Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on sodium carboxymethyl cellulose as a feed additive for all animal species. Sodium carboxymethyl cellulose is intended for use as a technological additive (functional groups: emulsifier, stabiliser, thickener, gelling agent and binder) in premixtures and feedingstuffs for all animal species with no minimum and maximum content. A proper identification and characterisation of sodium carboxymethyl cellulose as required for a feed additive is not available and the occurrence of potential toxic impurities cannot be assessed. The following conclusions apply only to sodium carboxymethyl cellulose meeting the food additive specifications. The FEEDAP Panel concluded that sodium carboxymethyl cellulose is considered safe for all animal species. The use of sodium carboxymethyl cellulose in animal nutrition is of no concern for consumer safety. In the absence of data, the FEEDAP Panel was not in the position to conclude on the safety of sodium carboxymethyl cellulose for the user. The use of sodium carboxymethyl cellulose as a feed additive is considered safe for the environment. The additive is considered to be efficacious in feedingstuffs for all animal species.
Keywords: Sodium carboxymethyl cellulose, E 466, technological additives, emulsifier, stabiliser, binder, thickener, gelling agents, all animal species
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 10(2) of that Regulation also specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, at the latest one year before the expiry date of the authorisation given pursuant to Directive 70/524/EEC for additives with a limited authorisation period, and within a maximum of seven years after the entry into force of this Regulation for additives authorised without a time limit or pursuant to Directive 82/471/EEC.
The European Commission received a request from Organisation des Fabricants de produits Cellulosiques Alimentaires (OFCA)2 for re‐evaluation of the product sodium carboxymethyl cellulose (E464), when used as a feed additive for all animal species (category: technological; functional group: emulsifier, stabiliser, binder, thickener and gelling agents).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 26 April 2019.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product sodium carboxymethyl cellulose, when used under the proposed conditions of use (see Section 3.1.1).
1.2. Additional information
The additive under assessment is sodium carboxymethyl cellulose. It is intended to be used as a technological additive in feed for all animal species.
Sodium carboxymethyl cellulose (E 466) is currently authorised as a feed additive for all animal species, without a minimum and a maximum content. It is also authorised, quantum satis, for use as a food additive.
Sodium carboxymethyl cellulose is authorised to be used as a food additive in accordance with Annex II to Regulation (EC) No (1333/2008)3 with specific purity criteria defined in Commission Regulation (EU) No 231/20124. Before, it was evaluated, together with other cellulose, by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1989 (JECFA, 1990) and 1998 (JECFA, 1999a,b). An ‘ADI not specified’ was established for each modified cellulose E 461–E 466 and E 469 (JECFA, 1990, 1999a,b).
Sodium carboxymethyl cellulose safety and efficacy was evaluated by EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) Panel, for use in active food contact materials, in 2018 (EFSA CEF Panel, 2018). The additive has been re‐evaluated as a food additive in 2018, together with other celluloses (EFSA ANS Panel, 2018).
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier5 in support of the authorisation request for the use of sodium carboxymethyl cellulose as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of methyl cellulose in animal feed. The Executive Summary of the EURL report can be found in Annex A.6
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of sodium carboxymethyl cellulose is in line with the principles laid down in Regulation (EC) No 429/20087 and the relevant guidance documents: Guidance on technological additives (EFSA FEEDAP Panel, 2012a), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012b), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The additive consists of pure sodium carboxymethyl cellulose and is free from any other added components. It is intended to be used as a technological additive (category: emulsifier, stabiliser, binder, thickener and gelling agents) in feedingstuffs and premixture for all animal species.
3.1. Characterisation
Sodium carboxymethyl cellulose is identified with the single Chemical Abstracts Service (CAS) number 9000‐32‐4, and the European Inventory of Existing Chemical Substances (EINECS) number 618‐378‐6. It is the partial sodium salt of a carboxymethyl ether of cellulose. The cellulose is obtained directly from natural strains of fibrous plant material. It is prepared by the etherification reaction between the alkali‐cellulose complex and monochloroacetic acid with consequent formation of the additive and sodium chloride as a by‐product.
Sodium carboxymethyl cellulose is marketed in the form of white or slightly yellowish or greyish odourless and tasteless granules or fibrous powder. It is almost insoluble in ethanol. It yields a viscous colloidal solution with water. A generalised structure of sodium carboxymethyl cellulose is shown in Figure 11.
Figure 1.
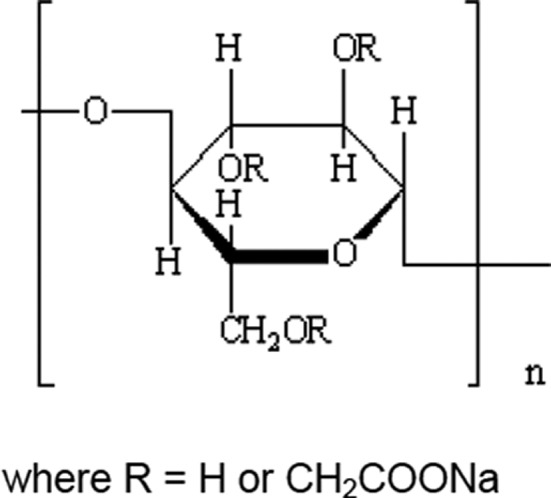
Chemical structure of sodium carboxymethyl cellulose
No analytical data that would support the identification of the active substance and the batch‐to‐batch consistency of the additive was provided.
The applicant claims that the feed additive sodium carboxymethyl cellulose is specified to be manufactured to meet the specifications set for its use as a food additive. The main specifications as food and feed additive are: carboxymethyl groups > 0.2 and < 1.5%, loss on drying < 12%, sodium glycolate < 0.4%, sodium < 12.4% and pH 5–8 (1% colloidal solution).
The analysis of five batches of the additive8 resulted in: carboxymethyl groups between 0.81 and 0.92%, loss on drying 4.3−5.7%, sodium glycolate 0.09−0.21%, sodium 7.6–9% and pH between 7.5 and 8.3. Only statements, without figures, of compliance with the specifications for some impurities (heavy metals, solvents, microbial purity) were provided. Information on other impurities (arsenic, aldehydes, pesticides, dioxins, dioxin‐like and non‐dioxin‐like polychlorinated biphenyls, mycotoxins, botanical impurities) was not provided. No information on the dusting potential or the particle size distribution of the additive was made available.
Sodium carboxymethyl cellulose is specified to have a shelf life of several years. However, no analytical evidence was provided. No specific information on the stability of sodium carboxymethyl cellulose or its capacity to homogeneously distribute in feed was made available.
3.1.1. Conditions of use
Sodium carboxymethyl cellulose is intended to be used as a technological additive (functional group: emulsifier, stabilizer, binder, thickener and gelling agents) in feedingstuffs and premixture for all animal species, with no recommendation of a minimum or maximum content.
3.2. Safety
The applicant did not provide new studies on the safety of sodium carboxymethyl cellulose, but made reference to previous assessment of celluloses (as a group) performed by other scientific bodies. Cellulose and cellulose derivatives were evaluated for their safety by JECFA (1990), which allocated a group acceptable daily intake (ADI) of ‘not specified’. The Scientific Committee for Food (SCF, 1994, 1999) also assessed five closely related cellulose derivatives and allocated a group ADI of ‘not specified’. The last comprehensive evaluation of cellulose and cellulose derivatives, including sodium carboxymethyl cellulose, for their use as food additives was done in 2017 by the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) (EFSA ANS Panel, 2018), which concluded that there was no need to set a numerical ADI. Although the data set available for the different celluloses is not complete and most of the studies were old and do not meet the current requirements of toxicological testing, the ANS Panel considered that the physico‐chemical, structural, biological and kinetic similarities between the modified celluloses make it possible to apply a read‐across approach among the different celluloses.
The main findings of the studies evaluated in the previous assessments, in particular in the ANS Panel opinion (EFSA ANS Panel, 2018) are summarised below.
3.2.1. Absorption, distribution, metabolism and excretion
3.2.1.1. Cellulose
Cellulose is a linear homopolymer consisting of repeating β‐d‐glucopyranosyl units linked via (1,4) glycosidic bonds. In its pure form the straight chains are bound closely together by multiple intermolecular hydrogen bonds and van der Waals forces, producing a water insoluble fibrous or crystalline substance which is relatively inert. The EFSA ANS Panel (2018) assessed recently the absorption, distribution, metabolism and excretion (ADME) of celluloses and draw the following conclusions concerning non‐herbivore mammals: cellulose is not absorbed intact in the gastrointestinal tract of animals and humans but is fermented during its passage through the large intestine by the microbiota, with the limited production (9% of the administered dose in the rat) of short‐chain fatty acids (mainly acetic acid and succinic acid), hydrogen, carbon dioxide and methane.
In ruminants, cellulose is first hydrolysed by ruminal microorganisms into cellobiose, then is fermented to pyruvate and finally volatile fatty acids. The changes of forage to concentrate ratios in the diet significantly affect the number and type of rumen microorganisms and then affect the end products of fermentation. Moreover, the extent of cellulose digestion is a compromise between the rate of hydrolysis and the retention time in the rumen related to the particle size of the forage. The intrinsic digestibility of cellulose depends on the origin and treatment of the forage. As far as cellulose is associated to lignin, hemicelluloses and cutin in natural forages, a wide range of digestibility is observed (30–90%). Crystallinity of cellulose decreases the rate but not the extent of digestibility that may reach 80% (Van Soest, 1994).
Marine and freshwater fish harbour an intestinal microbiota less abundant than in mammals, made of aerobic and facultative anaerobic bacteria. Limited and conflicting data have shown either the complete lack of cellulose degradation in the trout or tilapia, or a limited (13%) activity in the trout. A digestibility study carried out in the trout and the carp administered a purified (devoid of lignin and reduced amount of hemicelluloses) crystalline cellulose extracted from wood (fibre length ˂ 150 μm, diameter ˂ 45 μm), showed the practical absence of cellulose degradation in both species (Bergot and Breque, 1983).
In poultry, most data in the literature tend to demonstrate that the cellulose complex from plant feedingstuffs that exhibits high crystallinity and water insolubility is not digested (Janssen and Carré, 1985).
In the rabbit hindgut, fermentation occurs through a wide prevalence of Bacteroides that do not allow an extended digestibility of fibre. Digestibility of cellulose was shown to amount to 16% of the administered dose, whereas values of 14% and 18% were reported for fibre (cellulose being the main component) (review from the NRC, 1977). Later studies reported values comprised between 15% and 25% in rabbits administered different plant sources of cellulose (Gidenne and Perez, 1996; Chiou et al., 1998). In the horse, digestion of plant structural carbohydrates (including cellulose) occurs in the hindgut (colon and overall caecum). The microbiota of the caecum comprises bacteria similar to those of the rumen, but specific protozoa. The resulting digestibility is about two‐third that measured in ruminants.
3.2.1.2. Modified celluloses
The etherification of cellulose disrupts the hydrogen bonding and the resulting compounds are not ionized and more water soluble. The EFSA ANS Panel (2018) concluded that modified celluloses including ethyl, methyl, hydroxypropyl celluloses, would not be absorbed intact and not fermented in the gastrointestinal tract of animals (rat) or humans; they are excreted essentially unchanged mainly via the faeces (more than 90% of the administrated doses), while only minor amounts of metabolites and derived‐products are excreted via urine or expired air (as 14CO2) and there is no indication for accumulation in the body.
No data are available concerning the ADME of modified celluloses in the target animal species. However, the absence of significant metabolism in the rat and the human indicates that, despite an increase of water solubility, the etherification of cellulose would strongly limit the action of microbial cellulases. Consequently, the FEEDAP Panel considers likely the lack of digestibility of these compounds in monogastric mammals, poultry and fish. In ruminants and hindgut animals (rabbit and horse), it cannot be excluded that the rich and complex microbiota would allow a limited enzymatic attack of these structures.
3.2.2. Toxicological studies
3.2.2.1. Genotoxicity
Overall, the data set for genotoxicity is not complete for all the substances and several studies were not in line with the current standard. However, it should be considered that the chemical structure of unmodified and modified cellulose does not show any alert for genotoxicity and that no indication of genotoxicity was found for any of these substances in several in vitro and in vivo genotoxicity studies.
Concerning modified celluloses, methyl cellulose was negative in the bacterial reverse mutation assay, in the in vitro chromosomal aberration test in mammalian cells and in host‐mediated assays with yeast and bacteria. In vivo methyl cellulose was also negative in a chromosome aberration assay in rat bone marrow and in the dominant lethal assay in male rats. Sodium carboxymethyl cellulose was negative in the bacterial reverse mutation assay, in the in vitro chromosomal aberration test in mammalian cells, performed only without metabolic activation, and in host‐mediated assays.
The ANS Panel considered that the read‐across from methyl cellulose and sodium carboxymethyl cellulose to other modified celluloses bearing similar simple substituents (including methyl cellulose) was justified.
Moreover, the FEEDAP Panel also noted that methyl cellulose and sodium carboxymethyl cellulose have been used for a long time as vehicles for non‐water‐soluble substances in several in vivo genotoxicity assays and are recommended for this use by the current OECD test guidelines (e.g. TGs 474, 475, 478 and 483). Based on the available experimental data, neither microcrystalline cellulose nor modified cellulose raise concern for genotoxicity.
3.2.2.2. Short‐term and subchronic toxicity
The majority of the available studies have been performed in rats, just few of them in rabbits and dogs. Among those meeting the current criteria for toxicological testing, the no observed adverse effect level (NOAEL) for the different modified celluloses were identified most often corresponding to the highest tested level or changes in body or organ weights. For microcrystalline cellulose (E 460(i)) the identified NOAELs in rats ranged from 3,769 mg/kg body weight (bw) per day to 9,000 mg/kg bw per day and in all cases corresponded to the highest levels of the test substance. For methyl cellulose (E 461), the dose level of 3% in rats (equivalent to 2,700 mg/kw bw per day) was selected as the NOAEL based on a decrease in body and organ weight displayed in male rats administered with the top additive level (10%, i.e. 9,000 mg/kg bw per day and day).
For hydroxypropyl cellulose (E 463), the identified NOAEL corresponded to the highest dose 6,000 mg hydroxypropyl cellulose/kg bw and day (by gavage). The most relevant feeding studies with hydroxypropyl methyl cellulose (E 464) (HPMC) were performed in rats which tolerated up to 10%, corresponding to 9,000 mg test item/kg bw per day. Rabbits tolerated up to 7,500 mg HPMC/kg bw per day administered via the diet (30 days exposure) and dogs up to 1,500 mg HPMC/kg bw and day, in either case being the highest tested dosages. More studies were conducted using sodium carboxymethyl cellulose (E 466). The most relevant ones were conducted in rats, with NOAEL values ranging from 4,500 to 9,000 mg test item/kg bw per day (highest tested dosages). In these studies, some effects (caecum and colonic enlargement, urothelial hyperplasia, nephrocalcinosis, diffuse epithelial hyperplasia in the urinary bladder) were observed, however, not considered of toxicological concern: the findings in the gastrointestinal tract were considered to be a consequence of the accumulation of poorly absorbed water‐soluble material and the findings in kidneys and urinary bladder were attributed to the up to fourfold higher concentration of sodium in the test diet compared with the basal diet. In one further study, rats were daily exposed (gavage) to doses equivalent to 0, 500, 2,500 or 5,000 mg/kg bw per day. Animals treated with ≥ 2,500 mg/kg bw per day had soft and pale faeces, which was attributed to the presence of test material and not considered of toxicological relevance. In the absence of any other adverse effects, also for this study the identified NOAEL was the highest dose (5,000 mg/kg bw).
3.2.2.3. Chronic toxicity and carcinogenicity
Data on chronic toxicity and carcinogenicity are available for microcrystalline cellulose (E 460 (i)), methyl cellulose (E 461) hydroxypropyl cellulose (E 463), HPMC (E 464), and sodium carboxymethyl cellulose (E 466). Some studies were unfit for evaluation due to methodological shortcomings. In the only relevant study, the dietary administration of even high doses of microcrystalline cellulose (E 460 (i)) (30%, 15,000 mg/kg bw) to rats for 72 weeks did not affect survival, feed efficiency or haematology. Apart from some dystrophic calcification in renal tubules, no other relevant lesions were noted and tumour incidence did not differ with that of controls. Several studies were conducted in rats with methyl cellulose (E 461) via feed or drinking water or by gavage at concentrations up to 5% (2,500 mg methyl cellulose/kg bw per day) and for up to 2 years. For all examined parameters, no adverse effects were reported and also the observed tumours did not differ in type and number in treated and control groups. In the only identified study, the daily dosing of male and female rats (0, 1,500, 3,000 or 6,000 mg hydroxypropyl cellulose/kg bw) via gavage for 6 months did not cause adverse effects (including carcinogenicity) apart from a decrease in body weight in high‐dosed rats (statistically significant in females only). Apart from a decrease in body weights of high‐dosed males, no other significant adverse findings were reported and there was no indication of a carcinogenic effect in rats of either sex dietary exposed to HPMC (E464) up to 20% (10,000 mg/kg bw per day) for 1 year. Sodium carboxymethyl cellulose (E 466) was tested in mice and rats at dosages of 0, 10,000 or 100,000 mg/kg diet (equivalent to 0, 1,500 or 15,000 mg/kg bw per day for mice and to 0, 500 or 5,000 mg/kg bw per day for rats) for up to 104 weeks. Despite the increase in feed intake, a treatment related decrease in body weight was noted at the end of the treatment. Histological examination revealed no intestinal abnormality or evidence of the passage of the additive across the intestinal wall in either species and the tumour incidences were comparable among groups.
In conclusion, based on a limited data set, the chronic toxicity studies revealed growth retardation for some modified celluloses mostly at the highest dosage level. There was no indication for carcinogenic effects for all tested compounds.
3.2.2.4. Reproductive and developmental studies
There are data for microcrystalline cellulose (E 460 (i)), methyl cellulose (E 461), hydroxypropyl cellulose (E 463) and sodium carboxymethyl cellulose (E 466), which were tested in mice, rats, hamsters and/or rabbits with oral dosing or via gavage. As regards microcrystalline cellulose (E 460 (i)) studies have been conducted in rats (dietary exposure) with a mixture including guar gum or sodium carboxymethyl cellulose (E 466) (15% in either case). The NOAEL for both maternal and developmental toxicity were the highest experimental dosages, i.e. 4,500 mg/kg bw (for mixture with guar gum) and 4,600 mg/kg bw (for mixture with sodium carboxymethyl cellulose). Methyl cellulose (E 461) was examined in mice, rats, hamsters and rabbits. In two different studies, pregnant mice were exposed via gavage (vehicle corn oil) to a dose range of 16–1,600 mg methyl cellulose (E 461)/kg bw per day from day 6 to 15 of gestation, followed by a caesarean section at day 17 of gestation. In the first study, maternal toxicity (increase in mortality and reduced pregnancy rate in the survivors) as well as retarded ossification in fetuses were noticed at the highest tested level, pointing to a NOAEL of 345 mg methyl cellulose (E 461)/kg bw per day (the last but one highest dosage) in mice. In the second study, no maternal toxicity and fetal abnormalities were observed in mice exposed up to 700 mg methyl cellulose (E 461)/kg bw per day. Rat studies (n = 2) were performed in pregnant dams exposed via gavage (vehicle corn oil) to a dose range of 16‐1,320 mg methyl cellulose (E 461)/kg bw per day from day 6 to 15 of gestation followed by a caesarean section at day 20. In the first study (0, 13, 51, 285 or 1,320 mg methyl cellulose (E 461)/kg bw per day) the highest tested dosage resulted in no maternal toxicity but also in increased incidence of extra centres of ossification in vertebrae of fetuses from high‐dose dams; in a second rat study, the incidence of such alteration slightly increased in fetuses from the highest dosed group (1,200 mg methyl cellulose (E 461)/kg bw per day). Based on the above results, a NOAEL of 285 mg methyl cellulose (E 461) mg/kg bw per day could be identified in rats. No maternal or fetal toxicity was detected in Golden hamsters exposed via gavage (vehicle corn oil) up to 1,000 mg methyl cellulose (E 461)/kg bw per day from day 6 to 10 of gestation followed by a caesarean section at day 20. The study on rabbits was discarded due to poor experimental design. The only relevant developmental toxicity study with hydroxypropyl cellulose (E 463) (dissolved in 1% gum arabic solution) was performed in pregnant rats exposed via gavage from day 7 to 17 of gestation to 0, 200, 1,000 or 5,000 mg/kg bw test item and some of them subjected to caesarean sections at day 20. No treatment‐related adverse effects were detected in dams or in the examined fetuses. A number of dams were allowed to deliver and no clinical, behavioural or morphological changes were observed in the examined pups. Their reproductive ability was seemingly not affected and no abnormalities were found in the F1‐derived fetuses. The in utero exposure to the highest dose (5,000 mg/kg bw per day) may be considered as the NOAEL of methyl cellulose (E 461) for this study. No mortality, and no adverse effects were observed on implantation or on fetal survival in pregnant mice or rats dosed via gavage with up to 1,600 mg sodium carboxymethyl cellulose (E 466)/kg bw per day.
3.2.2.5. Conclusions on toxicological properties of celluloses
The FEEDAP Panel agrees with the approach of the ANS Panel that, although the data set available for the different celluloses is not complete and most of the studies were old and do not meet the current requirements of toxicological testing, the physico‐chemical, structural, biological and kinetic similarities between the modified celluloses make it possible to apply a read‐across approach among the different celluloses. Overall, the available information allows to conclude that the celluloses (as a group) are of low toxicological concern.
3.2.3. Safety for the target species
Cellulose is the most frequent polysaccharide in nature consisting of (some hundreds up to ten thousands) β‐glycosidic linked glucose molecules. It is the main constituent of plant cell walls and vegetable fibre. It occurs mostly associated with hemicelluloses and lignin. It is therefore a common component of plant‐based feed for all food producing and companion animals. However, these animals are not capable to digest cellulose enzymatically due to the lack of cellulases. The monomer element of cellulose, glucose, will not be released from cellulose. But gastrointestinal microbes can split cellulose, the main degradation products are short‐chain fatty acids. In a simplified view, non‐ruminant animals cannot digest cellulose, small amounts are microbially degraded in the large intestine. Minor amounts of cellulose may be absorbed as such by paracellular transport (passing through the intercellular space) or by transcytosis (transcellular transport of macromolecules captured in vesicles). On the other side, animals with large fermentation in the gastrointestinal tract, such as ruminants, horses and rabbits, utilize large amounts of cellulose as energy source. In summary, cellulose is a natural part of feed and plays a physiological role in nutrition of animals (see Section 3.2.1).
Substitution of cellulose with ethyl‐, methyl‐, hydroxypropyl‐, hydroxypropyl‐methyl‐ and carboxymethyl groups may increase the resistance of cellulose to degradation. Resistance increases with the degree of substitution and is greatest when the substituent groups are evenly dispersed along the polymer chain. Most cellulose of the additive under assessment will therefore pass the intestine undigested and will excreted unchanged via faeces. Even when a high cellulolytic activity is present, as in the rumen, ethyl cellulose remains sufficiently resistant to degradation to be used as enteric coatings designed to protect methionine from rumen release (EFSA FEEDAP Panel, 2012c). Subsequent degradation in the post‐ruminal tract is most likely to lead to high molecule weight breakdown products, with little probability of absorption.
Sodium carboxymethyl cellulose meeting the food additive specification is consequently considered safe for all animal species. Setting a maximum content in complete diets is not considered necessary. The low toxicity of celluloses shown in the toxicological studies (see Section 3.2.2) support this conclusion.
3.2.4. Safety for the consumer
JECFA (1990), the SCF (1994) and the EFSA ANS Panel (2018) all considered it unnecessary to set an ADI for celluloses, including sodium carboxymethyl cellulose, based on a low toxicity and, if any, negligible absorption in the human gastrointestinal tract.
Residues of cellulose and breakdown products in edible tissues and products from animals fed sodium carboxymethyl cellulose are not expected. Although the (partial) degradation of sodium carboxymethyl cellulose would eventually occur in some species (ruminants, hindgut fermenters), breakdown products would still likely be of high molecular weight and poorly or not absorbed; the short‐chain fatty acids resulting from microbial breakdown of cellulose in the rumen or hindgut will enter the physiological pools of the animals. The consumer would therefore not be exposed to the additive or its degradation products when consuming edible tissues and products from animals given diets containing methyl cellulose. Consequently, the FEEDAP Panel concludes that the use of sodium carboxymethyl cellulose in animal nutrition is of no concern for consumer safety.
3.2.5. Safety for user
No specific information was submitted. In the absence of data, the FEEDAP Panel is not in the position to conclude on the safety of sodium carboxymethyl cellulose for the user.
3.2.6. Safety for the environment
Cellulose is a natural component of plants and occurs abundantly in the environment. The microbial degradation of cellulose and its derivatives (including sodium carboxymethyl cellulose) in the environment is expected. Therefore, the use of sodium carboxymethyl cellulose as a feed additive is considered safe for the environment.
3.3. Efficacy
No specific data on the efficacy of sodium carboxymethyl cellulose in feedingstuffs were provided. Carboxymethyl cellulose is authorised for use as a food additive. The effect seen when used in food could reasonably be expected to be seen when sodium carboxymethyl cellulose is used as an additive in feed.
4. Conclusions
A proper identification and characterisation of sodium carboxymethyl cellulose as required for a feed additive is not available and the occurrence of potential toxic impurities cannot be assessed.
The following conclusions apply only to sodium carboxymethyl cellulose meeting the food additive specifications.
Sodium carboxymethyl cellulose is considered safe for all animal species. Setting a maximum content in complete diets is not considered necessary.
The use of sodium carboxymethyl cellulose in animal nutrition is of no concern for consumer safety.
In the absence of data, the FEEDAP Panel is not in the position to conclude on the safety of carboxymethyl cellulose for the user.
The use of sodium carboxymethyl cellulose as a feed additive is considered safe for the environment.
The additive is considered to be efficacious in feedingstuffs for all animal species.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 12/03/2019 | Dossier received by EFSA. Sodium carboxymethyl cellulose for all animal species. Submitted by Organisation des Fabricants de produits Cellulosiques Alimentaires (OFCA) |
| 12/03/2019 | Reception mandate from the European Commission |
| 26/04/2019 | Application validated by EFSA – Start of the scientific assessment |
| 26/07/2019 | Comments received from Member States |
| 18/10/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety for the consumer |
| 24/01/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 02/07/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ADI
acceptable daily intake
- ADME
absorption, distribution, metabolism and excretion
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstracts Service
- CEF
EFSA Scientific Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- EINECS
European Inventory of Existing Chemical Substances
- EURL
European Union Reference Laboratory
- FEEDAP
EFSA Scientific Panel on European Inventory of Existing Chemical Substances
- HPMC
hydroxypropyl methyl cellulose
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- SCF
Scientific Committee on Food
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for carboxymethyl cellulose
1.
In the current application authorisation is sought under Article 10 for microcrystalline cellulose and sodium carboxymethyl cellulose under the category/functional group 1 (c,d,e,f) “technological additives”/“emulsifiers, stabilisers, thickeners and gelling agents” according to the classification system of Annex I of Regulation (EC) No 1831/2003. Specifically, authorisation is sought for the use of the feed additives for all animal species. Microcrystalline cellulose is a material of white to off‐white hygroscopic granules or powder of fine fibers. Sodium carboxymethyl cellulose is a white to off‐white hygroscopic powder or granules. The Applicant states that the specific purity criteria set in Commission Regulation (EU) 231/2012 for the use of microcrystalline cellulose and sodium carboxymethyl cellulose as the food additives are also applicable when using them as the feed additives. The feed additives are intended to be included into feedingstuffs through premixtures with no minimum or maximum dose indicated by the Applicant. For the identification/characterisation of the feed additives, the Applicant referred to Commission Regulation (EU) 231/2012, where the criteria and specific qualitative and quantitative tests/methods are indicated for checking the compliance with the criteria specified for microcrystalline cellulose and sodium carboxymethyl cellulose. For microcrystalline cellulose the identity tests for solubility and suspension, including colour reaction and analysis by infrared spectrometry, have to be performed. In addition, the methods for purity measurements include the determination of the loss on drying, water‐soluble matter, sulfated ash, pH of a 10% suspension and the presence/absence of starch. Finally, the determination of the cellulose content is specified by the above mentioned Regulation. For sodium carboxymethyl cellulose the following tests for the identity check are outlined: solubility, foam and precipitate formation together with a colour reaction. The methods for purity checking include measurements of the degree of substitution, loss on drying, total glycolate and sodium contents. Finally, the determination of the sodium carboxymethyl cellulose content is required according to the above mentioned Regulation. All of the above mentioned tests/methods are described in the FAO JECFA ‘microcrystalline cellulose’ and ‘sodium carboxymethyl cellulose’ monographs and the ‘volume 4’ of the FAO JECFA combined compendium for food additives specifications. The EURL recommends for the identification/characterisation of the feed additives the above mentioned methods described in the FAO JECFA ‘microcrystalline cellulose’ and ‘sodium carboxymethyl cellulose’ monographs and the ‘volume 4’ of FAO JECFA combined compendium for food additives specifications.
As the accurate quantification of microcrystalline cellulose and sodium carboxymethyl cellulose added to premixtures or feedingstuffs is not achievable experimentally the EURL cannot evaluate nor recommend any method for official control to quantify microcrystalline cellulose and sodium carboxymethyl cellulose in premixtures or feedingstuffs. Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005, as last amended by Regulation (EU) 2015/1761) is not considered necessary
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Bories G, Gropp J, Nebbia C, Innocenti ML and Aquilina G, 2020. Scientific Opinion on the safety and efficacy of sodium carboxymethyl cellulose for all animal species. EFSA Journal 2020;18(7):6211, 13 pp. 10.2903/j.efsa.2020.6211
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00179
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Kos Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgments: The Panel wishes to acknowledge the contribution of Angelica Amaduzzi to this opinion.
Adopted: 2 July 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Organisation des Fabricants de produits Cellulosiques Alimentaires (OFCA), Kerkweide 27, 2265DM, Leidschendam, The Netherlands.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:354:0016:0033:en:PDF
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0231&from=EN
FEED dossier reference: FAD‐2016‐0064.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep-fad-2016-00620064-microcrystallinecarboxymethyl-cellulose.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annexes Sect. II/Annex II_4_COA_ E466.
References
- Bergot F and Breque J, 1983. Digestibility of starch by rainbow trout: effects of the physical state of starch and of the intake level. Aquaculture, 34, 203–212. [Google Scholar]
- Chiou PWS, Bi Yu B and Lin C, 1998. The effect of different fibre components on growth rate, nutrient digestibility, rate of digesta passage and hindgut fermentation in domesticated rabbits. Laboratory Animals, 32, 276–283. [DOI] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Younes M, Aggett P, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Filipič M, Jose Frutos M, Galtier P, Gott D, Gundert‐Remy U, Georg Kuhnle G, Lambré C, Leblanc J‐C, Lillegaard IT, Moldeus P, Mortensen A, Oskarsson A, Stankovic I, Tobback P, Waalkens‐Berendsen I, Wright M, Tard A, Tasiopoulou S and Woutersen RA, 2018. Scientifi c Opinion on the re‐evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives. EFSA Journal 2018;16(1):5047, 104 pp. 10.2903/j.efsa.2018.5047 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavouringsand Processing Aids), Silano V, Bolognesi C, Chipma n K, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Mennes W, Milana MR, Pfaff K, Riviere G, Srinivasan J, Tavares Pocßas MF, Tlustos C, Wlöfle D, Zorn H, Kolf‐Clauw M, Lampi E, Svensson K, Van Haver E and Castle L, 2018. Scientific Opinion on the safety assessment of the active substances carboxymethyl cellulose, acetylated distarch phosphate, bentonite, boric acid and aluminium sulfate, for use in active food contact materials. EFSA Journal 2018;16(2):5121, 7 pp. 10.2903/j.efsa.2018.5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for technological additives. EFSA Journal 2012;10(1):2528, 23 pp. 10.2903/j.efsa.2012.2528 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Scientific Opinion on DL‐methionine, DL‐methionine sodium salt, the hydroxy analogue of methionine and the calcium salt of methionine hydroxy analogue in all animal species; on the isopropyl ester of methionine hydroxy analogue and DL‐methionine technically pure protected with copolymer vinylpyridine/styrene in dairy cows; and on DL‐methionine technically pure protected with ethylcellulose in ruminants. EFSA Journal 2012;10(3):2623, 42 pp. 10.2903/j.efsa.2012.2623. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidenne T and Perez JM, 1996. Apports de cellulose dans the Rabbit. Apports de cellulose dan l'alimentation du lapin en croissance. I. Consequences sur la digestion et le transit. Annales De Zootechnie, 45, 289–298. [Google Scholar]
- Janssen WMMA and Carré B, 1985. Influence of fibre on digestibility of poultry feeds. Recent Advances in Animal Nutrition, Butterworths, London, UK.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1990. Toxicological evaluation of certain food additives and contaminants. 687. Modified celluloses. WHO Food Additives Series, No. 26, 81–124.
- JECFA (Joint FAO/WHO Committee on Food Additives), 1999a. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Committee on Food Additives. WHO Technical Report No. 884. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999b. Safety evaluation of certain food additives. 936. Sodium carboxymethyl cellulose enzymatically hydrolysed. WHO Food Additives Series, No. 42.
- NRC (National Research Council), 1977. Committee on animal nutrition. Nutritional requirements of rabbits, Second revisited version, Edition 1977. The National Academies Press, Washington, DC: 10.17226/35 [DOI] [Google Scholar]
- SCF (Scientific Committee for Food), 1994. Food science and techniques. Reports of the Scientific Committee for Food, 32nd Series.
- SCF (Scientific Committee for Food), 1999. Food science and techniques. Reports of the Scientific Committee for Food, 44th series. 88 pp.
- Van Soest PJ, 1994. Nutritional Ecology of the Ruminant. Cornell University Press, Ithaca, USA. 476 pp. [Google Scholar]


