Central Illustration
Key Words: cardiovascular disease, innate immune system, NLRP3, NOD1, nucleotide-binding oligomerization domain-like receptors, toll-like receptors
Abbreviations and Acronyms: AMI, acute myocardial infarction; Ca2+, calcium ion; CARD, caspase activation and recruitment domain; CVD, cardiovascular disease; DAMPs, danger-associated molecular patterns; DAP, D-glutamyl-meso-diaminopimelic acid; ER, endoplasmic reticulum; HF, heart failure; IL, interleukin; I/R, ischemia/reperfusion; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; NLR, nucleotide-binding oligomerization domain-like receptors; NLRP, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing receptor; NOD, Nucleotide-binding oligomerization domain-containing protein; PAMP, pathogen-associated molecular pattern; ROS, reactive oxygen species; SR, sarcoplasmic reticulum; TLR, toll-like receptor
Highlights
-
•
The present review recapitulates relevant findings related to the role of the receptors of the innate immune system (TLRs and NLRs) in the progression of the most prevalent CVDs.
-
•
TLRs and NLRs play a key role in the progression of atherosclerosis, acute myocardial infarction or heart failure.
-
•
The development of new specific strategies to impair exacerbated TLR and NLR activation in CVDs are strong candidates for therapy and opens a new research field.
Summary
Cardiovascular diseases (CVDs) are the leading cause of death in the industrialized world. Most CVDs are associated with increased inflammation that arises mainly from innate immune system activation related to cardiac damage. Sustained activation of the innate immune system frequently results in maladaptive inflammatory responses that promote cardiovascular dysfunction and remodeling. Much research has focused on determining whether some mediators of the innate immune system are potential targets for CVD therapy. The innate immune system has specific receptors—termed pattern recognition receptors (PRRs)—that not only recognize pathogen-associated molecular patterns, but also sense danger-associated molecular signals. Activation of PRRs triggers the inflammatory response in different physiological systems, including the cardiovascular system. The classic PRRs, toll-like receptors (TLRs), and the more recently discovered nucleotide-binding oligomerization domain-like receptors (NLRs), have been recently proposed as key partners in the progression of several CVDs (e.g., atherosclerosis and heart failure). The present review discusses the key findings related to the involvement of TLRs and NLRs in the progression of several vascular and cardiac diseases, with a focus on whether some NLR subtypes (nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing receptor 3 and nucleotide-binding oligomerization domain-containing protein 1) can be candidates for the development of new therapeutic strategies for several CVDs.
The danger theory of immunology proposed by Polly Matzinger in 1994 (1) reshaped our understanding of the immune system. This theory posited that the innate immune system does not discriminate between self and nonself, but rather responds to “danger signals” that can be endogenous or exogenous in nature. Exogenous danger signals, often referred to as pathogen-associated molecular patterns (PAMPs), are highly conserved motifs in microbial pathogens, whereas endogenous danger signals, or danger-associated molecular patterns (DAMPs), are proteins, cytokines, chemokines, and other molecules from distressed and injured cells. Both PAMPs and DAMPs can activate the innate immune response through pattern recognition receptors (PRRs) on immune responsive cells, which activate different signaling cascades to ultimately trigger a wide array of responses against cell damage (2).
PRRs can be classified into 2 major groups based on their cellular location: 1) the transmembrane protein families—toll-like receptors (TLRs) and C-type lectin receptors; and 2) the cytoplasmic protein families—retinoic acid−inducible gene-like receptors, absent in melanoma 2-like receptors, cytosolic DNA receptors, and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (3, 4, 5, 6, 7, 8, 9). Although innate immune response is orchestrated mainly by classic immune cells, including macrophages and dendritic cells, other nonprofessional cells (e.g., endothelial cells, cardiomyocytes, and fibroblasts) also express these receptors and can actively contribute to immune response via PRR signaling (7,10).
Growing evidence supports the notion that innate immunity is closely related to the development of several CVDs, particularly when the heart responds to ischemia or mechanical stress (10, 11, 12, 13, 14, 15). In this regard, there are extensive data that have documented a crucial role for some TLRs and NLRs, which are present on immune cardiac resident cells, cardiac fibroblasts and cardiomyocytes, in the initiation, development, and maintenance of inflammatory response during heart failure (HF) or atherosclerosis (Central Illustration) (12,16, 17, 18). In this review, we summarize the key characteristics of TLRs and NLRs, with a particular focus of the role of TLRs and NLRs (nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing receptor [NLRP]3 and nucleotide-binding oligomerization domain-containing protein 1 [NOD1]) in the development of cardiovascular-related pathologies.
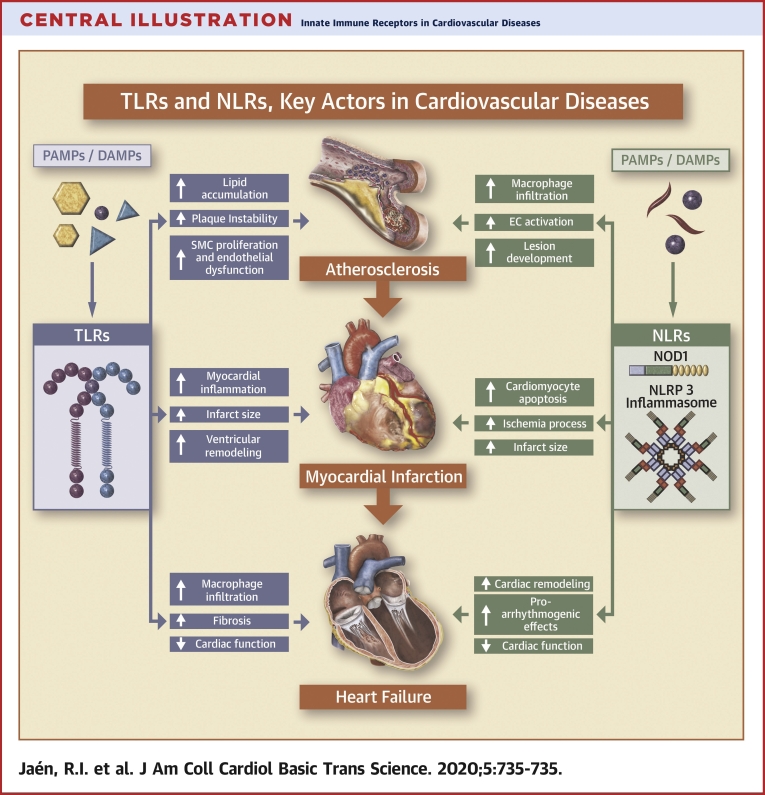
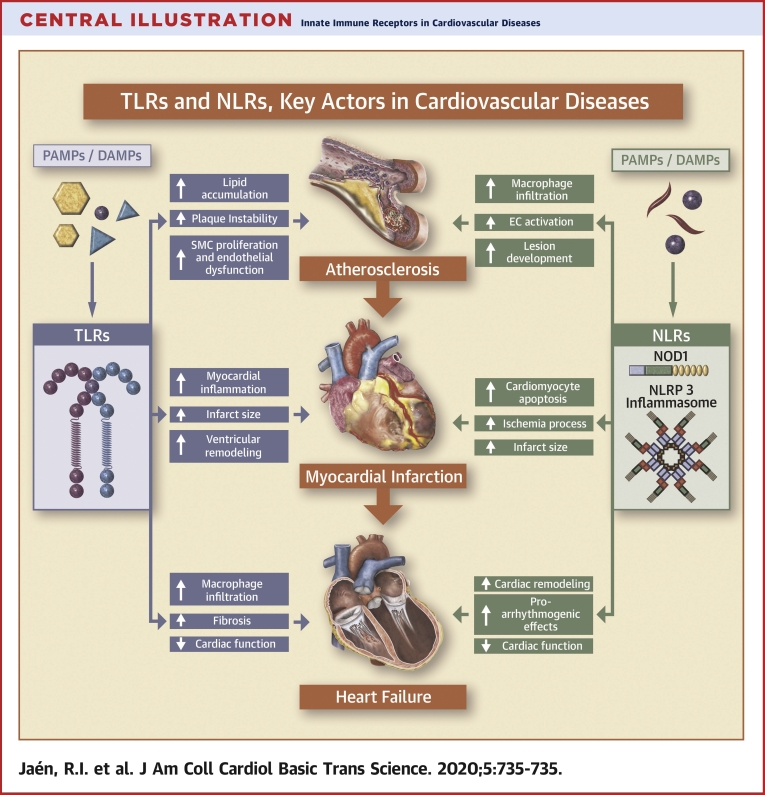
Central Illustration.
Innate Immune Receptors in Cardiovascular Diseases
Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) play a key role on the onset of cardiovascular diseases. This figure illustrates the potential detrimental action of the exacerbated activation of TLRs and NLRs in cardiovascular diseases, focusing on atherosclerosis, acute myocardial infarction, and heart failure as the most prevalent pathologies worldwide. DAMP = danger-associated molecular pattern; EC = excitation-contraction coupling; NLRP = nucleotide-binding oligomerization domain, leucine rich Repeat and pyrin domain-containing receptor; NOD 1 = nucleotide-binding oligomerization domain-containing protein 1; PAMP = pathogen-associated molecular pattern; SMC = smooth muscle cell.
Toll-Like Receptors
The toll-like family of receptors is a key component of the innate immune system that constitute 13 different receptors that are evolutionarily conserved from plants to humans (19,20). The precise number of TLRs differs across mammalian species; 10 types have been described in humans, whereas TLR11 is not functional and TLR12 and TLR13 are not expressed (Figure 1A) (19). They are mainly present on immune cells, such as macrophages, dendritic cells, natural killer cells, and lymphocytes, but they are also found on nonimmune cells, including fibroblasts and even cardiovascular and nerve cells. The engagement of PAMPs and DAMPs with TLRs induces key molecular events that ultimately lead to innate immune responses and the development of antigen-specific acquired immunity (21,22).
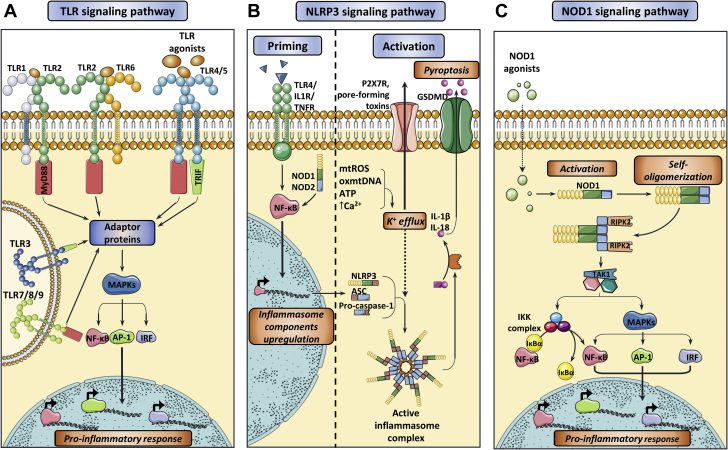
Figure 1.
Activation of the Main Innate Immune Receptors Involved in CVDs
(A) Toll-like receptor (TLR) signaling pathway. The interaction of TLR agonists with their corresponding TLRs induce a downstream signaling pathway that are mainly mediated by adaptor proteins that are capable of activating mitogen-activated protein kinase (MAPKs) and finally induce the nuclear translocation of the transcription factors nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), Activator protein 1 (AP-1), and interferon regulatory factor (IRF), promoting the activation of pro-inflammatory molecules. Main effectors molecules upstream to this pathway are myeloid differentiation primary response protein 88 (MyD88) or TIR domain-containing adaptor protein inducing IFN-β (TRIF). TLR4/TRIF binding is depicted to happen on the cell surface for simplicity reasons, although it is believed to occur in endosomes after receptor internalization. (B) Nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing receptor 3 (NLRP3) inflammasome priming and activation signaling. Appropriate NLRP3 inflammasome activation requires an initial priming step triggered by several inflammatory cytokines, which, in turn, upregulates the transcription of the different inflammasome components. Next, a plethora of stimuli ultimately causing potassium (K+) efflux induce inflammasome assembly, which is then capable of activating interleukin (IL)-1β and IL-18 as well as gasdermin D (GSDMD), leading to pyroptosis and exacerbating the inflammatory response. (C) Nucleotide-binding oligomerization domain-containing protein 1 (NOD1) signaling pathway. NOD1 agonists mainly consist of pathogen-associated molecular patterns (PAMPs) and/or danger-associated molecular patterns (DAMPs) that induce conformational changes on NOD1, leading to receptor self-oligomerization and recruitment of Receptor-interacting serine/threonine-protein kinase 2 (RIPK2). This adaptor protein then triggers a signaling cascade that subsequently activates NF-κB and MAPKs, eventually upregulating the transcription of pro-inflammatory genes. CVD = cardiovascular disease.
TLRs can be classified into 2 subfamilies based on their subcellular localization (23): plasma membrane TLRs include TLR1, TLR2, TLR4 to 6, and TLR10; and endosomal TLRs include TLR3 and TLR7 to 9. Cell surface TLRs mainly recognize microbial membrane components such as lipids, lipoproteins, and proteins, whereas intracellular TLRs recognize nucleic acids derived from bacteria and viruses, and also self-nucleic acids in disease (23). Key ligands of the TLRs are summarized in Table 1.
Table 1.
Classification of the Identified Types of Toll-Like Receptors and Their Main Activators
| TLR | Main Ligands | Associated Pathogen |
|---|---|---|
| Location: plasma membrane | ||
| TLR1 | Triacylated Lipoproteins | Bacteria Mycobacteria |
| TLR2 | Lipoproteins Zymosan Mannan Others |
Bacteria Mycobacteria Fungi Protists Nematodes |
| TLR4 | Lipopolysaccharide Heat-shock proteins Oligosaccharides |
Bacteria Nematodes Viruses |
| TLR5 | Flagellin | Bacteria |
| TLR6 | Lipoproteins Zymosan |
Bacteria Fungi |
| TLR10 | Unknown | Unknown |
| Location: endosomes | ||
| TLR3 | dsRNA | Viruses |
| TLR7 | ssRNA Synthetic compounds |
Viruses |
| TLR8 | ssRNA Synthetic compounds |
Viruses |
| TLR9 | Unmethylated CpG oligos | Viruses Bacteria |
dsRNA = double-stranded RNA; ssRNA = single-stranded RNA; TLR = toll -like receptor.
Each TLR is composed of an ectodomain with leucine-rich repeats that mediate PAMP recognition, a transmembrane domain, and a cytoplasmic toll/interleukin (IL)-1 receptor (TIR) domain that initiates downstream signaling (24,25). The interaction of TLRs with their cognate ligands triggers their dimerization. Subsequently, they can interact with several adaptor proteins, such as myeloid differentiation primary response protein 88 (MyD88) or TIR domain−containing adaptor protein-inducing interferon (IFN)-β (TRIF) (26), which leads to the downstream activation of mitogen-activated protein kinases (MAPKs) (27). The crucial endpoint of the cascade reaction is the activation of several transcription factors, including nuclear factor−kappa light chain enhancer of activated B cells (NF-κB), activator protein-1 (AP-1), and interferon regulatory factors (IRFs). After their translocation into the nucleus, they activate multiple pro-inflammatory genes and enhance the levels of nitric oxide and interferons, which are directly toxic to invading micro-organisms (Figure 1). Moreover, TLR stimulation contributes to the maturation of dendritic cells, and, in some cases, induces the adaptive immune system.
Although PAMP recognition by TLRs is crucial for host defense responses to pathogen infection, aberrant activation of TLR signaling by PAMPs and/or DAMPs and mutations in TLR signaling molecules are related to the development of several diseases (e.g., autoimmune, chronic inflammatory, and allergic diseases), as well as cancer and CVDs (28,29).
Role of TLRs in the progression of CVDs
TLRs are expressed on most of the cells of the cardiovascular system, including endothelial cells, smooth muscle cells, and cardiomyocytes (30,31). Human heart tissue expresses all TLR isoforms, although the relative mRNAs levels of TLR2, 3, and 4 are approximately 10-fold higher than other isoforms. Growing evidence indicates the involvement of innate immune activation mediated by myocardial TLRs in CVDs (25,32). Short-term activation of TLRs appears to have cytoprotective effects on the cardiovascular system, whereas prolonged or excessive activation of TLRs induces chronic low-grade inflammation, which leads to endothelial dysfunction, increased cell death, and adverse cardiac remodeling. In the following sections we discuss the role of the different TLRs in vascular and cardiac diseases.
Involvement of TLRs in atherosclerosis and other vascular diseases
Atherosclerosis is a chronic inflammatory disease that is triggered by accumulation of lipid particles (mainly oxidized low-density lipoproteins), endothelial cells, and macrophage-derived foam cells on the arterial wall, which creates an atheromatous plaque or atheroma (33). Atheromatous plaques may progress into unstable plaques, which are characterized by a highly inflammatory and necrotic core infiltrated with macrophages and lymphocytes and a thin fibrous cap that renders these plaques prone to rupture and destabilization.
Because of these processes, overall vascular homeostasis and hemodynamics are perturbed, which increases the risk of thrombosis and ultimately leads to severe CVDs (e.g. coronary artery disease or acute myocardial infarction [AMI]) (34).
Because inflammation plays a crucial role in the atherogenic process (35), TLRs and their signaling cascades have emerged as important mediators in this pathology. Oxidized low-density lipoprotein has been found to be one of the most crucial TLR ligands during atherogenesis, influencing macrophage differentiation to foam cells and inducing the secretion of pro-inflammatory cytokines (36, 37, 38).
Most studies in the area of TLRs in atherosclerosis have focused on TLR4 and TLR2 because they are overexpressed in atheromas (39); certain atherosclerosis treatments like statins are able to reduce their expression (40,41). Knockdown of Tlr4 or Tlr2 in atherosclerosis-prone, apolipoprotein E−deficient (Apoe−/−) mice results in a reduction in foam cell accumulation and less severe atherosclerosis compared with control Apoe−/− mice (42,43). Similarly, genetic deletion of Tlr4 has been found to reduce atherosclerotic lesions by 24% and macrophage infiltration by 65% (42). Stewart et al. (44) described that oxidized low-density lipoprotein could elicit inflammation through activation of a newly identified TLR4/TLR6 heterodimer by regulation of the CD36 scavenger receptor. Consequently, most results suggest that TLR4 is responsible for specific atherogenic processes, such as foam cell formation, lipid accumulation, plaque instability and rupture, and arterial remodeling, whereas TLR2 apparently does not contribute to atheroma destabilization and is involved in other processes (39).
The influence of TLR2 on atherosclerosis seems to be complex because it typically establishes a heterodimer with TLR1 or TLR6, and TLR2/1 and TLR2/6 heterodimers have been observed to be overexpressed in atheromas (39). Generally, TLR2 activation tends to stimulate atheroma development and thickening and to induce lipid accumulation (45), endothelial cell inflammation (39), and smooth muscle cell migration and proliferation (46,47). Accordingly, genetic deletion of Tlr2 in atherosclerotic mice reduced the severity of disease and aggregation of foam cells in the aorta (48). Tlr1−/− and Tlr6−/− mice with atherosclerosis exhibited the same degree of disease severity as control atherosclerotic mice (49), which indicated that TLR2 might play a role in atherosclerosis as a heterodimer in contrast to TLR1 and TLR6, which do not seem to be atherogenic per se. In this context, it would be interesting to evaluate the consequences of the inhibition of both TLR1 and TLR6 to test whether a compensatory mechanism exists between the heterodimers.
Endosomal TLRs have also been associated with atherosclerosis, but published studies are contradictory or inconclusive as to whether they play protective or deleterious roles. For instance, TLR3 expression seems to be upregulated in atheromas, and its activation increases plaque size (50). However, Tlr3−/−Apoe−/− mice develop atherosclerosis earlier than Tlr3+/+Apoe−/− mice (24), and further stimulation of TLR3 in this model reduces the formation of scar tissue (51), which suggests a protective effect for this TLR. Likewise, TLR7 and TLR9 appear to play protective roles because lipid aggregation, macrophage infiltration and accumulation, and production of pro-inflammatory cytokines are exacerbated in both Tlr7−/−Apoe−/− and Tlr9−/−Apoe−/− mice (52, 53, 54). In contrast, other groups have reported that TLR7 or TLR9 blockade may have beneficial effects in other murine atherosclerosis models by reducing scar tissue, lipid accumulation, macrophage infiltration, and plaque instability (55, 56, 57).
Beyond atherosclerosis, TLRs have been associated with several vascular diseases, including hypertension (58) and abdominal aortic aneurysm (59). The involvement of TLRs in hypertension became evident from the observation that angiotensin II can activate TLR4 signaling, thus upregulating TLR4, myeloid differentiation primary response protein 88, and NF-κB (60). Several experimental models broadly described the contribution of TLR4 to hypertension progression (61,62). In addition to TLR4, TLR9 activation was found to increase blood pressure, leading to vascular alterations in normotensive rats (63) and triggering hypertension in pregnant rats (64). TLR9 inhibition was also found to reduce blood pressure in hypertensive rats (65).
TLR4 upregulation was also widely associated with abdominal aortic aneurysm; its inhibition prevented aortic dilation and aneurysm progression in several experimental models (59,66,67). Inhibition of TLR2 also ameliorated abdominal aortic aneurysm in mice (68), and its genetic deletion was found to prevent abdominal aortic aneurysm aggravated by periodontal bacterial infection (69). TLR3 also seems to be upregulated in aortic wall tissue during abdominal aortic aneurysm development; however, its specific involvement in this pathology remains unclear (70).
Role of TLRs in cardiac diseases
Acute Myocardial Infarction
Despite great advances in treatment strategies over the last several years, AMI, which is caused by a sudden obstruction of the coronary arteries from an acutely formed thrombus, remains a major cause of death and morbidity (71). Restoration of blood flow to ischemic myocardium is associated with cardiac injury mainly due to the toxic effects of reactive oxygen intermediates that are generated during heart reperfusion (72). Although the cause of this injury is multifactorial, increasing experimental evidence suggests a pivotal role for the innate immune system in initiating the inflammatory cascade that leads to tissue injury. Thus, it has been described that in patients with unstable angina or with AMI, endogenous DAMPs (e.g., such as the heat shock proteins), high mobility group box 1 protein, and even genomic DNA or adenosine triphosphate (among other biomolecules) are released from damaged cardiac cells and signal through TLR receptors (73,74). In the same line, it has been reported that the increased presence of heat shock protein-60 on the surface of cardiomyocytes after ischemic heart failure can activate TLR4, leading to tumor necrosis factor-α−mediated apoptosis and increasing cardiac injury (75,76). Similarly, TLRs activated during myocardial ischemia and/or reperfusion (I/R) injury induce rapid NF-κB activation with the subsequent release of inflammatory cytokines, which mediate left ventricular dysfunction after injury (77).
Recent studies have revealed that TLR2 or TLR4 deficiency attenuates myocardial inflammation, reduces infarct size, and preserves ventricular function after transient ischemic injury in mice. This is supported by the findings that TLR2 (78) and TRL4 (79,80) antagonists have demonstrated efficacy in reducing NF-κB activation and infarct size and also improve cardiac function in mouse models of AMI. Using an AMI model of coronary artery ligation, Shishido et al. (81) reported that Tlr2−/− mice presented a significant reduction in mortality, left ventricular dysfunction and less ventricular remodeling compared with wild-type mice. These data indicate that in the context of cardiac ischemic injury, loss of cardiac TLR2 signaling is beneficial for cardiac function. Using a similar model, Timmers et al. (82) reported that Tlr4-defective mice showed reduced left ventricular remodeling and preserved left ventricular function after myocardial infarction. Other TLRs have been analyzed in this context. For example, TLR3-TIR domain-containing adaptor protein inducing IFN-β signaling was found to represent an injurious pathway during cardiac I/R (83). TLR9 appeared to play a protective role against cardiac rupture in the early phase after AMI, but its stimulation at onset of ischemia did not alter infarct size (84).
Despite these results, it has been also reported in animal models that activation of TLR2, 4, and 9 before I/R can lead to reduced infarct size and improved cardiac function through pre-conditioning mechanisms (85). These protective effects are mediated mainly by the phosphoinositide 3-kinase/protein kinase B pathway (PI3K/PKB), which prevents apoptotic signaling in cardiomyocytes and reduces the NF-κB inflammatory cardiac response (86). These results thus support an alternative therapy in AMI through a pre-conditioning approach.
Heart Failure
In many cases, patients with AMI ultimately develop HF, which is a complex clinical syndrome that occurs because of structural or functional impairment of ventricular filling or ejection of blood in the heart, and a failure to pump blood efficiently to meet the body's needs. HF is a leading cause of morbidity and mortality worldwide and increasingly affects millions of people (87).
Cardiac inflammation plays a critical role in the development of HF, and TLRs are actively involved in this process. TLR2 has been described to be upregulated in murine HF models, both in cardiomyocytes and in vascular endothelial cells, and HF progression in significantly reduced in Tlr2−/− mice (81). Inhibitory therapy using anti-TLR2 antibodies has been recently found to block angiotensin II−induced cardiac fibrosis by suppressing macrophage recruitment and inflammation in the heart (88). TLR4 is also upregulated in patients with chronic HF and induces a robust production of pro-inflammatory cytokines that favors the progression of myocardial dysfunction and fibrosis (89,90). Pharmacological blockade of TLR4 using different molecules has been found to attenuate myocardial I/R injury and the development of cardiac hypertrophy (79,91). There is also emerging evidence for the involvement of TLR9 in HF, which can be activated by endogenous DAMPs, including mitochondrial DNA, to modulate the progression of the disease (92). It has been reported that plasma levels of mitochondrial DNA are elevated in patients with HF and are associated with increased mortality (92).
Related to the role of TLRs in the modulation of cardiac function, chronic stimulation of TLRs can lead to cardiac dysfunction, at least in part through the modulation of cardiac ion channel activity. It is well known that cardiac function and calcium ion (Ca2+) homeostasis are closely coupled in the heart (93). Cardiac contraction is tightly regulated by changes in intracellular Ca2+ levels, and myocyte mishandling of this ion is a central cause of both cardiac dysfunction and arrhythmias in the pathophysiological mechanisms of CVD. In this context, Ca2+ is a key mediator of cardiac excitation−contraction coupling, the process from electrical excitation of the myocyte to contraction of the heart that is initiated by an action potential in cardiomyocytes (93). Specifically, the initial depolarization phase of the action potential activates sarcolemma L-type Ca2+ channels, firing Ca2+ entry into the cytosol and triggering a large release of Ca2+ from the sarcoplasmic reticulum (SR) by ryanodine receptors, which allows cell contraction. Relaxation is achieved by Ca2+ removal from the cytosol by SR-Ca2+ adenosine triphosphatase 2a and the sodium−calcium exchanger (93).
In relation to these processes, the direct effect of TLR2 activation by heat shock protein-70 on contractile performance has been confirmed in vitro using a murine cardiomyocyte cell line (94). Likewise, cardiac TLR4 activation by lipopolysaccharide has been shown to increase both the Ca2+ efflux via the sodium−calcium exchanger and action potential duration, which leads to arrhythmogenic events (95,96). In addition, the inflammatory cytokine high-mobility group box 1 protein can induce TLR4-dependent activation of nicotinamide adenine dinucleotide phosphate oxidase, which leads to reactive oxygen species (ROS) overproduction and oxidative stress (97,98). Importantly, oxidative stress activates ryanodine receptor type 2 during diastole, impairing both cardiac contraction and function. In contrast, TLR4 inhibition appears to prevent the occurrence of these abnormal events, restoring the contractile capacity of the heart and improving cardiac function (99). It has also been reported that Tlr4−/− mice are protected against lethal anthrax toxin−induced cardiac dysfunction and suppressed intracellular Ca2+ mishandling (100,101).
Nucleotide-Binding Oligomerization Domain-Like Receptors
The NLRs are modular cytosolic sensors of intracellular DAMPS and PAMPs and can be divided into 4 subfamilies based on their N-terminal domain configuration: NLRA, NLRB/ NAIP, NLRC, and NLRP. A conserved tripartite structure is common to the human NLR family, consisting of an amino-terminal, effector-binding domain, a central domain, and a carboxy-terminal, ligand-recognition domain, which mediates intracellular ligand sensing (102). The central NOD regulates its self-oligomerization, which is crucial for downstream effector activation. The amino-terminal effector-binding domain mediates binding to effector molecules, which determines the final activated signaling pathway and varies depending on whether the protein interaction is homo- or heterophilic. In this sense, the caspase-recruitment domain (CARD) and the pyrin domain mediate homophilic protein−protein interactions, which are associated with death effectors. Other NOD proteins have a heterophilic amino-terminal effector-binding domain, such as the neuronal apoptosis inhibitory protein or the major histocompatibility complex class II transactivator (103,104). The main activators of the different types of NLRs are listed in Table 2.
Table 2.
Classification of the Identified Types of NOD-Like Receptors (NLRs) and Their Main Activators
| NLR | Main Activators |
|---|---|
| NLR A | |
| CIITA | IFN-γ |
| NLR B | |
| NAIP1/2 | Injectisome |
| NAIP5/6 | Flagellin |
| NLR C | |
| NOD1 (NLRC1) | Peptidoglycan iE-DAP |
| NOD2 (NLRC2) | Peptidoglycan Muramyl dipeptide ssRNA |
| NLRC3 | Undetermined |
| IPAF (NLRC4) | Injectisome/flagellin (mediated by NAIPs) |
| NLRC5 | IFNγ LPS Poly I:C |
| NLRX1 | LPS Viral RNA |
| NLRP | |
| NLRP1 | Anthrax lethal toxin Toxoplasma gondii ATP depletion Muramyl dipeptide |
| NLRP2 | LPS/TNF-α |
| NLRP3 | K+ efflux Bacterial toxins and RNA Nigericin Aluminum hydroxide ATP Cholesterol and uric acid crystals Particulate matter Mitochondrial damage |
| NLRP4 | Viral DNA/RNA |
| NLRP5/8/13/14 | Undetermined |
| NLRP6 | Viral RNA TLR ligands Lipotheicoic acid |
| NLRP7 | Bacterial acylated lipoproteins Lysosomal proteases? K+ flux? |
| NLRP9 | dsRNA |
| NLRP10 | NOD1 activation |
| NLRP11 | TLR4 ligands |
| NLRP12 | ATP TLR2 ligands Yersinia pestis |
ATP = adenosine triphosphate; CIITA = major histocompatibility complex class II transactivator; iE-DAP = γ-D-glutamyl-meso-diaminopimelic acid; IFN = interferon; K = potassium; IPAF = interleukin-1b (IL-1b)-converting enzyme-protease-activating factor; LPS = lipopolysaccharide; NLR = nucleotide-binding oligomerization domain-like receptors; NAIP = Baculoviral IAP repeat-containing protein 1; NLRP = nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain-containing receptor; NOD1 = Nucleotide-binding oligomerization domain-containing protein 1; TNF = tumor necrosis factor; other abbreviations as in Table 1.
NLRs regulate a broad range of molecular functions, including NF-κB signaling, retinoic acid−inducible gene-I−like receptor signaling, autophagy, major histocompatibility complex gene regulation, reproduction, and development (105). Several reports related to some NLRs, especially NLRP3 and NOD1, have been recently published and are discussed in the following sections.
NLRP receptors
NLRP receptors have recently garnered attention due to their association with inflammasomes. Inflammasomes are macroprotein complexes that mediate the inflammatory response upon DAMP and PAMP signaling, by processing and activating pro−caspase-1 to caspase-1. Caspase-1 further activates a series of pro-inflammatory cytokines, such as IL-1β, IL-18, and high-mobility group box 1 protein, unleashing a potent inflammatory reaction (106,107). In addition, recent data have shown that caspase-1 can also elicit pyroptosis, a specific pro-inflammatory type of cell death mediated by gasdermin D (108). Once cleaved by the inflammasome complex, gasdermin D is capable of forming nonselective large oligomeric pores within the cell membrane that induce cell death and the release of intracellular content, particularly IL-1β, which further enhances the inflammatory reaction (Figure 1B) (106,108,109).
Assembly of the inflammasome involves the oligomerization of 3 essential proteins: a sensor molecule consisting of a PRR that recognizes a specific signal; an apoptosis-associated, speck-like protein containing CARD that serves as a scaffold; and pro−caspase-1 (106,107). Thus far, 5 types of PRRs have been described to establish inflammasomes: NLRP1, NLRP3, NLRC1/NOD1, pyrin and absent in melanoma 2 (106). Other PRRs, including IFI16, NLRP6, NLRP7, NLRP12, and NLRP14, are also able to form inflammasomes; however, their structures are yet to be determined. Of these PRRs, research has mainly focused on NLRP3 due to its association with several diseases including Alzheimer’s, asthma, rheumatoid arthritis, and CVDs (107).
NLRP3 inflammasome
Activation of the NLRP3 inflammasome has been described to occur in a 2-step process—an initial priming step followed by an activation step. During priming, PAMPs and endogenous cytokines signal through specific receptors and activate the NF-κB pathway, ultimately inducing expression of inflammasome components (NLRP3, an apoptosis-associated speck-like protein containing CARD, and pro–caspase-1) and targets (pro-IL-1β and pro-IL-18) (106). Priming also entails different post-translational modifications on NLRP3, including deubiquitination (110) and phosphorylation (111), which trigger conformational changes that favor the formation of the inflammasome.
The activation step involves the assembly of the complex and activation of pro-caspase-1. Signaling is initiated by cellular indicators of instability and damage, including cation mobilization (potassium and Ca2+ efflux), adenosine triphosphate, pore-forming toxins, mitochondrial dysfunction, lysosomal rupture, and particulate matter (108). This plethora of stimuli ultimately induce potassium efflux, which is believed to be the pivotal step for NLRP3 inflammasome activation (Figure 1B) (112). New modulators of the activation step are still being discovered, such as NEK7, a never in mitosis A-related serine/threonine kinase that acts as a positive regulator of the NLRP3 inflammasome (113,114). NEK7 deficiency reduces IL-1β production and disease severity in murine models (106,114).
Role of NLRP3 inflammasome in CVDs
The first evidence of the involvement of the NLRP3 inflammasome in CVDs was described by Duewell et al. (115), who observed that cholesterol crystals can activate this complex. This discovery complemented previous evidence of the role of both IL-1β and IL-18 in cardiac inflammation (116, 117, 118) and fibrosis (119,120). Accordingly, the NLRP3 inflammasome and its targets represents one of the major mediators of the deleterious immune response responsible in part for many CVDs. They have been linked to hypertension (121), HF (122,123), atrial fibrillation (124), AMI (125), diabetes (126), and vascular diseases, including atherosclerosis (127).
Involvement of NLRP3 in atherosclerosis and vascular diseases
Great progress has been made in characterizing the involvement of NLRP3 in atherosclerosis because pro-atherogenic components such as free fatty acids, oxidized low-density lipoprotein, and cholesterol crystals can elicit NLRP3 inflammasome assembly (115,128). In particular, cholesterol crystals cannot be completely phagocytosed by macrophages, which causes lysosome destabilization and cathepsin B release, which, in turn, trigger inflammasome activation (115). In the same line, atherosclerosis is characterized by an increase in ROS, endoplasmic reticulum (ER) stress, and mitochondrial dysfunction, all of which also contribute to NLRP3 inflammasome priming and activation (129).
The crucial role played by the NLRP3 inflammasome in atherosclerosis has been demonstrated with regard to both its components and its targets. For example, NLRP3, an apoptosis-associated speck-like protein containing CARD (Asc), caspase-1, IL-1β, and IL-18 levels have all been found to be elevated in the plaques of patients with atherosclerosis (130,131), particularly in unstable plaques (132). This increase has been observed both in foam cells and in endothelial cells (132). In addition, NLRP3 appears to be upregulated in the aorta of patients with atherosclerosis (133). However, studies performed in atherosclerosis-prone mice yielded conflicting results as to the participation of the NLRP3 inflammasome. Although some studies reported that the deletion of Nlrp3, Asc, Casp1, or Il1a/b attenuated lesion development (116,134), diminished macrophage infiltration (135), and decreased the severity of the disease (136), another study failed to observe any significant change in atherosclerosis development or macrophage infiltration upon deletion of any of these genes (137). These findings seem to be highly dependent on the animal model chosen (Ldlr−/− or Apoe−/−) and the content of the high-fat diet used; thus, more research is needed to consider the implication of these 2 variables on inflammasome activation and atherogenesis. In the same line, studies with statins—a common medication for atherosclerosis and other CVDs— exhibited contradictory evidence in relation to NLRP3 function, with some studies describing inhibition (138,139), activation (140,141), or lack of effect (139) on the activation of the inflammasome complex and downstream cytokine production.
Regarding the pathogenesis of aortic aneurysm, aortas from patients showed increased Nlrp3 and Il1b expression levels (142). Inhibition of this cytokine or its receptor in a murine model resulted in a preserved aorta structure and reduced inflammation (143). Interestingly, Erthart et al. (144) recently reported that patients with a more severe form of aortic aneurysm show attenuated inflammasome expression, possibly due to a change in the immune response as the disease progressed.
NLRP3 inflammasome in cardiac diseases
Acute Myocardial Infarction
Studies in models of myocardial I/R injury revealed that NLRP3, caspase-1 activity, IL-1β, and IL-18 were all upregulated in the ischemic heart (145). Moreover, knocking out or inhibiting NLRP3 reduced infarct size and the overall I/R injury (145, 146, 147), decreased macrophage and neutrophil infiltration (145), and diminished cardiac fibrosis and left ventricular dysfunction (146). Similar results were obtained in mice lacking Asc or Casp1, which was accompanied by a decrease in IL-1β levels (148). The activation of NLRP3 was proven to occur in both cardiac cell types (cardiomyocytes and cardiac fibroblasts) and in infiltrating cells (mainly macrophages and neutrophils) (148,149). Cardiomyocytes do not seem to produce consistently high levels of IL-1β, yet they are capable of cleaving caspase-1 and inducing pyroptosis, indicating that cardiac fibroblasts might be the chief cardiac cell population involved in inflammasome activation in the context of myocardial infarction and subsequent cardiac remodeling (148).
Toldo et al. (150) hypothesized that mitochondrial damage generated during the ischemic episode served as the priming signal for inflammasome, whereas the increase in ROS during the subsequent reperfusion provided the activating signal. In this line, several studies that treated I/R injury with antioxidant compounds before or at reperfusion reported a reduction in infarct size, in NLRP3 levels, in active caspase-1, and in IL-1β, which supported the link between ROS production and inflammasome activation in AMI (151, 152, 153).
Based on the results of these pre-clinical studies, 2 clinical trials were designed to investigate targeting the NLRP3 inflammatory cascade in patients with AMI: 1) a trial on anakinra, which is a recombinant IL-1β receptor antagonist; and 2) a trial on canakinumab, which is a monoclonal antibody targeting IL-1β. Both trials are in phase II and phase III, respectively (116,154). Both trials reported a decrease in cardiovascular risk with treatment; however, further research is needed to determine effective doses and to ascertain and reduce the associated side effects (116,154).
Heart failure
Research on the involvement of the NLRP3 inflammasome in HF is more scarce, with only a few studies describing an increase in both IL-1β (155,156) and IL-18 (157) in heart and plasma from patients with HF. Mallat et al. (157) also identified IL-18 expression in cardiomyocytes, macrophages, and endothelial cells in these patients. In the same line, Toldo et al. (158) demonstrated that inflammasomes were highly formed in leukocytes, cardiomyocytes, fibroblasts, and endothelial cells in heart biopsies from patients with acute myocarditis, especially in those with severe HF.
There is a general consensus that the NLRP3 inflammasome, mainly via the activation and release of IL-1β and IL-18, is strongly implicated in the adverse cardiac remodeling processes that provoke cardiac dysfunction and subsequent HF (159). Accordingly, the available evidence points to the inflammasome complex as a new potential target to treat CVDs and opens a door to the discovery of new mechanisms that regulate cardiac function.
As previously mentioned, both cardiac function and Ca2+ homeostasis are closely coupled in the heart. In addition, Ca2+ signaling has been suggested to be a key regulator in NLRP3 inflammasome activation (160). Administration of activators of the Ca2+-sensing receptor, a member of the 7-transmembrane receptor super family (161) implicated in Ca2+ release from ER stores and thus involved in NLRP3 activation, activates the phospholipase C signaling pathway in mouse bone marrow−derived macrophages. This leads to inositol phosphate accumulation and Ca2+ release from ER stores, which increases intracellular Ca2+ that activates NLRP3 (162). Also, the administration of a Ca2+-sensing receptor activator has been shown to induce myocardial fibrosis in Wistar rats (163). Supporting the notion that Ca2+ signaling is involved in inflammasome activation, the constitutive activation of the NLRP3 inflammasome in cardiomyocytes enhances the expression of RyR2, which leads to increased pro-arrhythmogenic SR Ca2+ release events (124). Conversely, genetic deletion of Nlrp3 reduces the incidence of atrial fibrillation episodes (164).
Nucleotide-binding oligomerization containing protein 1
In the early 2000s, 2 NOD-containing molecules, NOD1 and NOD2, were discovered by a database search for a homologue for the apoptosis regulator Apaf-1 (165, 166, 167). Since then, the NOD family has widened; currently, it consists of >20 human proteins, together with a large number of proteins from animals, plants, bacteria, and fungi (167).
As alluded to earlier, Nod1 encodes an intracellular scaffolding protein that consists of CARD, NOD, and leucine-rich repeat domains (Figure 1C). NOD1 exists as an inactive monomer in cytosol, and, upon ligand recognition, it undergoes a conformational change that promotes its activation. Once activated, NOD1 self-oligomerizes and recruits receptor-interacting serine/threonine-protein kinase 2 (RIPK2) through homotypic CARD−CARD interactions (168). Receptor-interacting serine/threonine-protein kinase 2 subsequently mediates the recruitment and activation of the serine/threonine kinase TAK1 that, in turn, activates the IκB kinase complex and the MAPK pathway. IκB kinase then phosphorylates the NF-κB inhibitor IκBα, which releases NF-κB, allowing it to translocate to the nucleus and modulate the expression of downstream target genes (Figure 1C) (169).
NOD1 mainly detects D-glutamyl-meso-diaminopimelic acid (DAP), which is a dipeptide present in peptidoglycan primarily found in Gram-negative bacteria, but is also found in specific groups of Gram-positive bacteria. However, NOD1 signal transduction can also be stimulated in the absence of direct cellular infection by a bacterial pathogen. Keestra-Gounder et al. (170) recently demonstrated that pro-inflammatory responses induced by ER stress are mediated through the NOD1/NOD2 pathway, which suggests a potential role for NOD1 in inflammatory diseases associated with this perturbation. The link between NOD1 and ER stress appears to be the unfolded protein response, as inhibiting IRE1α, a kinase implicated in this pathway, attenuates the NOD1-associated inflammatory reaction (170). Some studies have associated ER stress with an imbalance in cellular Ca2+ and the activation of NOD1 signaling (171,172). A recent study revealed that NOD1 activation both by bacterial pathogens and the NOD ligand C12-iE-DAP induced unfolded protein response activation through the ER kinase PERK, as well as Ca2+ flux from the ER membrane Ca2+ channel IP3R, which exacerbated the inflammatory response via NOD1 signaling (171). In the same line, Molinaro et al. (172) demonstrated that the increase in intracellular Ca2+ concentration in intestinal epithelial cells triggered NOD-dependent inflammatory signaling, including NF-κB. All these data supported a link between the activation of NOD1 and the perturbation of the homeostasis of cellular Ca2+, which suggested that the NOD1 inflammatory pathway could be responsible for several cardiac pathologies, in part through Ca2+ signaling.
Role of NOD1 in atherosclerosis and other vascular diseases
The endothelium represents the first barrier against blood-borne bacterial PAMPs and is therefore an important component of the innate immune system response to pathogens. In this regard, Moreno et al. (173) found that NOD1 is selectively expressed in vascular smooth muscle and its activation triggers the expression of the inflammatory mediators NOS2 and COX2. Similarly, specific NOD1 ligands can activate inflammatory responses in intact human vessels in vitro (174) and induce a significant number of pro-inflammatory responses in endothelial cells, which points to NOD1 as a crucial partner in vascular diseases (174).
The migration and proliferation of vascular smooth muscle cells that occurs in atherosclerosis contributes to vascular inflammation through the release of pro-inflammatory cytokines. The pathogenesis of diabetes mellitus accelerates these atherosclerotic vascular events, which have been related to NOD-mediated innate immunity signaling pathways. Human aortic vascular smooth muscle cells treated with insulin or iE-DAP showed a significant increase in Nod1 expression, together with increased levels of IL-8 and IL-1β (175).
In relation to the role of NOD1 in atherosclerosis, Kanno et al. (176) demonstrated, for the first time, that long-term oral administration of a synthetic NOD1 ligand accelerated the development and progression of atherosclerosis in Apoe−/− mice. In addition, the complete loss of NOD1 significantly decreased the size of atherosclerotic lesions, providing robust evidence of a relationship between NOD1 and atherosclerosis progression (176). Also, NOD1 expression was increased in endothelial cells of human and mouse atheromatous plaques, and the endothelial expression of the adhesion molecule VCAM-1 was reduced in atheroma lesions in Nod1−/− Apoe−/− mice compared with Apoe−/− mice (14). Furthermore, treatment of endothelial cells from Apoe−/− mice with a pharmacological inhibitor of NOD1 prevented the increased expression of VCAM-1 (17), which suggested a crucial role of NOD1 in the onset of atherosclerosis.
NOD1 involvement in cardiac diseases: acute myocardial infarction and heart Failure
NOD1 has been associated with several cardiovascular-related diseases, such as HF (16) and diabetic cardiomyopathy (177). Recently, Yang et al. (178) demonstrated that activation of NOD1 with DAP (a synthetic activator of NOD1) significantly aggravated cardiac I/R injury in mice and enhanced cardiomyocyte apoptosis and inflammation. In a similar line, Delgado et al. (179) demonstrated that NOD1 activation in mice promoted a significant decrease in cardiac function. NOD1 is functional in cardiomyocytes, and isolated cardiomyocytes from iE-DAP-treated mice showed diminished ICaL density, depressed Ca2+ transients, and a slower time decay of intracellular Ca2+ transients, which correlated with the reduced expression of SR-Ca2+ adenosine trisphosphatase 2a (179), features that are common in CVDs. In addition, systolic Ca2+ mishandling was accompanied by diastolic release events, such as increasing Ca2+ sparks frequency and occurrence of Ca2+ waves. Supporting these results, iE-DAP treatment of Nod1−/− mice failed to modify the cardiac excitation−contraction coupling parameters (179).
Nod1 genetic deletion or pharmacological inhibition also prevents Ca2+ mishandling associated with HF in ventricular cells. In this context, Val- Blasco et al. (16) demonstrated that Nod1 deletion in a murine HF model improved systolic Ca2+ release and SR-Ca2+ load and prevented the occurrence of pro-arrhythmogenic events. Moreover, Nod1 deletion also prevented the β-adrenergic response in failing cardiac murine cardiomyocytes (180).
In light of these studies, NOD1 emerges as an important regulator of Ca2+ dynamics in cardiac excitation−contraction coupling and can be the starting point for a new research line for Ca2+ handling impairment in specific CVDs, such as dilated cardiomyopathy or I/R injury, where NLR activation has already been described (178,179).
Conclusions
The present review summarizes relevant findings related to the role of the classic and the more recently discovered PRRs in the progression of the most prevalent cardiac and vascular diseases. In this regard, both TLRs and NLRs are clearly involved in the progression of some CVD pathologies, such as atherosclerosis, AMI, or HF. All or most of these actions are mediated by immune cells, but also by cardiomyocytes, fibroblasts, as well as endothelial and vascular cells, pointing to cardiovascular cells as new targets for TLR and NLR actions. The sustained activation of TLR2/4, NLRP3, or NOD1 are widely related to cardiac remodeling because they contribute to promotion of the progression of pathological processes, mainly by the induction of pro-inflammatory mediators. In contrast, the specific inhibition or the genetic deletion of these PRRs prevents cardiovascular damage in most CVDs. Accordingly, the development of new specific strategies to impair exacerbated PRR activation in these pathologies arise as strong candidates for therapy and opens a new field in CVD research.
Footnotes
This work was supported by the Spanish Ministry of Economy and Competitiveness (AEI/EU) and FEDER: SAF2017-82436R and RTC2017-6283), S2017/BMD-3686 from Comunidad de Madrid, ISCIII (PI17/01344), Fondo Social Europeo (FSE), and CIBERCV, a network funded by ISCIII. Dr. Fernández-Velasco is a Miguel Servet II researcher of ISCIII (MSII16/00047 Carlos III Health Institute). Dr. Jaén holds a FPU fellowship from MECD (Spain). Dr. López-Sendón has received research grants from Sanofi, Boehringer Ingelheim, Amgen, Pfizer, Bayer, and Merck. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Patricia Prieto, Email: patriciaprieto@ucm.es.
María Fernández-Velasco, Email: maria.fernandez@idipaz.es.
References
- 1.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 2.Hayden M.S., West A.P., Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Broz P., Monack D.M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:55–65. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Liang C. Innate recognition of microbial-derived signals in immunity and inflammation. Sci China Life Sci. 2016;59:1210–1217. doi: 10.1007/s11427-016-0325-6. [DOI] [PubMed] [Google Scholar]
- 6.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16:35–50. doi: 10.1038/nri.2015.8. [DOI] [PubMed] [Google Scholar]
- 7.Chen G.Y., Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong T., Liu L., Jiang W. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 9.Lu F., Lan Z., Xin Z. Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol. 2019:1–15. doi: 10.1002/jcp.29268. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Huang Z., Li H. Insights into innate immune signalling in controlling cardiac remodelling. Cardiovasc Res. 2017;113:1538–1550. doi: 10.1093/cvr/cvx130. [DOI] [PubMed] [Google Scholar]
- 11.Lin L., Knowlton A.A. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014;100:1–8. doi: 10.1016/j.lfs.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann D.L. Innate immunity and the failing heart. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anzai A., Choi J.L., He S. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. 2017;214:3293–3310. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sager H.B., Heidt T., Hulsmans M. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation. 2015;132:1880–1890. doi: 10.1161/CIRCULATIONAHA.115.016160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta P., Sager H.B., Stengel K.R. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitor cells. Cell Stem Cell. 2015;16:477–487. doi: 10.1016/j.stem.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Val-Blasco A., Piedras M.J.G.M., Ruiz-Hurtado G. Role of NOD1 in heart failure progression via regulation of Ca(2+) handling. J Am Coll Cardiol. 2017;69:423–433. doi: 10.1016/j.jacc.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 17.González-Ramos S., Paz-García M., Rius C. Endothelial NOD1 directs myeloid cell recruitment in atherosclerosis through VCAM-1. FASEB J. 2019;33:3912–3921. doi: 10.1096/fj.201801231RR. [DOI] [PubMed] [Google Scholar]
- 18.Yu L., Feng Z. The role of toll-like receptor signaling in the progression of heart failure. Mediators Inflamm. 2018;2018:9874109. doi: 10.1155/2018/9874109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidya M.K., Kumar V.G., Sejian V. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol. 2018;37:20–36. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majer O., Liu B., Barton G.M. Nucleic acid-sensing TLRs: trafficking and regulation. Curr Opin Immunol. 2017;44:26–33. doi: 10.1016/j.coi.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda K., Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 24.Falck-Hansen M., Kassiteridi C., Monaco C. Toll-like receptors in atherosclerosis. Int J Mol Sci. 2013;14:14008–14023. doi: 10.3390/ijms140714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 26.Deguine J., Barton G.M. MyD88: a central player in innate immune signaling. F1000Prime Rep. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown J., Wang H., Hajishengallis G.N. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ospelt C., Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol. 2010;42:495–505. doi: 10.1016/j.biocel.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Drexler S.K., Foxwell B.M. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:506–518. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell J.A., Ryffel B., Quesniaux V.F.J. Role of pattern-recognition receptors in cardiovascular health and disease. Biochem Soc Trans. 2007;35:1449–1452. doi: 10.1042/BST0351449. [DOI] [PubMed] [Google Scholar]
- 31.Vallejo J.G. Role of toll-like receptors in cardiovascular diseases. Clin Sci (Lond) 2011;121:1–10. doi: 10.1042/CS20100539. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S., Garg I., Ashraf M.Z. TLR signalling and association of TLR polymorphism with cardiovascular diseases. Vascul Pharmacol. 2016;87:30–37. doi: 10.1016/j.vph.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Bäck M., Hansson G.K. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol. 2015;12:199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 34.Bäck M. Omega-3 fatty acids in atherosclerosis and coronary artery disease. Futur Sci OA. 2017;3:FSO236. doi: 10.4155/fsoa-2017-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby P., Okamoto Y., Rocha V.Z. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 36.Endo A. A gift from nature: the birth of the statins. Nat Med. 2008;14:1050–1052. doi: 10.1038/nm1008-1050. [DOI] [PubMed] [Google Scholar]
- 37.Miller Y.I. Toll-like receptors and atherosclerosis: oxidized LDL as an endogenous toll-like receptor ligand. Future Cardiol. 2005;1:785–792. doi: 10.2217/14796678.1.6.785. [DOI] [PubMed] [Google Scholar]
- 38.Li M., Qian M., Kyler K. Endothelial-vascular smooth muscle cells interactions in atherosclerosis. Front Cardiovasc Med. 2018;5:151. doi: 10.3389/fcvm.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edfeldt K., Swedenborg J., Hansson G.K. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 40.Katsargyris A., Klonaris C., Tsiodras S. Statin treatment is associated with reduced toll-like receptor 4 immunohistochemical expression on carotid atherosclerotic plaques: a novel effect of statins. Vascular. 2011;19:320–326. doi: 10.1258/vasc.2011.oa0306. [DOI] [PubMed] [Google Scholar]
- 41.Kapelouzou A., Giaglis S., Peroulis M. Overexpression of toll-like receptors 2, 3, 4, and 8 is correlated to the vascular atherosclerotic process in the hyperlipidemic rabbit model: the effect of statin treatment. J Vasc Res. 2017;54:156–169. doi: 10.1159/000457797. [DOI] [PubMed] [Google Scholar]
- 42.Michelsen K.S., Wong M.H., Shah P.K. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoneveld A.H., Oude Nijhuis M.M., van Middelaar B. Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc Res. 2005;66:162–169. doi: 10.1016/j.cardiores.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 44.Stewart C.R., Stuart L.M., Wilkinson K. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazemi M.R., McDonald C.M., Shigenaga J.K. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol. 2005;25:1220–1224. doi: 10.1161/01.ATV.0000159163.52632.1b. [DOI] [PubMed] [Google Scholar]
- 46.de Graaf R., Kloppenburg G., Kitslaar P.J.H.M. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through toll-like receptors 2 and 4. Microbes Infect. 2006;8:1859–1865. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Lee G.-L., Chang Y.-W., Wu J.-Y. TLR 2 induces vascular smooth muscle cell migration through cAMP response element-binding protein-mediated interleukin-6 production. Arterioscler Thromb Vasc Biol. 2012;32:2751–2760. doi: 10.1161/ATVBAHA.112.300302. [DOI] [PubMed] [Google Scholar]
- 48.Higashimori M., Tatro J.B., Moore K.J. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:50–57. doi: 10.1161/ATVBAHA.110.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curtiss L.K., Black A.S., Bonnet D.J. Atherosclerosis induced by endogenous and exogenous toll-like receptor (TLR)1 or TLR6 agonists. J Lipid Res. 2012;53:2126–2132. doi: 10.1194/jlr.M028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmer S., Steinmetz M., Asdonk T. Activation of endothelial toll-like receptor 3 impairs endothelial function. Circ Res. 2011;108:1358–1366. doi: 10.1161/CIRCRESAHA.111.243246. [DOI] [PubMed] [Google Scholar]
- 51.Cole J.E., Navin T.J., Cross A.J. Unexpected protective role for toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A. 2011;108:2372–2377. doi: 10.1073/pnas.1018515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niessner A., Sato K., Chaikof E.L. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 53.Salagianni M., Galani I.E., Lundberg A.M. Toll-like receptor 7 protects from atherosclerosis by constraining ‘inflammatory’ macrophage activation. Circulation. 2012;126:952–962. doi: 10.1161/CIRCULATIONAHA.111.067678. [DOI] [PubMed] [Google Scholar]
- 54.Koulis C., Chen Y.-C., Hausding C. Protective role for toll-like receptor-9 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:516–525. doi: 10.1161/ATVBAHA.113.302407. [DOI] [PubMed] [Google Scholar]
- 55.Karper J.C., Ewing M.M., Habets K.L.L. Blocking toll-like receptors 7 and 9 reduces postinterventional remodeling via reduced macrophage activation, foam cell formation, and migration. Arterioscler Thromb Vasc Biol. 2012;32:e72–e80. doi: 10.1161/ATVBAHA.112.249391. [DOI] [PubMed] [Google Scholar]
- 56.Fukuda D., Nishimoto S., Aini K. Toll-like receptor 9 plays a pivotal role in angiotensin II–induced atherosclerosis. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma C., Ouyang Q., Huang Z. Toll-like receptor 9 inactivation alleviated atherosclerotic progression and inhibited macrophage polarized to M1 phenotype in ApoE-/- mice. Dis Markers. 2015;2015:909572. doi: 10.1155/2015/909572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goulopoulou S., McCarthy C.G., Webb R.C. Toll-like receptors in the vascular system: sensing the dangers within. Pharmacol Rev. 2016;68:142–167. doi: 10.1124/pr.114.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y., Ma Z., Yang G. Alginate oligosaccharide indirectly affects toll-like receptor signaling via the inhibition of microRNA-29b in aneurysm patients after endovascular aortic repair. Drug Des Devel Ther. 2017;11:2565–2579. doi: 10.2147/DDDT.S140206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biancardi V.C., Bomfim G.F., Reis W.L. The interplay between angiotensin II, TLR4 and hypertension. Pharmacol Res. 2017;120:88–96. doi: 10.1016/j.phrs.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Hernanz R., Martínez-Revelles S., Palacios R. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol. 2015;172:3159–3176. doi: 10.1111/bph.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bomfim G.F., Echem C., Martins C.B. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 2015;122:1–7. doi: 10.1016/j.lfs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarthy C.G., Wenceslau C.F., Goulopoulou S. Circulating mitochondrial DNA and toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res. 2015;107:119–130. doi: 10.1093/cvr/cvv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goulopoulou S., Matsumoto T., Bomfim G.F. Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci (Lond) 2012;123:429–435. doi: 10.1042/CS20120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adamczak D.M. The role of toll-like receptors and vitamin D in cardiovascular diseases-a review. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang T., Ran F., Qiao Q. Tanshinone IIA attenuates elastase-induced AAA in rats via inhibition of MyD88-dependent TLR-4 signaling. Vasa. 2014;43:39–46. doi: 10.1024/0301-1526/a000326. [DOI] [PubMed] [Google Scholar]
- 67.Pirianov G., Torsney E., Howe F. Rosiglitazone negatively regulates c-Jun N-terminal kinase and toll-like receptor 4 proinflammatory signalling during initiation of experimental aortic aneurysms. Atherosclerosis. 2012;225:69–75. doi: 10.1016/j.atherosclerosis.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 68.Yan H., Cui B., Zhang X. Antagonism of toll-like receptor 2 attenuates the formation and progression of abdominal aortic aneurysm. Acta Pharm Sin B. 2015;5:176–187. doi: 10.1016/j.apsb.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aoyama N., Suzuki J., Ogawa M. Toll-like receptor-2 plays a fundamental role in periodontal bacteria-accelerated abdominal aortic aneurysms. Circ J. 2013;77:1565–1573. doi: 10.1253/circj.cj-12-1191. [DOI] [PubMed] [Google Scholar]
- 70.Jabłońska A., Neumayer C., Bolliger M. Analysis of host toll-like receptor 3 and RIG-I-like receptor gene expression in patients with abdominal aortic aneurysm. J Vasc Surg. 2018;68:39S−46S. doi: 10.1016/j.jvs.2017.10.087. [DOI] [PubMed] [Google Scholar]
- 71.Jayaraj C., Davatyan K., Chadwick J. Epidemiology of myocardial infarction. In: Pamukçu Burak., editor. Myocardial Infarction. IntechOpen; London: 2018. pp. 9–19. [Google Scholar]
- 72.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valen G., Hansson G.K., Dumitrescu A. Unstable angina activates myocardial heat shock protein 72, endothelial nitric oxide synthase, and transcription factors NFkappaB and AP-1. Cardiovasc Res. 2000;47:49–56. doi: 10.1016/s0008-6363(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 74.Amanvermez R., Acar E., Günay M. Hsp 70, hsCRP and oxidative stress in patients with acute coronary syndromes. Bosn J Basic Med Sci. 2012;12:102–107. doi: 10.17305/bjbms.2012.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heiserman J.P., Chen L., Kim B.S. TLR4 mutation and HSP60-induced cell death in adult mouse cardiac myocytes. Cell Stress Chaperones. 2015;20:527–535. doi: 10.1007/s12192-015-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S.-C., Stice J.P., Chen L. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009;105:1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen H., Zhang R.-Q., Wei X.-G. Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac J Trop Med. 2016;9:503–507. doi: 10.1016/j.apjtm.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Arslan F., Smeets M.B., O’Neill L.A.J. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation. 2010;121:80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 79.Shimamoto A., Chong A.J., Yada M. Inhibition of toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270−4. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 80.Teshima Y., Yufu K., Akioka H. Early atorvastatin therapy improves cardiac function in patients with acute myocardial infarction. J Cardiol. 2009;53:58–64. doi: 10.1016/j.jjcc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Shishido T., Nozaki N., Yamaguchi S. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 82.Timmers L., Sluijter J.P.G., van Keulen J.K. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–264. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 83.Chen C., Feng Y., Zou L. Role of extracellular RNA and TLR3-Trif signaling in myocardial ischemia-reperfusion injury. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohm I.K., Gao E., Belland Olsen M. Toll-like receptor 9-activation during onset of myocardial ischemia does not influence infarct extension. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dong J.-W., Vallejo J.G., Tzeng H.-P. Innate immunity mediates myocardial preconditioning through toll-like receptor 2 and TIRAP-dependent signaling pathways. Am J Physiol Heart Circ Physiol. 2010;298:H1079−87. doi: 10.1152/ajpheart.00306.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao Z., Ren D., Ha T. CpG-ODN, the TLR9 agonist, attenuates myocardial ischemia/reperfusion injury: involving activation of PI3K/Akt signaling. Biochim Biophys Acta. 2013;1832:96–104. doi: 10.1016/j.bbadis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Savarese G., Lund L.H. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao W., Xiong Y., Li Q. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol. 2017;8:508. doi: 10.3389/fphys.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frantz S., Kobzik L., Kim Y.D. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Birks E.J., Felkin L.E., Banner N.R. Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J Heart Lung Transplant. 2004;23:228–235. doi: 10.1016/S1053-2498(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 91.Ehrentraut H., Weber C., Ehrentraut S. The toll-like receptor 4-antagonist eritoran reduces murine cardiac hypertrophy. Eur J Heart Fail. 2011;13:602–610. doi: 10.1093/eurjhf/hfr035. [DOI] [PubMed] [Google Scholar]
- 92.Oka T., Hikoso S., Yamaguchi O. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bers D.M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 94.Mathur S., Walley K.R., Wang Y. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J. 2011;75:2445–2452. doi: 10.1253/circj.cj-11-0194. [DOI] [PubMed] [Google Scholar]
- 95.Milberg P., Pott C., Fink M. Inhibition of the Na+/Ca2+ exchanger suppresses torsades de pointes in an intact heart model of long QT syndrome-2 and long QT syndrome-3. Heart Rhythm. 2008;5:1444–1452. doi: 10.1016/j.hrthm.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 96.Monnerat-Cahli G., Alonso H., Gallego M. Toll-like receptor 4 activation promotes cardiac arrhythmias by decreasing the transient outward potassium current (Ito) through an IRF3-dependent and MyD88-independent pathway. J Mol Cell Cardiol. 2014;76:116–125. doi: 10.1016/j.yjmcc.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Fan J., Li Y., Levy R.M. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 98.Tsung A., Klune J.R., Zhang X. HMGB1 release induced by liver ischemia involves toll-like receptor 4–dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang C., Mo M., Ding W. High-mobility group box 1 (HMGB1) impaired cardiac excitation–contraction coupling by enhancing the sarcoplasmic reticulum (SR) Ca2 + leak through TLR4–ROS signaling in cardiomyocytes. J Mol Cell Cardiol. 2014;74:260–273. doi: 10.1016/j.yjmcc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 100.Kandadi M.R., Frankel A.E., Ren J. Toll-like receptor 4 knockout protects against anthrax lethal toxin-induced cardiac contractile dysfunction: role of autophagy. Br J Pharmacol. 2012;167:612–626. doi: 10.1111/j.1476-5381.2012.02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kandadi M.R., Hua Y., Ma H. Anthrax lethal toxin suppresses murine cardiomyocyte contractile function and intracellular Ca2+ handling via a NADPH oxidase-dependent mechanism. PLoS One. 2010;5:e13335. doi: 10.1371/journal.pone.0013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Franchi L., Muñoz-Planillo R., Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanneganti T.-D., Lamkanfi M., Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Inohara N., Nuñez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3:371–382. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 105.Mason D.R., Beck P.L., Muruve D.A. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun. 2011;4:16–30. doi: 10.1159/000334247. [DOI] [PubMed] [Google Scholar]
- 106.He Y., Hara H., Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mangan M.S.J., Olhava E.J., Roush W.R. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 108.Ramos-Junior E.S., Morandini A.C. Gasdermin: a new player to the inflammasome game. Biomed J. 2017;40:313–316. doi: 10.1016/j.bj.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu X., Zhang H., Qi W. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Juliana C., Fernandes-Alnemri T., Kang S. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song N., Liu Z.-S., Xue W. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68:185–197.e6. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 112.Muñoz-Planillo R., Kuffa P., Martínez-Colón G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He Y., Zeng M.Y., Yang D. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi H., Wang Y., Li X. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duewell P., Kono H., Rayner K.J. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 117.Van Tassell B.W., Toldo S., Mezzaroma E. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang M., Markel T.A., Meldrum D.R. Interleukin 18 in the heart. Shock. 2008;30:3–10. doi: 10.1097/SHK.0b013e318160f215. [DOI] [PubMed] [Google Scholar]
- 119.Gurantz D., Cowling R.T., Varki N. IL-1beta and TNF-alpha upregulate angiotensin II type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J Mol Cell Cardiol. 2005;38 doi: 10.1016/j.yjmcc.2004.12.015. 505−5. [DOI] [PubMed] [Google Scholar]
- 120.Xiao H., Li H., Wang J.-J. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. Eur Heart J. 2018;39:60–69. doi: 10.1093/eurheartj/ehx261. [DOI] [PubMed] [Google Scholar]
- 121.Krishnan S.M., Sobey C.G., Latz E. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014;171:5589–5602. doi: 10.1111/bph.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van Tassell B.W., Raleigh J.M.V., Abbate A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr Heart Fail Rep. 2015;12:33–41. doi: 10.1007/s11897-014-0231-7. [DOI] [PubMed] [Google Scholar]
- 123.Yamaoka-Tojo M., Tojo T., Inomata T. Circulating levels of interleukin 18 reflect etiologies of heart failure: Th1/Th2 cytokine imbalance exaggerates the pathophysiology of advanced heart failure. J Card Fail. 2002;8:21–27. doi: 10.1054/jcaf.2002.31628. [DOI] [PubMed] [Google Scholar]
- 124.Yao C., Veleva T., Scott L. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang L., Qu P., Zhao J. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch Med Sci. 2014;10:791–800. doi: 10.5114/aoms.2014.44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee H.-M., Kim J.-J., Kim H.J. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karasawa T., Takahashi M. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb. 2017;24:443–451. doi: 10.5551/jat.RV17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wen H., Gris D., Lei Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu D., Zeng X., Li X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol. 2018;113:5. doi: 10.1007/s00395-017-0663-9. [DOI] [PubMed] [Google Scholar]
- 130.Paramel Varghese G., Folkersen L., Strawbridge R.J. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Afrasyab A., Qu P., Zhao Y. Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessels. 2016;31:1218–1229. doi: 10.1007/s00380-015-0723-8. [DOI] [PubMed] [Google Scholar]
- 132.Jin Y., Fu J. Novel insights into the NLRP 3 inflammasome in atherosclerosis. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng F., Xing S., Gong Z. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ. 2013;22:746–750. doi: 10.1016/j.hlc.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 134.Gage J., Hasu M., Thabet M. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol. 2012;28:222–229. doi: 10.1016/j.cjca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 135.Usui F., Shirasuna K., Kimura H. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2012;425:162–168. doi: 10.1016/j.bbrc.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 136.Kirii H., Niwa T., Yamada Y. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 137.Menu P., Pellegrin M., Aubert J.-F. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S., Xie X., Lei T. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelial cells through a PXR-dependent mechanism. Mol Pharmacol. 2017;92:256–264. doi: 10.1124/mol.116.108100. [DOI] [PubMed] [Google Scholar]
- 139.Satoh M., Tabuchi T., Itoh T. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin Sci (Lond) 2014;126:233–241. doi: 10.1042/CS20130043. [DOI] [PubMed] [Google Scholar]
- 140.Henriksbo B.D., Schertzer J.D. Is immunity a mechanism contributing to statin-induced diabetes? Adipocyte. 2015;4:232–238. doi: 10.1080/21623945.2015.1024394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Henriksbo B.D., Lau T.C., Cavallari J.F. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes. 2014;63:3742–3747. doi: 10.2337/db13-1398. [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez-Hidalgo C., De Haro J., Bleda S. Differential mRNA expression of inflammasome genes NLRP1 and NLRP3 in abdominal aneurysmal and occlusive aortic disease. Ther Adv Cardiovasc Dis. 2018;12:123–129. doi: 10.1177/1753944717750338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnston W.F., Salmon M., Pope N.H. Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130:S51−9. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Erhart P., Cakmak S., Grond-Ginsbach C. Inflammasome activity in leucocytes decreases with abdominal aortic aneurysm progression. Int J Mol Med. 2019;44:1299–1308. doi: 10.3892/ijmm.2019.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu Y., Lian K., Zhang L. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:415. doi: 10.1007/s00395-014-0415-z. [DOI] [PubMed] [Google Scholar]
- 146.van Hout G.P.J., Bosch L., Ellenbroek G.H.J.M. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. 2017;38:828–836. doi: 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- 147.Marchetti C., Chojnacki J., Toldo S. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63:316–322. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kawaguchi M., Takahashi M., Hata T. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 149.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 150.Toldo S., Marchetti C., Mauro A.G. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 151.Sun W., Lu H., Lyu L. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway. J Physiol Biochem. 2019;75:531–547. doi: 10.1007/s13105-019-00702-7. [DOI] [PubMed] [Google Scholar]
- 152.Jun J.H., Shim J.-K., Oh J.E. Protective effect of ethyl pyruvate against myocardial ischemia reperfusion injury through regulations of ROS-related NLRP3 inflammasome activation. Oxid Med Cell Longev. 2019;2019:4264580. doi: 10.1155/2019/4264580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y., Yan X., Mi S. Naoxintong attenuates ischaemia/reperfusion injury through inhibiting NLRP3 inflammasome activation. J Cell Mol Med. 2017;21:4–12. doi: 10.1111/jcmm.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Van Tassell B.W., Lipinski M.J., Appleton D. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): a randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol. 2018;41:1004–1008. doi: 10.1002/clc.22988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Szekely Y., Arbel Y. A Review of interleukin-1 in heart disease: where do we stand today? Cardiol Ther. 2018;7:25–44. doi: 10.1007/s40119-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Tassell B.W., Arena R.A., Toldo S. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mallat Z., Heymes C., Corbaz A. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J. 2004;18:1752–1754. doi: 10.1096/fj.04-2426fje. [DOI] [PubMed] [Google Scholar]
- 158.Toldo S., Kannan H., Bussani R. Formation of the inflammasome in acute myocarditis. Int J Cardiol. 2014;171:e119–e121. doi: 10.1016/j.ijcard.2013.12.137. [DOI] [PubMed] [Google Scholar]
- 159.Butts B., Gary R.A., Dunbar S.B. The importance of NLRP3 inflammasome in heart failure. J Card Fail. 2015;21:586–593. doi: 10.1016/j.cardfail.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35:253–261. doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brown E.M., Gamba G., Riccardi D. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 162.Lee G.S., Subramanian N., Kim A.I. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu F.-H., Fu S.-B., Leng X. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell Physiol Biochem. 2013;31:728–743. doi: 10.1159/000350091. [DOI] [PubMed] [Google Scholar]
- 164.Chen G., Chelu M.G., Dobrev D. Cardiomyocyte inflammasome signaling in cardiomyopathies and atrial fibrillation: mechanisms and potential therapeutic implications. Front Physiol. 2018;9:1115. doi: 10.3389/fphys.2018.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Q.A., Hengartner M.O. The molecular mechanism of programmed cell death in C. elegans. Ann N Y Acad Sci. 1999;887:92–104. doi: 10.1111/j.1749-6632.1999.tb07925.x. [DOI] [PubMed] [Google Scholar]
- 166.Derry W.B., Putzke A.P., Rothman J.H. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science. 2001;294:591–595. doi: 10.1126/science.1065486. [DOI] [PubMed] [Google Scholar]
- 167.Inohara N., Nuñez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. 2001;20:6473–6481. doi: 10.1038/sj.onc.1204787. [DOI] [PubMed] [Google Scholar]
- 168.Inohara N., Koseki T., Del Peso L. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 169.Caruso R., Warner N., Inohara N. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908. doi: 10.1016/j.immuni.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Keestra-Gounder A.M., Byndloss M.X., Seyffert N. NOD1 and NOD2 signalling links ER stress with inflammation. Nature. 2016;532:394–397. doi: 10.1038/nature17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mendez J.M., Kolora L.D., Lemon J.S. Activation of the endoplasmic reticulum stress response impacts the NOD1 signaling pathway. Infect Immun. 2019;87 doi: 10.1128/IAI.00826-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Molinaro R., Mukherjee T., Flick R. Trace levels of peptidoglycan in serum underlie the NOD-dependent cytokine response to endoplasmic reticulum stress. J Biol Chem. 2019;294:9007–9015. doi: 10.1074/jbc.RA119.007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moreno L., McMaster S., Gatheral T. Nucleotide oligomerization domain 1 is a dominant pathway for NOS2 induction in vascular smooth muscle cells: comparison with Toll-like receptor 4 responses in macrophages. Br J Pharmacol. 2010;160:1997–2007. doi: 10.1111/j.1476-5381.2010.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Navarro R., Delgado-Wicke P., Nuñez-Prado N. Role of nucleotide-binding oligomerization domain 1 (NOD1) in pericyte-mediated vascular inflammation. J Cell Mol Med. 2016;20:980–986. doi: 10.1111/jcmm.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shiny A., Regin B., Mohan V. Coordinated augmentation of NFAT and NOD signaling mediates proliferative VSMC phenotype switch under hyperinsulinemia. Atherosclerosis. 2016;246:257–266. doi: 10.1016/j.atherosclerosis.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 176.Kanno S., Nishio H., Tanaka T. Activation of an innate immune receptor, Nod1, accelerates atherogenesis in Apoe −/− mice. J Immunol. 2015;194:773–780. doi: 10.4049/jimmunol.1302841. [DOI] [PubMed] [Google Scholar]
- 177.Prieto P., Vallejo-Cremades M.T., Benito G. NOD1 receptor is up-regulated in diabetic human and murine myocardium. Clin Sci (Lond) 2014;127:665–677. doi: 10.1042/CS20140180. [DOI] [PubMed] [Google Scholar]
- 178.Yang H., Li N., Song L.-N. Activation of NOD1 by DAP contributes to myocardial ischemia/reperfusion injury via multiple signaling pathways. Apoptosis. 2015;20:512–522. doi: 10.1007/s10495-015-1089-1. [DOI] [PubMed] [Google Scholar]
- 179.Delgado C., Ruiz-Hurtado G., Gómez-Hurtado N. NOD1, a new player in cardiac function and calcium handling. Cardiovasc Res. 2015;106:375–386. doi: 10.1093/cvr/cvv118. [DOI] [PubMed] [Google Scholar]
- 180.Val-Blasco A., Navarro-García J.A., Tamayo M. Deficiency of NOD1 improves the β-adrenergic modulation of Ca2+ handling in a mouse model of heart failure. Front Physiol. 2018;9:702. doi: 10.3389/fphys.2018.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]