Abstract
Objective
Recent evidence indicates that inhibition of prolyl hydroxylase domain (PHD) proteins can exert beneficial effects to improve metabolic abnormalities in mice and humans. However, the underlying mechanisms are not clearly understood. This study was designed to address this question.
Methods
A pan-PHD inhibitor compound was injected into WT and liver-specific hypoxia-inducible factor (HIF)-2α KO mice, after onset of obesity and glucose intolerance, and changes in glucose and glucagon tolerance were measured. Tissue-specific changes in basal glucose flux and insulin sensitivity were also measured by hyperinsulinemic euglycemic clamp studies. Molecular and cellular mechanisms were assessed in normal and type 2 diabetic human hepatocytes, as well as in mouse hepatocytes.
Results
Administration of a PHD inhibitor compound (PHDi) after the onset of obesity and insulin resistance improved glycemic control by increasing insulin and decreasing glucagon sensitivity in mice, independent of body weight change. Hyperinsulinemic euglycemic clamp studies revealed that these effects of PHDi treatment were mainly due to decreased basal hepatic glucose output and increased liver insulin sensitivity. Hepatocyte-specific deletion of HIF-2α markedly attenuated these effects of PHDi treatment, showing PHDi effects are HIF-2α dependent. At the molecular level, HIF-2α induced increased Irs2 and cyclic AMP-specific phosphodiesterase gene expression, leading to increased and decreased insulin and glucagon signaling, respectively. These effects of PHDi treatment were conserved in human and mouse hepatocytes.
Conclusions
Our results elucidate unknown mechanisms for how PHD inhibition improves glycemic control through HIF-2α-dependent regulation of hepatic insulin and glucagon sensitivity.
Keywords: Obesity-induced glucose intolerance, Type 2 diabetes, Liver glucagon sensitivity, Prolyl hydroxylase domain (PHD) enzymes, Insulin
Highlights
-
•
PHD inhibitor treatment improves glycemic control in obese glucose-intolerant mice.
-
•
PHD inhibitor treatment decreases liver glucagon sensitivity in obese mice.
-
•
The effects of PHD inhibition on glycemic control is hepatocyte HIF-2α-dependent.
-
•
PHD inhibitor treatment stimulates HIF-2α-dependent cAMP-specific PDE expression.
-
•
In human and mouse hepatocytes, PHD inhibitor treatment suppresses glucagon signaling.
1. Introduction
The global prevalence of type 2 diabetes mellitus (T2DM) is increasing rapidly and is now an epidemic. Therapeutic options for T2DM are available but cannot adequately to fully control this increasingly common disease. Moreover, except for thiazolidinediones, infrequently used because of potential side effects, there is no insulin-sensitizing anti-diabetes drug, even though insulin resistance is a principal etiologic cause of T2DM. One of the main actions of insulin is to suppress hepatic glucose production, and hepatic insulin resistance is a key feature of T2DM. Glucagon has the opposite effect and directly stimulates increases in hepatic glucose production, and insulin can counteract these hepatic effects of glucagon. Thus, postprandial increases in plasma insulin levels normally suppress pancreatic glucose secretion and inhibit hepatic glucagon action. In T2DM, hepatic insulin resistance combined with hyperglucagonemia is a key factor leading to the well-known increase in hepatic glucose production in this condition.
One approach to lowering plasma glucose levels is to antagonize glucagon action, and the success of this approach has been shown with various glucagon receptor antagonists in preclinical and clinical studies [[1], [2], [3], [4], [5], [6]]. For example, inhibition of the glucagon receptor normalizes blood glucose levels in streptozotocin-treated insulin-deficient mice [7]. Moreover, hepatocyte-specific deletion of the glucagon receptor has beneficial effects on glucose tolerance [8]. Most importantly, several early-stage clinical trials using different glucagon receptor antagonists have supported the concept that glucagon receptor inhibition improves glycemic control in T2DM patients [[3], [4], [5]].
Tissue oxygen tension is an important modulator of tissue metabolism, growth, and function. Cellular responses to hypoxia are largely mediated by HIFs [9]. HIFs are a group of heterodimeric transcription factors composed of oxygen-sensitive HIF-1α or HIF-2α subunits, which dimerize with constitutively expressed β subunits. Several lines of evidence have indicated that HIFs regulate glucose and lipid metabolism in normal and insulin-resistant states [[10], [11], [12], [13], [14], [15], [16]]. In obesity, expression of HIF-1α increases in adipocytes and hepatocytes, contributing to the development of insulin resistance and/or glucose intolerance [[10], [11], [12],15]. In normal mice, liver HIF-2α expression acutely increases after refeeding, and adipocyte and hepatocyte HIF-2α plays a protective role against obesity-induced glucose intolerance and insulin resistance [12,16,17]. Thus, deletion of adipocyte HIF-2α exaggerates obesity-induced increases in body weight, adipose tissue inflammation, and insulin resistance [12]. Moreover, liver-specific overexpression of a constitutively active form of HIF-2α improves glucose tolerance in db/db obese/diabetic mice by increasing Irs2 expression [17].
Cellular levels and activities of HIFα proteins are largely regulated by prolyl hydroxylase domain (PHD) enzymes [9]. Under normoxic conditions, PHD enzymes target HIFα proteins, leading to ubiquitin-dependent proteosomal degradation. In hypoxia, PHDs are inactivated, causing HIFα stabilization, leading to increased HIFα protein expression. In mice and humans, there are 3 PHD isoforms: PHD1, 2, and 3. Deletion of Phd1 or Phd2, or pharmacological inhibition of all 3 PHD isoforms, improves glucose tolerance and insulin resistance in high-fat diet (HFD)/obese mice [[18], [19], [20]]. However, the underlying mechanisms are not clearly understood.
In this study, we use a potent small molecule pan-PHD inhibitor compound (PHDi) and investigate its effects on in vivo glycemic control, as well as insulin and glucagon sensitivity in both mouse and human hepatocytes. Our results show that PHDi treatment can improve glycemic control by increasing insulin and decreasing glucagon sensitivity through induction of HIF-2α-dependent increases in Irs2 and cAMP-specific PDE gene expression in hepatocytes.
2. Materials and methods
2.1. Animals and treatments
Male C57BL6 mice were purchased from Jackson laboratory and used as WT mice. Hepatocyte-specific HIF-2α KO mice were generated by breeding Alb-Cre mice with Hif2afl/fl mice, as described in the literature [15]. To obtain relatively equal numbers of KO and WT littermates, breeding pairs were set up by mating Hif2afl/fl:Cre+/− and Hif2afl/fl:Cre−/− mice. All mice were on the C57BL6 background, male, and housed in colony cages in 12-hour light/12-hour dark cycles. At the age of 7 to 8-weeks, mice were subjected to 60% HFD (Research Diets Inc., catalog no. D12492) for 12 weeks. The PHDi compound (JNJ-42905343) was generated and provided by Janssen Pharmaceuticals, Inc. For the treatment studies, PHDi or vehicle (saline) was ip injected every day for 3 weeks or as indicated in figure legends. Glucose and glucagon tolerance tests were performed as described in the literature [12,15]. Briefly, for oral glucose tolerance tests (OGTTs), mice were fasted for 6 h and basal blood samples were taken, followed by oral glucose gavage (2 g/kg). Blood samples were collected at 10, 30, 60, 90, and 120 min after gavage to measure glucose and/or insulin levels. The calculation for HOMA-IR was based on the formula adjusted for mice: HOMA-IR = Fasting glucose level (mM) x fasting insulin level (mU/L)/22.5 [21]. For glucagon tolerance tests, mice were fasted for 6 h and intraperitoneally injected with glucagon (15 μg/kg). Blood glucose levels were measured at indicated time points. Mice were randomly allocated to groups, and WT and HIF-2α KO mice (littermates) were caged together.
2.2. Hyperinsulinemic euglycemic clamp studies
Hyperinsulinemic euglycemic clamp studies were performed as described in the literature [22]. Briefly, mice were surgerized for jugular vein cannulation. After 5 days of recovery, mice were fasted for 6 h and infused with D-[3H] glucose (PerkinElmer) for 60 min. After tracer equilibration, blood samples were collected at −10 and 0 min (basal). Glucose (50% dextrose) and tracer (5 μCi/h) plus insulin (8.0 mU/kg/min) were then infused into the jugular vein. Blood glucose levels were monitored every 10 min, and the glucose infusion rate was adjusted as necessary. Steady-state blood glucose levels were maintained at 120 ± 5 mg/dl for the last 20 min or longer, without changing the glucose infusion rate, and blood samples were collected at 110 and 120 min (clamped). Specific activity and insulin levels were measured from the basal and clamped plasma samples. Insulin-stimulated glucose disposal rate (IS-GDR) was calculated as follows: IS-GDR = glucose disposal rate (GDR; during the clamp) − basal GDR. Therefore, this value represents a measurement of the increase in GDR from the basal value (basal hepatic glucose production [HGP] = basal GDR = basal Ra) as a result of the insulin infused in the clamp. Because basal GDR is the same as basal HGP, the equation could also be IS-GDR = GDR (during the clamp) − basal HGP. We calculated total GDR during the clamp in the traditional manner as glucose infusion rate (GIR) + HGP (during the clamp).
2.3. Plasma protein measurements
Plasma insulin levels were measured by enzyme-linked immunosorbent assay (ELISA) in accordance with the manufacturer's instruction (ALPCO).
2.4. Liver lipid contents
Levels of tissue lipid contents were measured as described in the literature [22].
2.5. Primary hepatocyte isolation
Primary mouse hepatocytes were isolated as described in the literature [23]. Briefly, WT or HIF-2α KO mice were infused through the inferior vena cava with a calcium-free Hepes-phosphate buffer (pH 7.4) for 10 min followed by a collagenase solution (Liberase TM, Roche) for 10 min. The digested livers were excised, and hepatocytes were collected and washed 5 times in buffer by centrifuging at 70 g for 5 min. Cells were further purified by centrifugation (2,400 g for 10 min) over a Percoll density gradient (1.06 g/ml). Primary mouse hepatocytes were allowed to attach for 6 h on collagen-coated plates in Williams’ Medium E (Life Technologies, catalog no. 12551–032) fortified with nonessential amino acids, GlutaMAX (Life Technologies, catalog no. 35050–061), antibiotics, 10% fetal bovine serum, and dexamethasone (10 nM) and cultured overnight in the same medium without serum. Primary human hepatocytes were isolated and purified by using the collagenase perfusion method followed by centrifugation through 30% Percoll.
2.6. Intracellular cAMP levels
Intracellular cAMP levels were measured as described in the literature [23]. Briefly, hepatocytes were isolated, plated in 24-well plates, and pretreated with 10 μg/ml PHDi for 24 h. Cells were incubated with or without 50 ng/ml glucagon or 100 nM insulin for 7 min and subjected to cAMP assays using Bridge-It Cyclic AMP Designer Assay kit (catalog no.122934; Mediomics LLC) or cAMP direct immunoassay kit (catalog no. K371-100; Biovision) in accordance with the manufacturer's instructions except the addition of isobutyl methyl xanthine.
2.7. In vitro glucose production assay
Glucose production activities of primary hepatocytes were measured as described in the literature [23]. Briefly, cells were washed in Hepes phosphate-salt-bicarbonate buffer (10 mM Hepes, 4 mM KCl, 125 mM NaCl, 0.85 mM KH2PO4, 1.25 mM Na2HPO4, 1 mM MgCl2, 1 mM CaCl2, and 15 mM NaHCO3) containing 0.2% FFA-free bovine serum albumin (BSA) and incubated in the same buffer containing PHDi (10 μg/ml), insulin (10 nM), and/or glucagon (10 ng/ml) and substrates for 3 h in a 5% CO2 incubator. 14C-pyruvate (2 mM, 0.5 μCi pyruvate per incubation) was used as a substrate. Incubations were conducted in 0.5-ml buffer in 24-well plates containing 0.25 million cells per well. At the end of incubation, the buffer solutions were transferred to 1.7-ml microcentrifuge tubes and added with 0.25 ml of 5% ZnSO4 and 0.25 ml of 0.3 N Ba(OH)2 suspensions to each tube, followed by the addition of 0.5 ml of water. After centrifugation, supernatants were transferred to a fresh set of tubes and assayed for radiolabeled glucose released into the medium by separation of radiolabeled glucose by mixed-bed ion exchange resins, AG 501-X8 resins (Bio-Rad). Two hundred milligrams of resins was added to each tube, vortexed intermittently for 15 min, and centrifuged, and the supernatants were transferred to scintillation vials for counting radioactivity. Cells on the plates were dissolved in 1 N NaOH for protein estimation.
2.8. Irs2 knockdown in primary hepatocytes
To knockdown Irs2, Irs2–specific siRNAs (SMARTpool, Dharmacon) or non-specific negative control siRNAs were transfected into primary hepatocytes by using Lipofectamine RNA iMAX (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after isolation, cells were incubated for 6 h with Lipofectamine-siRNA complex, and the medium was replaced with a fresh medium (Williams' medium) before the cells were incubated for an additional 30 h. Thirty-six hours after transfection, cells were treated with or without a pan-PHD inhibitor for 16 h. Ten min before harvesting the cells, the cells were acutely treated with or without 100 nM insulin.
2.9. Western blot analysis
Tissues and cells were lysed with Mammalian Protein Extraction Reagent (Thermo Scientific) containing an EDTA-free protease and phosphatase inhibitor mixture (Roche Diagnostics) and then centrifuged at 13,000 r.p.m. for 10 min at 4 °C. The supernatants were separated in acrylamide gels (Bio-Rad) and electrotransferred to polyvinylidene difluoride membranes. The membranes were blocked for 2 h in Tris-buffered saline with Tween 20 (TBST; 10 mM Tris–HCl, 0.1% Tween 20, pH 7.4) containing 5% BSA and then incubated with antibodies to HIF-1α (Abcam, ab-2185), HIF-2α (Novus Biologicals, NB100-122), HSP90 (Santa Cruz Biotechnology, SC-13119), Akt (Cell Signaling Technology, 4685S), phosphorylated Akt (Ser473, Cell Signaling Technology, 4060S), CREB (Abcam, ab31387), and phosphorylated (Ser133) CREB (Cell Signaling Technology, 9198S), actin (Sigma–Aldrich, A2228), IRS-2 (Cell Signaling Technology, 4502S), FOXO1 (Cell Signaling Technology, 9454), and phosphorylated (Ser256) FOXO1 (Cell Signaling Technology, 9461S) at 4 °C overnight. After washing with fresh TBST, the membrane was incubated with secondary antibodies conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories; 1:5,000 dilution) and visualized by using the ECL system (Thermo Scientific) and then autoradiography or the Bio-Rad ChemiDoc XRS + imaging system. Intensity of the bands in the autoradiograms was measured by using Image J software. To assess the effect of PHDi treatment on PDE expression, isolated hepatocytes were plated in 6-well plates. After 24 h stabilization, the cells were treated with or without 1 or 10 ug/ml PHDi. After 24 h, the cells were washed with phosphate-buffered saline and harvested for Western blot analysis. Antibodies against PDE7 (ab14616), PDE4 (21754-1-AP), and PDE8 (13956-1-AP) were purchased from Abcam or Proteintech.
2.10. 2-Deoxy glucose uptake assays
Primary mouse adipocytes isolation was performed as described in the literature [24,25]. Briefly, mouse epididymal adipose tissue was minced and digested in collagenase buffer (1 mg/ml) for 30 min at 37 °C with shaking. The digests were filtered through 100 μm cell strainers and centrifuged at 500 g for 5 min. The floating adipocyte layer was saved and washed with fresh PBS 3 times before being subjected to glucose uptake assays. For glucose uptake assays, isolated primary mouse adipocytes were incubated in Hepes-Salt buffer (20 mM Hepes, 40 mM potassium chloride, 125 mM sodium chloride, 0.85 mM potassium phosphate monobasic, 1.25 mM sodium phosphate dibasic, 1 mM magnesium chloride, 1 mM calcium chloride, 0.1% fatty acid-free BSA) +/− 100 nM insulin for 20 min at 37 °C. The cells were then incubated with [14C] 2-deoxy-d-glucose at a final concentration of 3 μmol/L for 20 min. After 3 washings in ice-cold PBS, the cells were lysed in lysis buffer with 0.1% sodium dodecyl sulfate and subjected to scintillation counting to determine their 14C radioactivity. The protein concentrations were determined by using a bicinchoninic acid assay kit (Pierce Chemical Co., Rockford, IL), and the radioactivities were normalized by total protein contents.
2.11. Quantitative realtime RT-PCR and primer sequences
Total RNA was extracted by TRIzol reagent (Invitrogen) or RNeasy Mini Kit (QIAGEN). Synthesis of cDNA was performed by using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative realtime PCR (qRT-PCR) was performed by using Power SYBR Green PCR Master Mix (Applied Biosystems). Primer sequences are shown in Supplementary Table 1.
2.12. Study approval
All animal procedures were performed in accordance with an Institutional Animal Care and Use Committee–approved protocol and the research guidelines for the use of laboratory animals of the University of California San Diego. The preparation and the use of primary human hepatocytes were reviewed and approved by the Institutional Review Board of the University of California San Diego Human Research Protections Program.
2.13. Statistics
Statistical methods were not used to predetermine necessary sample size, but sample sizes were selected based on estimates from pilot experiments and published results such that appropriate statistical tests could yield significant results. Statistical analyses used in the data presented are justified and described in all legends. Parametric tests were used that assume normal distribution, which we showed to be the case when data were plotted as frequencies. Variances were tested by Levene's test for homogeneity of variance, and variances in the data were not significantly different. Experiments were not performed in a blinded fashion. The results are shown as means ± SEM. All statistical analysis was performed by 2-tailed Student's t test or ANOVA, unless indicated; P less than 0.05 was considered significant. A representative figure for each experiment is presented without combining of data from different batch experiments, unless indicated in the figure legend.
3. Results
3.1. PHD inhibition improves hyperglycemia by modulating hepatic insulin and glucagon sensitivity in obese mice
To investigate whether pharmacological increases of liver HIF-2α expression can improve glycemic control in obesity, we treated chronic (12 weeks) HFD/obese mice with a vehicle or a pan-PHD inhibitor (PHDi) at 1 or 3 mg/kg. PHDi treatment increased liver HIF-1α and HIF-2α expression (Figure 1A,B) along with mRNA expression of common target genes such as Glut1 and Pdk1 (Figure 1C). Moreover, mRNA and protein expression of an HIF-2α-specific target gene Irs2 was increased by PHDi treatment (Figure 1C,D). mRNA expression of another HIF-2α-specific target gene, Epo, was also increased in liver (Figure 1C) and kidney (Supplementary Fig. 1A) of PHDi-treated mice; this was accompanied by increased plasma Epo levels and hematocrit, compared with those of vehicle-treated mice (Supplementary Figs. 1B and 1C). Bodyweight and food intake were not affected by PHDi treatment (Figure 1E,F).
Figure 1.
Inhibition of PHDs improves glycemic control in obese mice. Twelve-week HFD male C57Bl6J mice were ip injected with 1 or 3 mg/kg PHDi every day. Fed glucose levels were measured at days 5 and 11, and OGTTs were performed at day 9. After 12 days of recovery, mice were sacrificed for tissue analysis. (A) Western blot analysis of liver HIF-1α expression (n = 4 mice per group). (B) Western blot analysis of liver HIF-2α expression (n = 4 mice per group). (C) mRNA expression of HIF target genes in the liver of vehicle or PHDi-treated mice (n = 4 mice per group). (D) Western blot analysis of liver IRS-2 expression (n = 4 mice per group). (E) Daily food intake. (F) Bodyweight at sacrifice. (G) Fed glucose levels at day 5. (H) Oral glucose tolerance tests at day 9. (I) Plasma insulin levels during OGTTs. (J) HOMA-IR insulin resistance index. n = 10 mice per group for panels E–J. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. vehicle by the 1-way or 2-way ANOVA with post hoc t tests between the individual groups in all panels.
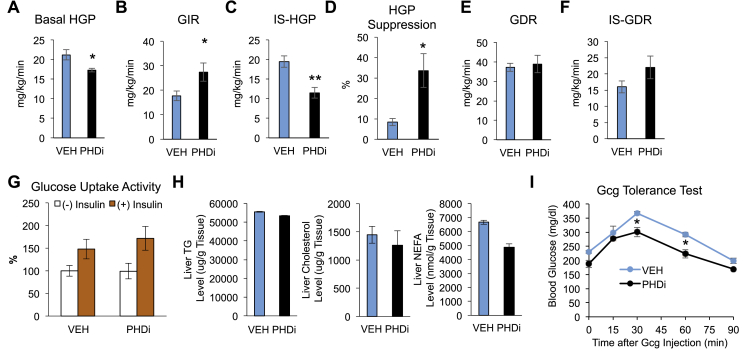
When measured at day 5, fed (ad libitum) blood glucose levels were decreased (Figure 1G). Moreover, glucose tolerance was significantly improved by 3 mg/kg PHDi treatment (Figure 1H), without changes in plasma insulin levels (Figure 1I). The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index was decreased in PHDi-treated mice compared with vehicle-treated mice (Figure 1J), suggesting that PHDi treatment increased insulin sensitivity. To quantitatively measure changes in in vivo glucose flux and insulin sensitivity by PHDi treatment, we performed hyperinsulinemic euglycemic clamp studies. Consistent with the view that fasting blood glucose levels are determined by basal HGP, basal HGP was reduced in PHDi-treated mice (Figure 2A). After insulin infusion, the GIR was increased by 54% in PHDi-treated mice compared with vehicle-treated mice, suggesting that PHDi treatment increased systemic insulin sensitivity (Figure 2B). These effects were attributed to decreased IS-HGP, indicating increased hepatic insulin action (IS-HGP; Figure 2C,D). GDR and IS-GDR were unchanged by PHDi treatment (Figure 2E,F). Consistent with this finding, basal and insulin-stimulated glucose uptake into adipocytes was unaltered by PHDi treatment in HFD/obese mice (Figure 2G). The insulin-sensitizing effect of PHDi treatment was not associated with changes in liver triglyceride, NEFA, or cholesterol accumulation in obese mice (Figure 2H).
Figure 2.
Inhibition of PHDs increases insulin and decreases glucagon sensitivity in obese insulin-resistant mice. (A–F) Hyperinsulinemic euglycemic clamp studies. C57BL6 male mice were fed HFD for 12 weeks and treated with PHDi for 9 days. Basal HGP (A), glucose infusion rate (GIR) (B), insulin-stimulated HGP (IS-HGP) (C), insulin-dependent suppression of HGP (D), glucose disposal rate (GDR) (E), and IS-GDR (F) were determined as described in Methods. n = 4 mice per group. (G) 2-deoxy glucose uptake assays in primary adipocytes isolated from HFD mice treated with vehicle (VEH) or PHDi. n = 6 mice per group. (H) Triglyceride (TG), cholesterol, and nonesterified fatty acid (NEFA) contents in liver of vehicle (VEH) or PHDi-treated mice. n = 4 mice per group. (I) Glucagon (Gcg) tolerance tests in HFD WT mice treated with vehicle (VEH) or PHDi for 9 days. n = 4 mice per group. ∗P < 0.05 and ∗∗P < 0.01 by the Student's t tests (A–F) or by the 2-way ANOVA with post hoc t tests between the individual groups (I).
Along with insulin resistance, hyperglucagonemia contributes to obesity-induced increased basal HGP [2]. Because basal HGP was decreased by PHDi treatment, we tested whether PHDi treatment also affected systemic glucagon action. In Figure 2I, the glucagon-stimulated glucose excursion was significantly decreased in PHDi-treated compared with vehicle-treated HFD/obese mice. Taken together, these results suggest that PHDi treatment improves glycemic control mainly by decreasing HGP because of decreased glucagon and increased insulin action.
3.2. PHD inhibition improves insulin resistance and suppresses glucagon action in hepatocytes
We tested whether the effects of in vitro PHDi treatment on liver insulin and glucagon sensitivity are mediated through hepatocytes. Incubation of primary hepatocytes from normal lean mice with PHDi increased the expression of HIF-1α and HIF-2α, as well as target gene mRNA expression exemplified by Vegfa and Pdk1 (Figure 3A–C). This induced a moderate but significant (P = 0.02) increase in Irs2 mRNA expression along with a robust increase in insulin-stimulated Akt phosphorylation (Figure 3C,D). Moreover, PHDi treatment increased insulin-stimulated inhibitory phosphorylation (Ser256) of a key gluconeogenic transcription factor, FOXO1 (Figure 3E), which can be mediated by activated Akt [26,27]. We also assessed whether PHDi treatment can improve in vitro insulin resistance. Twenty four hour palmitic acid (PA) treatment of hepatocytes induced insulin resistance with decreased insulin-stimulated Akt phosphorylation (Figure 3D). Additionally, consistent with the in vivo results, PHDi treatment increased insulin-stimulated Akt phosphorylation and IRS-2 expression in PA-treated cells (Figure 3D,F). To test whether IRS-2 is necessary for PHDi-induced increased insulin signaling, we measured the effect of PHDi treatment to increase insulin-stimulated Akt phosphorylation in mock or Irs2-specific siRNA-transfected hepatocytes. In Figure 3G, siRNA-mediated Irs2 knockdown blocked the effect of PHDi to increase insulin-stimulated Akt phosphorylation, indicating that IRS2 is necessary for the effect of PHDi to increase insulin sensitivity.
Figure 3.
Inhibition of PHDs increases insulin and decreases glucagon sensitivity in primary mouse hepatocytes. (A–C) Primary mouse hepatocytes were isolated from NCD/lean WT mice, treated with 10 μg/ml (or indicated doses of) PHDi for 24 h, and subjected to Western blot and qRT-PCR analyses for determining protein expression of HIF-1α (A) and HIF-2α (B) and mRNA expression of HIF target genes (C; n = 3 mice for vehicle and 4 mice for PHDi-treated groups). (D) Insulin-stimulated Akt phosphorylation. Primary WT mouse hepatocytes were treated with or without 500 μM PA, +/− 10 μg/ml PHDi for 24 h, and then stimulated with or without 100 nM insulin for 15 min. Total (tAkt) and phosphorylated (pAkt) Akt levels were determined by Western blots. Samples from 3 independent experiments were ran in a single gel, and the pAkt:tAkt ratio in each sample was calculated and graphed. (E) Western blot analysis of insulin-stimulated FOXO1 phosphorylation in primary hepatocytes treated with or without PHDi. Mouse primary hepatocytes were treated with 10 μg/ml PHDi for 24 h and then stimulated with insulin for 10 min. (F) HIF-1α, HIF-2α, and IRS-2 protein levels were determined by Western blots in primary WT mouse hepatocytes treated with or without 500 μM PA, +/− 10 μg/ml PHDi for 24 h. (G) Western blot analysis of insulin-stimulated Akt phosphorylation. Primary mouse hepatocytes transfected with mock or Irs2-specific siRNAs. Twenty-four hours after siRNA transfection, cells were treated with 500 μM palmitic acid (PA) + vehicle or PA + PHDi for an additional 24 h, followed by 10 min insulin simulation. (H) Intracellular cAMP levels. Primary WT mouse hepatocytes were pretreated with or without PHDi for 24 h. After washing, cells were stimulated with or without 50 ng/ml glucagon (Gcg) for 7 min and subjected to cAMP assays (n = 3 wells per group). AU, arbitrary unit. (I) Primary WT mouse hepatocytes were pretreated with or without 10 μg/ml PHDi for 24 h. Cells were washed and treated with or without glucagon for 7 min. Total (tCREB) and phosphorylated (pCREB) CREB levels were determined by Western blots. Samples from 3 independent experiments were ran in a single gel and the pCREB:tCREB ratios were calculated and graphed. (J) mRNA expression of Pepck and G6pase in primary hepatocytes treated with or without 10 μg/ml PHDi for 24 h. ∗P < 0.05 by the Student's t tests (F, J) or by the 1-way ANOVA with post hoc t tests between the individual groups (C, D, and H).
We also determined whether PHDi treatment suppresses glucagon signaling. Glucagon-stimulated intracellular 3′, 5′-cyclic adenosine monophosphate (cAMP) levels were decreased by PHDi treatment in the untreated and PA-treated WT hepatocytes (Figure 3H and Supplementary Fig. 2), suggesting that PHDi treatment suppresses glucagon signaling regardless of insulin sensitivity. Consistent with the cAMP levels, phosphorylated cyclic AMP response element-binding protein (pCREB) levels were decreased by PHDi treatment (Figure 3I). Moreover, mRNA expression of gluconeogenic genes such as G6pase and Pepck was decreased by PHDi treatment (Figure 3J). Taken together, these results suggest that inhibition of PHD activity can confer hepatocyte autonomous effects to decrease glucagon and increase insulin sensitivity.
3.3. HIF-2α mediates PHDi-induced Irs2 and phosphodiesterase expression in hepatocytes
To determine whether HIF-2α mediates the effects of PHDi to increase insulin and decrease glucagon sensitivity, we measured insulin-stimulated Akt phosphorylation and Irs2 expression in WT (Hif2afl/fl:Cre−) and HIF-2α KO (Hif2afl/fl:Cre+) hepatocytes treated with PA for 24 h. Figure 4A,B, in contrast to its effects in WT hepatocytes, PHDi treatment did not increase in Irs2 mRNA expression or insulin-stimulated Akt phosphorylation in HIF-2α KO hepatocytes. The effect of PHDi treatment to suppress glucagon-stimulated cAMP level was also blunted in HIF-2α KO hepatocytes (Figure 4C). These results suggest that HIF-2α mediates the effect of PHDi treatment on increasing insulin and decreasing glucagon sensitivity in hepatocytes.
Figure 4.
Hepatocyte HIF-2α mediates the effects of PHDi treatment to increase insulin and decrease glucagon sensitivity by inducing Irs2 and cAMP-specific PDE gene expression. (A) Western blot analysis of total (tAkt) and phosphorylated (pAkt) Akt levels in primary mouse hepatocytes. WT hepatocytes were pretreated with 500 μM PA, +/− PHDi for 24 h. After washing, cells were stimulated by 100 nM insulin for 15 min and subjected to the Western blots. (B) mRNA expression of Hif2a and its target genes in WT and HIF-2α KO hepatocytes treated with or without 10 μg/ml PHDi for 24 h. n = 3 mouse per group. (C) Intracellular cAMP levels. HIF-2α KO hepatocytes were treated with or without PHDi for 24 h. After washing, cells were stimulated with or without 50 ng/ml glucagon (Gcg) for 7 min and subjected to cAMP assays. AU, arbitrary unit. (D) mRNA expression of PDE genes in WT and HIF-2α KO hepatocytes treated with or without 10 μg/ml PHDi for 24 h (n = 2 wells per group). (E) Western blot analysis of cAMP-specific PDE isoforms in primary hepatocytes treated with or without 10 μg/ml PHDi for 24 h. (F–G) Intracellular cAMP levels. To knockdown Irs2, primary mouse hepatocytes were transfected with mock or Irs2-specific siRNAs. 36 h after transfection, cells were treated with vehicle or 10 μg/ml PHDi. 18 h later, cells were treated with PHDi and/or 200 μM IBMX for an additional 6 h, which was followed by 50 ng/ml glucagon (Gcg), +/− 100 nM insulin (Ins) stimulation for 7 min. In all panels, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001, VEH-WT vs. PHDi-WT (or as indicated). #<0.05, VEH-WT vs. VEH–HIF–2α KO. ¶P < 0.05 and ¶¶P < 0.01, PHDi-WT vs. PHDi-H2HKO. Statistical analysis was performed by the 1-way ANOVA with post hoc t tests between the individual groups in all panels.
Intracellular cAMP levels are tightly regulated by adenylate cyclase and phosphodiesterases (PDEs) [28]. Upon glucagon stimulation, Gαs-dependent activation of adenylate cyclase increases intracellular cAMP levels, which are rapidly degraded by PDEs. Because PHDi treatment reduced glucagon-stimulated intracellular cAMP levels in a HIF-2α-dependent manner, we tested whether PHDi treatment increases PDE gene expression. In Figure 4D, mRNA expression of several PDE isoforms, including cAMP-specific PDEs such as Pde4b, 4d, 7a, and 8a [28] were increased by PHDi treatment; among these, protein expression of PDE4 and 7 but not PDE8 was increased by PHDi treatment (Figure 4E). By contrast, PHDi treatment did not increase Pde4d, 7a, and 8a in HIF-2α KO hepatocytes (Figure 4D), suggesting that HIF-2α mediates PHDi-induced PDE expression. To test whether the increase in cAMP-specific PDE expression/activity is necessary for the effect of PHDi to decrease glucagon-stimulated cAMP levels, we measured cAMP levels in PHDi-treated hepatocytes in the presence or absence of the non-selective PDE inhibitor, isobutyl methyl xanthine (IBMX). IBMX blocks all PDE isoforms except PDE8 and 9 [29]. In Figure 4F, IBMX blocked the effect of PHDi to decrease glucagon-stimulated cAMP levels. Knockdown of Irs2 did not affect PHDi-induced decreased cAMP levels but did reduce insulin-dependent suppression of glucagon-stimulated increased cAMP levels (Figure 4G). These results suggest that PHDi increases insulin sensitivity and decreases glucagon sensitivity by inducing HIF-2α-dependent increases in Irs2, which are independent of IRS2, by increased PDE gene expression.
3.4. Hepatocyte HIF-2α mediates the beneficial effects of PHDi treatment on glycemic control
We next hypothesized that the effect of PHDi treatment to improve glucose tolerance is mainly mediated by hepatocyte-specific HIF-2α stabilization. To address this, we treated 12 week-HFD-fed hepatocyte-specific HIF-2α KO (H2HKO; Hif2afl/fl: Cre+/−) and Hif2afl/fl: Cre−/− (WT) control mice with PHDi and measured glucose and glucagon tolerance. Consistent with reports in the literature [15,16], glucose tolerance was comparable in vehicle-treated HFD HIF-2α KO mice and Cre- control mice (Figure 5A). In HFD Cre- control mice, fasting blood glucose levels were decreased and glucose tolerance was improved by PHDi treatment (Figure 5A). However, in HIF-2α KO mice, PHDi treatment did not improve glucose tolerance. Moreover, glucagon tolerance was not improved by PHDi treatment in HIF-2α KO mice (Figure 5B). PHDi treatment increased HIF-1α and HIF-2α expression in Cre- control mice and increased HIF-1α and not HIF-2α expression in HIF-2α KO mice (Figure 5C). Last, we tested whether obesity affects postprandial increases in liver HIF-2α expression. In Figure 5D, in contrast to NCD/lean mice, postprandial increases in liver HIF-2α expression was blunted in HFD/obese mice, suggesting that obesity suppresses physiological increases in liver HIF-2α expression necessary for hemostatic regulation of liver insulin and glucagon sensitivity. These results suggest that hepatocyte HIF-2α mediates the in vivo effects of PHDi treatment to improve glucose and glucagon tolerance in obese mice.
Figure 5.
Hepatocyte HIF-2α mediates the effects of PHDi treatment on improving glucose and glucagon tolerance in mice. (A) Glucose tolerance tests. Twelve week HFD WT and HIF-2α KO mice were treated with or without 3 mg/kg PHDi for 9 days (n = 4 VEH-WT, 4 PHDi-WT, 8 VEH–HIF–2α KO, and 8 PHDi–HIF–2α KO mice). ∗P < 0.05 VEH-WT vs. PDHi-WT. #P < 0.05 and ##P < 0.01 PHDi-WT vs. PHDi KO. (B) Glucagon tolerance tests. Twelve week HFD H2HKO mice were treated with vehicle or 3 mg/kg PHDi for 15 days (n = 5 and 4 mice per group). (C) Western blot analysis of HIF-1α and HIF-2α in the liver of WT and HIF-2α KO mice treated with or without 3 mg/kg PHDi for 3 weeks. (D) Western blot analysis of HIF-1α and HIF-2α in the liver of NCD and HFD WT mice after 24 h fasting, +/− 1 h refeeding. In all panels, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001, VEH-WT vs. PHDi-WT. #<0.05, VEH-WT vs. VEH–HIF–2α KO. ¶¶P < 0.01. N.S., not significant. Statistical analysis was performed by the Student's t tests (D) or the 1-way (A-right) or 2-way (A-left) ANOVA with post hoc t tests between the individual groups.
3.5. PHDi treatment suppresses glucagon-stimulated glucose production in normal and T2DM human hepatocytes
We also treated primary human hepatocytes with PHDi and measured insulin and glucagon sensitivity. PHDi treatment increased HIF-1α and HIF-2α and induced target gene expression including PDK1 and IRS2 (Figure 6A,B). IRS-2 protein levels were also significantly increased by PHDi treatment (Figure 6C). Consistent with our results in mouse hepatocytes, PHDi treatment suppressed glucagon-stimulated glucose production in normal human hepatocytes even in the absence of insulin (Figure 6D). Basal glucose production was not affected by PHDi or insulin in normal hepatocytes. In hepatocytes from a T2DM subject, PHDi treatment decreased basal glucose production, as well as glucagon-stimulated glucose production (Figure 6E). These effects were associated with decreased cAMP levels and increased expression of cAMP-specific PDE isoforms such as PDE4c, 7a, 8a, and 8b (Figure 6F,G). Glucagon-stimulated phosphorylated CREB levels were also decreased by PHDi treatment (Figure 6H; Supplementary Fig. 4). Furthermore, consistent with the results in mouse hepatocytes, insulin-stimulated Akt phosphorylation was also increased by PHDi treatment (Figure 6I). These results suggest that PHD-dependent regulation of hepatocyte glucagon and insulin sensitivity is well conserved in humans.
Figure 6.
Inhibition of PHDs induces increased Irs2 and cAMP-specific PDE expression, and increases insulin and decreases glucagon sensitivity in human hepatocytes. (A–C) Primary human hepatocytes isolated from 3 normal individual donors were treated with vehicle or 10 μg/ml PHDi for 24 h. Protein expression HIF-1α (A-upper), HIF-2α (A-lower), and IRS-2 (C) and mRNA expression of HIF target genes (B) were determined by Western blots and qRT-PCR analyses. n = 3 wells per group. ∗P < 0.05. (D–E) Gluconeogenic activities in normal human (D) and T2DM (E) hepatocytes. Hepatocytes were treated with vehicle or 10 μg/ml PHDi for 24 h and then incubated with or without 50 ng/ml glucagon (Gcg), +/− 10 nM Insulin (Ins) for 3 h. Glucose levels were measured in the media as described in Methods. n = 6–8 wells per group. For normal/nondiabetic hepatocyte experiments, we used hepatocytes from 2 individual donors, and the experiments with each individual donor hepatocytes were performed independently. Representative results are presented in panel D. For T2DM hepatocyte experiments, we tested PHDi effects in hepatocytes from one donor who was diagnosed for 6 years. BMI, gender, and age of this donor were 32.08, male, and 42 years, respectively. #P < 0.05 and ##P < 0.01 vs. Gcg-treated group. &P < 0.05 vs. Gcg + Ins group. (F) Intracellular cAMP levels. Primary human hepatocytes from the normal donor were treated with or without PHDi for 24 h. After washing, cells were stimulated with or without 50 ng/ml glucagon (Gcg), +/− 100 nM Insulin (Ins) for 7 min and subjected to cAMP assays. n = 3 wells per group. ∗P < 0.05. (G) mRNA expression of PDE genes in normal human hepatocytes with or without PHDi for 24 h. n = 3 wells per group. ∗P < 0.05 and ∗∗P < 0.01, vehicle vs. 10 μg/ml PHDi-treated groups. ¶P < 0.05, vehicle vs. 1 μg/ml PHDi-treated groups. §P < 0.05, vehicle vs. 0.1 μg/ml PHDi-treated groups. P < 0.05, vehicle vs. 0.1 μg/ml PHDi-treated groups. (H) Western blot analysis of total (tCREB) and phosphorylated (pCREB) CREB levels in normal human hepatocytes treated with vehicle or PHDi for 24 h. Similar results were obtained in 3 independent experiments with hepatocytes from 3 different individual donors. CREB phosphorylation was increased by 1 and 10 ng/ml glucagon (see also Supplementary Fig. 4). One hundred ng/ml glucagon did not induce increased CREB phosphorylation probably because of negative feedback inhibition. (I) Western blot analysis of basal and insulin-stimulated total (tAkt) and phosphorylated (pAkt) Akt levels in normal human hepatocytes treated with vehicle or PHDi for 24 h. Statistical analysis was performed by the Student's t test (B, C) or 1-way (D–H) ANOVA with post hoc t tests between the individual groups.
4. Discussion
Our studies show that inhibition of PHDs improves glycemic control in HFD/obese insulin-resistant mice by decreasing glucagon and increasing insulin sensitivity in the liver. Using the hyperinsulinemic euglycemic clamp technique, we revealed that PHDi treatment improved glycemic control by decreasing basal HGP and increasing hepatic insulin sensitivity. These effects of PHDi treatment were markedly attenuated in hepatocyte-specific HIF-2α KO mice, suggesting that hepatocyte HIF-2α largely mediates these effects. Ex vivo studies revealed that PHDi treatment stimulated HIF-2α-dependent increases in Irs2 and cAMP-specific PDE expression in hepatocytes. Consistent with this finding, PHDi treatment increased insulin-stimulated Akt and Foxo1 phosphorylation and decreased glucagon-stimulated cAMP levels and CREB phosphorylation. Knockdown of IRS2 blocked the effect of PHDi treatment to increase insulin-stimulated Akt phosphorylation. Moreover, PDE inhibitor treatment blocked the effect of PHDi to decrease glucagon-stimulated increased cAMP levels. This indicates that the effect of PHDi to increase insulin and decrease glucagon sensitivity is largely mediated by HIF-2α-dependent IRS2, as well as PDE expression. Notably, the effect of PHDi treatment to decrease cAMP levels occurred independent of insulin and was unchanged by IRS2 knockdown, suggesting that PHDi can partly suppress glucagon signaling independent of insulin or increased IRS2 expression. Our results provide a novel mechanism for how PHDs regulate hepatocyte glucose metabolism (Supplementary Fig. 5).
Notably, deletion of HIF-2α did not decrease basal levels of Irs2 but did block the PHDi-induced increase in Irs2 expression. These results suggest that HIF-2α is only responsible for inducible but not basal Irs2 expression. Consistent with this idea, other studies have shown that deletion of hepatocyte HIF-2α does not decrease insulin sensitivity in mice [15,16] and that overexpression of hepatocyte HIF-2α enhances insulin sensitivity [14,17]. Because decreased Irs2 expression is a feature of obesity-induced hepatic insulin resistance [30], our results suggest PHDi treatment reversed insulin resistance by increasing Irs2 expression.
We also found that PHDi treatment increased mRNA expression of several PDE isoforms in a HIF-2α-dependent mechanism. The literature reported that postprandial increases in hepatocyte HIF-2α stimulate PDE activity and block glucagon signaling [16]. However, the mechanism has not been defined. We found that HIF-2α regulates mRNA and protein expression of cAMP-specific PDE isoforms including PDE4 and 7, attenuating glucagon-induced cAMP and phosphorylated CREB levels. PHDi treatment increased cAMP-specific PDE expression in both mouse and human hepatocytes, suggesting that PHD-dependent PDE regulation through HIF-2α is an evolutionarily conserved mechanism. The literature reported that deletion of hepatocyte HIF-2α does not exacerbate obesity-induced glucagon intolerance in HFD/obese insulin resistance mice [15], although beneficial effects of increased hepatocyte HIF-2α expression on glycemic control have been reported [16]. These seemingly conflicting reports raised the possibility that obesity suppresses liver HIF-2α expression and/or activity. Consistent with this idea, we observed that the postprandial increase in liver HIF-2α expression was absent in HFD/obese mice. Our results suggest that this effect of obesity can be reversed by PHDi treatment.
Small molecule PHD inhibitors have been under development for use in the treatment of anemia in chronic kidney disease. Some of these compounds have shown positive results in early phase clinical trials with unexpected beneficial metabolic effects, including decreased low density lipoprotein (LDL) cholesterol levels [[31], [32], [33]]. Several lines of evidence in mice support this concept. For example, pharmacological inhibition of PHDs [18,19,34] or genetic deletion of PHD1 or PHD2 [19,20] attenuates HFD-induced weight gain and obesity-associated metabolic abnormalities, such as glucose intolerance, insulin resistance, and atherosclerosis in HFD WT or LDL receptor KO mice. Our study was designed to test whether inhibition of PHDs after the onset of obesity, insulin resistance, and glucose intolerance could treat these abnormalities. To avoid confounding effects of obesity, we initiated PHDi treatment after 12 weeks of HFD, when the increases in body weight had plateaued. We found that PHDi administration improved glucose tolerance without changing body weight or food intake, suggesting that PHDi treatment can reverse glucose intolerance and insulin resistance independent of effects on obesity.
Indeed, PHDs are multifunctional proteins. Besides HIF-αs, PHDs interact with some proteins, including GCN5, IKKβ, MAPK6, p53, and FOXO3a, and regulate their functions by hydroxylase activity-dependent and independent mechanisms [35,36]. For example, PHD3 suppresses IKKβ/NF-kB signaling in a mechanism not requiring its hydroxylase activity [36], and PHD-dependent regulation of HIF-αs is dependent of hydroxylase activity. Probably because of this multifunctional nature of the PHDs, although the beneficial metabolic effects of systemic PHD inhibition have been reported in several clinical and preclinical studies, the underlying mechanism remains unclear. In this study, by using a hyperinsulinemic euglycemic clamp technique, we found that PHD inhibition improved glycemic control mainly by targeting the liver: decreased HGP with increased insulin sensitivity and decreased glucagon sensitivity. By using hepatocyte-specific HIF-2α KO mice, we could also trace that these hepatic effects were mediated mainly by HIF-2α. Although the PHD–HIF–2α-Irs2 pathway was relatively well-defined in the regulation of liver insulin sensitivity [14,17], whether and how PHDs control hepatic glucagon action was unknown. Therefore, our results provide a novel mechanism by which systemic PHD inhibition improves glycemic control.
Although all PHD isoforms regulate HIF-α expression [37], the specificity of a PHD isoform to HIF-1α and HIF-2α can vary across organs or cell types [14,38,39]. In liver, PHD3 exhibits relative specificity to HIF-2α, and PHD2 is specific to HIF-1α. Because the beneficial effects of PHDi treatment were hepatocyte HIF-2α-dependent, a plausible speculation is that PHD3-selective inhibition would also exert positive glycemic effects with lower risks for potential adverse effects. For example, in contrast to the beneficial effects of hepatocyte HIF-2α on glycemic control, increased hepatocyte HIF-1α expression can promote glucose intolerance. Moreover, overexpression of HIF-1α alone or HIF-1α and HIF-2α in combination can induce liver steatosis in normal mice [14,40]. Although we did not observe further increases in liver lipid accumulation in obese mice after PHDi treatment, further research is required to address this issue.
In summary, we report that inhibition of PHDs can improve glucose tolerance in obese mice by reversing insulin resistance and suppressing hepatic glucagon action. These effects were independent of changes in body weight or food intake but were mediated by HIF-2α-dependent increases in liver Irs2 and cAMP-specific PDE expression. According to our review of the literature, we are the first to describe the effect of PHD inhibition on glucagon sensitivity and cAMP-specific PDE expression. Although we only tested the effect of PHDi in obese insulin-resistant mice, because the glucose-lowering effects of PHDi treatment were potentially independent of insulin, a possiblity is that PHDs can be targeted for a broader range of indications.
Author contributions
M.R. performed and analyzed in vivo mouse studies including the glucose and glucagon tolerance tests, hyperinsulinemic euglycemic clamp studies, and tissue analyses. J.-S.M. performed and analyzed in vitro insulin and glucagon signaling and effects in mouse and human hepatocytes for intracellular mechanism studies. G.K.B measured in vitro HGP. S.Y. measured plasma erythropoietin levels. K.L. and X.L. isolated primary human hepatocytes. T.K. and D.B. supported the study design and participated in the data discussion. Y.S.L. conceived and designed the study, supervised the project, analyzed and interpreted the data, and wrote the paper. All authors discussed the results and commented on the manuscript.
Acknowledgements
We thank Dr. Jerrold Olefsky, Thomas Gustafson, Jean Whaley, Joseph Hedrick, Raymond Patch, and Raul Camacho for their critical comments and discussions on this paper. This study was supported by a P&F grant from the University of California San Diego/University of California Los Angeles Diabetes Research Center (DK063491), a grant from the National Institutes of Health (NIH) (DK124298), UCSD Health Sciences Research Grant (RG084153), and a grant from the Janssen Pharmaceuticals. M.R. was supported by a postdoctoral fellowship from the American Heart Association (16POST29990015).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.101039.
Conflict of Interest
S.Y. was employed by Janssen Pharmaceuticals, Inc. T.K., D.B., and Y.S.L. have received research funding from Janssen Pharmaceuticals.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Figure 1. Subchronic PHDi administration increases serum Epo levels and hematocrit. (A) mRNA expression of Epo in the kidney of the mice treated with vehicle (VEH) or PHDi for 3 weeks (n=4 mice per group). (B) Serum Epo levels in vehicle or PHDi-treated mice for 8 days or 3 weeks (n=10 mice per group). (C) Hematocrit in vehicle or PHDi-treated mice (n=10 mice per group). ∗P<0.05 vs. vehicle-treated group. Figure 2. PHDi treatment suppresses basal and glucagon-stimulated cAMP levels in PA-treated insulin-resistant hepatocytes. Primary WT mouse hepatocytes were pretreated with 500 μM PA, +/- PHDi for 24h. After washing, cells were stimulated with or without 50 ng/ml glucagon (Gcg) for 7 min and subjected to cAMP assays (n=3 wells per group). AU, arbitrary unit. ∗P<0.05 and ∗∗P<0.01 by the 1-way ANOVA with post hoc t tests between the individual groups. Figure 3. Obesity suppresses postprandial increases in liver HIF-1α and HIF-2α expression. C57BL6J male mice were fasted for 18h and then separated into 2 groups. One group of mice were sacrificed promptly for liver harvest. The other group of mice were refed for 30 min and then sacrificed for liver harvest. To measure HIF-1α and HIF-2α expression the liver samples, Western blots were performed. (A) HIF-1α and 2α expression in NCD mice. (B) HIF-1α and 2α expression in HFD mice. Figure 4. Western blot analysis of total (tCREB) and phosphorylated (pCREB) CREB levels in normal human hepatocytes from 3 individual donors. One day after isolation, cells were treated with vehicle or PHDi, +/- 400 μM PA. After 24h, cells were stimulated by insulin for 10 min. Figure 5. Schematic representation of how PHD inhibitor treatment improves glycemic control in obese insulin-resistant mice. Our results suggest that obesity suppresses physiological increases (e.g., postprandial increases) in hepatic HIF-2α expression. Pharmacologically induced increased hepatocyte HIF-2α promotes cAMP-specific PDE isoforms and Irs2 expression, causing decreased hepatic glucagon and increased insulin signaling. This decreases HGP and improves glycemic control.
References
- 1.Lee Y.H., Wang M.Y., Yu X.X., Unger R.H. Glucagon is the key factor in the development of diabetes. Diabetologia. 2016;59(7):1372–1375. doi: 10.1007/s00125-016-3965-9. [DOI] [PubMed] [Google Scholar]
- 2.Unger R.H., Cherrington A.D. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation. 2012;122(1):4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pettus J., Reeds D., Cavaiola T.S., Boeder S., Levin M., Tobin G. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: a randomized controlled trial. Diabetes, Obesity and Metabolism. 2018;20(5):1302–1305. doi: 10.1111/dom.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly R.P., Garhyan P., Raddad E., Fu H., Lim C.N., Prince M.J. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes, Obesity and Metabolism. 2015;17(4):414–422. doi: 10.1111/dom.12446. [DOI] [PubMed] [Google Scholar]
- 5.Kazda C.M., Ding Y., Kelly R.P., Garhyan P., Shi C., Lim C.N. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care. 2016;39(7):1241–1249. doi: 10.2337/dc15-1643. [DOI] [PubMed] [Google Scholar]
- 6.Pearson M.J., Unger R.H., Holland W.L. Clinical trials, triumphs, and tribulations of glucagon receptor antagonists. Diabetes Care. 2016;39(7):1075–1077. doi: 10.2337/dci15-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M.Y., Yan H., Shi Z., Evans M.R., Yu X., Lee Y. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proceedings of the National Academy of Sciences of the U S A. 2015;112(8):2503–2508. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longuet C., Robledo A.M., Dean E.D., Dai C., Ali S., McGuinness I. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: evidence for a circulating alpha-cell growth factor. Diabetes. 2013;62(4):1196–1205. doi: 10.2337/db11-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palazon A., Goldrath A.W., Nizet V., Johnson R.S. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang C., Qu A., Matsubara T., Chanturiya T., Jou W., Gavrilova O. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60(10):2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K.Y., Gesta S., Boucher J., Wang X.L., Kahn C.R. The differential role of Hif1beta/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metabolism. 2011;14(4):491–503. doi: 10.1016/j.cmet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y.S., Kim J.W., Osborne O., Oh D.Y., Sasik R., Schenk S. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun K., Halberg N., Khan M., Magalang U.J., Scherer P.E. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Molecular and Cellular Biology. 2013;33(5):904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi C.M., Finger E.C., Krieg A.J., Wu C., Diep A.N., LaGory E.L. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nature Medicine. 2013;19(10):1325–1330. doi: 10.1038/nm.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y.S., Riopel M., Cabrales P., Bandyopadhyay G.K. Hepatocyte-specific HIF-1alpha ablation improves obesity-induced glucose intolerance by reducing first-pass GLP-1 degradation. Science Advance. 2019;5(7) doi: 10.1126/sciadv.aaw4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramakrishnan S.K., Zhang H., Takahashi S., Centofanti B., Periyasamy S., Weisz K. HIF2alpha is an essential molecular brake for postprandial hepatic glucagon response independent of insulin signaling. Cell Metabolism. 2016;23(3):505–516. doi: 10.1016/j.cmet.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei K., Piecewicz S.M., McGinnis L.M., Taniguchi C.M., Wiegand S.J., Anderson K. A liver Hif-2alpha-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nature Medicine. 2013;19(10):1331–1337. doi: 10.1038/nm.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito H., Tanaka T., Sugahara M., Tanaka S., Fukui K., Wakashima T. Inhibition of prolyl hydroxylase domain (PHD) by JTZ-951 reduces obesity-related diseases in the liver, white adipose tissue, and kidney in mice with a high-fat diet. Laboratory Investigation. 2019;99(8):1217–1232. doi: 10.1038/s41374-019-0239-4. [DOI] [PubMed] [Google Scholar]
- 19.Rahtu-Korpela L., Karsikas S., Horkko S., Blanco Sequeiros R., Lammentausta E., Makela K.A. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes. 2014;63(10):3324–3333. doi: 10.2337/db14-0472. [DOI] [PubMed] [Google Scholar]
- 20.Marsch E., Demandt J.A., Theelen T.L., Tullemans B.M., Wouters K., Boon M.R. Deficiency of the oxygen sensor prolyl hydroxylase 1 attenuates hypercholesterolaemia, atherosclerosis, and hyperglycaemia. European Heart Journal. 2016;37(39):2993–2997. doi: 10.1093/eurheartj/ehw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S., Muniyappa R., Yan X., Chen H., Yue L.Q., Hong E.G. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. American Journal of Physiology. Endocrinology and Metabolism. 2008;294(2):E261–E270. doi: 10.1152/ajpendo.00676.2007. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y.S., Li P., Huh J.Y., Hwang I.J., Lu M., Kim J.I. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60(10):2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riopel M., Seo J.B., Bandyopadhyay G.K., Li P., Wollam J., Chung H. Chronic fractalkine administration improves glucose tolerance and pancreatic endocrine function. Journal of Clinical Investigation. 2018;128(4):1458–1470. doi: 10.1172/JCI94330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y.S., Kim A.Y., Choi J.W., Kim M., Yasue S., Son H.J. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Molecular Endocrinology. 2008;22(9):2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo J.B., Riopel M., Cabrales P., Huh J.Y., Bandyopadhyay G.K., Andreyev A.Y. Knockdown of ANT2 reduces adipocyte hypoxia and improves insulin resistance in obesity. Nature Metabolism. 2018 doi: 10.1038/s42255-018-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong X.C., Copps K.D., Guo S., Li Y., Kollipara R., DePinho R.A. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metabolism. 2008;8(1):65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakae J., Kitamura T., Silver D.L., Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. Journal of Clinical Investigation. 2001;108(9):1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurice D.H., Ke H., Ahmad F., Wang Y., Chung J., Manganiello V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nature Reviews Drug Discovery. 2014;13(4):290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soderling S.H., Beavo J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Current Opinion in Cell Biology. 2000;12(2):174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T., Kubota N., Kadowaki T. Imbalanced insulin actions in obesity and type 2 diabetes: key mouse models of insulin signaling pathway. Cell Metabolism. 2017;25(4):797–810. doi: 10.1016/j.cmet.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Joharapurkar A.A., Pandya V.B., Patel V.J., Desai R.C., Jain M.R. Prolyl hydroxylase inhibitors: a breakthrough in the therapy of anemia associated with chronic diseases. Journal of Medicinal Chemistry. 2018;61(16):6964–6982. doi: 10.1021/acs.jmedchem.7b01686. [DOI] [PubMed] [Google Scholar]
- 32.Provenzano R., Besarab A., Sun C.H., Diamond S.A., Durham J.H., Cangiano J.L. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clinical Journal of the American Society of Nephrology. 2016;11(6):982–991. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson E., Demopoulos L., Haws T.F., Hu E., Fang Z., Mahar K.M. Short-term treatment with a novel HIF-prolyl hydroxylase inhibitor (GSK1278863) failed to improve measures of performance in subjects with claudication-limited peripheral artery disease. Vascular Medicine. 2014;19(6):473–482. doi: 10.1177/1358863X14557151. [DOI] [PubMed] [Google Scholar]
- 34.Rahtu-Korpela L., Maatta J., Dimova E.Y., Horkko S., Gylling H., Walkinshaw G. Hypoxia-inducible factor prolyl 4-hydroxylase-2 inhibition protects against development of atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(4):608–617. doi: 10.1161/ATVBAHA.115.307136. [DOI] [PubMed] [Google Scholar]
- 35.Strowitzki M.J., Cummins E.P., Taylor C.T. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: unique or ubiquitous? Cells. 2019;8(5) doi: 10.3390/cells8050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yano H., Sakai M., Matsukawa T., Yagi T., Naganuma T., Mitsushima M. PHD3 regulates glucose metabolism by suppressing stress-induced signalling and optimising gluconeogenesis and insulin signalling in hepatocytes. Scientific Reports. 2018;8(1):14290. doi: 10.1038/s41598-018-32575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myllyharju J., Koivunen P. Hypoxia-inducible factor prolyl 4-hydroxylases: common and specific roles. Biological Chemistry. 2013;394(4):435–448. doi: 10.1515/hsz-2012-0328. [DOI] [PubMed] [Google Scholar]
- 38.Matsuura H., Ichiki T., Inoue E., Nomura M., Miyazaki R., Hashimoto T. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation. 2013;127(21):2078–2087. doi: 10.1161/CIRCULATIONAHA.113.001742. [DOI] [PubMed] [Google Scholar]
- 39.Kim M., Neinast M.D., Frank A.P., Sun K., Park J., Zehr J.A. ERalpha upregulates Phd3 to ameliorate HIF-1 induced fibrosis and inflammation in adipose tissue. Molecular Metabolism. 2014;3(6):642–651. doi: 10.1016/j.molmet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nath B., Levin I., Csak T., Petrasek J., Mueller C., Kodys K. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53(5):1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. Subchronic PHDi administration increases serum Epo levels and hematocrit. (A) mRNA expression of Epo in the kidney of the mice treated with vehicle (VEH) or PHDi for 3 weeks (n=4 mice per group). (B) Serum Epo levels in vehicle or PHDi-treated mice for 8 days or 3 weeks (n=10 mice per group). (C) Hematocrit in vehicle or PHDi-treated mice (n=10 mice per group). ∗P<0.05 vs. vehicle-treated group. Figure 2. PHDi treatment suppresses basal and glucagon-stimulated cAMP levels in PA-treated insulin-resistant hepatocytes. Primary WT mouse hepatocytes were pretreated with 500 μM PA, +/- PHDi for 24h. After washing, cells were stimulated with or without 50 ng/ml glucagon (Gcg) for 7 min and subjected to cAMP assays (n=3 wells per group). AU, arbitrary unit. ∗P<0.05 and ∗∗P<0.01 by the 1-way ANOVA with post hoc t tests between the individual groups. Figure 3. Obesity suppresses postprandial increases in liver HIF-1α and HIF-2α expression. C57BL6J male mice were fasted for 18h and then separated into 2 groups. One group of mice were sacrificed promptly for liver harvest. The other group of mice were refed for 30 min and then sacrificed for liver harvest. To measure HIF-1α and HIF-2α expression the liver samples, Western blots were performed. (A) HIF-1α and 2α expression in NCD mice. (B) HIF-1α and 2α expression in HFD mice. Figure 4. Western blot analysis of total (tCREB) and phosphorylated (pCREB) CREB levels in normal human hepatocytes from 3 individual donors. One day after isolation, cells were treated with vehicle or PHDi, +/- 400 μM PA. After 24h, cells were stimulated by insulin for 10 min. Figure 5. Schematic representation of how PHD inhibitor treatment improves glycemic control in obese insulin-resistant mice. Our results suggest that obesity suppresses physiological increases (e.g., postprandial increases) in hepatic HIF-2α expression. Pharmacologically induced increased hepatocyte HIF-2α promotes cAMP-specific PDE isoforms and Irs2 expression, causing decreased hepatic glucagon and increased insulin signaling. This decreases HGP and improves glycemic control.