Abstract
MicroRNA (miR)-137 is highly expressed in the brain and plays a crucial role in the development and prognosis of glioma. In this review, we aim to summarize the latest findings regarding miR-137 in glioma cell apoptosis, proliferation, migration, invasion, angiogenesis, drug resistance, and cancer treatment. In addition, we focus on the identified miR-137 targets and pathways in the occurrence and development of glioma. Finally, future implications for the diagnostic and therapeutic potential of miR-137 in glioma were discussed.
Keywords: glioma, miR-137, target genes, diagnosis, treatment
Graphical Abstract
miR-137 is highly expressed in the brain and plays a crucial role in the development and prognosis of glioma. In this review, Ma and colleagues summarize the latest findings regarding miR-137 in glioma cell apoptosis, proliferation, migration, invasion, angiogenesis, drug resistance, and in cancer treatment.
Main Text
Glioma is the most common malignant and invasive primary brain tumor.1 It is characterized by high recurrence and mortality rates due to difficulty in complete resection by surgery and low sensitivity to chemoradiotherapy.2 Although substantial improvements in the diagnosis and treatment of glioma have been made over the past several decades, the prognosis is still poor. The 5-year overall survival rate of patients is less than ten percent.3 To date, the molecular mechanism involved in the development of glioma remains unclear. Therefore, it is necessary to develop new therapeutic strategies and improve the clinical outcome.
In recent decades, microRNAs (miRNAs) have started a revolution in molecular biology and are regarded as key players in tumors. miRNAs can block the expression of multiple target genes by sequence-specific binding to the 3′ untranslated region (3′UTR).4,5 Emerging studies have shown that dysregulated miRNAs can rewire multiple critical cellular and biological processes that are deeply involved in glioma initiation and malignant progression.4,6 In particular, microRNA-137 (miR-137), most abundantly expressed in the brain, may act as a central mediator to regulate the expression of over 1,000 predicted target genes.7 Subtle changes in miR-137 may have profound impacts on the development of many kinds of tumors, including glioma.6,8, 9, 10 This review provides a succinct but comprehensive summary of the literature on recent advances about the roles and mechanisms of miR-137 in glioma initiation and development, as well as its values in glioma diagnosis and treatment.
miR-137 Dysregulation Is Related to the Malignant Progression of Glioma
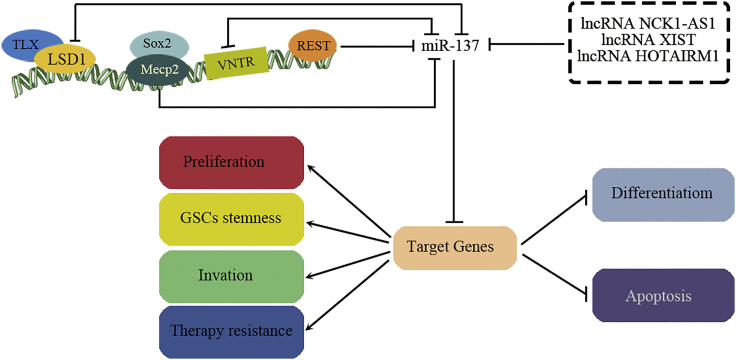
miR-137 is located on chromosome 1p21.3 within the nonprotein-coding RNA (ncRNA) gene AK094607.11 As a brain-enriched miRNA, miR-137 is not only expressed in neurons but also present in glial cells. miR-137 regulation is driven by multiple mechanisms (Figure 1), including transcriptional, epigenetic, and long noncoding RNA (lncRNA) regulation. The main regulatory mechanism of miR-137 in glioma is transcriptional control. Of note, it is negatively regulated by repressor element-1 silencing transcription repressor (REST).12,13 Given the potential involvement of REST in glioma pathology, REST-mediated miR-137 regulation could be an important molecular mechanism.14 Moreover, there are 15 bp variable number tandem repeat (VNTR) elements near the 5′ end of the pre-miR-137 region.15 In the human brain, the number of repeats at the VNTR locus ranges from 3 to 13, and the number of repeats is negatively associated with the expression level of miR-137.16 However, whether altered VNTR in miR-137 affects glioma risk in patients remains unknown. Epigenetic transcriptional regulation constitutes another mechanism of miR-137 expression control. The genome region encoding miR-137 lies across a large density of CpG islands,17 and its expression is inversely regulated by DNA methylation.18,19 DNA hypermethylation of miR-137 has been reported in different kinds of solid tumors,20, 21, 22 including glioma,6,23 with loss of miR-137 expression and function. In mouse adult neuronal stem cells (NSCs), miR-137 is regulated by methyl-CpG-binding protein 2 (MeCP2)- and transcription factor (TCF) Sox2-mediated DNA methylation.24 Sun et al.25 demonstrated that nuclear receptor Tailless (TLX) recruits histone lysine-specific demethylase 1 (LSD1) to the miR-137 promoter and suppresses its transcription. In addition, LSD1 is also targeted by miR-137, creating a double-negative regulatory feedback loop.26,27 More recently, lncRNAs negatively regulating the expression of miR-137 have also been identified. Several lncRNAs, such as lncRNA X-inactive-specific transcript (XIST),28,29 lncRNA HOXA transcript antisense RNA, myeloid-specific 1 (HOTAIRM1),30 and lncRNA noncatalytic kinase 1 antisense RNA 1 (NCK1-AS1),31 are reported to act as sponges or antagomirs that competitively regulate the expression and function of miR-137 in glioma cells. Together, these studies suggest that a complicated epigenetic modulation network is involved in miR-137 regulation, and imbalance of this regulatory circuit involving miR-137 and epigenetic changes may contribute to glioma pathology.
Figure 1.
The miR-137 Regulators and Functions in Glioma Cells
→, upregulation or activation; ┥, downregulation or inhibition.
miR-137 expression is strictly regulated during brain development. More intriguingly, miR-137 dysregulation is associated with many brain disorders, suggesting that miR-137 is critical for brain functions.11,32 Downregulation of miR-137 is associated with epithelial-mesenchymal transition (EMT) in a variety of tumors, such as breast cancer,33, 34, 35 cervical cancer,36 ovarian cancer,37 non-small cell lung cancer,38,39 and gastrointestinal stromal tumors.40 However, EMT in glioma has not been reported to be associated with miR-137. Other related evidence shows that miR-137 expression is decreased in both glioma samples and cell lines compared to normal controls17,41, 42, 43, 44, 45 and that miR-137 levels are inversely correlated with clinical stage and overall survival.6,41 We speculate that miR-137 dysregulation may be related to glioma EMT, but further studies are needed.
miR-137 Is a Suppressor of Glioma
Gliomas are infiltrative tumors derived from glial cells and represent deadly and the most common type of primary malignant central nervous system tumor1,2 Increasing evidence has indicated that miR-137 has great inhibitory effects on the proliferation, metastasis, and invasion of glioma cells.6,17,41
miR-137 Inhibits Glioma Cell Proliferation and Induces Glioma Stem Cell (GSC) Differentiation
Accelerated proliferation is a hallmark of cancer cells that contributes to the development of malignant tumors. This biological characteristic is thought to be even more important in glioma, as the uncontrolled growth of this brain cancer is key to its high lethality.
miR-137 regulates cell proliferation, mainly by affecting the cell cycle and promoting stem-cell differentiation. Transfection of miR-137 into glioma cell lines and tumor-derived neural stem cells leads to cell-cycle exit, whereas silencing miR-137 leads to the opposite outcome. It has been proven that miR-137 triggers cell-cycle arrest in the G1 phase by suppressing the phosphorylation of cyclin-dependent kinase 6 (CDK6) and retinoblastoma-associated protein-1 phosphorylation and the expression of nuclear casein kinase and CDK substrate 1 (NUCKS1) protein. Overexpression of miR-137 inhibits proliferation, migration, and survival and arrests the cell cycle in G1 phase inglioma cells, whereas repression of miR-137 exerts the opposite effects.46,47 In contrast, lncRNA XIST promotes glioma cell growth by acting as an endogenous sponge by competing with miR-137, thereby upregulating the level of the miR-137 target gene Rac1 (the small GTP-binding protein Ras-related C3 botulinum toxin substrate 1).29 In vitro, miR-137 levels increase gradually during the differentiation of NSCs. Ectopic expression of miR-137 in human induced pluripotent stem cell (iPSC)-derived NSCs reduces proliferation and accelerates neuronal differentiation and migration.48 Moreover, miR-137 induces the differentiation of adult NSCs, oligodendroglioma-derived stem cells, and GSCs.17 Recently, miR-137 was also reported to enhance neuronal differentiation by inducing mitochondrial biogenesis, fusion, fission, and oxidative phosphorylation (OXPHOS).48 miR-137 accelerates mitochondrial biosynthesis by upregulating the expression of TCF A of mitochondria (TFAM) and nuclear factor erythroid 2-related factor 2 (NRF2). In addition, miR-137 regulates mitochondrial dynamics by inducing mitochondrial fusion and fission events, resulting in increased mitochondrial content and activation of OXPHOS and oxygen consumption.48
miR-137 Induces Glioma Cell Apoptosis
Almost all cancer types feature suppression of apoptosis. In glioma, it has been extensively shown that the antiapoptotic capability of tumor cells is associated with disease progression and resistance to therapies. A study showed that miR-137 is involved in inducing apoptosis in noncancerous diseases.49 miR-137 induces caspase-3 activity in hippocampal neural stem cells by targeting BCL2L13 (a B cell chronic lymphocytic leukemia/lymphoma 2 [BCL-2] family member).49 In this way, miR-137 also enhances the apoptotic process in glioblastoma (GBM) cells by directly targeting Bcl-2.50 In addition, glutamine transporter solute carrier 1 family member 5 (SLC1A5) plays an important role in regulating cell metabolism by mediating glutamine uptake.51 Since glutamine is considered to be a conditionally essential amino acid in rapidly proliferating cancer cells, SLC1A5 has favorable effects on tumor development.52 Accordingly, it was also reported that direct inhibition of SLC1A5 by miR-137 enhances glioma cell oxidative stress and triggers lipid peroxidation, which eventually induces tumor cell death.51 Thus, miR-137 could be a very promising target for the treatment of glioma.
miR-137 Inhibits Glioma Cell Invasion
The high lethality of glioma is closely correlated with its ability to infiltrate the surrounding tissue, which leads to rapid postsurgery recurrence owing to incomplete resection. Molecular mechanisms underlying glioma cell invasion and migration usually involve factors that induce degradation of the extracellular matrix (ECM) or cell motility. Recent studies have proven that miR-137 contributes to the above processes. In vitro, miR-137 exerts inhibitory effects on glioma cell migration and invasion, reducing matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9) levels and reducing ECM degradation, whereas inhibition of miR-137 expression enhances glioma cell invasiveness.53 miR-137 is expressed at low levels in glioma tissues, particularly in high-grade glioma, which might contribute to the acquisition of invasive potential. Emerging evidence has shown that miR-137 directly targets C-X-C motif ligand 12 (CXCL12), epidermal growth factor receptor (EGFR) and cyclooxygenase-2 (COX-2), which are highly expressed in high-grade tumors and correlate with glioma cell growth and metastasis and poor prognosis.41,54,55 Moreover, it has been demonstrated that lncRNA HOTAIRM1 promotes glioblastoma cell proliferation and invasion by upregulating transcriptional factor specificity protein 1 (SP1) expression by sponging miR-137.30 Recently, it was reported that stanniocalcin-1 (STC1), a secreted glycoprotein hormone, may act as a novel metastasis/metastatic dissemination-promoting factor regulated by miR-137 in glioblastoma.56,57 STC1 is closely related to the degree of glioma malignancy. The mRNA and protein levels of STC1 in high-grade glioma tissues are much higher than those in low-grade glioma tissues. The overall survival of patients with high STC1 was significantly lower than that of patients with low STC1.56 miR-137 has a predicted binding site in STC1 mRNA, and the miR-137 analog is involved in downregulating STC1 mRNA expression in glioblastoma cells.57 Therefore, the inhibitory effect of miR-137 on glioma invasion and metastasis may be partly implemented by suppressing STC1.
miR-137 Blocks Glioma Angiogenesis
Angiogenesis is another important process involved in the malignant progression of glioma. Increasing investigations have shown that miR-137 is linked to glioma angiogenesis by modulating a variety of angiogenic targets. For example, lncRNA XIST was observed to regulate angiogenesis positively in vivo by directly modulating the expression of miR-137 in glioma, which targets tight junction protein gene zonula occludens-2 (ZO-2) and forkhead box C1 (FOXC1), and FOXC1 is able to induce chemokine (C-X-C motif) receptor 7b (CXCR7) expression and activate angiogenesis.28 In addition, the aberrant high expression of FOXC1 in glioma-associated endothelial cells (GECs) leads to a decreased permeability of the blood tumor barrier (BTB) by increasing the expression of tight junction proteins (ZO-1, claudin-5, and occludin).28 In addition, miR-137 was recently shown to regulate the proliferation and angiogenesis of glioblastoma cell lines in vivo by directly targeting polycomb group protein enhancer of zeste 2 (EZH2).58 Therefore, miR-137 and the related molecules of the angiogenic pathway could be a potential therapeutic target in the treatment of glioma.
miR-137 Suppresses GSC Development and Stemness Maintenance
The GSC hypothesis proposes that tumors harbor a small subpopulation of cells characterized by self-renewal capability, high migration rates, and unlimited growth capacity to drive gliomagenesis; this population of cells is also responsible for tumor aggressiveness, recurrence, and therapy resistance. The roles of miR-137 in regulating GSCs have received much attention in recent years. For example, the origin of gliomas is largely unknown, but they are supposed to originate from GSCs, which might consist of transformed NSCs. Silber et al.17 demonstrated that miR-137 was much lower in GSCs than in NSCs, whereas overexpression of miR-137 induced neuronal differentiation in both cell types. In particular, ectopic expression of miR-137 significantly suppresses the self-renewal of GSCs by decreasing OCT4, SOX2, NANOG, and sonic hedgehog (SHH) levels.6 Furthermore, miR-137 inhibits glioblastoma stemness through various targets, such as glioma pathogenesis-related protein 1 (GLIPR1) and Musashi-1 (Msi1), which are related to testes-specific vespid and pathogenesis protein 1 (RTVP-1) and STC1.6,57,59 For example, miR-137 reduces the transcriptional expression of RTVP-1, while RTVP-1 facilitates the self-renewal of GSCs by enhancing CXXR4.6 miR-137 is involved in regulating the expression of STC1, whereas the latter enhances the stem-like characteristics of glioblastomacells by activating the NOTCH1-SOX2 signaling pathway.57,59 Msi1 is a stem-cell protein involved in self-renewal that has the opposite expression pattern and function to miR-137, and these proteins inhibit each other. Msi1 and miR-137 are regulators of molecular conversion between self-renewal and differentiation, and they share 141 target genes related to differentiation, development, and morphogenesis. In gliomas, miR-137 has an anticancer effect, whereas Msi1 is a proto-oncogene. The balance between Msi1 and miR-137 is a key determinant of cell fate, and the disruption of this balance may lead to glioma development.60
Given their critical roles in glioma initiation, propagation, and maintenance, GSCs offer an attractive therapeutic target. miR-137 is essential for the canonical differentiation of GSCs and neural progenitor cells. Abrogation of its expression could lead to GSC formation and gliomagenesis. Upregulation of miR-137 in GSCs could be a therapeutic method for GSC differentiation, thereby eliminating their stem-cell properties. Thus, the understanding of the roles of miR-137 in GSCs may offer an innovative clinical strategy for the early diagnosis and treatment of glioma.
The Underlying Molecular Mechanisms by which miR-137 Inhibits Gliomagenesis
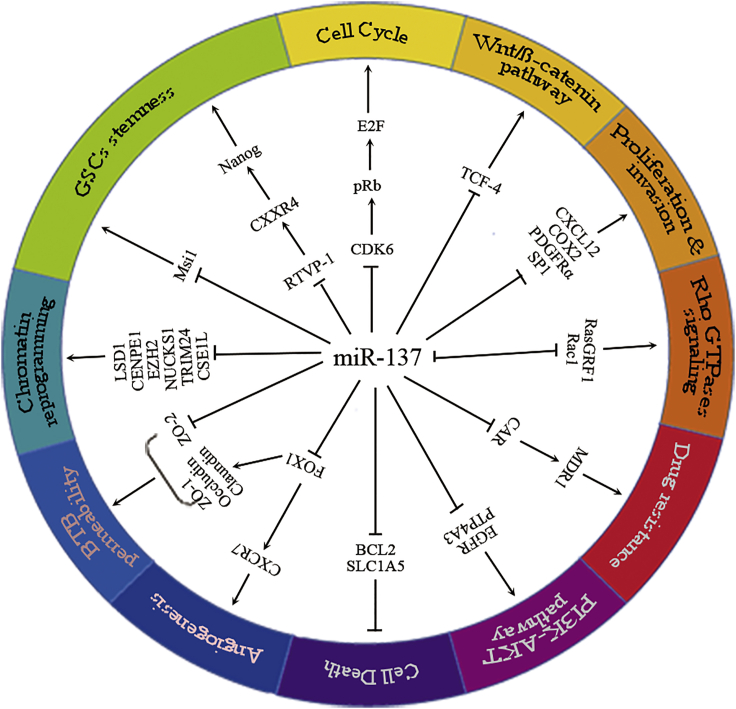
miR-137 acts as a gene-network hub by blocking the expression of multiple target genes. miR-137 has over 1,300 predicted target genes,46 and a number of downstream target genes of miR-137 have been experimentally verified in glial cells (summarized in Table 1 and Figure 2). These target genes, to a certain extent, illustrate the molecular mechanism underlying the inhibition of gliomagenesis by miR-137. In particular, miR-137 is located at a node of several essential cellular signaling networks of glioblastoma cell aggressiveness. Moreover, miR-137 could potentially alter basic cell biological processes by regulating chromatin-state reprogramming and inhibiting tumorigenesis.
Table 1.
mRNA Targets of miR-137 in Glioma Development and Function
| Targets | Major Functions | References |
|---|---|---|
| LSD1 | promoting NSC or GSC proliferation and inhibiting differentiation | 25 |
| EZH2 | promoting proliferation and angiogenesis of glioma cells | 58 |
| RTVP-1 | promoting GSCs’ self-renewal and inhibiting their differentiation | 6 |
| TCF4 | binding to β-catenin and triggering Wnt/β-catenin signaling | 61 |
| CDK6 | cell-cycle regulator | 17 |
| COX2 | promoting glioma cell proliferation and invasion | 41 |
| EGFR | increasing cell growth and decreasing cell apoptosis | 55 |
| FOXK1 | promoting proliferation and cell-cycle transition and inhibiting apoptosis | 62 |
| PDGFRα | increasing cell growth and reducing cell apoptosis | 60 |
| ZO-2 | angiogenesis and blood tumor barrier permeability | 28 |
| CSE1L | inhibiting glioma cell proliferation, invasion | 63 |
| Msi1 | promoting self-renewal, proliferation, tumorigenesis | 60 |
| RasGRF1 | promoting glioma cell proliferation and inhibiting apoptosis | 23 |
| Rac1 | promoting glioma cell proliferation and inhibiting apoptosis | 50 |
| CENPE | promoting pediatric high-grade glioma cell proliferation | 45 |
| PTP4A3 | promoting GBM cell proliferation, migration, and invasion | 64 |
| SP1 | promoting cell proliferation and invasion | 30 |
| FOXC1 | angiogenesis and blood tumor barrier permeability | 28 |
| TRIM24 | drug resistance | 31 |
| CAR | drug resistance | 65 |
| STC1 | promoting metastasis and cell proliferation | 57 |
| CXCL12 | promoting cell proliferation and invasion | 54 |
| NUCKS1 | promoting cell proliferation and drug resistance | 66 |
| BCL2 | inhibiting apoptosis | 50 |
| SLC1A5 | regulating cell growth, survival, and proliferation | 51 |
GBM, glioblastoma.
Figure 2.
The Functions of miR-137 in Glioma Cell Development and the Underlying Molecular Mechanisms
→, upregulation or activation; ┥, downregulation or inhibition.
miR-137 Targets Multiple Chromatin-Modifying Proteins in Glioma
Gliomagenesis is a multistep process involving both genetic and epigenetic mechanisms. Histone modifications and chromatin structure alterations play important roles in chromatin-based gene regulation. An increasing number of studies have demonstrated that histone-modifying enzymes and chromatin modifiers affect genome stability in gliomas.67,68 Accumulated evidence has shown that miR-137 acts as an epigenetic regulator that contributes to chromatin-state changes in glioma. First, miR-137 affects histone methylation levels by directly inhibiting LSD1 and EZH2.24,58,69 LSD1 specifically catalyzes the removal of methyl groups from histone H3 at lysine 4 to activate transcription, whereas EZH2 is a histone methyltransferase that mediates gene silencing by catalyzing histone methylation.70 Notably, as one of the master epigenetic proteins, LSD1 plays an important role in GSC stemness.71,72 miR-137 also inhibits proliferation and angiogenesis of glioblastoma cells by directly regulating the level of EZH2.58 Second, tripartite motif-containing 24 (TRIM24) is a member of the transcription intermediary factor family and has been reported to bind with H3K23ac to regulate the development of a variety of tumors.31 Recently, it was reported that miR-137 directly targets TRIM24 to suppress glioma development and improve chemosensitivity by activating the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway.73,74 Third, miR-137 regulates chromosome assembly and chromosome positioning at the metaphase plate by targeting chromosome segregation 1-like (CSE1L) and centromere-associated protein-E (CENP-E), respectively. CSE1L is critical in maintaining genomic stability and is involved in apoptosis, cell survival, nucleocytoplasmic transport, microvesicle formation, and cancer metastasis.63 CENP-E is essential in mitosis and exhibits periodic accumulation and loss with cell-cycle stages.75 Additionally, there is now a substantial body of evidence validating that the loss of CENP-E function causes cell-cycle arrest in mitosis.45 Fourth, nuclear casein kinase and NUCKS1, a chromatin-associated nuclear DNA-binding protein, have been documented to play pivotal roles in cell-cycle progression and proliferation.18,76 NUCKS1 affects tumor development by influencing the cellular response to DNA damage, homologous recombination, and DNA repair.76 The increased expression of NUCKS1 has been documented in several kinds of cancers.77,78 Recently, Giunti et al.66 demonstrated that the tumor-suppressive effects of miR-137 are mediated by the negative regulation of NUCKS1 protein expression in human pediatric glioblastoma tissues.
miR-137 Modulates Multiple Signaling Pathways in Glioma Cells
Emerging studies demonstrate that miR-137 acts as a gene network hub modulating key nodes of various essential tumor signaling pathways. First, miR-137 regulates glioma cell proliferation through the Akt/mammalian target of rapamycin (mTOR) signaling pathway. miR-137 inhibits the expression of protein tyrosine phosphatase 4A3 (PTP4A3), which regulates the activity of the Akt/mTOR signaling pathway by inducing Akt and mTOR dephosphorylation.64 Second, researchers also demonstrated that miR-137 blocks cell growth by inhibiting the Wnt/β-catenin pathway and negatively regulates FOXK1 expression in glioma cells.62 Wnt signaling is one of the key cascades in the regulation of human tumor growth and development, especially cell proliferation. Studies have confirmed that the Wnt/β-catenin signaling pathway regulates the growth of glioma.79 FOXK1 activates the Wnt/β-catenin signaling pathway and promotes glioma cell proliferation and cell-cycle transformation and inhibits apoptosis.62 miR-137 directly binds to the 3′ UTR of FOXK1 and suppresses its expression, therefore inhibiting its effect on glioma cell proliferation and apoptosis.62 In addition, TCF4 controls critical steps of glioma development by interacting with β-catenin80 and was reported to be regulated by miR-137.11 Therefore, miR-137 may restrain Wnt/β-catenin signaling by disrupting TCF4 binding to β-catenin in glioma cells. Third, Rho GTPase signaling is another well-known pathway closely related to glioma development.81 Rac1 is one of the most characteristic Rho GTPases in the regulation of cell migration and plays a key role in regulating cytoskeleton reorganization as well as the cell cycle and apoptosis of tumor cells.18,50 In gliomas, increased Rac1 facilitates the growth and invasion behavior of glioma cells by regulating cyclin D1, MMP2, Bcl-2, and BCL-2-associated X protein (Bax).82,83 miR-137 suppresses the Rac1 gene by binding directly to the 3′ -UTR, thereby inhibiting the development of gliomas.50 On the other hand, guanine nucleotide exchange factors (GEFs) control the activation and inactivation of Rho GTPase, whereas Ras protein-specific guanine nucleotide-releasing factor 1 (RasGRF1) is one of the GEFs that regulates cell proliferation and apoptosis.84,85 miR-137 also directly inhibits RasGRF1 expression by binding to the 3′ -UTR, therefore leading to cell proliferation suppression and inducing apoptosis in astrocytoma.23
miR-137 May Be a Biomarker of Glioma
Tumor biomarkers provide important information for diagnosis, therapy response, and prognosis. The role of miR-137 in glioma development and the extensive alterations in miR-137 expression make it an important candidate biomarker.42 miR-137 signatures have been identified in both glioma tissue and the blood of glioma patients. Li et al.86 recently demonstrated that serum miR-137 is downregulated in glioma patients compared to healthy controls. In addition, low serum miR-137 levels are strongly associated with high glioma clinical grades and poorer survival in glioblastoma patients, highlighting the potential of miR-137 as a useful, noninvasive diagnostic marker for glioma.
Thus far, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation has been the only confirmed clinical molecular predictive factor in glioma.87 miR-137 transcription is regulated by the promoter hypomethylated CpG island.88 A recent study indicated that the promoter methylation status of miR-137 may be another possible biomarker, although replication of this finding is needed.6 Thus, miR-137 may be used as a valuable indicator for pathological diagnosis and prognosis evaluation of malignant glioma.
miR-137 Boosts the Chemosensitivity of Glioma Cells
Chemotherapy is the most attractive cancer therapy method. Multidrug resistance (MDR) remains the leading cause of glioma treatment failure. Recently, an increasing amount of evidence has shown that miR-137 exerts important effects on chemotherapy resistance in a variety of tumors.89, 90, 91 For example, Zhu et al.92 demonstrated that miR-137 is involved in MDR in breast cancer by the regulation of MDR1 (P-glycoprotein) by targeting Y-box binding protein-1 (YB-1), indicating that miR-137 might be a valuable target for preventing and reversing MDR in cancer cells. In GSCs, EGFR activity is elevated and is required to maintain chemotherapy resistance, and EGFR is a direct target of miR-137.93 Interestingly, miR-137 expression is upregulated in glioma cells after treatment with temozolomide (TMZ) and correlated with reduced resistance to TMZ.94 Most importantly, it was reported that miR-137 negatively regulates constitutive androstane receptor (CAR), and CAR upregulates MDR1 and reduces intracellular drug accumulation and cellular sensitivity to doxorubicin.65 Remarkably, Chen et al.31 demonstrated that lncRNA NCK1-AS1 could increase the drug resistance of glioma cells to TMZ by modulating the miR-137/transcription intermediary factor 1a (TRIM24) axis.
These results provide new theoretical support for miR-137 in glioma treatment and prognosis. We assume that miR-137 facilitates the resensitization of drug-resistant glioma cells and contributes to better prognosis and survival in glioma patients.
Exosomes May Be an Effective Vehicle Enabling the Use of miR-137 in the Diagnosis and Treatment of Glioma
Exosomes are 40–100 nm diameter lipid bilayer vesicles secreted by most mammalian cells. The composition of exosomes varies slightly depending on the cell type from which they are derived. Glioma cell-derived exosomes are rich in the oncogenic proteins EGFR variant III,95 angiogenic factors,96 and noncoding RNAs,96, 97, 98, 99 which may facilitate the transmission of carcinogens between cancer cells, thus forming a positive-feedback loop or facilitating communication between cancer cells and adjacent stromal cells.
Characteristic carcinogens carried by cancer cell-derived exosomes can be used as biomarkers for disease diagnosis. Exosomes from cancer cells have been identified as important transporters of carcinogenic miRNAs.96,100 For example, exosomal miR-21 was one of the first miRNAs recommended for use in the diagnosis of patients with glioblastoma multiforme.101 miR-137-containing exosomes were found in the serum of healthy people and patients with dementia or Parkinson’s disease.102,103 However, there have been no reports related to miR-137-containing exosomes in glioma patients.
In addition to functioning in diagnosis, exosomes have unique therapeutic advantages that can be applied to glioma therapy: small exosomes can penetrate the blood-brain barrier,104 facilitate immune escape,105 increase molecule half-life,106,107 and enable the ability to target specific types of cancer cells. 108 Exosomes not only protect therapeutic agents, including small-molecule inhibitors, therapeutic small interfering RNAs (siRNAs), and even peptides, but also improve their bioavailability and overall efficacy.109, 110, 111, 112 Based on our understanding of miR-137, exosomal miR-137 may play a good therapeutic role in glioma. Further studies of exosomal miR-137 will very possibly facilitate the diagnosis and treatment of glioma.
Summary and Prospect
In this review, we highlighted the function and mechanisms of miR-137 in glioma cell proliferation, apoptosis, invasion, and angiogenesis and in glioma treatment to provide evidence for further investigations. miR-137 may be implicated in the core pathophysiologic mechanisms underlying glioma pathogenesis, and targeting miR-137 offers great therapeutic potential for improving the outcome of glioma patients.
Emerging evidence strongly suggests that targeting miR-137 is of great value in glioma therapy, but so far, research on inhibiting glioma by regulating miR-137 has remained in the experimental stage at the cell level. One of the greatest challenges regarding the efficacy of miRNA-based glioma therapies is the absence of effective delivery methods to the brain. Currently, the development and optimization of novel delivery methods, such as exosomes, adeno-associated viruses, and nanoparticles, will pave the way for the clinical application of miR-137 as a weapon against glioma. We hope that our review will attract the attention of the scientific research community and clinical practitioners and encourage them to carry out treatments targeting miR-137 as soon as possible.
Author Contributions
Y.W. wrote the manuscript. R.C. contributed to critical revision/review of the manuscript. X.Z., R.G., and J.Y. edited the manuscript. Y.L. and G.M. were responsible for the conception and final approval of the manuscript. All authors approved the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
Support for this work includes funding from the National Natural Science Foundation of China (81670252, 81770034, 81571157, and 81873649), Guangdong Basic and Applied Basic Foundation (2019A1515011306, 2019A1515011424, and 2020A1515010240), and 2016 Talent Assistance Project of Guangdong (4YF17006G).
Contributor Information
You Li, Email: dreamly2001@126.com.
Guoda Ma, Email: sihan1107@126.com.
References
- 1.Morgan L.L. The epidemiology of glioma in adults: a “state of the science” review. Neuro-oncol. 2015;17:623–624. doi: 10.1093/neuonc/nou358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., Burger P.C., Jouvet A., Scheithauer B.W., Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Yang H., Gong B., Jiang C., Yang L. Combined gene expression and protein interaction analysis of dynamic modularity in glioma prognosis. J. Neurooncol. 2012;107:281–288. doi: 10.1007/s11060-011-0757-4. [DOI] [PubMed] [Google Scholar]
- 4.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Bier A., Giladi N., Kronfeld N., Lee H.K., Cazacu S., Finniss S., Xiang C., Poisson L., deCarvalho A.C., Slavin S. MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1. Oncotarget. 2013;4:665–676. doi: 10.18632/oncotarget.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright C., Turner J.A., Calhoun V.D., Perrone-Bizzozero N. Potential Impact of miR-137 and Its Targets in Schizophrenia. Front. Genet. 2013;4:58. doi: 10.3389/fgene.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith A.R., Marquez R.T., Tsao W.C., Pathak S., Roy A., Ping J., Wilkerson B., Lan L., Meng W., Neufeld K.L. Tumor suppressive microRNA-137 negatively regulates Musashi-1 and colorectal cancer progression. Oncotarget. 2015;6:12558–12573. doi: 10.18632/oncotarget.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y., Li F., Saha M.N., Abdi J., Qiu L., Chang H. miR-137 and miR-197 Induce Apoptosis and Suppress Tumorigenicity by Targeting MCL-1 in Multiple Myeloma. Clin. Cancer Res. 2015;21:2399–2411. doi: 10.1158/1078-0432.CCR-14-1437. [DOI] [PubMed] [Google Scholar]
- 10.Liu L.L., Lu S.X., Li M., Li L.Z., Fu J., Hu W., Yang Y.Z., Luo R.Z., Zhang C.Z., Yun J.P. FoxD3-regulated microRNA-137 suppresses tumour growth and metastasis in human hepatocellular carcinoma by targeting AKT2. Oncotarget. 2014;5:5113–5124. doi: 10.18632/oncotarget.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin J., Lin J., Luo X., Chen Y., Li Z., Ma G., Li K. miR-137: a new player in schizophrenia. Int. J. Mol. Sci. 2014;15:3262–3271. doi: 10.3390/ijms15023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soldati C., Bithell A., Johnston C., Wong K.Y., Stanton L.W., Buckley N.J. Dysregulation of REST-regulated coding and non-coding RNAs in a cellular model of Huntington’s disease. J. Neurochem. 2013;124:418–430. doi: 10.1111/jnc.12090. [DOI] [PubMed] [Google Scholar]
- 13.Warburton A., Breen G., Rujescu D., Bubb V.J., Quinn J.P. Characterization of a REST-Regulated Internal Promoter in the Schizophrenia Genome-Wide Associated Gene MIR137. Schizophr. Bull. 2015;41:698–707. doi: 10.1093/schbul/sbu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C., Zou H., Wang Z., Tang X., Fan X., Zhang K., Liu J., Li Z. REST, not REST4, is a risk factor associated with radiotherapy plus chemotherapy efficacy in glioma. Drug Des. Devel. Ther. 2018;12:1363–1371. doi: 10.2147/DDDT.S161602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bemis L.T., Chen R., Amato C.M., Classen E.H., Robinson S.E., Coffey D.G., Erickson P.F., Shellman Y.G., Robinson W.A. MicroRNA-137 targets microphthalmia-associated transcription factor in melanoma cell lines. Cancer Res. 2008;68:1362–1368. doi: 10.1158/0008-5472.CAN-07-2912. [DOI] [PubMed] [Google Scholar]
- 16.Pacheco A., Berger R., Freedman R., Law A.J. A VNTR Regulates miR-137 Expression Through Novel Alternative Splicing and Contributes to Risk for Schizophrenia. Sci. Rep. 2019;9:11793. doi: 10.1038/s41598-019-48141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silber J., Lim D.A., Petritsch C., Persson A.I., Maunakea A.K., Yu M., Vandenberg S.R., Ginzinger D.G., James C.D., Costello J.F. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitfield M.L., Sherlock G., Saldanha A.J., Murray J.I., Ball C.A., Alexander K.E., Matese J.C., Perou C.M., Hurt M.M., Brown P.O., Botstein D. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaguer F., Link A., Lozano J.J., Cuatrecasas M., Nagasaka T., Boland C.R., Goel A. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–6618. doi: 10.1158/0008-5472.CAN-10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan Y., Guan X., An H., Baihetiya A., Wang W., Shao W., Yang H., Wang Y. Epigenetic silencing of miR-137 induces resistance to bicalutamide by targeting TRIM24 in prostate cancer cells. Am. J. Transl. Res. 2019;11:3226–3237. [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Y., Zhang S., Deng S., An G., Qin X., Li F., Xu Y., Hao M., Yang Y., Zhou W. Epigenetic silencing of miR-137 induces drug resistance and chromosomal instability by targeting AURKA in multiple myeloma. Leukemia. 2017;31:1123–1135. doi: 10.1038/leu.2016.325. [DOI] [PubMed] [Google Scholar]
- 22.Steponaitiene R., Kupcinskas J., Langner C., Balaguer F., Venclauskas L., Pauzas H., Tamelis A., Skieceviciene J., Kupcinskas L., Malfertheiner P., Link A. Epigenetic silencing of miR-137 is a frequent event in gastric carcinogenesis. Mol. Carcinog. 2016;55:376–386. doi: 10.1002/mc.22287. [DOI] [PubMed] [Google Scholar]
- 23.Deng D., Xue L., Shao N., Qu H., Wang Q., Wang S., Xia X., Yang Y., Zhi F. miR-137 acts as a tumor suppressor in astrocytoma by targeting RASGRF1. Tumour Biol. 2016;37:3331–3340. doi: 10.1007/s13277-015-4110-y. [DOI] [PubMed] [Google Scholar]
- 24.Szulwach K.E., Li X., Smrt R.D., Li Y., Luo Y., Lin L., Santistevan N.J., Li W., Zhao X., Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun G., Ye P., Murai K., Lang M.F., Li S., Zhang H., Li W., Fu C., Yin J., Wang A. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat. Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang K., Ren C., Nair V.D. MicroRNA-137 represses Klf4 and Tbx3 during differentiation of mouse embryonic stem cells. Stem Cell Res. (Amst.) 2013;11:1299–1313. doi: 10.1016/j.scr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Tarantino C., Paolella G., Cozzuto L., Minopoli G., Pastore L., Parisi S., Russo T. miRNA 34a, 100, and 137 modulate differentiation of mouse embryonic stem cells. FASEB J. 2010;24:3255–3263. doi: 10.1096/fj.09-152207. [DOI] [PubMed] [Google Scholar]
- 28.Yu H., Xue Y., Wang P., Liu X., Ma J., Zheng J., Li Z., Li Z., Cai H., Liu Y. Knockdown of long non-coding RNA XIST increases blood-tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis. 2017;6:e303. doi: 10.1038/oncsis.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Yuan J., Li L., Yang Y., Xu X., Wang Y. Long non-coding RNA XIST exerts oncogenic functions in human glioma by targeting miR-137. Am. J. Transl. Res. 2017;9:1845–1855. [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Y., Li X., Chen H., Huo H., Liu Z., Chai E. Over-expression of long noncoding RNA HOTAIRM1 promotes cell proliferation and invasion in human glioblastoma by up-regulating SP1 via sponging miR-137. Neuroreport. 2020;31:109–117. doi: 10.1097/WNR.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 31.Chen M., Cheng Y., Yuan Z., Wang F., Yang L., Zhao H. NCK1-AS1 Increases Drug Resistance of Glioma Cells to Temozolomide by Modulating miR-137/TRIM24. Cancer Biother. Radiopharm. 2020;35:101–108. doi: 10.1089/cbr.2019.3054. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoudi E., Cairns M.J. MiR-137: an important player in neural development and neoplastic transformation. Mol. Psychiatry. 2017;22:44–55. doi: 10.1038/mp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du F., Yu L., Wu Y., Wang S., Yao J., Zheng X., Xie S., Zhang S., Lu X., Liu Y., Chen W. miR-137 alleviates doxorubicin resistance in breast cancer through inhibition of epithelial-mesenchymal transition by targeting DUSP4. Cell Death Dis. 2019;10:922. doi: 10.1038/s41419-019-2164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying X., Sun Y., He P. MicroRNA-137 inhibits BMP7 to enhance the epithelial-mesenchymal transition of breast cancer cells. Oncotarget. 2017;8:18348–18358. doi: 10.18632/oncotarget.15442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y., Bi Y., Bi H., Diao C., Zhang G., Cheng K., Yang Z. miR-137 suppresses the invasion and procedure of EMT of human breast cancer cell line MCF-7 through targeting CtBP1. Hum. Cell. 2016;29:30–36. doi: 10.1007/s13577-015-0124-4. [DOI] [PubMed] [Google Scholar]
- 36.Miao H., Wang N., Shi L.X., Wang Z., Song W.B. Overexpression of mircoRNA-137 inhibits cervical cancer cell invasion, migration and epithelial-mesenchymal transition by suppressing the TGF-β/smad pathway via binding to GREM1. Cancer Cell Int. 2019;19:147. doi: 10.1186/s12935-019-0852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong P., Xiong Y., Watari H., Hanley S.J., Konno Y., Ihira K., Yamada T., Kudo M., Yue J., Sakuragi N. MiR-137 and miR-34a directly target Snail and inhibit EMT, invasion and sphere-forming ability of ovarian cancer cells. J. Exp. Clin. Cancer Res. 2016;35:132. doi: 10.1186/s13046-016-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Zhang G., Cheng Z., Dai L., Jia L., Jing X., Wang H., Zhang R., Liu M., Jiang T. Knockdown of LncRNA-XIST Suppresses Proliferation and TGF-β1-Induced EMT in NSCLC Through the Notch-1 Pathway by Regulation of miR-137. Genet. Test. Mol. Biomarkers. 2018;22:333–342. doi: 10.1089/gtmb.2018.0026. [DOI] [PubMed] [Google Scholar]
- 39.Chang T.H., Tsai M.F., Gow C.H., Wu S.G., Liu Y.N., Chang Y.L., Yu S.L., Tsai H.C., Lin S.W., Chen Y.W. Upregulation of microRNA-137 expression by Slug promotes tumor invasion and metastasis of non-small cell lung cancer cells through suppression of TFAP2C. Cancer Lett. 2017;402:190–202. doi: 10.1016/j.canlet.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Liu S., Cui J., Liao G., Zhang Y., Ye K., Lu T., Qi J., Wan G. MiR-137 regulates epithelial-mesenchymal transition in gastrointestinal stromal tumor. Tumour Biol. 2014;35:9131–9138. doi: 10.1007/s13277-014-2177-5. [DOI] [PubMed] [Google Scholar]
- 41.Chen L., Wang X., Wang H., Li Y., Yan W., Han L., Zhang K., Zhang J., Wang Y., Feng Y. miR-137 is frequently down-regulated in glioblastoma and is a negative regulator of Cox-2. Eur. J. Cancer. 2012;48:3104–3111. doi: 10.1016/j.ejca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Koshkin F.A., Chistyakov D.A., Nikitin A.G., Konovalov A.N., Potapov A.A., Usachyov D.Y., Pitskhelauri D.I., Kobyakov G.L., Shishkina L.V., Chekhonin V.P. Profile of microRNA expression in brain tumors of different malignancy. Bull. Exp. Biol. Med. 2014;157:794–797. doi: 10.1007/s10517-014-2669-8. [DOI] [PubMed] [Google Scholar]
- 43.Koshkin P.A., Chistiakov D.A., Nikitin A.G., Konovalov A.N., Potapov A.A., Usachev D.Y., Pitskhelauri D.I., Kobyakov G.L., Shishkina L.V., Chekhonin V.P. Analysis of expression of microRNAs and genes involved in the control of key signaling mechanisms that support or inhibit development of brain tumors of different grades. Clin. Chim. Acta. 2014;430:55–62. doi: 10.1016/j.cca.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Visani M., de Biase D., Marucci G., Cerasoli S., Nigrisoli E., Bacchi Reggiani M.L., Albani F., Baruzzi A., Pession A., PERNO Study Group Expression of 19 microRNAs in glioblastoma and comparison with other brain neoplasia of grades I-III. Mol. Oncol. 2014;8:417–430. doi: 10.1016/j.molonc.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang M.L., Hsieh T.H., Ng K.H., Tsai Y.N., Tsai C.F., Chao M.E., Liu D.J., Chu S.S., Chen W., Liu Y.R. Downregulation of miR-137 and miR-6500-3p promotes cell proliferation in pediatric high-grade gliomas. Oncotarget. 2016;7:19723–19737. doi: 10.18632/oncotarget.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamim S., Vo D.T., Uren P.J., Qiao M., Bindewald E., Kasprzak W.K., Shapiro B.A., Nakaya H.I., Burns S.C., Araujo P.R. Genomic analyses reveal broad impact of miR-137 on genes associated with malignant transformation and neuronal differentiation in glioblastoma cells. PLoS ONE. 2014;9:e85591. doi: 10.1371/journal.pone.0085591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhaskaran V., Nowicki M.O., Idriss M., Jimenez M.A., Lugli G., Hayes J.L. The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma. Nat. Commun. 2019;10:422. doi: 10.1038/s41467-019-08390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Channakkar A.S., Singh T., Pattnaik B., Gupta K., Seth P., Adlakha Y.K. MiRNA-137-mediated modulation of mitochondrial dynamics regulates human neural stem cell fate. Stem Cells. 2020;38:683–697. doi: 10.1002/stem.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schouten M., Fratantoni S.A., Hubens C.J., Piersma S.R., Pham T.V., Bielefeld P., Voskuyl R.A., Lucassen P.J., Jimenez C.R., Fitzsimons C.P. MicroRNA-124 and -137 cooperativity controls caspase-3 activity through BCL2L13 in hippocampal neural stem cells. Sci. Rep. 2015;5:12448. doi: 10.1038/srep12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun G., Cao Y., Shi L., Sun L., Wang Y., Chen C., Wan Z., Fu L., You Y. Overexpressed miRNA-137 inhibits human glioma cells growth by targeting Rac1. Cancer Biother. Radiopharm. 2013;28:327–334. doi: 10.1089/cbr.2012.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo M., Wu L., Zhang K., Wang H., Zhang T., Gutierrez L., O’Connell D., Zhang P., Li Y., Gao T. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Geldermalsen M., Wang Q., Nagarajah R., Marshall A.D., Thoeng A., Gao D., Ritchie W., Feng Y., Bailey C.G., Deng N. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35:3201–3208. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakrabarti M., Ray S.K. Direct transfection of miR-137 mimics is more effective than DNA demethylation of miR-137 promoter to augment anti-tumor mechanisms of delphinidin in human glioblastoma U87MG and LN18 cells. Gene. 2015;573:141–152. doi: 10.1016/j.gene.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 54.Li D., Shan W., Fang Y., Wang P., Li J. miR-137 acts as a tumor suppressor via inhibiting CXCL12 in human glioblastoma. Oncotarget. 2017;8:101262–101270. doi: 10.18632/oncotarget.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z., Song X., Tian H., Miao Y., Feng X., Li Y., Wang H. MicroRNA-137 inhibits growth of glioblastoma through EGFR suppression. Am. J. Transl. Res. 2017;9:1492–1499. [PMC free article] [PubMed] [Google Scholar]
- 56.Su J., Guo B., Zhang T., Wang K., Li X., Liang G. Stanniocalcin-1, a new biomarker of glioma progression, is associated with prognosis of patients. Tumour Biol. 2015;36:6333–6339. doi: 10.1007/s13277-015-3319-0. [DOI] [PubMed] [Google Scholar]
- 57.Sakata J., Sasayama T., Tanaka K., Nagashima H., Nakada M., Tanaka H., Hashimoto N., Kagawa N., Kinoshita M., Nakamizo S. MicroRNA regulating stanniocalcin-1 is a metastasis and dissemination promoting factor in glioblastoma. J. Neurooncol. 2019;142:241–251. doi: 10.1007/s11060-019-03113-2. [DOI] [PubMed] [Google Scholar]
- 58.Sun J., Zheng G., Gu Z., Guo Z. MiR-137 inhibits proliferation and angiogenesis of human glioblastoma cells by targeting EZH2. J. Neurooncol. 2015;122:481–489. doi: 10.1007/s11060-015-1753-x. [DOI] [PubMed] [Google Scholar]
- 59.Li Y., He Z.C., Zhang X.N., Liu Q., Chen C., Zhu Z., Chen Q., Shi Y., Yao X.H., Cui Y.H. Stanniocalcin-1 augments stem-like traits of glioblastoma cells through binding and activating NOTCH1. Cancer Lett. 2018;416:66–74. doi: 10.1016/j.canlet.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 60.Velasco M.X., Kosti A., Guardia G.D.A., Santos M.C., Tegge A., Qiao M., Correa B.R.S., Hernández G., Kokovay E., Galante P.A.F., Penalva L.O.F. Antagonism between the RNA-binding protein Musashi1 and miR-137 and its potential impact on neurogenesis and glioblastoma development. RNA. 2019;25:768–782. doi: 10.1261/rna.069211.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon E., Wang W., Tsai L.H. Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol. Psychiatry. 2013;18:11–12. doi: 10.1038/mp.2011.170. [DOI] [PubMed] [Google Scholar]
- 62.Ji Z.G., Jiang H.T., Zhang P.S. FOXK1 promotes cell growth through activating wnt/beta-catenin pathway and emerges as a novel target of miR-137 in glioma. Am. J. Transl. Res. 2018;10:1784–1792. [PMC free article] [PubMed] [Google Scholar]
- 63.Li K.K., Yang L., Pang J.C., Chan A.K., Zhou L., Mao Y. MIR-137 suppresses growth and invasion, is downregulated in oligodendroglial tumors and targets CSE1L. Brain Pathol. 2013;23:426–439. doi: 10.1111/bpa.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L., Liu J., Zhong Z., Gong X., Liu W., Shi L. PTP4A3 is a target for inhibition of cell proliferatin, migration and invasion through Akt/mTOR signaling pathway in glioblastoma under the regulation of miR-137. Brain Res. 2016;1646:441–450. doi: 10.1016/j.brainres.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Takwi A.A., Wangle Y.M., Wu J., Michaelis M., Cinatl J., Chen T. miR-137 regulates the constitutive androstane receptor and modulates doxorubicin sensitivity in parental and doxorubicin-resistant neuroblastoma cells. Oncogene. 2014;33:3717–3729. doi: 10.1038/onc.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giunti L., Da Ros M., De Gregorio V., Magi A., Landini S., Mazzinghi B. A microRNA profile of pediatric glioblastoma: The role of NUCKS1 upregulation. Mol. Clin. Oncol. 2019;10:331–338. doi: 10.3892/mco.2019.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciechomska I.A., Jayaprakash C., Maleszewska M., Kaminska B. Histone Modifying Enzymes and Chromatin Modifiers in Glioma Pathobiology and Therapy Responses. Adv. Exp. Med. Biol. 2020;1202:259–279. doi: 10.1007/978-3-030-30651-9_13. [DOI] [PubMed] [Google Scholar]
- 68.Maleszewska M., Kaminska B. ). Deregulation of histone-modifying enzymes and chromatin structure modifiers contributes to glioma development. Future Oncol. 2015;11:2587–2601. doi: 10.2217/fon.15.171. [DOI] [PubMed] [Google Scholar]
- 69.Li Z., Ren X., Bai X., Zhang X., Tang L., Zhao X. Quantitative nuclear proteomics identifies that miR-137-mediated EZH2 reduction regulates resveratrol-induced apoptosis of neuroblastoma cells. Mol. Cell Proteomics. 2015;14:316–328. doi: 10.1074/mcp.M114.041905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vire E., Brenner C., Deplus R., Blanchon L., Fraga M., Didelot C. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 71.Pereira J.D., Sansom S.N., Smith J., Dobenecker M.W., Tarakhovsky A., Livesey F.J. ). Ezh2, the histone methyltransferase of PRC2, regulates the balance between self-renewal and differentiation in the cerebral cortex. Proc. Natl. Acad. Sci. USA. 2010;107:15957–15962. doi: 10.1073/pnas.1002530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang W.W., Salinas R.D., Siu J.J., Kelley K.W., Delgado R.N., Paredes M.F. Distinct and separable roles for EZH2 in neurogenic astroglia. Elife. 2014;3:e02439. doi: 10.7554/eLife.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L.H., Yin A.A., Cheng J.X., Huang H.Y., Li X.M., Zhang Y.Q. TRIM24 promotes glioma progression and enhances chemoresistance through activation of the PI3K/Akt signaling pathway. Oncogene. 2015;34:600–610. doi: 10.1038/onc.2013.593. [DOI] [PubMed] [Google Scholar]
- 74.Lv D., Jia F., Hou Y., Sang Y., Alvarez A.A., Zhang W. Histone Acetyltransferase KAT6A Upregulates PI3K/AKT Signaling through TRIM24 Binding. Cancer Res. 2017;77:6190–6201. doi: 10.1158/0008-5472.CAN-17-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood K.W., Chua P., Sutton D., Jackson J.R. Centromere-associated protein E: a motor that puts the brakes on the mitotic checkpoint. Clin Cancer Res. 2008;14:7588–7592. doi: 10.1158/1078-0432.CCR-07-4443. [DOI] [PubMed] [Google Scholar]
- 76.Sharma N., Parplys A.C., Zhao W., Groesser T., Liang F., Maranon D.G. NUCKS1 is a novel RAD51AP1 paralog important for homologous recombination and genome stability. Nucleic Acids Res. 2015;43:9817–9834. doi: 10.1093/nar/gkv859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheong J.Y., Kim Y.B., Woo J.H., Kim D.K., Yeo M., Yang S.J. Identification of NUCKS1 as a putative oncogene and immunodiagnostic marker of hepatocellular carcinoma. Gene. 2016;584:47–53. doi: 10.1016/j.gene.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 78.Kikuchi A., Ishikawa T., Mogushi K., Ishiguro M., Iida S., Mizushima H. Identification of NUCKS1 as a colorectal cancer prognostic marker through integrated expression and copy number analysis. Int. J. Cancer. 2013;132:2295–2302. doi: 10.1002/ijc.27911. [DOI] [PubMed] [Google Scholar]
- 79.Gao L., Chen B., Li J., Yang F., Cen X., Liao Z. Wnt/β-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PLoS One. 2017;12:e0181346. doi: 10.1371/journal.pone.0181346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu X., Yao L., Ma G., Cui L., Li Y., Liang W. TCTP promotes glioma cell proliferation in vitro and in vivo via enhanced beta-catenin/TCF-4 transcription. Neuro Oncol. 2014;16:217–227. doi: 10.1093/neuonc/not194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fortin Ensign S.P., Mathews I.T., Symons M.H., Berens M.E., Tran N.L. Implications of Rho GTPase Signaling in Glioma Cell Invasion and Tumor Progression. Front. Oncol. 2013;3:241–245. doi: 10.3389/fonc.2013.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Savitch B.A., Tran N.L., McDonough W.S., Fortin S.P., Winkles J.A., Symons M. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–9542. doi: 10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 83.Chan A., Salhia B., Tran N.L., Wolf A., Nakada M., Rutka F. The guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate the invasive behavior of glioblastoma. Am. J. Pathol. 2008;173:1828–1838. doi: 10.2353/ajpath.2008.080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Innocenti M., Zippel R., Brambilla R., Sturani E. CDC25(Mm)/Ras-GRF1 regulates both Ras and Rac signaling pathways. FEBS Lett. 1999;460:357–362. doi: 10.1016/s0014-5793(99)01374-5. [DOI] [PubMed] [Google Scholar]
- 85.Kiyono M., Satoh T., Kaziro Y. G protein beta gamma subunit-dependent Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm) Proc. Natl. Acad. Sci. U S A. 1999;96:4826–4831. doi: 10.1073/pnas.96.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li H.Y., Li Y.M., Li Y., Shi X.W., Chen H. Circulating microRNA-137 is a potential biomarker for human glioblastoma. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3599–3604. [PubMed] [Google Scholar]
- 87.Riemenschneider M.J., Hegi M.E., Reifenberger G. MGMT promoter methylation in malignant gliomas. Target Oncol. 2010;5:161–165. doi: 10.1007/s11523-010-0153-6. [DOI] [PubMed] [Google Scholar]
- 88.Kozaki K., Imoto I., Mogi S., Omura K., Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–2105. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- 89.Sun J., Cai X., Yung M.M., Zhou W., Li J., Zhang Y. miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer. Oncogene. 2019;38:564–580. doi: 10.1038/s41388-018-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma Y., Bu D., Long J., Chai W., Dong J. LncRNA DSCAM-AS1 acts as a sponge of miR-137 to enhance Tamoxifen resistance in breast cancer. J. Cell. Physiol. 2019;234:2880–2894. doi: 10.1002/jcp.27105. [DOI] [PubMed] [Google Scholar]
- 91.Cheng Y., Shen X., Zheng M., Zou G., Shen Y. Knockdown Of lncRNA NCK-AS1 Regulates Cisplatin Resistance Through Modulating miR-137 In Osteosarcoma Cells. Onco Targets Ther. 2019;12:11057–11068. doi: 10.2147/OTT.S228199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu X., Li Y., Shen H., Li H., Long L., Hui L. MiR-137 restoration sensitizes multidrug-resistant MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim. Biophys. Sin. (Shanghai). 2013;45:80–86. doi: 10.1093/abbs/gms099. [DOI] [PubMed] [Google Scholar]
- 93.Mazzoleni S., Politi L.S., Pala M., Cominelli M., Franzin A., Sergi Sergi L. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res. 2010;70:7500–7513. doi: 10.1158/0008-5472.CAN-10-2353. [DOI] [PubMed] [Google Scholar]
- 94.Tunca B., Tezcan G., Cecener G., Egeli U., Ak S., Malyer H. Olea europaea leaf extract alters microRNA expression in human glioblastoma cells. J. Cancer Res. Clin. Oncol. 2012;138:1831–1844. doi: 10.1007/s00432-012-1261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 96.Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu T., Wang X., Zhi T., Zhang J. Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett. 2018;433:210–220. doi: 10.1016/j.canlet.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 98.Lang H.L., Hu G.W., Zhang B., Kuang W., Chen Y. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017;38:785–798. doi: 10.3892/or.2017.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C.C., Eaton S.A., Young P.E., Lee M., Shuttleworth R. Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 2013;10:1333–1344. doi: 10.4161/rna.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salido-Guadarrama I., Romero-Cordoba S., Peralta-Zaragoza O., Hidalgo-Miranda A., Rodríguez-Dorantes M. MicroRNAs transported by exosomes in body fluids as mediators of intercellular communication in cancer. Onco Targets Ther. 2014;7:1327–1338. doi: 10.2147/OTT.S61562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akers J.C., Ramakrishnan V., Kim R., Skog J., Nakano I. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One. 2013;8:e78115. doi: 10.1371/journal.pone.0078115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei H., Xu Y., Xu W., Zhou Q., Chen Q. Serum Exosomal miR-223 Serves as a Potential Diagnostic and Prognostic Biomarker for Dementia. Neuroscience. 2018;379:167–176. doi: 10.1016/j.neuroscience.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Y., Liu J., Chen L., Jin Y., Zhang G., Lin Z. Serum secreted miR-137-containing exosomes affects oxidative stress of neurons by regulating OXR1 in Parkinson’s disease. Brain Res. 2019;1722:146331. doi: 10.1016/j.brainres.2019.146331. [DOI] [PubMed] [Google Scholar]
- 104.Wood M.J., Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes.: Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 105.Hood J.L. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine (Lond) 2016;11:1745–1756. doi: 10.2217/nnm-2016-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rupert D.L.M., Claudio V., Lässer C., Bally M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim. Biophys. Acta Gen. Subj. 2017;1861:3164–3179. doi: 10.1016/j.bbagen.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 107.Kumar S., Malhotra H., Sheokand N., Chauhan A.S., Kumar M., Jakhar P. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J. Biomed. Nanotechnol. 2016;12:1101–1114. doi: 10.1166/jbn.2016.2229. [DOI] [PubMed] [Google Scholar]
- 108.Turturici G., Tinnirello R., Sconzo G., Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 109.Zhuang X., Sun D., Xiang X., Liu Y., Zhang S., Liu C. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhuang X., Xiang X., Grizzle W., Sun D., Zhang S., Axtell R.C. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed S.F., Das N., Sarkar M., Chatterjee U., Chatterjee S., Ghosh M.K. Exosome-mediated delivery of the intrinsic C-terminus domain of PTEN protects it from proteasomal degradation and ablates tumorigenesis. Mol Ther. 2015;23:255–269. doi: 10.1038/mt.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei Z., Zeng A., Yan W., Yin J., Huang X., Zhou X. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10–21. doi: 10.1016/j.canlet.2018.08.004. [DOI] [PubMed] [Google Scholar]