Summary
TLR3, a major innate immune pattern recognition receptor of RNA viruses, triggers inflammatory response through the transcription factor NF-κB. However, a genome-wide understanding of the genes and mechanisms regulating TLR3-mediated NF-κB activation is incomplete. We herein report the results of a human genome-wide RNAi screen that identified 591 proteins regulating TLR3-mediated NF-κB response. Bioinformatics analysis revealed several signaling modules including linear ubiquitination assembly complex and mediator protein complex network as regulators of TLR3 signaling. We further characterized the kinase ATM as a previously unknown positive regulator of TLR3 signaling. TLR3 pathway stimulation induced ATM phosphorylation and promoted interaction of ATM with TAK1, NEMO, IKKα, and IKKβ. Furthermore, ATM was determined to coordinate the assembly of NEMO with TAK1, IKKα, and IKKβ during TLR3 signaling. This study provided a comprehensive understanding of TLR3-mediated inflammatory signaling regulation and established a role for ATM in innate immune response.
Subject Areas: Biological Sciences, Immunology, Cell Biology
Graphical Abstract

Highlights
-
•
TLR3 is an antiviral innate immune pattern recognition receptor
-
•
ATM kinase regulates TLR3-mediated inflammatory response
-
•
ATM kinase facilitates assembly of NEMO with TAK1, IKKα, and IKKβ during TLR3 signaling
Biological Sciences; Immunology; Cell Biology
Introduction
Mammalian cells have evolved highly efficient receptors called pattern recognition receptors (PRRs) for detecting and eliciting strong antiviral response against RNA viruses (Brencicova and Diebold, 2013; Goubau et al., 2013; Gurtler and Bowie, 2013; Kawai and Akira, 2009). Toll-like receptor 3 (TLR3) is an endosome localized PRR recognizing double-stranded RNA, the replication intermediate of RNA viruses (Alexopoulou et al., 2001; Matsumoto et al., 2011; Schroder and Bowie, 2005; Uematsu and Akira, 2006). Engagement of TLR3 by viruses leads to the activation of both NF-κB-mediated inflammatory response and type I interferon induction (Alexopoulou et al., 2001; Sen and Sarkar, 2005). The inflammatory response is critical for the subsequent immune cell recruitment and adaptive immunity development.
Some of the core regulators of TLR3-mediated NF-κB induction is known (Alexopoulou et al., 2001; Jiang et al., 2003). Ligand primed TLR3 recruits its adaptor TRIF, followed by its association with the E3 ubiquitin ligase TRAF6 (Oshiumi et al., 2003; Sasai et al., 2010). Subsequently, this complex will activate the kinase TAK1 through its phosphorylation. Activated TAK1 will promote the association of the IKK complex comprising NEMO, IKKα, and IKKβ (Jiang et al., 2003; Shim et al., 2005). TAK1 is known to promote phosphorylation of IKKβ (Israel, 2010; Wang et al., 2001). IKKβ upon stimulation will phosphorylate IkB, leading to the release of NF-κB from its inhibition, and subsequent nuclear migration resulting transcription initiation. NEMO is known to undergo ubiquitination during NF-κB activation signaling, and the ubiquitin bound NEMO serves as a scaffold for the assembly of IKK components (Clark et al., 2013; Ni et al., 2008).
Apart from the core regulators of NF-κB activation, several molecules and mechanisms were previously identified to regulate upstream steps of TLR3 signaling leading NF-κB pathway. Autophagy has recently shown as essential for NF-κB induction from TLR3. Proteins such as Gab1, 14-3-3-zeta, WDFY1, S100A9, SREC-1, GSK3β, LRRC59, and UNC93B1 were previously reported as positive regulators of NF-κB signaling from TLR3 (Brinkmann et al., 2007; Hu et al., 2015b; Ko et al., 2015; Murshid et al., 2015; Tatematsu et al., 2015; Tsai et al., 2015; Zheng et al., 2010). TRIM38, WWP2, PP1, and ADAM15 were identified by earlier studies as negative regulators of TLR3-driven NF-κB stimulation (Ahmed et al., 2013; Gu et al., 2014; Hu et al., 2015a; Yang et al., 2013).
Thus, although some of the regulators of TLR3 pathway are known, systems-level information on the regulation of the signaling cascade triggered by TLR3 leading to NF-κB activation is still lacking. Such a genome-wide understanding on the regulators of NF-κB activation is available for other TLRs such as TLR7 and TLR8 (Chiang et al., 2012). Systems-level information will facilitate a better understanding of antiviral innate immune response regulation mechanisms and enable us to devise strategies to counter viral block of antiviral response. Although application of large-scale genetic screen approaches offers a viable approach to unravel regulators of TLR3 signaling, this has not been performed hitherto to interrogate TLR3 signaling.
In this report, we present results of a human genome-wide RNA interference (RNAi)-based genetic screen to identify regulators of TLR3-mediated NF-κB response. Moreover, we describe the kinase ATM as a regulator of TLR3 signaling.
Results
Human Genome-wide RNA-Interference Screen for Identifying Regulators of TLR3-Mediated NF-κB Activation
To identify the human genes regulating TLR3-induced NF-κB activation (TLR3-to-NF-κB), we performed an in vitro reverse genetic screen by silencing 18,121 human genes using arrayed small interfering RNAs (siRNAs) (Figure 1A). We used human epithelial cell line HEK293T, a cell that naturally expresses TLR3, as the model system for genetic screen. To monitor NF-κB activation during TLR3 stimulation, we optimized a reporter assay using an NF-κB binding site-driven GFP reporter that was stably integrated into cells (see Transparent Methods). As TLR3 senses double-stranded RNA viruses, we used synthetic poly inosinic:poly cytidilic acid (poly(I:C)), a well-known mimic of double-stranded RNA widely used for TLR3 activation. We reasoned that use of virus as a source for activating TLR3 will yield a large number of false positives because silencing of several genes might reduce infection itself, yielding poor TLR3 activation; however, use of poly(I:C) would likely reveal more TLR3-specific effects. Silencing of TLR3 and its adaptor TRIF, but not the cytosolic nucleic acid sensor RIG-I, led to a reduction (up to 10-fold, p < 0.01) of TLR3-triggered NF-κB-GFP reporter activity (Figure 1B). The GFP reporter activity was recorded using high-throughput fluorescent microscopy and expressed as the percentage of GFP-positive cells/siRNA treatment. A single image was captured from each well of a 384-well plate in which each well corresponded to a unique siRNA treatment. The screen was performed in duplicate, and each gene was targeted by a pool of four unique siRNAs. The hits were selected using a statistical approach involving calculation of mean-based Z scores. Any siRNA treatment that changed GFP signal by three standard deviations from the plate mean signal was selected as hit. Any silencing that resulted in alteration of cell number or lactate dehydrogenase release by more than 30% of the siNT-treated cells was eliminated as potentially confounding toxicity-related phenotypic effects.
Figure 1.
RNAi Screening for Identifying TLR3-Mediated NF-κB Activation
(A) Schematic showing RNAi screening methodology. Images correspond to the GFP signal in negative control siRNA and positive control siRNA targeting (si-TRIF).
(B) GFP reporter assay efficiency for measuring NF-κB activation induced by poly(I:C) with and without nucleic acid sensing innate immune pathway genes. NF-κB-GFP reporter containing HEK293T cells were stimulated with poly(I:C) for 24 h, and images were quantified and expressed as percentage of GFP-positive cells. The values shown are mean ± SE of three independent experiments performed in triplicates. The statistical significance of the difference in values between groups was analyzed using unpaired two-tailed Student's t test, and p values <0.05 was considered statistically significant. ∗∗p value <0.01. Knockdown efficiencies of relevant siRNAs determined by western blot are shown in the inset; left and right panel of western blots, respectively, show scrambled siRNA or gene-specific siRNA-treated cells.
(C–E) Enriched Molecular Function, Biological Processes, and Cellular Compartment categories among the hit genes obtained from RNAi screening, respectively.
(F) Enrichment of disease categories among the screening hits. The plot was generated using Ingenuity Pathway Analysis software. GFP, green fluorescent protein. siNT, negative control siRNA.
RNAi Screen Revealed Known Components of TLR3 and NF-κB Signaling
The RNAi screening identified a total of 591 genes as hits, with 514 positive and 77 negative regulators of TLR3-mediated NF-κB activation (Table S1). We initially analyzed the screening results to assess the correlation with previously existing knowledge on TLR3 and NF-κB signaling regulation, as a test to verify the accuracy of the approach. The hits identified in our screen included both TLR3 and its adaptor TRIF, thus validating the specificity of our approach. In addition, our study also re-identified core known components of NF-κB pathway such as CHUK, TRAF6, IKBKG (NEMO), and RELA. Autophagy was previously reported as a positive regulator of TLR3-induced NF-κB activation. Consistent with this, our screen identified key autophagy regulator ATG13 as a hit. Besides these known pathway-specific regulators, our genetic screen also identified multiple subunits of several generically acting cellular multiprotein complexes. For example, we identified 21 components of ribosomes (e.g., RPL14), 4 components of mRNA translation machinery (e.g., POLR2D), and 6 subunits of proteasome (e.g., PSMD2). These data provide strong evidences for the success and efficiency of the genetic screening employed in this study to discover regulators of TLR3 signaling.
We also compared the results of current study with previously published RNAi screening performed to identify regulators of NF-κB activation from TLR7 and TLR8 (Chiang et al., 2012). There were 10 genes (HECTD1, IKBKG, MAP3K7, NME1, PCTK3, POLR2C, RELA, TM4SF18, TRAF6, USP33) common between our screen and the previous RNAi screen performed to discover TLR7/8-mediated NF-κB activation.
Bioinformatics Analysis Identified Several Genes Associated with Human Diseases as NF-κB Regulators
We performed an integrated analysis to identify systems-level information embedded in the genes identified as TLR3 regulators in this study. Bioinformatics analysis identified the molecular processes, biological processes, and cell compartment terms enriched among the screen hits (Figures 1C–1E, respectively).
Because NF-κB and inflammation are widely implicated in both cell and organism-level physiology, it is likely that several regulators of NF-κB signaling play roles in various diseases. Previous studies, including genome-wide association studies, have identified various genetic loci associated with several human diseases. Using database searches, we interrogated whether any of the identified genes are associated with human diseases. Remarkably, we identified that a strikingly large number of the genes identified in our study are associated with several diseases (Table S2). A subset of these genes showed specific association with infection, immunity, and inflammation-related diseases, consistent with the role of NF-κB in inflammatory and immune processes (Figure 1F). There were 24 genes (e.g., CORO1A, CXCR6, FGA) with known role in susceptibility to infections, 15 genes (ACO1, ADORA1, PTPN22) associated with inflammatory disease, 134 genes (e.g., CORO1A, CHUK, MEFV) implicated in immune-related diseases, and 25 genes (HAVCR2, LRBA, PDCD1) associated with autoimmune diseases. In addition, several of the identified NF-κB regulator genes were previously shown associated with other diseases such as asthma (MS4A2, MUC7, PPP2CA), cancer (ATM, ATR, BRAF), and cardiovascular (BRAF, CD163, CES3) and metabolic (COMT, CORO1A, FGF23) diseases. This study thus sheds light on the potential association of innate immune TLR3 pathway regulators with several diseases. Further studies on the results obtained from this study may help to elucidate the underlying mechanism by which specific genes contribute to human diseases.
Multiple Signaling Modules Regulating TLR3 Signaling Is Identified by the RNAi Screen
Integrated analysis identified that a considerable number of the identified genes have previously known physical or functional interaction with other hit genes (Figure 2A). Among these, there were several signaling modules regulating TLR3 signaling. Some of these included LUBAC, myosin complex, and mediator complex, respectively (Figures 2B–2D).
Figure 2.
Bioinformatics Analysis Identifies Gene Networks Regulating TLR3-Mediated NF-κB Activation
(A) Interactome analysis using all hit genes revealed several known protein-protein associations.
(B–D) Respectively shows signaling networks of linear ubiquitination complex, myosin, and mediator complex. The network was generated by STRING. The thickness of the connecting lines indicates the confidence level of known functional interactions between indicated proteins.
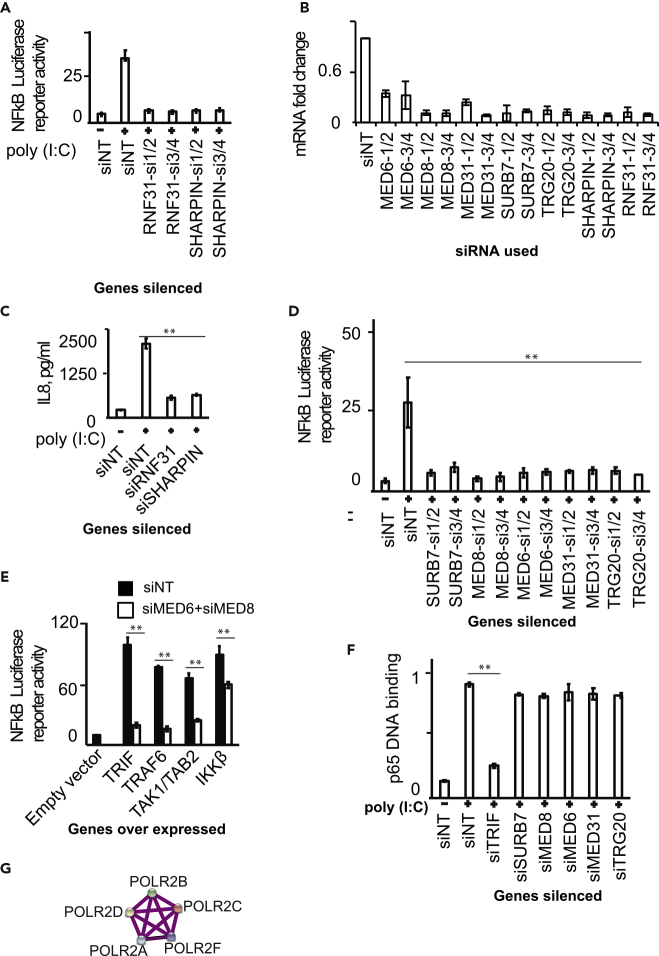
There was a striking enrichment of ubiquitination-associated processes among the obtained hits. Among the ubiquitination-related hits identified in this screen, there were two components of the linear ubiquitin chain assembly complex (LUBAC), RNF31 (HOIP) and SHARPIN (Gerlach et al., 2011; Ikeda et al., 2011). Although LUBAC was previously identified as important for NF-κB activation from innate immune pathways such as NOD2 and TNF-α signaling by targeting NEMO for posttranslational modification, it is not previously known whether LUBAC is involved in TLR3 stimulation-dependent NF-κB activation (Boisson et al., 2012; Damgaard et al., 2012; Gerlach et al., 2011; Ikeda et al., 2011; Niu et al., 2011). As shown in Figure 3A, silencing of RNF31 (HOIP) and SHARPIN by two independent pairs of siRNAs resulted in abrogation of poly(I:C)-driven NF-κB reporter activity in HEK293T cells. Knockdown was confirmed by quantitative real-time PCR (Figure 3B). In addition, knockdown of RNF31 (HOIP) and SHARPIN resulted in reduced secretion of IL8 cytokine by human primary monocytes stimulated with poly(I:C) (Figure 3C).
Figure 3.
LUBAC and Mediator Complex Are Needed for TLR3 Signaling
(A) Silencing of RNF31 and SHARPIN using two pairs of unique siRNAs reduced NF-κB luciferase reporter activation driven by poly(I:C) stimulation in HEK293T cells.
(B) Efficiency of gene silencing is shown. Genes were targeted using pairs of siRNAs. The gene transcript levels were determined using qRT-PCR. The values correspond to mean ± SD of a triplicate experiment and are expressed as fold change of mRNA level relative to scrambled siNT sample (siNT value is taken as 1). The qRT-PCR results were computed through determination of relative Ct value, using the formula (Fold-change = 2(Ct of unstimulated – Ct of stimulated) Target gene/2(Ct of unstimulated – Ct of stimulated) Reference gene).
(C) Silencing of RNF31 and SHARPIN reduced IL8 secretion driven by poly(I:C) stimulation in human primary monocytes.
(D) Silencing of five component genes of mediator complex using two pairs of unique siRNAs reduced NF-κB luciferase reporter activation driven by poly(I:C) stimulation in HEK293T cells.
(E) Mediator complex silencing did not affect the ability of HEK293T cells to support NF-κB luciferase reporter activation induced by ectopic expression of 50 ng each of TRIF, TRAF6, TAK1/TAB2, IKKβ for 24 h.
(F) Mediator complex silencing did not affect the ability of p65 to bind to target DNA in poly(I:C) treated HEK293T cells.
(G) A network of RNA polymerase II subunits uncovered in the current RNAi screening as hits.
The values shown for (A and C–F) are mean ± SE of three independent experiments performed in triplicates. The statistical significance of the difference in values between groups was analyzed using an unpaired two-tailed Student's t test, and p values <0.05 were considered statistically significant. ∗∗p value <0.01.
Mediator complex is a multi-subunit complex of around 31 proteins and is known to be involved in transcription by RNA polymerase II. Our RNAi screen identified five genes (SURB7, MED8, MED6, MED31, and TRG20) of mediator complex as positive regulators of TLR3-mediated NF-κB reporter activity. Silencing of each one of these five genes using two unique siRNAs attenuated NF-κB reporter activation (Figure 3D; knockdown verification Figure 3B). We subsequently aimed to identify the stage of TLR3 signaling in which mediator complex plays a role. Ectopic expression of TLR3 pathway components TRIF, TRAF6, TAK1, and IKKβ are known to drive NF-κB activation, and integrating this with mediator complex genes could indicate potential site of action. For this, as a representative, we silenced MED6 and MED8 together in HEK293T cells; then ectopically expressed TRIF, TRAF6, TAK1, and IKKβ; and measured the activation of NF-κB luciferase reporter activity. As shown in Figure 3E, expression of the mediator complex was essential for NF-κB reporter activity driven by ectopically expressed TRIF, TRAF6, TAK1, and IKKβ. This indicated that the mediator complex acted very downstream in the pathway. We also assessed the DNA-binding activity of NF-κB in mediator complex-silenced cells, using NF-κB target DNA sequence containing ELISA-based reporter assay. It was determined that silencing of the mediator complex did not affect the ability of activated NF-κB to bind to target DNA sequence (Figure 3F). This result clearly demonstrated that the mediator complex regulated TLR3 signaling likely at a step after the binding of NF-κB to its target DNA sequence. This is consistent with the known role of mediator complex at the level of recruitment of transcription initiation complex components to the target gene promoter region. Accordingly, our genetic screen identified multiple components of RNA polymerase II as positive regulators of TLR3 signaling (Figure 3G). However, it should be noted that the ectopic expression of IKKβ in mediator complex-silenced cells (Figure 3E) resulted in only a moderate reduction of NF-κB reporter (unlike that by TRIF, TRAF6, and TAK1), although the mediator complex appears to be acting further downstream in the pathway. Although the cause for this is unclear, the potential contribution of the artificially enhanced effects of the ectopic expression system cannot be ignored.
ATM Kinase Is a Positive Regulator of TLR3 Signaling
We observed that silencing of the gene ATM, a key component of a network of proteins associated with DNA double-strand break repair, impacted on TLR3 signaling as a positive regulator. Although a role for ATM in mediating NF-κB activation during genotoxicity is known, the potential dependence of innate immune antiviral signaling on ATM is unappreciated. Therefore, we decided to further validate and mechanistically investigate this.
Silencing of ATM in HEK293T cells using two independent pairs of siRNAs significantly reduced the activation of NF-κB luciferase reporter induced by poly(I:C)-mediated TLR3 stimulation (Figure 4A). Accordingly, ATM siRNA-treated HEK293T cells displayed diminished secretion of IL8, an NF-κB-dependent cytokine (Figure 4B). We subsequently determined the physiological relevance of ATM in TLR3 signaling using human primary cells. For this, ATM was silenced in human primary monocytes, stimulated with poly(I:C), and the expression of NF-κB-dependent cytokine IL8 was determined by ELISA. It was observed that ATM-silenced primary monocytes showed reduced secretion of IL8 upon poly(I:C) stimulation (Figure 4C). Noticeably, ATM silencing did not affect TLR3-mediated type-I interferon beta reporter activity triggered by poly(I:C) treatment in HEK293T cells (Figure 4D).
Figure 4.
ATM Kinase Is a Positive Regulator of TLR3-Mediated NF-κB Activation
(A) Silencing of ATM using two pairs of unique siRNAs reduced NF-κB luciferase reporter activation driven by poly(I:C) stimulation in HEK293T cells.
(B and C) Silencing of ATM reduced IL8 secretion driven by poly(I:C) stimulation, respectively, in HEK293T cells and human primary monocytes.
(D) Type-I interferon beta reporter activity was unaffected by ATM silencing.
(E) Poly(I:C) stimulation induced phosphorylation of ATM in HEK293T cells. The data are representative of at least three independent experiments.
(F) ATM silencing reduced the ability of HEK293T cells to support NF-κB luciferase reporter activation induced by ectopic expression of 50 ng each of TRIF, TRAF6, TAK1/TAB2, IKKβ for 24 h.
(G) ATM silencing reduced phosphorylation of Ikβ upon poly(I:C) stimulation. The data are representative of at least three independent experiments.
(H) ATM silencing reduced the ability of p65 to bind to target DNA in poly(I:C)-treated HEK293T cells.
(I) ATM silencing reduced NOD2-mediated NF-κB luciferase reporter activation driven by muramyl dipeptide (MDP) stimulation in HEK293T cells.
The values shown for (A–F, H, and I) are mean ± SE of three independent experiments performed in triplicates. The statistical significance of the difference in values between groups was analyzed using an unpaired two-tailed Student's t test, and p values <0.05 were considered statistically significant. ∗∗p value <0.01, ∗p value <0.05. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
ATM Undergoes Phosphorylation during TLR3 Pathway Activation
It was shown before that ATM undergoes phosphorylation during activation upon genotoxic stress. We wondered whether TLR3 stimulation also induces phosphorylation of ATM. To test this, we stimulated HEK293T cells with poly(I:C) and potential pATM formation was assayed by western blot. As shown in Figure 4E, poly(I:C) stimulation was found to induce phosphorylation of ATM as early as 15 min after stimulation of TLR3. This result demonstrated that the activating phosphorylation status of ATM is sensitive to stimulation of TLR3 pathway, further indicating the direct functional association between ATM and antiviral innate immune pathway.
ATM Is Needed for IKKβ Activation
We also investigated the relative position of ATM in the known hierarchy of PRR signaling cascade leading to NF-κB activation. To determine where ATM acts, we activated NF-κB reporter in ATM-silenced cells through ectopic expression of TRIF, TRAF6, TAK1/TAB2, and IKKβ. It was found that ectopically expressed TRIF, TRAF6, and TAK1/TAB2 failed to activate NF-κB reporter in ATM-silenced cells with strong defect in signal (Figure 4F). Contrary to this, ATM knockdown caused only a low reduction of NF-κB activation driven by ectopic expression of IKKβ (Figure 4F). These data indicated that ATM likely regulates PRR pathway signaling at the level of IKKβ, downstream of TAK1. It should be noted that the slight but statistically significant reduction of NF-κB reporter induced by ectopic expression of IKKβ in ATM-silenced cells (Figure 4F) may indicate that there are some effects of IKKbeta that are partially influenced by the absence of ATM.
Activation of NF-κB pathway often leads to the phosphorylation of Iκβ, a signal for the latter's degradation and release of NF-κB proteins. We also investigated whether ATM is needed for Iκβ phosphorylation. For this, ATM-silenced HEK293T cells were stimulated with poly(I:C) and pIKβ formation was detected by western blot. Consistent with the reporter assay results, ATM silencing reduced the induction of Iκβ phosphorylation upon poly(I:C) stimulation of HEK293T cells (Figure 4G). This experiment pointed that ATM regulates a step in TLR3 signaling downstream of TAK1 activation but upstream of Iκβ phosphorylation.
As an additional proof for the role of ATM in NF-κB activation, we also compared the ability of NF-κB from ATM-sufficient and -deficient conditions to bind to target DNA sites, using ELISA. As given in Figure 4G, poly(I:C) treatment resulted in enhanced DNA binding by NF-κB p65 in siNT-treated cells. However, consistent with reporter assay results, NF-κB p65 from ATM-silenced poly(I:C) cells displayed reduced DNA-binding activity (Figure 4H).
ATM Is Needed for NF-κB Activation by Multiple PRRs
There are several innate immune signaling pathways that activate NF-κB. We therefore investigated whether the role of ATM in PRR signaling is specific to TLR3 or not. For this, we silenced ATM expression in HEK293T cells and induced NF-κB activation from another major innate immune receptor NOD2. As shown in Figure 4I, the data revealed that indeed silencing of ATM mitigated NF-κB activation driven by activation of NOD2. These data strongly demonstrated that ATM has a wider role in NF-κB activation from multiple innate immune pathways. In addition, consistent with the above-described results, these data also indicated that ATM is part of the core machinery orchestrating NF-κB activation from diverse triggers.
ATM Interacts with Multiple Components of TLR3 Signalosome
We next attempted to determine how ATM regulates TLR3 signaling. ATM was previously known to interact with NEMO, IKKα, and IKKβ during genotoxic stress (Wu et al., 2010). Therefore, we investigated whether TLR3 activation also promotes the interaction of ATM with NEMO, TAK1, IKKα, and IKKβ at their endogenous levels. For this, we stimulated human primary monocytes with poly(I:C), immuno-precipitated endogenous ATM, and assessed the interaction of ATM with these pathway proteins through western blot. It was determined that endogenous ATM interacted with TAK1, NEMO, IKKα, and IKKβ (Figure 5A). TLR3 pathway activation enhanced the interaction of ATM with other components of TL3 signalosome.
Figure 5.
ATM Interacts with and Facilitates NEMO Signaling Complex Assembly
(A) Endogenous ATM interacted with endogenous NEMO, TAK1, IKKα, and IKKβ upon stimulation of human primary monocytes with poly(I:C).
(B) ATM expression was needed for NEMO to recruit TAK1, IKKα, and IKKβ upon stimulation of human primary monocytes with poly(I:C). ATM-silenced cells were stimulated with poly(I:C) for 120 min, and NEMO co-immunoprecipitation assay was performed.
The data are representative of at least three independent experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WCL, whole-cell lysate; IP, immunoprecipitation; IB, immunoblot.
ATM Is Needed for NEMO Signalosome Assembly
We also investigated whether ATM has any role in regulating or relaying signaling through TLR3 signalosome. We systematically investigated how the absence of expression of ATM affects the interaction of various modules of TLR3 signaling pathway. During TLR3 stimulation in normal cells, NEMO interacts with TAK1, IKKα, and IKKβ. We therefore investigated whether ATM plays any role in the assembly of NEMO signaling complex. For this, we silenced ATM in human primary monocytes, stimulated the cells with poly(I:C), and assessed the ability of endogenous NEMO to interact with TAK1, IKKα, and IKKβ through co-immunoprecipitation. As expected, poly(I:C) stimulation promoted the NEMO-TAK1-IKKα-IKKβ complex formation. Notably, we observed that the ability of NEMO to recruit multiple components of TLR3 signaling was adversely impacted by the absence of ATM. As shown in Figure 5B, silencing of ATM reduced the ability of NEMO to recruit TAK1, IKKα, and IKKβ, upon TLR3 signaling activation by poly(I:C). These data demonstrated that ATM is needed for TLR3-mediated assembly of NEMO signalosome, as essential step in NF-κB activation.
Discussion
Through a genome-wide loss-of-function genetic screen, we identified the compendium of human genes regulating inflammatory NF-κB activation induced by TLR3 signaling. We also identified ATM kinase as a positive regulator of TLR3 signaling. Given the widely established roles of TLR3 in infection control, vaccine response, and inflammatory diseases, the results of study will form a valuable resource for detailed mechanistic understanding of TLR3 signaling regulation.
The identification of several gene networks from the current RNAi screen implies the modular nature of regulation of NF-κB activation pathways. Whether these regulatory modules regulate NF-κB activation in a TLR3-specific mechanism or have conserved role across multiple PRRs is yet to be determined. A previous study had identified an endosomal dsRNA transporter that could expose extracellularly added dsRNA (e.g., poly (I:C)) to cytosol (Nguyen et al., 2017). Although our assay optimization established that the primary receptor for poly (I:C)-mediated NFkB activation in our assay is TLR3, we do not exclude the potential for any minor contribution by other pathways that could sense poly (I:C). Our results also provided potential clues about the functional role of several genes identified as disease susceptibility loci of several inflammatory diseases. Given that NF-κB is a key mechanism regulating inflammation, mutations in these genes could result in altered activation of NF-κB, leading to aberrant inflammatory responses and disease.
Previous studies demonstrated that LUBAC is needed for NF-κB activation triggered by NOD2, TLR4, and TNFR (Boisson et al., 2012; Damgaard et al., 2012; Gerlach et al., 2011; Ikeda et al., 2011; Niu et al., 2011). Our identification of LUBAC as a regulator of TLR3-mediated NF-κB activation further supports its role of inflammatory pathways. Consistent with our results, recently another study also reported a role for LUBAC in TLR3 signaling (Zinngrebe et al., 2016). LUBAC is known to attach linear ubiquitin to NEMO to create a scaffold for recruiting IKKα, IKKβ, and TAK1 (Niu et al., 2011). The mediator complex is known to aid in the assembly of RNA polymerase transcription complex assembly on target promoters. In fact, MED17 subunit of the mammalian mediator complex was previously shown to bind to NF-κB p65 and facilitate selective transcription of a subset of NF-κB target genes upon stimulation with TNF-α (Van Essen et al., 2009). Our study further proved the wider involvement of mediator complex in innate immune inflammatory response. Although the mediator complex has around 30 subunits, our RNAi screen identified only five subunits as hits. Similarly, the RNAi screening study by Chanda and colleagues identified that only MED12 and MED19 are required for TLR7/8-mediated NF-κB activation (Chiang et al., 2012). Although incomplete gene knockdown could have prevented other subunits from manifesting a phenotypic effect in these assays, future studies should investigate whether all subunits alike are required for NF-κB target gene transcription induced from TLR3 and various other PRRs. An original study in this direction systematically silenced all components of the mediator complex of fruit fly and assessed how depletion of each subunit affects transcription induced by selected ligands and found differential requirement of specific subunits of mediator complex for heat shock and LPS-induced transcription (Kim et al., 2004).
A key finding of our study is the identification of ATM as an important positive regulator of TLR3-mediated NF-κB activation. In addition, we also established ATM as a common regulator of NF-κB activation from multiple signaling pathways such as NOD2, strongly demonstrating ATM as a key component of the core NF-κB activation machinery. Our data unambiguously identified that mechanistically ATM expression is essential for the assembly of NEMO with IKKα, IKKβ, and TAK1. The association of NEMO with IKKβ and TAK1 is an essential core step underlying the phosphorylation of Ikβ triggered by nearly all canonical NF-κB activating pathways (Israel, 2010). Although our experiments clearly revealed the specific defect in the signaling in ATM-silenced cells, the underlying mechanism by which ATM triggers the association of TAK1 and IKKα and IKKβ to NEMO is unclear. It can be reasoned from our results that ATM regulates innate immune signaling downstream of TAK1 signaling. Several previous studies identified that genotoxic stress induces NF-κB activation in a manner dependent on ATM (Fang et al., 2014; Hinz et al., 2010; Panta et al., 2004; Wu et al., 2006, 2010; Yang et al., 2011). It was also further demonstrated that genotoxic stress promotes association of ATM with NEMO, IKKα and IKKβ, and TAK1 (Wu et al., 2010). It is still unclear how ATM regulates TLR3 signaling. In one previous study, it was demonstrated that the presence of ATM facilitated the interaction of NEMO with IKKα, IKKβ, and TAK1 during genotoxic stimulation (Wu et al., 2010). This effect was very comparable with our results, in which ATM silencing resulted in inefficient assembly of NEMO interactome during TLR3 stimulation. The protein ELKS was previously shown to be essential for the activation of NF-κB by ATM during genotoxic stress (Wu et al., 2010). In a related context, another earlier study identified that ATM is essential for NF-κB activation and type I interferon induction during paramyxovirus infection (Fang et al., 2015). In our genetic screen, however, ELKS was not identified as a hit. It was previously known that humans with genetic mutations in ATM tend to have immunological deficiency. Our study provides one potential mechanistic explanation for the immuno-deficiencies of ATM mutations.
In summary, this study identified a regulatory mechanism of TLR3 signaling pathway by ATM and has also revealed the larger set of genes involved in NF-κB response regulation, which will help to generate a systems-level view of inflammatory signaling cascades.
Limitations of the Study
Although the cell-based model system used in this study displayed some of the well-known core characteristics of the TLR3 signaling pathway, it is also important to acknowledge the incompleteness of the used model system as well as the tools and approaches. First, whether the immortalized HEK293 system has all the genes regulating TLR3 signaling in primary epithelial cells is unknown. Similarly, whether all of the genes identified in this study using RNAi screen play a role in the regulation of TLR3 signaling in the primary cells is yet to be determined. In addition, our approach of using RNAi also has its own limitations. For example, whether all of the hit genes selected from the primary RNAi screening were resulting from on-target activities of the siRNAs employed in the study remains undetermined.
Resource Availability
Lead Contact
Manoj N. Krishnan (manoj.krishnan@duke-nus.edu.sg).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
All data are included in the published article and the supplementary materials, and any additional information will be available from the lead contact upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
M.N.K. received funding from National Research Foundation, Singapore. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author Contributions
U.U., A.C., and M.N.K. performed experiments. U.U., A.C., and M.N.K. wrote the manuscript.
Declaration of Interests
Authors declare no competing interests.
Published: August 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101356.
Supplemental Information
References
- Ahmed S., Maratha A., Butt A.Q., Shevlin E., Miggin S.M. TRIF-mediated TLR3 and TLR4 signaling is negatively regulated by ADAM15. J. Immunol. 2013;190:2217–2228. doi: 10.4049/jimmunol.1201630. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Boisson B., Laplantine E., Prando C., Giliani S., Israelsson E., Xu Z., Abhyankar A., Israel L., Trevejo-Nunez G., Bogunovic D. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencicova E., Diebold S.S. Nucleic acids and endosomal pattern recognition: how to tell friend from foe? Front. Cell Infect. Microbiol. 2013;3:37. doi: 10.3389/fcimb.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann M.M., Spooner E., Hoebe K., Beutler B., Ploegh H.L., Kim Y.M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.Y., Engel A., Opaluch A.M., Ramos I., Maestre A.M., Secundino I., De Jesus P.D., Nguyen Q.T., Welch G., Bonamy G.M. Cofactors required for TLR7- and TLR9-dependent innate immune responses. Cell Host Microbe. 2012;11:306–318. doi: 10.1016/j.chom.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K., Nanda S., Cohen P. Molecular control of the NEMO family of ubiquitin-binding proteins. Nat. Rev. Mol. Cell Biol. 2013;14:673–685. doi: 10.1038/nrm3644. [DOI] [PubMed] [Google Scholar]
- Damgaard R.B., Nachbur U., Yabal M., Wong W.W., Fiil B.K., Kastirr M., Rieser E., Rickard J.A., Bankovacki A., Peschel C. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol. Cell. 2012;46:746–758. doi: 10.1016/j.molcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Fang L., Choudhary S., Tian B., Boldogh I., Yang C., Ivanciuc T., Ma Y., Garofalo R.P., Brasier A.R. Ataxia telangiectasia mutated kinase mediates NF-kappaB serine 276 phosphorylation and interferon expression via the IRF7-RIG-I amplification loop in paramyxovirus infection. J. Virol. 2015;89:2628–2642. doi: 10.1128/JVI.02458-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Choudhary S., Zhao Y., Edeh C.B., Yang C., Boldogh I., Brasier A.R. ATM regulates NF-kappaB-dependent immediate-early genes via RelA Ser 276 phosphorylation coupled to CDK9 promoter recruitment. Nucleic Acids Res. 2014;42:8416–8432. doi: 10.1093/nar/gku529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach B., Cordier S.M., Schmukle A.C., Emmerich C.H., Rieser E., Haas T.L., Webb A.I., Rickard J.A., Anderton H., Wong W.W. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- Goubau D., Deddouche S., Reis E Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Ouyang C., Lin W., Zhang T., Cao X., Xia Z., Wang X. Phosphatase holoenzyme PP1/GADD34 negatively regulates TLR response by inhibiting TAK1 serine 412 phosphorylation. J. Immunol. 2014;192:2846–2856. doi: 10.4049/jimmunol.1302537. [DOI] [PubMed] [Google Scholar]
- Gurtler C., Bowie A.G. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21:413–420. doi: 10.1016/j.tim.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M., Stilmann M., Arslan S.C., Khanna K.K., Dittmar G., Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol. Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Hu M.M., Xie X.Q., Yang Q., Liao C.Y., Ye W., Lin H., Shu H.B. TRIM38 negatively regulates TLR3/4-mediated innate immune and inflammatory responses by two sequential and distinct mechanisms. J. Immunol. 2015;195:4415–4425. doi: 10.4049/jimmunol.1500859. [DOI] [PubMed] [Google Scholar]
- Hu Y.H., Zhang Y., Jiang L.Q., Wang S., Lei C.Q., Sun M.S., Shu H.B., Liu Y. WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep. 2015;16:447–455. doi: 10.15252/embr.201439637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F., Deribe Y.L., Skanland S.S., Stieglitz B., Grabbe C., Franz-Wachtel M., Van Wijk S.J., Goswami P., Nagy V., Terzic J. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Zamanian-Daryoush M., Nie H., Silva A.M., Williams B.R., Li X. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.W., Kwon Y.J., Kim J.M., Song Y.H., Kim S.N., Kim Y.J. MED16 and MED23 of Mediator are coactivators of lipopolysaccharide- and heat-shock-induced transcriptional activators. Proc. Natl. Acad. Sci. U S A. 2004;101:12153–12158. doi: 10.1073/pnas.0401985101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko R., Park J.H., Ha H., Choi Y., Lee S.Y. Glycogen synthase kinase 3beta ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat. Commun. 2015;6:6765. doi: 10.1038/ncomms7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Oshiumi H., Seya T. Antiviral responses induced by the TLR3 pathway. Rev. Med. Virol. 2011;21:67–77. doi: 10.1002/rmv.680. [DOI] [PubMed] [Google Scholar]
- Murshid A., Gong J., Ahmad R., Borges T.J., Calderwood S.K. Scavenger receptor SREC-I promotes double stranded RNA-mediated TLR3 activation in human monocytes. Immunobiology. 2015;220:823–832. doi: 10.1016/j.imbio.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.A., Smith B.R.C., Tate M.D., Belz G.T., Barrios M.H., Elgass K.D., Weisman A.S., Baker P.J., Preston S.P., Whitehead L. SIDT2 transports extracellular dsRNA into the cytoplasm for innate immune recognition. Immunity. 2017;47:498–509.e6. doi: 10.1016/j.immuni.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni C.Y., Wu Z.H., Florence W.C., Parekh V.V., Arrate M.P., Pierce S., Schweitzer B., Van Kaer L., Joyce S., Miyamoto S. Cutting edge: K63-linked polyubiquitination of NEMO modulates TLR signaling and inflammation in vivo. J. Immunol. 2008;180:7107–7111. doi: 10.4049/jimmunol.180.11.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J., Shi Y., Iwai K., Wu Z.H. LUBAC regulates NF-kappaB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. EMBO J. 2011;30:3741–3753. doi: 10.1038/emboj.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiumi H., Matsumoto M., Funami K., Akazawa T., Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- Panta G.R., Kaur S., Cavin L.G., Cortes M.L., Mercurio F., Lothstein L., Sweatman T.W., Israel M., Arsura M. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol. Cell. Biol. 2004;24:1823–1835. doi: 10.1128/MCB.24.5.1823-1835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai M., Tatematsu M., Oshiumi H., Funami K., Matsumoto M., Hatakeyama S., Seya T. Direct binding of TRAF2 and TRAF6 to TICAM-1/TRIF adaptor participates in activation of the Toll-like receptor 3/4 pathway. Mol. Immunol. 2010;47:1283–1291. doi: 10.1016/j.molimm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Schroder M., Bowie A.G. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Sen G.C., Sarkar S.N. Transcriptional signaling by double-stranded RNA: role of TLR3. Cytokine Growth Factor Rev. 2005;16:1–14. doi: 10.1016/j.cytogfr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Shim J.H., Xiao C., Paschal A.E., Bailey S.T., Rao P., Hayden M.S., Lee K.Y., Bussey C., Steckel M., Tanaka N. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Funami K., Ishii N., Seya T., Obuse C., Matsumoto M. LRRC59 regulates trafficking of nucleic acid-sensing TLRs from the endoplasmic reticulum via association with UNC93B1. J. Immunol. 2015;195:4933–4942. doi: 10.4049/jimmunol.1501305. [DOI] [PubMed] [Google Scholar]
- Tsai S.Y., Segovia J.A., Chang T.H., Shil N.K., Pokharel S.M., Kannan T.R., Baseman J.B., Defrene J., Page N., Cesaro A. Regulation of TLR3 activation by S100A9. J. Immunol. 2015;195:4426–4437. doi: 10.4049/jimmunol.1500378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S., Akira S. Toll-like receptors and innate immunity. J. Mol. Med. (Berl) 2006;84:712–725. doi: 10.1007/s00109-006-0084-y. [DOI] [PubMed] [Google Scholar]
- Van Essen D., Engist B., Natoli G., Saccani S. Two modes of transcriptional activation at native promoters by NF-kappaB p65. PLoS Biol. 2009;7:e73. doi: 10.1371/journal.pbio.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Deng L., Hong M., Akkaraju G.R., Inoue J., Chen Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Wu Z.H., Shi Y., Tibbetts R.S., Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- Wu Z.H., Wong E.T., Shi Y., Niu J., Chen Z., Miyamoto S., Tergaonkar V. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol. Cell. 2010;40:75–86. doi: 10.1016/j.molcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liao B., Wang S., Yan B., Jin Y., Shu H.B., Wang Y.Y. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc. Natl. Acad. Sci. U S A. 2013;110:5115–5120. doi: 10.1073/pnas.1220271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xia F., Hermance N., Mabb A., Simonson S., Morrissey S., Gandhi P., Munson M., Miyamoto S., Kelliher M.A. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol. Cell. Biol. 2011;31:2774–2786. doi: 10.1128/MCB.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., An H., Yao M., Hou J., Yu Y., Feng G., Cao X. Scaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophages. J. Immunol. 2010;184:6447–6456. doi: 10.4049/jimmunol.0901750. [DOI] [PubMed] [Google Scholar]
- Zinngrebe J., Rieser E., Taraborrelli L., Peltzer N., Hartwig T., Ren H., Kovacs I., Endres C., Draber P., Darding M. --LUBAC deficiency perturbs TLR3 signaling to cause immunodeficiency and autoinflammation. J. Exp. Med. 2016;213:2671–2689. doi: 10.1084/jem.20160041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the published article and the supplementary materials, and any additional information will be available from the lead contact upon request.