Dear Editor,
The main clinical features of severe Corona Virus Disease 2019 (COVID-19) are hypoxaemia and respiratory failure [1]. Some COVID-19 patients may benefit from high-flow oxygen through nasal cannula (HFNC) [2]. However, it is critical not to delay intubation when it becomes necessary, otherwise increased mortality may be observed [3]. The “ROX index”, dividing the oxygen saturation by the inspired oxygen fraction and the respiratory rate (SpO2/FiO2/RR), has been proposed to monitor patients treated with HFNC [4, 5].
We conducted a monocentric prospective observational study to assess the accuracy of several parameters, including the ROX, to detect HFNC failure in the specific setting of SARS-CoV-2-related severe pneumonia. All the patients admitted in our intensive care unit with proven COVID-19 requiring HFNC during March and April 2020 were included. Clinical parameters were collected within the 4 h before, and 30 min, 2 and 6 h after HFNC initiation. HFNC was systematically initiated at 60 L min−1/FiO2 1. Then, FiO2 was decreased hourly, maintaining 92% ≤ SpO2 ≤ 98%, down to 0.4, at which point flow was progressively reduced until weaning. “HFNC failure” was defined as the need for invasive mechanical ventilation within 7 days of HFNC onset.
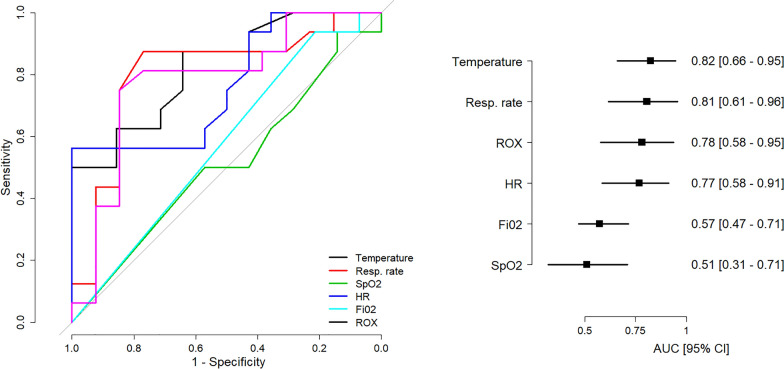
Thirty patients were included (Table S1 in the eSupplement). Prior to HFNC, the median [IQR] RR was 30 [26–36]/min and O2 flow was 10 [8–15] L/min. Sixteen patients met the outcome “HFNC failure” after 1 [0.9–2.5] day. The remaining 14 patients were weaned after 5 [4–7] days. Although not different before HFNC onset, RR was significantly lower at H0.5 in the “weaned” compared to the “failure” group (24 [20–24] vs. 31 [27–34]/min, p = 0.004). The area under the receiver operating characteristic curve (AUROC) of RR at H0.5 was 0.81 95% CI [0.61–0.96] (Fig. 1), with a best cut-off value at 26/min (sensitivity 75%, specificity 85%, positive likelihood ratio 4.9). RR at H2 and H6 was less informative (Table S2). ROX H0.5 had an AUROC of 0.78 [0.58–0.95]. Performance characteristics of ROX H0.5 using the previous published cut-off value of 4.88 [4, 5] were 81% sensitivity, 38% specificity and a positive likelihood ratio of 1.3. Neither the ROX at H2 and H6, nor its changes between H0 and H0.5, H0.5–H2, and H2–H6, had better diagnostic performance than RR at H0.5 (Tables S1 and S2). Results for the other parameters are reported in Fig. 1 and in the eSupplement.
Fig. 1.
Receiver Operating Characteristic (ROC) curves for the principal clinical parameters for the diagnosis of high-flow nasal cannula failure. SpO2 oxygen saturation, HR heart rate, FiO2 inspired oxygen fraction, ROX “Respiratory rate-Oxygenation” index
The main limitations of this derivation cohort are its monocentric design and the small number of patients included. These results should be confirmed in future validation cohorts before proposing to intubate patients who are still very tachypneic as early as 30 min after HFNC onset. However, our results suggest that monitoring COVID-19 patients requiring HFNC with the ROX index did not add value to RR alone. This is in agreement with a possible lower diagnostic value of the ROX in viral pneumonia [4]. This may be because the ROX was mostly dependent on RR, as FiO2 was persistently high during the first hours of HFNC [6] and as COVID-19 patients may present higher dead space due to diffuse pulmonary thrombi [7]. In addition, one-third of the patients had 100% SpO2 despite the 92% ≤ SpO2 ≤ 98% target, which may have decreased the contribution of the SpO2/FiO2 in the diagnostic accuracy of the ROX. Our results highlight the need for continuous monitoring of COVID-19 patients requiring HFNC, and suggested reinforcing the surveillance of patients with a RR ≥ 26/min half an hour after HFNC onset, as it may be associated with a high risk of intubation.
In conclusion, among the respiratory parameters available for monitoring COVID-19 patients treated with HFNC, using the RR is accurate and simple, thus “being most likely the right solution” according to Occam’s razor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This work has not been funded by any external source.
Availability of data and material
Data are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflicts of interest
All the authors declare that they do not have any competing interest with the current work.
Ethics approval
This work has been approved by the French Anesthesiology and Intensive Care Medicine Society (SFAR) ethical committee (IRB 00010254-2020-096).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China : summary of a report of 72 314 cases from the Chinese center of disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Wang K, Zhao W, Li J, Shu W, Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10(1):37. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 4.Roca O, Messika J, Caralt B, García-de-Acilu M, Sztrymf B, Ricard J-D, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016;35:200–205. doi: 10.1016/j.jcrc.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernandez G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 6.Tatkov S. Nasal high-flow therapy: role of FiO2 in the ROX index. Am J Respir Crit Care Med. 2019;200(1):115–116. doi: 10.1164/rccm.201902-0376LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poor HD, Ventetuolo CE, Tolbert T, Chun G, Serrao G, Zeidman A, et al. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clin Transl Med. 2020 doi: 10.1002/ctm2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on reasonable request.