Abstract
Pathogenic variants in RANBP2 cause autosomal dominant familial and recurrent Acute Necrotizing Encephalopathy of Childhood (ANEC). Affected children typically experience a 3-stage disease: a 3 to 5 days prodrome of non-specific febrile illness, acute encephalopathy, and recovery with or without neurological sequelae or death. Neuroradiological finding of bilateral symmetrical thalamic lesions raise the suspicion of this diagnosis. A devastating disease, reported mortality approaches 1/3 of those affected and only approximately 10% of patients recover completely without sequelae. We report a Malaysian family with RANBP2 pathogenic variant c.1754C>T (p.Thr585Met). The clinical presentation and course over a maximum of 7 years, as well as neuroradiological features of the 3 affected children are described. In contrast to the reported high mortality and morbidity, our patients have recovered with minor sequelae. We would like to highlight the absence of pathogenic variants in both parents' blood, raising the possibility of germline mosaicism in one of the parents as the underlying genetic mechanism of inheritance. To our knowledge, this is the first report of germline mosaicism in RANBP2 Susceptibility to Infection-induced Encephalopathy.
Keywords: Bilateral symmetrical thalamic hyperintensities, ANEC, RANBP2
1. Introduction
Acute Necrotizing Encephalopathy of Childhood (ANEC) is an immune mediated, para-infectious inflammatory encephalitis that typically affect healthy children younger than 6 years old. It is usually sporadic and does not recur [8,9]. In 2009, a separate entity of familial and recurrent ANEC have been reported to be associated with pathogenic variants in the RAN-binding protein 2 (RANBP2), a gene encoding nuclear pore components [10,11]. These proteins have important functions in nuclear and pro-inflammatory signaling, as well as mitochondrial trafficking. A loss of function of RANBP2 results in disruption of cell processes, leading to cytokine dysregulation, breakdown of blood brain barrier and disordered oxidative phosphorylation and energy metabolism in neuronal cells. These translate clinically as increased risk of stress-induced neurotoxicity in the affected individual [5,7,11]. This disease is synonymously known as RANBP2 Susceptibility to Infection-induced Encephalopathy, Infection-induced Acute Encephalopathy 3 (OMIM 608033) and ANE1. Clinical presentation, as in ANEC, involves a prodrome of common childhood febrile illness, progressing rapidly to neurologic dysfunction that manifests as altered sensorium, seizures, ataxia, decerebrate or decorticate posturing. Investigations exclude intracranial but may uncover non-neuronopathic infections e.g. pneumonia. Implicated organisms include influenza, parainfluenza, herpes, enteroviruses, coxsackie, rotavirus and mycoplasma among others [[8], [9], [10]]. Distinctive pathologic lesions on magnetic resonance imaging (MRI) are bilateral symmetrical signal abnormalities in thalami, brainstem, temporal lobe, limbic system, external capsule and occasionally basal ganglia; these lesions may undergo hemorrhagic conversion [[2], [3],5,6,8,10,[13], [14],15]. ANE1 follows an autosomal dominant Mendelian inheritance pattern but with incomplete penetrance [[10], [11], [12]], which could contribute to underdiagnosis. Its prevalence and incidence are unknown; to date 85 cases have been reported worldwide [5].
2. Case series
The three sisters presented here are born of unrelated Malay parents. They achieved normal neurodevelopmental milestones and were healthy pre-morbidly.
2.1. Sibling 1
The proband [III-2] presented at 11 years old with acute onset of blurred vision, headache and disorientation. Leading up to this, she had a 3-day history of low-grade fever, lethargy, nausea and emesis. Physical examination revealed irritability, brisk deep tendon reflexes with extensor Babinski response, optic disc swelling, reduced vision and color vision in both eyes as well as right homonymous hemianopia on Bjerrum screen chart. Blood cell counts, renal and liver functions, serum calcium and phosphate, blood ammonia and lactate, plasma amino acids, urine organic acids, dried blood spot acylcarnitine profile were normal. Lumber puncture yielded dry tap. She was treated empirically with IV Ceftriaxone and Acyclovir for intracranial infection and oral prednisolone for possible optic neuritis. Brain MRI were suggestive of ANEC (Table 1 and Fig. 2). On day 7 of admission, she was started on Methylprednisolone (30 mg/kg/day for 5 days) and intravenous Immunoglobulin (1 g/kg × 2 doses). Blood culture and nasopharyngeal aspirate for virology were subsequently reported normal. Visual impairment improved significantly within one week of completing Methylprednisolone and IVIg. Headache and irritability had resolved completely at this stage. At 1 month after her presentation, neurological examination was normal except for a small visual field defect of right homonymous hemianopia detectable only on Bjerrum screen chart.
Table 1.
Summary of clinical features and investigation results of the three siblings.
| Sibling 1 (III-2) | Sibling 2 (III-3) | Sibling 3 (III-6) | |
|---|---|---|---|
| Age at presentation | 11 years old | 4½ years old | 2½ years old |
| Prodrome | 3 days of fever, nausea & vomiting | 4 days of chesty cough, fever & rigor | 5 days of fever & anorexia; 1 day of diarrhoea |
| Acute encephalopathy | |||
| Presenting symptoms | Blurring of vision, confusion | Impaired conscious level | Several short episodes of upward gaze |
| Physical signs | Hypertonia, hyper-reflexia and extensor Babinski response | Shock and acute kidney injury | Loss of vision |
| Impaired vision & color vision | Right hemiparesis | ||
| Right homonymous hemianopia | Impaired swallow | ||
| Infective screening | Normal | + Parainfluenza I (nasopharyngeal aspirate) | + Mycoplasma Pneumoniae titre 1:80 (blood) |
| No cerebral spinal fluid (CSF) analysis | CSF – normal | + Parainfluenza I (nasopharyngeal aspirate) | |
| CSF – normal | |||
| MRI brain | Bilateral symmetrical abnormal signal intensities in the lateral geniculate nuclei of the thalami, temporal lobes, midbrain and left side of the pons | Symmetrical diffuse involvement of thalami, midbrain (sparing medulla) and temporal lobes with hemorrhagic components | Diffuse hyperintensity in both temporal lobes, insula cortices, limbic systems (hippocampus and amygdala), pons, midbrain, thalami and external capsule with hemorrhagic components |
| Sequelae onfollow up | 2½ years follow-up: Mild learning disability (LD), right homonymous hemianopia | 7 years follow-up: Mild LD, clumsy, right sided hyper-reflexia but normal power | 3½ years follow-up: Normal development and neurology |
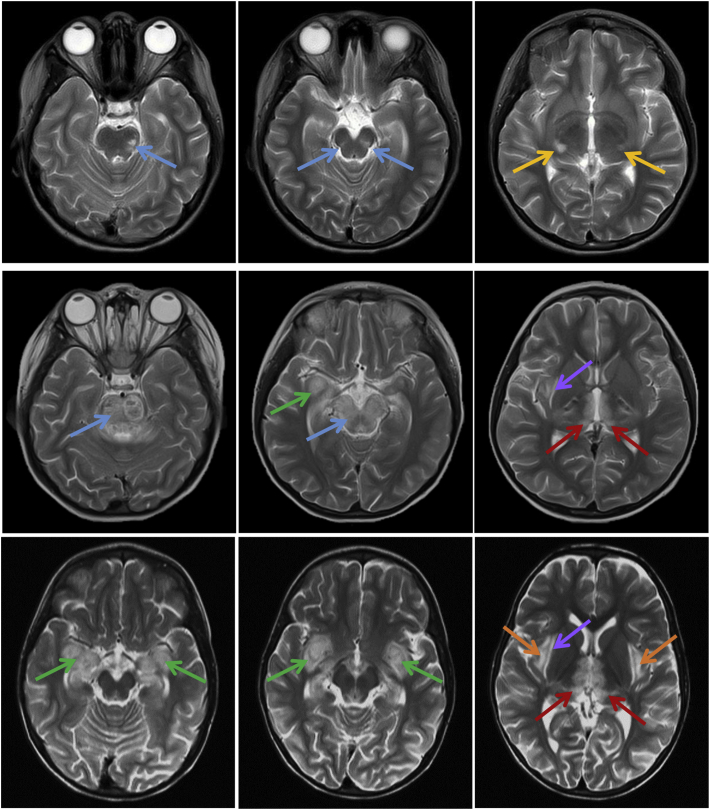
Fig. 2.
Brain MRI findings in the three siblings. T2-weighted images obtained during the acute encephalopathy (top row – Sibling 1, middle – Sibling 2, bottom – Sibling 3). Abnormal signals were visible in the brainstem [pons & midbrain] (blue arrow), lateral geniculate nuclei (yellow arrow), temporal lobes (green arrow), thalami (red arrow), external capsules (purple arrow) and insular cortices (orange arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At her current age of 13½ years old, her parents report that she has difficulty in concentration and has fallen below average in school. She has normal visual acuity and neurological examination except for the residual visual field defect as documented after the acute episode.
During her admission with acute encephalopathy, it came to light that in the past, her 3rd sister was treated for Acute Disseminated Encephalomyelitis (ADEM) and youngest sister for Herpes Encephalitis.
2.2. Sibling 2
The 3rd child of this family [III-3] is the first twin of dichorionic diamniotic pregnancy. She presented at 4½ years old with 4 days history of chesty cough and high-grade fever with rigors. Upon admission to the ward, she was found to have altered sensorium and required invasive ventilation for deteriorating Glasgow Coma Scale; GCS was E4V1M5. Upon admission to Paediatric Intensive Care Unit, she was treated for septic shock with unstable haemodynamic status, respiratory failure and end organ damage namely acute kidney injury. Cerebral spinal fluid (CSF) examination was normal, including PCR for Herpes Simplex 1, Dengue, Japanese Encephalitis and Parainfluenza 1 Viruses. MRI brain was suggestive of ADEM (Table 1 & Fig. 2). In intensive care, she received 1 week of IV Ceftriaxone, 21 days of IV Acyclovir, 5 days of IV Ciprofloxacin and 3 doses of IV Immunoglobulin. She was supported by mechanical ventilation for 10 days and stayed in the ward for a further 15 days. Nasopharyngeal aspirate immunofluorescence was positive for Parainfluenza 1. Upon discharge, renal impairment had resolved completely. She could not walk and had right lower limb weakness, hyper-reflexia and clonus, nystagmus on right lateral gaze and swallow incoordination. She required nasogastric tube feeding for a week post discharge. She regained independent ambulation in the few months after discharge with the help of physical and occupational therapy.
She has been followed up for 7 years since the event. Neurological examination reveals a narrow-based gait but inability to do Tandem gait, normal power and tone, and hyper-reflexia detected only in her right lower limb. Ophthalmology assessment is normal except for mild astigmatism. Her parents report that she has mild learning difficulty; she attends a normal national school.
2.3. Sibling 3
The youngest child [III-6] presented at 2½ years old with fever and anorexia for 5 days with 1 day of diarrhoea. While admitted to the ward, she had several short episodes of upward gaze suggestive of seizures. There was no jerking movement observed. She was febrile with temperature of 38 °C; neurological examination was normal except for inability to fix and follow objects and absence of light perception. CSF examination was normal, including PCR for Herpes Simplex 1 and 2, Parainfluenza and Human Herpes 6 Viruses. Electroencephalography was abnormal for diffuse background slow-wave forms suggestive of encephalopathy. Together with the documented MRI changes (Table 1 & Fig. 2), she was suspected to have Herpes Encephalitis. She was treated with Ceftriaxone for 1 week and Acyclovir for 3 weeks. Subsequently, nasopharyngeal aspirate immunofluorescence was positive for Parainfluenza 1 and blood IgM serology for Mycoplasma Pneumoniae was also positive at titre of 1:80. Upon discharge, she had visual impairment but recovered gradually and completely.
She has been followed up for 3½ years since the event. She does not have any neurological deficits and has normal cognition.
3. Molecular analysis
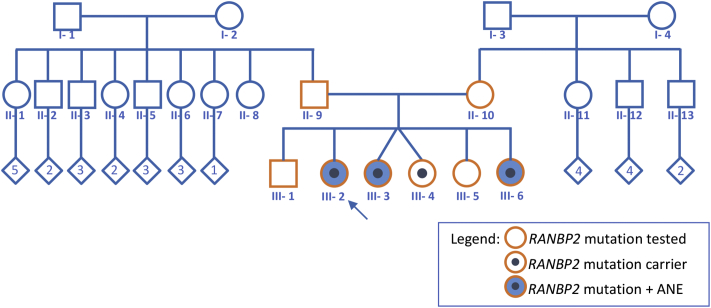
DNA isolated from the blood of the proband was submitted for Sanger sequencing of the RANBP2 gene. This yielded heterozygous pathogenic variant c.1754C>T (p.Thr585Met). This variant has been previously reported to be associated with ANE1 [1,4]. All first-degree family members were tested and this variant is found in the other two affected sisters and one other unaffected sibling (III-4) (Fig. 1).
Fig. 1.
Family pedigree.
4. Discussion and conclusion
We present here the first report of a family with ANE1 in Southeast Asia with the RANBP2 pathogenic variant c.1754C>T (p.Thr585Met) and relatively favorable neurological course.
ANE1 is a rare disease with no pathognomonic clinical or neuroradiologic signs. In the diagnostic evaluation of our patients, we have considered a myriad of differential diagnoses which we divide broadly into the following groups of disorders: (1) post-infectious demyelinating disorders e.g. ADEM or multiple sclerosis, (2) intracranial infections e.g. viral or bacterial meningoencephalitis, (3) mitochondrial cytopathy e.g. Leigh Syndrome or POLG-related disorders. The paramount clue of recurrence in siblings made sporadic conditions in the first two groups less likely. ‘Relevant negatives’ of normal premorbid cognition, unremarkable metabolic investigations and lack of subsequent neuro-regression post-encephalopathy pointed away from an inborn error of mitochondrial metabolism.
Acute encephalopathy of ANE1 may present as seizures, altered sensorium or any focal neurological deficit. We would like to highlight that two of our patients presented with loss of vision in the acute period, one of them with residual visual field defect. This is an unusual focal neurological sign for ANE1. The pathology can be correlated to the thalamic and more specifically lateral geniculate nuclei lesions as seen in the MRI brain of these patients. Sibling 2 had a dramatic presentation with acute encephalopathy and shock. Initially treated as septic shock, retrospectively we postulate the cause of shock to be due to brainstem injury, cytokine dysregulation with systemic inflammation that led to end organ damage, as can occur in ANE1.
Pathologic lesions seen in neuroimaging are strikingly similar in all 3 sisters. They demonstrated multifocal, symmetric lesions involving the thalami, temporal lobes and brainstem (pons and midbrain). MRI changes were more extensive in Sibling 3 as abnormal signal intensities can be seen in insula cortex, limbic system and external capsule. Hemorrhagic changes are also present in Sibling 2 and 3. Previous reports elucidated to a correlation between neuroradiologic lesions and outcome. Poor prognostic factors suggested by evidence at case series level are the presence of hemorrhage and cavitation, involvement of brainstem, bilateral as oppose to unilateral thalami and cerebral or cerebellar white matter [5,16]. It is worthwhile to mention that Sibling 3 who has all of these features recovered well without neurological sequelae. She was also the only sibling who did not receive immunomodulation therapy as she was treated with the presumed diagnosis of Herpes Encephalitis, 1 year before the proband (sibling 1) presented with encephalopathy and diagnosed with ANE1.
Uncovering the genetic diagnosis for this family has allowed accurate genetic counselling. It is interesting to note that no pathogenic variant was found in both parents' blood (Fig. 1). It would be extremely rare for a spontaneous mutation to occur in 4 of their children, therefore it is inferred that one parent is a germline mosaic for this mutation. Another possibility is genetic mosaicism that is not confined to germline but also affecting other somatic tissues. We have not tested any other tissues other than blood in this family. Genetic mosaicism has not been reported for ANE1 and there has been no documentation on whether genetic mosaic carriers are susceptible to acute encephalopathy.
The RANBP2 c.1754C>T (p.Thr585Met) is a known pathogenic variant associated with ANE1. It is usually associated with severe neurological sequelae and high mortality. However, full recovery has been reported, albeit making up the minority [1,4]. Two of our patients have minimal neurological sequelae and one complete recovery. None have had recurrence of encephalopathy to date. Therefore, our patients seem to fall into the milder end of the clinical spectrum. Twin sister of sibling 2 was identified to carry this variant but never had encephalopathy. It is possible that this individual will never be affected with encephalopathy as the penetrance is estimated to be 40% [11,12]. Nevertheless encephalopathy is also reported to occur for the first time in adolescent and adulthood [6,12]. As such, we have instituted expectant management for all individuals carrying this variant; they have been given a small booklet denoting their risk and need for prompt intervention at the first sign of neurological deficit.
All 3 of our patients were diagnosed retrospectively after the acute episode of encephalopathy has been treated. No correlation may be derived between treatment and outcome as they received different treatment regime. To date, there are no published guidelines on the treatment of ANE1. In view of the postulated pathogenesis of cytokine storm, immunomodulation with intravenous corticosteroids and immunoglobulin, and therapeutic hypothermia have been used by many [1,16]. Variable treatment outcomes have been reported and no consensus was reached on whether these modalities should be used individually or in combination, neither the dosages, timing and duration. Some authors suggested that influenza vaccination is important in ANE1 survivors as it is a common trigger and the recurrence risk of encephalopathy is high [1,3,7].
We conclude that ANE1 presents a variable disease course. Despite carrying a RANBP2 mutation that is frequently associated with a severe disease course and manifesting neuroradiological signs deemed negative prognosticators, our patients behaved contrary. With regards to optimum management, further research on the exact pathogenesis is awaited for this intriguing disease entity.
References
- 1.Howard A., Uyeki T.M., Fergie J. Influenza-associated encephalopathy in siblings. J. Pediatr. Infect. Dis. Soc. 2018;7(3):1–6. doi: 10.1093/jpids/piy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isikay S., Sahin Y. RANBP2 mutation in clinically undiagnosed acute necrotizing encephalopathy. Indian J. Pediatr. 2018;85(9):820–821. doi: 10.1007/s12098-018-2678-0. [DOI] [PubMed] [Google Scholar]
- 3.Larsh T., Hsich G. Temporal course of imaging and laboratory findings in a fulminant case of acute necrotizing encephalopathy of childhood. Pediatr. Neurol. 2020;102:74–75. doi: 10.1016/j.pediatrneurol.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y.J., Hwang S.K., Lee S.M., Kwon S. Familial acute necrotising encephalopathy with RANBP2 mutation: the first report in Northeast Asia. Brain Dev. 2017;39(7):625–628. doi: 10.1016/j.braindev.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine J.M., Ahsan N., Ho E., Santoro J.D. Genetic acute necrotizing encephalopathy associated with RANBP2: clinical and therapeutic implications in pediatrics. Mult. Scler. Relat. Disord. 2020;43:102194. doi: 10.1016/j.msard.2020.102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lönnqvist T., Isohanni P., Valanne L., Olli-Lähdesmäki T., Suomalainen A., Pihko H. Dominant encephalopathy mimicking mitochondrial disease. Neurology. 2011;76:101–103. doi: 10.1212/WNL.0b013e318203e908. [DOI] [PubMed] [Google Scholar]
- 7.Marco E.J., Anderson J.E., Neilson D.E., Strober J.B. Acute necrotizing encephalopathy in 3 brothers. Pediatrics. 2010;125(3):e693–e698. doi: 10.1542/peds.2009-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 9.Mizuguchi M., Yamanouchi H., Ichiyama T., Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol. Scand. 2007;115(Suppl. 186):45–56. [PubMed] [Google Scholar]
- 10.Neilson D.E., Adams M.D., Orr C.M.D., Schelling D.K., Eiben R.M., Kerr D.S., Anderson J., Bassuk A.G., Bye A.M., Childs A.M., Clarke A., Crow Y.J., Di Rocco M., Dohna-Schwake C., Dueckers G., Fasano A.E., Gika A.D., Gionnis D., Gorman M.P., Grattan-Smith P.J., Hackenberg A., Kuster A., Lentschig M.G., Lopez-Laso E., Marco E.J., Mastroyianni S., Perrier J., Schmitt-Mechelke T., Servidei S., Skardoutsou A., Uldall P., Van der Knaap M.S., Goglin K.C., Tefft D.L., Aubin C., de Jager P., Hafler D., Warman M.L. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am. J. Hum. Genet. 2009;84:44–51. doi: 10.1016/j.ajhg.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neilson D.E. The interplay of infection and genetics in acute necrotizing encephalopathy. Curr. Opin. Pediatr. 2010;22:751–757. doi: 10.1097/MOP.0b013e3283402bfe. [DOI] [PubMed] [Google Scholar]
- 12.Neilson D.E. Vol. 3. GeneReview; 2014. Susceptibility to Infection-Induced Acute Encephalopathy.www.ncbi.nlm.nih.gov/books/NBK258641 [Google Scholar]
- 13.Nishimura N., Higuchi Y., Kimura N., Nozaki F., Kumada T., Hoshino A., Saitoh M., Mizuguchi M. Familial acute necrotizing encephalopathy without RANBP2 mutation: poor outcome. Pediatr. Int. 2016 doi: 10.1111/ped.13119. [DOI] [PubMed] [Google Scholar]
- 14.Singh R.R., Sedani S., Lim M., Wassmer E., Absoud M. RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur. J. Paediatr. Neurol. 2015;19(2):106–113. doi: 10.1016/j.ejpn.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Vockley J., Dobrowolski S.F., Arnold G.L., Guerrero R.B., Derks T.G.J., Weinstein D.A. Complex patterns of inheritance, including synergistic heterozygosity, in IEM: implications for precision medicine driven diagnosis and treatment. Mol. Genet. Metab. 2019;128:1–9. doi: 10.1016/j.ymgme.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X.J., Wu W., Pan W., Wu L.M., Liu K.D., Zhang H.L. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediat. Inflamm. 2015:792578. doi: 10.1155/2015/792578. [DOI] [PMC free article] [PubMed] [Google Scholar]