Abstract
Snakebite envenoming is a neglected disease of public health concern. Most snakebite accidents occur in developing countries. In Ecuador, 17 viper species are responsible for 99% of official accidents, and ten species are in critical conservation states. This report analyzes the snakebite incident cases and mortality rates in Ecuador between 2014 and 2019. The data obtained from the national surveillance system suggests that the incidence and mortality rates remained constant. The geographic region with the highest incidence rates is the Amazonian region. National policies are urgently needed to prevent snakebite accidents and to protect snakes in danger of extinction.
Keywords: Epidemiology of snakebites, Viperidae, Snakebite envenoming, Ecuadorian vipers, Ecuador
Highlights
-
•
Snakebite national incidence (2014–2019): 7.7–11.0 cases/100.000 inhabitants/year.
-
•
Mortality national incidence (2014–2019): 0.03–0.10 deaths/100.000 inhabitants/year.
-
•
1400–1800 snakebites caused by Bothrops asper, Bothrops atrox and Lachesis muta.
-
•
Ten species of the Viperidae family in Ecuador are critically endangered.
Snakebite envenoming continues to be a neglected disease. This problem affects approximately five million people each year worldwide (Chippaux, 2017a; Perry et al., 2020) and constitutes a significant health problem in tropical Latin American regions (Chippaux, 2017b; Gutierrez et al., 2017; Marcussi et al., 2011). Particularly in Ecuador, the last available scientific publication estimated incidence and mortality rates of 9.5 and 0.058 cases per 100.000 inhabitants between 2014 and 2016 (Chippaux, 2017a). It is necessary to improve our understanding of this critical health problem to identify research gaps that can guide public health programs in Ecuador. This report comprises two sections: an updated summary of snakebite incident cases and deaths in Ecuador between 2014 and 2019, and an analysis of the snakes’ species distribution and conservation status in the country.
A literature review was carried out to summarize the available data regarding the snakes’ species distribution and conservation in the country. Snakebite incidence rates and mortality rates were calculated using secondary data. Cumulative data of snakebite cases of the last week of each year was obtained from the National Surveillance System webpage (Gaceta Epidemiológica Ecuador, n.d). Snakebite deaths data was obtained from the causes of death datasets in the general population publicly available on the National Institute of Statistics webpage (INEC, n.d.). The total population and population per region were obtained from the inter-census projections from the last census (INEC, n.d.). Data were analyzed and managed using Origin 8 v8.0891, Origin Lab Corporation, Northampthon, MA USA.
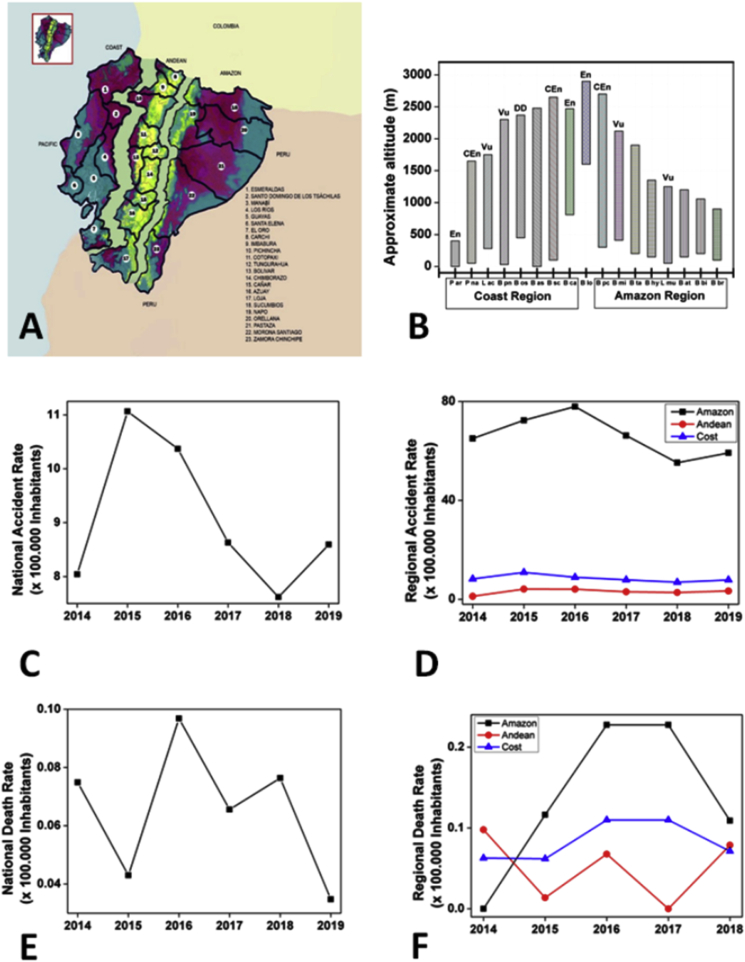
Ecuador is located in the northwestern region of South America; its continental territory comprises three geographic regions. The Coastal region concentrates 49% of the total population (altitude: 0–1200 m above sea level; temperature: 20–40 °C, and pluviometric indexes below 1000 mm/year). The Andean region houses 45% of the Ecuadorian population (altitude: 1200–6400 m above sea level; temperatures usually below 15 °C, and pluviometric indexes below 500 mm/year). Finally, the Amazonian region is the least populated, with 5% of the Ecuadorian inhabitants (altitude below 750 m above sea level; temperature above 28 °C, and pluviometric indexes between 2000 and 3000 mm/year) (INEC, n.d.; Derrotero, 2005) (Fig. 1A and 1B).
Fig. 1.
Human demographics and snake population distribution in Ecuador: A) Political map of Ecuador: the continental area comprises three regions with its provinces (Coast: provinces 1–7; Andean: provinces 8–17; and Amazon: provinces 18–23) (Modified from Yañez-Arenas et al., 2018), B) Viperidae Family snake species in the country and altitudes where they are found. The species in critical state of preservation are denoted: Endangered (En), Critically Endangered (CEn), Vulnerable (Vu), Date Deficient (DD) (Birskis-Barros, 2019; Carrillo et al., 2005). C) National snakebite incidence rate per year (per 100.000 inhabitants). D) Snakebite incidence rate per year per geographic region (per 100.000 inhabitants). E) National snakebite mortality rate per year (per 100.000 inhabitants). E) Snakebite mortality rate per year per geographic region per (100.000 inhabitants).
The Andean mountains shape the Ecuadorian landscape with heterogeneous environments, climatic conditions, and diverse flora and fauna in the lowlands compared to the highlands (INEC, n.d.; Derrotero, 2005). The last explains the country's vast biodiversity, considered as one of the most megadiverse in the world (Moura et al., 2014; Esquerre et al., 2019; Almeida et al., 2020).
Ecuador's exuberant biodiversity is also reflected in the variety of its venomous snakes, exemplified by the presence of Elapidae and Viperidae families in the country. The Elapidae family comprises two genera and 19 species distributed at different altitudes, from cero to 2100 m above sea level: Hydrophis platurus and 18 Micrurus species. Among the Micrurus species, seven (ancoralis, bocourti, mipartitus decussates, dumerilii trasandinus, multiculatus, tschudii olsoni, mertensi) are distributed in the Coastal region, and eleven in the Amazonian region (peruvianus, petersi, steindachneri, melanotus, obscurus, ortoni, scutiventris, langsdorffii, helleri, ornatissimus, surinamensis). In Ecuador, however, snakebite accidents caused by the Elapidae family are unusual (less than 1% of total accidents), with M. mipartitus decussates and M. helleri being the most common species responsible for these accidents (Torres-Carvajal et al., 2020; MSP, 2017; Valencia et al., 2016).
On the other hand, the Viperidae family is concentrated in Ecuador's Coastal (8 species) and the Amazonian regions (8 species). This family comprises five distinct genera and 17 species that can be found in several provinces at different altitudinal levels, ranging from sea level up to approximately 3000 m: Bothriechis (schlegelii), Bothrocophias (campbelli, hyoprora, microphthalmus), Bothrops (asper, atrox, bilineata, brazili, lojanus, osbornei, pulcher, punctatus, taeniatus), Lachesis (acrochorda, muta) Porthidium (arcosae, nasutum) (Table 1) (Torres-Carvajal et al., 2020; Valencia et al., 2016; Alencar et al., 2016). The majority of snakebite accidents in Ecuador are caused by the species B. asper, B. atrox, B. bilineata and L. muta (MSP, 2017; WHO, 2010; Yañez-Arenas et al., 2018). Fig. 1B presents the species distribution by geographic region. The species are separated from each other by the Andes and have no contact between them, except Bothrops lojanus, which can be found in these three Ecuadorian regions. It is possible that the movement of this species occurs through the Inter-Andean valley of Vilcabamba (Torres-Carvajal et al., 2020; Salazar-Valenzuela et al., 2018; Valencia et al., 2016) (Fig. 1B and Table 1).
Table 1.
Viperidae family species in Ecuador and their geographic location (see Fig. 1A) (Torres-Carvajal et al., 2020; Valencia et al., 2016).
| Genus | Species | Photo | Common Names | Province localizations (See Fig. 1A) |
|---|---|---|---|---|
| Bothriechis |
schlegelii |
 Photo by Omar Torres-Carvajal |
Eyelash palm-pit vipers; Cushili; Víbora papagayo; Zampiña; Campanita. | 1; 2; 3; 4; 5; 7; 9; 10; 11; 15; 16 |
| Bothrocophias | campbelli |
 Photo by David Valenzulea-Salazar |
Ecuadorian toad headed pit vipers; Boca de sapo; Curruncha. | 1; 2; 9; 10; 11; 13; 14 |
| hyoprora |
 Photo by Wolfgang Wuster |
Amazonian toad headed pit vipers; Nariz de Puerco; Cabeza de candado; Ushuali. | 18; 19; 20; 21; 22; 23 |
|
| microphthalmus |
 Photo by Museo de Zoología QCAZ |
Small-eyed toad headed pit vipers; Hoja podrida; Macanchilla; Núkamp; Pushlio. | 12; 19; 21; 22; 23 |
|
| Bothrops | asper |
 Photo by Wolfgang Wuster |
American Lancehead; Cuatronarices; Equis; Pudridora; Macanchi mariposa. | 1; 2; 3; 4; 5; 6; 7; 8; 9; 10; 11; 13; 14; 15; 16; 17 |
| atrox |
 Photo by Museo de Zoología QCAZ |
South American lanceheads; Equis negra; Shishi; Macanchi. | 18; 19; 20; 21; 22; 23 |
|
| bilineata |
 Photo by Museo de Zoología QCAZ |
Two-striped forest-pitvipers; Lorito; Palo verde; Nukam; Tobenaka. | 18; 19; 20; 21; 22 |
|
| brazili |
 Photo by Wolfgang Wuster |
Brazil's lanceheads; Equis de Brazil; Pitala; Kara napi; Yawayawaa. | 18; 20; 21; 22; 23 |
|
| lojanus |
 Photo by Museo de Zoología QCAZ |
Lojan lanceheads; Macanchi; Macucho. | 7; 16; 17; 23 |
|
| osbornei |
 Photo by Museo de Zoología QCAZ |
Osborne's lanceheads; Llucti negra. | 10; 11; 14 | |
| pulcher |
 Photo by Museo de Zoología QCAZ |
Andean forest-pitvipers; Culebra tigre; Lorito; Loro mashaco; Yaku pitalala. | 12; 17; 19; 20; 21; 22; 23 |
|
| punctatus |
 Photo by Andrés Calero |
Chocoan lanceheads; Equis de árbol; Equis manchada; Granita de oro | 1; 2; 8; 10 | |
| taeniatus |
 Photo by Museo de Zoología QCAZ |
Speckled forest pit vipers; Orito palo; Gunjintsin; Shishink; Wascapitalala. | 14; 18; 19; 20; 21; 22; 23 |
|
| Lachesis | acrochorda |
 Photo by David Valenzulea-Salazar |
Chocoan bushmasters; Guacama; Verrugosa | 1; 2; 3; 10 |
| muta |
 Photo by Museo de Zoología QCAZ |
Amazon bushmasters; Verrugosa; Sol; Motolo; Cofase. | 18; 19; 20; 21; 22; 23 |
|
| Porthidium | arcosae |
 Photo by Wolfgang Wuster |
Manabí hog nosed pit vipers; Sabanera | 3 |
| nasutum |
 Photo by Museo de Zoología QCAZ |
Rainforest hognosed pit viper; Guardacaminos; Vivora. | 1; 2; 3; 9; 10 |
In Ecuador, from 2014 to 2019 the average snakebite incident and mortality rates remained constant (average cases: 1506 [range: 1400–1800]; average incidence rate: 9.1 [range: 7.6–11.1] cases per 100.000 inhabitants; mortality rate: 0.07 [range: 0.03–0.10] per 100.000 inhabitants) as compared to the reported cases from Chippaux et al. (2017) from the period between 2014 and 2016 (Fig. 1C y 1E).
The Amazonian region still has the highest number of incident cases (55–78 bites per 100.000 inhabitants) followed by the Coastal (7–11 bites per 100.000 inhabitants) and the Andean (1–4 bites per 100.000 inhabitants) regions. These data highlight that snakebite envenoming is still a neglected condition in Ecuador. We were unable to identify any policy or program aiming to prevent snakebites or to improve the population's knowledge regarding this topic. Incidence rates remained constant over the years in the Andean and Coast regions but dropped since 2017 in the Amazon region. The reasons are still to be elucidated as reported Chippaux et al. (2017a; Chippaux, 2017b).
According to the models generated by Yañez-Arenas et al. (2018), the areas at the highest risk of snake envenoming are located in the central and northern Coastal and Amazonian regions of Ecuador: areas with the highest concentration of rural communities, which in turn, are highly vulnerable to snakebite accidents. Nevertheless, underreporting is a probable concern as a considerable number of rural inhabitants attend private or non-medical resources for emergency events (Eckhardt et al., 2018). In line with this finding, qualitative research has demonstrated how rural snakebite victims opt for traditional healers due to accessibility, cultural issues, and efficacy and access to antivenoms in formal health care centers (Schioldann et al., 2018).
Accidents and deaths per 100,000 inhabitants are much higher in the Amazonian region when compared to the Coastal region. Fig. 1D and F shows how from 2015 until 2017, there was an increase in accidents and deaths secondary to snakebite envenoming in the Amazonian region. This aspect reinforces the great number of questions that remain unanswered. New research is urgently needed to improve our understanding of human and snake behaviors, as well as the possible seasonal variations that can favor contact between the two populations (Gonzalez-Andrade and Chippaux, 2010).
Unfortunately, antivenoms are not produced in Ecuador; instead, polyvalent antivenoms are imported mostly from the Instituto Clodomiro Picado-University of Costa Rica, and less frequently from Colombia and Argentina (MSP, 2017). Concerning the lethal doses (LD50) of the venoms from the principal species responsible for snakebite accidents in Ecuador (B. asper and B. atrox), Teran and Lomonte (2016) demonstrated that the Ecuadorian B. atrox venom is similar to B. atrox from Peru, and the Ecuadorian B. asper venom is comparable to that from the same species from Guatemala, Costa Rica, and Colombia.
Laines et al. (2014) analyzed the LD50 of the Ecuadorian B. atrox and B. asper and verified that the LD50 of these species is much the same. The authors concluded that the polyspecific botropic antivenoms imported from Costa Rica are effective in neutralizing the LD50 and, therefore, hemorrhagic, coagulant, and defibrinogenic effects of the Ecuadorian B. atrox and B. asper.
Relative to the snakes' conservation state, the Elapidae family species in danger of extinction are similarly distributed in the Coastal (five species) and Amazonian regions (four species). However, the majority of Viperidae species in danger of extinction (except B. asper) are distributed in the Coastal region, where the majority of the Ecuadorian human population is concentrated. The high growth population rate leads human beings to invade natural environments and to kill snakes in an attempt to avoid snakebites. In fact, among the eight Viperidae species living in the Coastal region, seven are critically endangered (Fig. 1B). On the contrary, in the Amazonian Region, where only 5% of the national population lives, only one species is in grave danger of extinction (B. pucher), and two are in a vulnerable state (B. microphthalmus and L. Muta) (Fig. 1B) (Birskis-Barros, 2019; Carrillo et al., 2005). From a geographical perspective, the Coastal region is a highly limited strip of land in an east-west direction between the Pacific Ocean and the Andes. Therefore, both humans and snakes tend to move in the north-south direction, which increases the possibilities of interactions between them.
On the other hand, this geographic restriction observed in the Coastal region is absent in the Amazonian region. The only restriction that exists in the Ecuadorian Amazon is the Andes that limits it on the west side. Thus, both human and snake populations have greater freedom and can disperse to the Peruvian, Colombian, and Brazilian Amazon, decreasing the likelihood of contact. In the Amazonian environment, the multi-environmental complexity might protect biodiversity in general, including snake populations (Antonelli et al., 2018; Rittler et al., 2018).
Ecuador is extremely rich in biodiversity; thus, it must be treated with extreme care to avoid the loss of this valuable heritage, including snake environments. Nevertheless, a crucial aspect to consider is the species' conservation in the context of essential economic activities. Therefore, behavioral, ecological, and environmental research is needed to understand the population's dynamics to support the actions of environmental management without impacting economic growth. In this sense, this study offers an insight of the accidents and deaths caused by snakebites in Ecuador. Still, several questions remain unanswered. Future research should identify the real impact of snakebite envenoming in rural communities, and the perceptions of victims about health care access and pertinence. Programs are urgently needed to: (i) improve the national surveillance system reporting, by avoiding inconsistencies and providing a more detailed description (i.e., species involved), (ii) implement programs aimed at educating the population concerning the importance of snake conservation, (iii) evaluate the diagnosis and treatments provided by primary health care practitioners, and (iv) develop appropriate interventions to prevent snakebite accidents. The Ecuadorian Ministry of Health should invest in improving primary health care services and provide high-quality training to health professionals and community members.
Ethical statement
International ethical guidelines for scientific papers were followed in the preparation of this manuscript.
CRediT authorship contribution statement
Angélica Ochoa-Avilés: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing - original draft, Writing - review & editing. Odalys S. Heredia-Andino: Formal analysis. Samuel A. Escandón: Formal analysis. Cristopher A. Celorio-Carvajal: Investigation, Methodology. María C. Arias-Peláez: Investigation, Methodology. Fausto Zaruma-Torres: Investigation, Methodology. Cleópatra A. da S. Caldeira: Investigation, Methodology. Andreimar M. Soares: Investigation, Methodology. Saulo L. Da Silva: Conceptualization, Investigation, Methodology, Project administration, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
References
- Alencar L.R.V. Diversification in vipers: phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 2016;105:50–62. doi: 10.1016/j.ympev.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Almeida J.R. Assessing the stability of historical and desiccated snake venoms from a medically important Ecuadorian collection. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;230:108702. doi: 10.1016/j.cbpc.2020.108702. [DOI] [PubMed] [Google Scholar]
- Antonelli A. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. U.S.A. 2018;115:6034–6039. doi: 10.1073/pnas.1713819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birskis-Barros I. Ecological and conservation conservation of rarity in new world pitvipers. Diversity. 2019;11:147–162. [Google Scholar]
- Carrillo E. 2005. Lista roja de los reptiles del Ecuador. Fundación Novum Milenium, UICN-Sur, UICN-Comité Ecuatoriano, Ministerio de Educación y Cultura. Quito Ecuador. [Google Scholar]
- Chippaux J.-P. Incidence and mortality due to snakebite in the Americas. PLoS Neglected Trop. Dis. 2017;11(6) doi: 10.1371/journal.pntd.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017;23:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrotero . Insituto Oceanografico de la Armada INOCAR Retrieved from; 2005. De la Costa Continental e Insular del Ecuador. Quito Ecuador. [Google Scholar]
- Eckhardt M. Universal health coverage in rural Ecuador: a cross-sectional study of perceived emergencies. West. J. Emerg. Med. 2018;19(5):889–900. doi: 10.5811/westjem.2018.6.38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerre D. How mountains shape biodiversity: the role of the Andes in biogeography, diversification, and reproductive biology in South America's most species-rich lizard radiation (Squamata: liolaemidae) Evolution. 2019;73:214–230. doi: 10.1111/evo.13657. [DOI] [PubMed] [Google Scholar]
- G, EE Gaceta epidemiológica Ecuador (SIVE-ALERTA). (n.d.). Ministerio de Salud Pública. https://www.salud.gob.ec/gaceta-epidemiologica-ecuador-sive-alerta/ from.
- Gutierrez J.M. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: state-of-the-art and challenges ahead. Toxins. 2017;9 doi: 10.3390/toxins9050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INEC (Instituto Nacional de Estadística y Censos). (n.d.) Proyecciones poblacionales. Instituto nacional de Estadística Y censos. https://www.ecuadorencifras.gob.ec/proyecciones-poblacionales/ from.
- Laines J. Toxicity of Bothrops sp snake venoms from Ecuador and preclinical assessment of the neutralizing efficacy of a polyspecific antivenom from Costa Rica. Toxicon. 2014;88:34–37. doi: 10.1016/j.toxicon.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Marcussi S. Evaluation of the genotoxicity of Crotalus durissus terrificus snake venom and its isolated toxins on human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;274:59–63. doi: 10.1016/j.mrgentox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Moura A.A. Purification and biochemical characterization of three myotoxins from Bothrops mattogrossensis snake venom with toxicity against Leishmania and tumor cells. BioMed Res. Int. 2014 doi: 10.1155/2014/195356. Article number 195356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MSP (Ministerio de Salud Pública) 2017. Manejo clínico del envenenamineto por mordeduras de serpientes venenosas y picaduras de escorpiones. Protocolo basado en evidencia.https://www.coursehero.com/file/41672301/Manejo-clinico-del-envenenamiento1pdf/ [Google Scholar]
- Perry G., Lacy M., Das I. Snakes, snakebites, and humans. In: Angelici F.M., Rossi L., editors. Problematic Wildlife II: New Conservation and Management Challenges in the Human-Wildlife Interactions. Springer International Publishing; 2020. pp. 561–580. [Google Scholar]
- Rittler C.D. Locality or habitat? Exploring predictors of biodiversity in Amazonia. Ecography. 2018;42:321–333. [Google Scholar]
- Salazar-Valenzuela D. Divergence od tropical pitvipers promoted by independent colonizacion events of dry montane Andean habitats. J. Biogeogr. 2018;46:1826–1840. [Google Scholar]
- Schioldann E. Why snakebite patients in Myanmar seek traditional healers despite availability of biomedical care at hospitals? Community perspectives on reasons. PLoS Neglected Trop. Dis. 2018;12(2) doi: 10.1371/journal.pntd.0006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran M.C., Lomonte B. Actividad letal de seis venenos de serpientes de importancia médica en el Ecuador. Rev. Ecuat. Med. Cienc. Biol. 2016;37:25–30. [Google Scholar]
- Torres-Carvajal O. 2020. Reptiles del Ecuador.https://bioweb.bio/faunaweb/reptiliaweb/ListaEspeciesPorFamilia/115 from. [Google Scholar]
- Valencia J.H. Universidad de Texas Quito Ecuador; 2016. Serpientes Venenosas del Ecuador Fundación Herpetológica Gustavo Orcés. [Google Scholar]
- WHO (World Health Organization) 2010. Guide Lines for the Production Control and Regulation of Snake Antivenom Immunoglobulins; pp. 1–134. [Google Scholar]
- Yañez-Arenas C. Estimating geographic patterns of ophidism risk in Ecuador. Neotropical Biodiversity. 2018;4:55–61. [Google Scholar]