been linked with behavioral problems in pediatric and adolescent populations (Wolff et al., 2012; Koch et al., 2014). Prenatal and early postnatal periods are periods of heightened neural sensitivity to environmental influences (Weiss and Wagner, 1998), such as stress generated by low parental mood and subsequent poorer quality of caregiving (Field, 2010). One possibility is that in some infants, higher FA values in the newborn period reflect more rapid neural development and premature myelination, which in turn makes them more susceptible to the current environment. This view is supported by studies linking neurotrophic factors to brain maturation and neuroplasticity in the postnatal period (Dyer et al., 2016; Kowiański et al., 2018), as well as a growing number of studies reporting that early adversity is associated with both accelerated brain maturation and a risk for later developmental problems (Callaghan and Tottenham, 2016; Posner et al., 2016; Thijssen et al., 2017; Spann et al., 2019). Further, in line with our findings, one adult study has shown that lower FA values may reflect prolonged/treatment-resistant depression (less plasticity and response to environment) (De Diego-Adeliño et al., 2014). Thus, FA metrics may reflect sensitivity to environment across different age groups.
We detected the association using CC and CB FA, but against our hypothesis, not using UF FA as moderators. Interestingly, CC and CB were among areas indicated to have decreased FA in adults with treatment-resistant depression, that is, less sensitivity to intervention (De Diego-Adeliño et al., 2014). Moreover, white matter development is highly dynamic, and some studies have suggested that FA values in some areas may even decrease around birth (Gupta et al., 2005; Bockhorst et al., 2008). Thus, alternatively, higher FA values at a certain stage of infancy and in certain areas of the brain may also reflect atypical brain development and later risk for abnormal emotional development (Wolff et al., 2012).
One further possibility not analyzed in the current study is that the relation between parental symptoms of depression and infant white matter FA is genetically determined (Carballedo et al., 2012; Whalley et al., 2013; Kochunov et al., 2015). Additionally, epigenetic and other programming caused by parental symptoms of depression during pregnancy (Fatemi et al., 2009; El Marroun et al., 2018) and before conception could also affect newborn brain characteristics. As mentioned earlier, a growing number of studies have linked early life adversities to accelerated brain maturation (e.g. Gee et al., 2013; Spann et al., 2019). Thus, poorer parental mental health prior to and during pregnancy may program child brain development through epigenetic mechanisms toward more accelerated neurodevelopment reflected in white matter microstructure. The offspring with this neural phenotype may then also be programmed toward more susceptibility to the postnatal environment, and consequently, the behavior varies as a function of postnatal environment (parental distress) only within these offspring. In this study, the hypothesized interaction was found after controlling for several prenatal exposures. Given the lack of other similar studies, a more detailed understanding about the mechanisms underlying the findings is needed to make conclusions about the (epi)genetic basis of the findings.
In the present study, we had an adequate sample size for a newborn imaging study and wide coverage of validated questionnaire data. However, the findings of this study can only be considered preliminary for several reasons. First, we had relatively few mothers with low education and symptoms that could be considered clinically relevant, warranting ideally a replication in a sample with clinically depressed parents. Instead, our findings provide some insights into the neural mechanisms of normal variation in early negative reactivity, a precursor of later behavioral development. Second, postpartum depression is only a distant measure of environment and should optimally be considered together with measures of parenting or family well-being. Third, our analyses only focused on average FA values instead of larger brain networks or the other parameters of white matter microstructure, encouraging future studies to utilize simultaneous assessment of whole-brain connectivity. Fourth, major limitations are the maternal ratings of their own depressive symptoms as well as infant negative reactivity, leading to a possibility that mothers with higher symptoms are more prone to rate their infants’ distress (Richters and Pellegrini, 1989), although some studies also contradict the existence of this bias (Forman et al., 2003).
We conclude that the association between maternal depressive symptoms, especially continuously elevated levels of symptoms, and infant negative reactivity, a key trait predicting later psychiatric disorders, was modulated by higher newborn whole brain FA. The findings, although still very preliminary, have important implications for studies of environmental sensitivity and differential susceptibility, suggesting that inter-individual variation in white matter microstructure at birth may underlie infant sensitivity to parental distress.
Funding
This research work was supported by Alexander von Humboldt Foundation (S.N.), Emil Aaltonen Foundation (S.N. and J.J.T.), Hospital District of South-Western Finland State Research Grants for Clinical Research (J.J.T., H.M., N.S., L.K. and H.K.), Alfred Kordelin Foundation (J.J.T.), Turku University Foundation (J.J.T.), Sigrid Juselius Foundation (H.M.), Finnish Cultural Foundation (H.M.), Signe and Ane Gyllenberg Foundation (S.N., N.S., R.K., L.K. and HK), Yrjö Jahnsson Foundation (L.K.), the Brain and Behavior Research Foundation (YI Grant 19056; L.K.), Academy of Finland Research Council for Health (308176; L.K. and 253270, 264363 and 134950; H.K.), the Academy of Finland Research Council for Culture and Society (2608063; R.K.) and Jane and Aatos Erkko Foundation (H.K.).
Declaration of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgements
The FinnBrain staff and assisting personnel are acknowledged for their invaluable efforts for the logistics of the project.
References
- Aiken L.S., West S.G. (1991). Multiple Regression : Testing and Interpreting Interactions, Sage Publications. [Google Scholar]
- Andersson J., Sotiropoulos S.N. (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L., Sundström-Poromaa I., Wulff M., Åström M., Bixo M. (2006). Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstetricia et Gynecologica Scandinavica, 85, 937–44. [DOI] [PubMed] [Google Scholar]
- Arnone D., McIntosh A.M., Chandra P., Ebmeier K.P. (2008). Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatrica Scandinavica, 118, 357–62. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., et al. (2005). White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex, 15, 1848–54. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Chang K.D., Karchemskiy A., Howe M.E., Reiss A.L. (2009). Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biological Psychiatry, 66, 238–44. [DOI] [PubMed] [Google Scholar]
- Bellani M., Yeh P.-H., Tansella M., Balestrieri M., Soares J.C., Brambilla P. (2009). DTI studies of corpus callosum in bipolar disorder. Biochemical Society Transactions, 37, 1096–8. [DOI] [PubMed] [Google Scholar]
- Belsky J., Pluess M. (2009). The nature (and nurture?) of plasticity in early human development. Perspectives on Psychological Science, 4, 345–51. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Bollettini I., Poletti S., et al. (2015). White matter microstructure in bipolar disorder is influenced by the serotonin transporter gene polymorphism 5-HTTLPR. Genes, Brain and Behavior, 14, 238–50. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B, 57, 289–300. [Google Scholar]
- Bergink V., Kooistra L., Lambregtse-van den Berg M.P., et al. (2011). Validation of the Edinburgh depression scale during pregnancy. Journal of Psychosomatic Research, 70, 385–9. [DOI] [PubMed] [Google Scholar]
- Bockhorst K.H., Narayana P.A., Liu R., et al. (2008). Early postnatal development of rat brain: in vivo diffusion tensor imaging. Journal of Neuroscience Research, 86, 1520–8. [DOI] [PubMed] [Google Scholar]
- Boyce W.T. (2016). Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology, 41, 142–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb E.J., Metzler-Baddeley C., Aggleton J.P. (2018). The cingulum bundle: anatomy, function, and dysfunction. Neuroscience and Biobehavioral Reviews, 92, 104–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. (2016). The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology Reviews, 41, 163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S.B., Cohn J.F., Meyers T. (1995). Depression in first-time mothers: mother-infant interaction and depression chronicity. Developmental Psychology, 31, 349–57. [Google Scholar]
- Carballedo A., Amico F., Ugwu I., et al. (2012). Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159B, 537–48. [DOI] [PubMed] [Google Scholar]
- Charil A., Laplante D.P., Vaillancourt C., King S. (2010). Prenatal stress and brain development. Brain Research Reviews, 65, 56–79. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. (1987). Detection of postnatal depression. development of the 10-item Edinburgh postnatal depression scale. The British Journal of Psychiatry : The Journal of Mental Science, 150, 782–6. [DOI] [PubMed] [Google Scholar]
- De Diego-Adeliño J., Pires P., Gómez-Ansón B., et al. (2014). Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychological Medicine, 44, 1171–82. [DOI] [PubMed] [Google Scholar]
- De Pauw S.S.W., Mervielde I. (2010). Temperament, personality and developmental psychopathology: a review based on the conceptual dimensions underlying childhood traits. Child Psychiatry and Human Development, 41, 313–29. [DOI] [PubMed] [Google Scholar]
- Dean D.C., Planalp E.M., Wooten W., et al. (2018). Association of prenatal maternal depression and anxiety symptoms with infant white matter microstructure. JAMA Pediatrics, 53705, 973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane C., Vijayakumar N., Allen N.B., et al. (2019). Parenting × brain development interactions as predictors of adolescent depressive symptoms and well-being: differential susceptibility or diathesis-stress? Development and Psychopathology, 32, 1–12. [DOI] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Hüppi P.S., Hertz-Pannier L. (2014). The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. [DOI] [PubMed] [Google Scholar]
- Dyer A.H., Vahdatpour C., Sanfeliu A., Tropea D. (2016). The role of insulin-like growth factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience, 325, 89–99. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M., Eskild A., Tambs K., Schei B., Opjordsmoen S. (2001). The Edinburgh postnatal depression scale: validation in a Norwegian community sample. Nordic Journal of Psychiatry, 55, 113–7. [DOI] [PubMed] [Google Scholar]
- El Marroun H., Zou R., Muetzel R.L., et al. (2018). Prenatal exposure to maternal and paternal depressive symptoms and white matter microstructure in children. Depression and Anxiety, 35, 1–9. [DOI] [PubMed] [Google Scholar]
- Else-Quest N.M., Hyde J.S., Goldsmith H.H., Van Hulle C.A. (2006). Gender differences in temperament: a meta-analysis. Psychological Bulletin, 132, 33–72. [DOI] [PubMed] [Google Scholar]
- Fatemi S.H., Folsom T.D., Reutiman T.J., et al. (2009). Abnormal expression of myelination genes and alterations in white matter fractional anisotropy following prenatal viral influenza infection at E16 in mice. Schizophrenia Research, 112, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. (2010). Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behavior and Development, 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish M., Stifter C.A. (1993). Mother parity as a main and moderating influence on early mother-infant interaction. Journal of Applied Developmental Psychology, 14, 557–72. [Google Scholar]
- Forman D.R., O’Hara M.W., Larsen K., Coy K.C., Gorman L.L., Stuart S. (2003). Infant emotionality: observational methods and the validity of maternal reports. Infancy, 4, 541–65. [Google Scholar]
- Gee D.G., Gabard-durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. (2013). Early developmental emergence of human amygdala – prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110, 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallo R., Woolhouse H., Gartland D., Hiscock H., Brown S. (2015). The emotional–behavioural functioning of children exposed to maternal depressive symptoms across pregnancy and early childhood: a prospective Australian pregnancy cohort study. European Child and Adolescent Psychiatry, 24, 1233–44. [DOI] [PubMed] [Google Scholar]
- Giannelli M., Cosottini M., Michelassi M.C., et al. (2010). Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. Journal of Applied Clinical Medical Physics, 11, 176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Corouge I., et al. (2007). Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. American Journal of Neuroradiology, 28, 1789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Knickmeyer R.C., Gao W. (2018). Imaging structural and functional brain development in early childhood. Nature Reviews Neuroscience, 19, 123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault J.B., Cornea E., Goldman B.D., Knickmeyer R.C., Styner M., Gilmore J.H. (2019). White matter microstructural development and cognitive ability in the first 2 years of life. Human Brain Mapping, 40, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Lee P.S., Maisog J.M., et al. (2011). Strength of default mode resting-state connectivity relates to white matter integrity in children. Developmental Science, 14, 738–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven C.U., Lionetti F., Booth C., et al. (2019). Sensory processing sensitivity in the context of environmental sensitivity: a critical review and development of research agenda. Neuroscience and Biobehavioral Reviews, 98, 287–305. [DOI] [PubMed] [Google Scholar]
- Gupta R.K., Hasan K.M., Trivedi R., et al. (2005). Diffusion tensor imaging of the developing human cerebrum. Journal of Neuroscience Research, 81, 172–8. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. (2017). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach in Series Methodology in the Social Sciences, 2nd edn, New York, NY, US: Guilford Press. [Google Scholar]
- Horn A., Ostwald D., Reisert M., Blankenburg F. (2014). The structural-functional connectome and the default mode network of the human brain. NeuroImage, 102part, 142–51. [DOI] [PubMed] [Google Scholar]
- Jackowski A.P., Douglas-Palumberi H., Jackowski M., et al. (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Research: Neuroimaging, 162, 256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L.M., Barba A., Campbell M., et al. (2016). Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. NeuroImage. Clinical, 12, 1022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. (2012). Fsl. NeuroImage, 62, 782–90. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.D., Jones D.K., Sijbers J. (2013). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping, 34, 2747–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L., Tolvanen M., Scheinin N.M., et al. (2018). Cohort profile: the FinnBrain birth cohort study (FinnBrain). International Journal of Epidemiology, 47, 15–16j. [DOI] [PubMed] [Google Scholar]
- Kim J.-M., Stewart R., Kim S.-W., et al. (2007). Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry, 62, 423–8. [DOI] [PubMed] [Google Scholar]
- Kingston D., Tough S., Whitfield H. (2012). Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry and Human Development, 43, 683–714. [DOI] [PubMed] [Google Scholar]
- Koch K., Reeß T.J., Rus O.G., Zimmer C., Zaudig M. (2014). Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. Journal of Psychiatric Research, 54, 26–35. [DOI] [PubMed] [Google Scholar]
- Kochunov P., Jahanshad N., Marcus D., et al. (2015). Heritability of fractional anisotropy in human white matter: a comparison of human Connectome project and ENIGMA-DTI data. NeuroImage, 111, 300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyrka-Allchorne K., Wass S.V., Sonuga-Barke E.J.S. (2019). Research review: do parent ratings of infant negative emotionality and self-regulation predict psychopathology in childhood and adolescence? A systematic review and meta-analysis of prospective longitudinal studies. Journal of Child Psychology and Psychiatry, 61, 401–16. [DOI] [PubMed] [Google Scholar]
- Kowiański P., Lietzau G., Czuba E., Waśkow M., Steliga A., Moryś J. (2018). BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 38, 579–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpulainen V., Lehtola S.J., Tuulari J.J., et al. (2020). Prevalence and risk factors of incidental findings in brain MRIs of healthy neonates—the FinnBrain birth cohort study. Frontiers in Neurology, 10, 1347. doi: 10.3389/fneur.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A.L.T., Brambilla P., Sassi R.B., et al. (2005). Anatomical MRI study of corpus callosum in unipolar depression. Journal of Psychiatric Research, 39, 347–54. [DOI] [PubMed] [Google Scholar]
- Lebel C., Walton M., Letourneau N., Giesbrecht G.F., Kaplan B.J., Dewey D. (2016). Prepartum and postpartum maternal depressive symptoms are related to children’s brain structure in preschool. Biological Psychiatry, 80, 859–68. [DOI] [PubMed] [Google Scholar]
- Lehtola S.J., Tuulari J.J., Karlsson L., et al. (2019). Associations of age and sex with brain volumes and asymmetry in 2–5-week-old infants. Brain Structure and Function, 224, 501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kaaya S., Chai J., et al. (2017). Maternal depressive symptoms and early childhood cognitive development: a meta-analysis. Psychological Medicine, 47, 680–9. [DOI] [PubMed] [Google Scholar]
- Luoma I., Tamminen T., Kaukonen P., et al. (2001). Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child & Adolescent Psychiatry, 40, 1367–74. [DOI] [PubMed] [Google Scholar]
- Markant J.M., Thomas K.M. (2013). Postnatal brain development In: Zelazo P.D., editor. Oxford Handbook of Developmental Psychology, Vol. 1, Body and Mind, New York, NY, US: Oxford University Press, pp. 129–63. [Google Scholar]
- Meaney M.J. (2018). Perinatal maternal depressive symptoms as an issue for population health. American Journal of Psychiatry, 175, 1084–93. [DOI] [PubMed] [Google Scholar]
- Merisaari H., Tuulari J.J., Karlsson L., et al. (2019). Test-retest reliability of diffusion tensor imaging metrics in neonates. NeuroImage, 197, 598–607. [DOI] [PubMed] [Google Scholar]
- Moehler E., Brunner R., Wiebel A., Reck C., Resch F. (2006). Maternal depressive symptoms in the postnatal period are associated with long-term impairment of mother-child bonding. Archives of Women’s Mental Health, 9, 273–8. [DOI] [PubMed] [Google Scholar]
- Ni H., Kavcic V., Zhu T., Ekholm S., Zhong J. (2006). Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. American Journal of Neuroradiology, 27, 1776–81. [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K.J., Meaney M.J. (2017). Fetal origins of mental health: the developmental origins of health and disease hypothesis. American Journal of Psychiatry, 174, 319–28. [DOI] [PubMed] [Google Scholar]
- Oguz I., Farzinfar M., Matsui J., et al. (2014). DTIPrep: quality control of diffusion-weighted images. Frontiers in Neuroinformatics, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Mori S., Donohue P.K., et al. (2011). Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. NeuroImage, 56, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Cha J., Roy A.K., et al. (2016). Alterations in amygdala–prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry, 6, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenoveau J.M., Craske M.G., West V., et al. (2017). Maternal postnatal depression and anxiety and their association with child emotional negativity and behavior problems at two years. Developmental Psychology, 53, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulli E.P., Kumpulainen V., Kasurinen J.H., et al. (2018). Prenatal exposures and infant brain: review of magnetic resonance imaging studies and a population description analysis. Human Brain Mapping, 40, 1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam S.P., Helbig A.L., Gartstein M.A., Rothbart M.K., Leerkes E. (2014). Development and assessment of short and very short forms of the infant behavior questionnaire–revised. Journal of Personality Assessment, 96, 445–58. [DOI] [PubMed] [Google Scholar]
- Qiu A., Mori S., Miller M.I. (2015). Diffusion tensor imaging for understanding brain development in early life. Annual Review of Psychology, 66, 853–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A Language and Environment for Statistical Computing, Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramchandani P., Psychogiou L., Vlachos H., et al. (2011). Paternal depression: an examination of its links with father, child and family functioning in the postnatal period. Depression and Anxiety, 28, 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J.M., Kruggel F., Gilmore J.H., et al. (2017). A novel maturation index based on neonatal diffusion tensor imaging reflects typical perinatal white matter development in humans. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience, 56, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richters J., Pellegrini D. (1989). Depressed mothers’ judgments about their children: an examination of the depression-distortion hypothesis. Child Development, 60, 1068–75. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Meaney M.J., Chen H., et al. (2015). Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 313–21.e2. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Tan H.M., Shaun G.K.Y., et al. (2019). An initial investigation of neonatal neuroanatomy, caregiving, and levels of disorganized behavior. Proceedings of the National Academy of Sciences of the United States of America, 116, 16787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf D.R., Quarmley M., Elliott M.A., et al. (2016). The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. NeuroImage, 125, 903–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman G.I., Newman D.A., Fraley R.C., Haltigan J.D., Groh A.M., Haydon K.C. (2012). Distinguishing differential susceptibility from diathesis–stress: recommendations for evaluating interaction effects. Development and Psychopathology, 24, 389–409. [DOI] [PubMed] [Google Scholar]
- Roland J.L., Snyder A.Z., Hacker C.D., et al. (2017). On the role of the corpus callosum in interhemispheric functional connectivity in humans. Proceedings of the National Academy of Sciences of the United States of America, 114, 13278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Sinha R., Cross S.N., et al. (2017). Does prenatal stress alter the developing connectome? Pediatric Research, 81, 214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriber R.A., Anbari Z., Robins R.W., Conger R.D., Hastings P.D., Guyer A.E. (2017). Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clinical Psychological Science, 5, 632–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Johansen-Berg H., Jenkinson M., et al. (2007). Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols, 2, 499–503. [DOI] [PubMed] [Google Scholar]
- Spann M.N., Bansal R., Hao X., Rosen T.S., Peterson B.S. (2019). Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychology, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Carrasco M., Wiggins J.L., Thomason M.E., Monk C.S. (2014). Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. NeuroImage, 86, 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham E.L., Ramasubbu R., Gaxiola-Valdez I., et al. (2016). White matter integrity in major depressive disorder: implications of childhood trauma, 5-HTTLPR and BDNF polymorphisms. Psychiatry Research: Neuroimaging, 253, 15–25. [DOI] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., et al. (2017). Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Development and Psychopathology, 29, 505–18. [DOI] [PubMed] [Google Scholar]
- Tost H., Alam T., Geramita M., et al. (2013). Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology, 38, 525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Heide R., Vyas G., Olson I.R. (2013). The social network-network: size is predicted by brain structure and function in the amygdala and paralimbic regions. Social Cognitive and Affective Neuroscience, 9, 1962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulser H., Paillère Martinot M.-L., Artiges E., et al. (2018). Early variations in white matter microstructure and depression outcome in adolescents with subthreshold depression. American Journal of Psychiatry, 175, 1255–64. [DOI] [PubMed] [Google Scholar]
- Walterfang M., Wood A.G., Reutens D.C., et al. (2008). Morphology of the corpus callosum at different stages of schizophrenia: cross-sectional study in first-episode and chronic illness. British Journal of Psychiatry, 192, 429–34. [DOI] [PubMed] [Google Scholar]
- Weiss M.J.S., Wagner S.H. (1998). What explains the negative consequences of adverse childhood experiences on adult health? Insights from cognitive and neuroscience research. American Journal of Preventive Medicine, 14, 356–60. [DOI] [PubMed] [Google Scholar]
- West A.E., Newman D.L. (2003). Worried and blue: mild parental anxiety and depression in relation to the development of young children’s temperament and behavior problems. Parenting, 3, 133–54. [Google Scholar]
- Whalley H.C., Sprooten E., Hackett S., et al. (2013). Polygenic risk and white matter integrity in individuals at high risk of mood disorder. Biological Psychiatry, 74, 280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Sheeber L., et al. (2011). Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Development and Psychopathology, 23, 115–29. [DOI] [PubMed] [Google Scholar]
- Wolff J.J., Gu H., Gerig G., et al. (2012). Differences in white matter Fiber tract development present from 6 to 24 months in infants with autism. American Journal of Psychiatry, 169, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.B.H., Whittle S., Yücel M., et al. (2008). Interaction of parenting experiences and brain structure in the prediction of depressive symptoms in adolescents. Archives of General Psychiatry, 65, 1377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


