Abstract
Polycystic ovary syndrome (PCOS) continues to be one of the most complex reproductive and endocrine disorder among women of reproductive age. Recent reports have identified close interaction of Vitamin D deficiency and oxidative stress (OS) in exacerbating the pathophysiology of PCOS. This current study aims at assessing the combine effect of MitoQ10 and Vitamin D3 on dehydroepiandrosterone (DHEA) induced PCOS. Following successful induction of PCOS using DHEA, mice were organized into five groups (n = 8) namely: Negative Control (NC), Vitamin D3 Vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and DHEA, ethanol and distilled water, Vitamin D3, MitoQ10 and Vitamin D3 plus MitoQ10 were respectively administered for 20 consecutive days. The study also included positive control (PC) group (n = 8) in which no treatment was applied. Treatment effects were assessed using hormonal assays, biochemical assays, Real-Time PCR, western blotting and histological analysis. Combination of Vitamin D3 and MitoQ10 significantly reduced levels of estradiol, progesterone, FSH, LH, LH/FSH, SOD and MDA. The expression rate of mRNAs of 3β-HSD, Cyp19a1, Cyp11a1, StAR, Keap1, HO-1 and Nrf2 were also significantly low in V+M group. Moreover, the histomorphological inspection of ovaries from this group revealed many healthy follicles at various stages of development including few atretic follicles, pre-antral and antral follicles and many corpora lutea. The characteristics observed in this group were in many ways similar to that of the PC group. The combination of MitoQ10 and Vitamin D3 may be potential candidate to ameliorate PCOS.
Keywords: Biological sciences, Health sciences, Anatomy, Reproductive system, Endocrinology, Polycystic ovary syndrome (PCOS), Dehydroepiandrosterone (DHA), Vitamin D3, MitoQ10, Oxidative stress (OS), Steroidogenesis
Biological sciences; Health sciences; Anatomy; Reproductive system; Endocrinology; Polycystic ovary syndrome (PCOS); Dehydroepiandrosterone (DHA); Vitamin D3; mitoQ10, Oxidative stress (OS); Steroidogenesis
1. Introduction
PCOS is a complex endocrine and reproductive disorder affecting women of reproductive age which is primarily characterized by reproductive hormones dysregulation, polycystic ovarian morphology, anovulation, amenorrhea, infertility as well as many other conditions [1]. The etiology and pathophysiology of this disease remain a puzzle. However, most commonly discussed causes are insulin resistance and hyperinsulinemia, hyperandrogenemia and genetic factors [2].
Recent studies have shown that Vitamin D deficiency and oxidative stress (OS) and its consequence of low-grade inflammation may play a significant role in pathogenesis of PCOS. OS is an imbalance between oxidants and antioxidants resulting in an abnormal redox state of cell. OS has been found to be closely linked with hyperandrogenemia, insulin resistance and its consequence of hyperinsulinemia [3]. Many investigations have revealed that, OS markers such as malondialdehyde (MDA), Nitric oxide (NO) and Xanthine oxidase (XO) levels are significantly higher in patients with PCOS compared with the normal [4]. In accordance, enzymatic antioxidants including superoxide dismutase (SOD), catalase and Glutathione peroxidase (GPx) have also be found to be higher in serum of women with PCOS as compared with the normal [3]. High rate of expression of OS related genes such as nuclear factor erythroid 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1) heme-oxygenase-1(HO-1) has been reported PCOS animal model as well.
Vitamin D (ergocalciferol-D2, cholecalciferol-D3) is a fat-soluble vitamin that promote calcium and phosphorus absorption. Vitamin D3 deficiency is common in PCOS patients, with 67–85% of the patients having serum concentrations of 25-hydroxy Vitamin D3 (25OHD) less than 20 ng/ml [5, 6, 7]. Vitamin D deficiency may exacerbate symptoms of PCOS, with observational studies showing lower 25OHD levels associated with insulin resistance, ovulatory and menstrual irregularities, lower pregnancy success, hirsutism, hyperandrogenism, obesity and elevated CVD risk factors [8]. Vitamin D deficiency has long time been identified as critical factors in the pathophysiology of PCOS and many studies have been done to assess its therapeutic effect on the disorder but the results are inconsistent, possibly due to interplay with other factors. Meanwhile Vitamin D deficiency has been reported to have strong associated with OS and low grade inflammation in PCOS patient [9]. Thus in addressing Vitamin D deficiency in PCOS, the effect of OS must be taken into consideration.
MitoQ10 is a modified form of ubiquinone CoQ10 by conjugating it with lipophilic triphenylphosphonium cation (TPP+) that target mitochondria oxidative stress [10]. Magwere et al showed that MitoQ10 prolongs life span of SOD-impaired patients and improves pathologies associated with antioxidant deficiency [11]. Although, many studies have attested the efficacy of MitoQ10 in managing oxidative stress related disorders but result of MitoQ10 as therapeutic candidate of PCOS is scanty and as far as we know the effect of MitoQ10 and Vitamin D3 on PCOS has not been investigated.
Due to the strong association of OS and Vitamin D deficiency with the pathogenesis of PCOS, We therefore proposed that MitoQ10 and Vitamin D3 could be potential therapeutic candidates for the management of PCOS.
Several methods are used to create animal model for PCOS including prenatal or postnatal exposure to high dose of androgens such as testosterone (T), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA) and androstenedione (A4). However postnatal treatment of mice and rats with subcutaneous injections of DHEA for 15–20 days offer less invasive and sufficient means to induce PCOS [12]. Lee et al concluded that, postnatal treatment of rodents with DHEA induced almost all human PCOS characteristics and therefore offers one of the best methods in creating PCOS animal model [13].
Thus, this study employs DHEA for inducing PCOS mouse model followed by administration of MitoQ10 and Vitamin D3. The effects of these treatments are assessed by analysis of serum hormones (estradiol, progesterone, FSH, LH and LH/FSH ratio) levels in addition to OS markers (SOD and MDA) levels. Moreover, the effects of these treatments are also assessed by histomorphological analysis of ovaries as well as mRNA expression rate of steroidogenic and OS related genes including P450 side-chain cleavage enzyme (P450scc or Cyp11a1), 3β-hydroxysteroid dehydrogenase (3β-HSD), cytochrome P450 aromatase (Cyp19a1), steroidogenic acute regulatory protein (StAR), Nrf2, Keap1 and HO-1.
2. Materials and methods
All the animal handlings and procedures in this study were carried out in strict accordance with the 8th edition of the “Guide for the Care and Use of Laboratory Animals” The protocol for this study was evaluated and approved by Institutional Research Ethics Committee, Vice Chancellor in Research Affairs (Tehran University of Medical Sciences) with ethical certificate ID of IR.TUMS.VCR.REC.198.085.
This present study was conducted in two stages. The first stage was induction and verification of PCOS models and the second stage involved administration and assessment of the effect of MitoQ10 and Vitamin D3 on the induced PCOS model.
2.1. Induction of PCOS experimental animal model
Forty eight (48) females of 25-days old (12–13g) balb/C mice (in 6 sub-groups of 8 mice each) were injected with DHEA (6mg/100g body weight dissolved in 0.01ml 95% ethanol and mixed with 0.09ml sesame oil) for 20 continuous days (Dehydroepiandrosterone group) as reported in earlier study [14]. Eight female mice of the same age and weight were injected with 0.09ml sesame oil mixed with in 0.01 ml 95% ethanol for 20 continuous days (Vehicle group) while another 16 more balb/C mice (in 2 sub-groups of 8 mice each) of same age and weight range were not given any treatment (Control group). The animals were kept in a temperature (25 ± 1 °C) and illumination (12 h light and 12 h dark) controlled facility with free access to food and water. Daily inspection and weighing were performed throughout the experimental period in order to observe changes in weight. The estrous cycle was determined daily by vagina smear starting from 5th day after commencing the injection of DHEA. After 20 days, 8 mice (45 days old) were randomly selected from each of the DHEA and Control sub groups, and all the 8 mice in Vehicle group were anesthetized and sacrificed by cervical dislocation. Blood sera were collected and analyzed to determine the amount of estradiol, progesterone, LH and FSH. Six ovaries from each group were fixed in 10% formalin until later histological preparations. The remaining ovarian samples were cleared up in HBSS culture medium, washed in ice cold PBS and kept at -80 °C (New Brunswick Scientific U410-86, England) until further analysis.
2.1.1. Assessment of estrous cycle
The vaginal opening was flushed about 10 times with pipette containing double deionized water (ddH2O) to obtain the epithelia cells. The final flush was transferred onto glass slide and allowed to completely dry at room temperature. The smear was stained with crystal violet for 1 min and washed twice (1 min each) in dH2O and observed with microscope (Olympus IX71) equipped with camera (Olympus E-30). The identification of leukocytes, nucleated epithelial and cornified epithelial cells, was used as markers to determine the stage of estrous (proestrus, estrous, metestrus, or diestrus) cycle.
2.1.2. Histmorphological assessments of ovary
Six ovaries from each group (DHEA, Vehicle and Control) of 45-day old mice were fixed overnight at 4 °C using fresh 10% formalin. The ovarian tissues were dehydrated (with graded series of alcohol), embedded (in paraffin wax) and sectioned (5μm). The sections were then mounted on glass microscope slide deparaffinized using xylene and rehydrated in a graded series of alcohol. The tissues on the slide were then stained with hematoxylin and eosin and covered with cover slip by using resin as adhesive. The prepared slides were then examined to observe histomorphological features using microscope (Olympus IX71) equipped with camera (Olympus E-30).
2.1.3. Hormonal assay
Blood samples were collected from the mice in all the groups (Dehydroepiandrosterone, Vehicle and Control) by cardiac puncture immediately after cervical dislocation. The blood was put into 2 ml tubes to clot and centrifuged at 2500 rpm for 10 min. The serum was collected and froze (New Brunswick Scientific U410-86, England) until the next day when estrogen, progesterone, LH, and FSH concentrations were determined by using specific Chemiluminescence Immunoassay (CLIA) kit (Abnova, Taiwan) strictly according to the manufacturer's instructions.
2.2. Administration of Vitamin D3 and MitoQ10
After confirmation of PCOS in DHEA treated mice based on virginal smear, hormonal (estradiol, progesterone, LH, FSH and FH/FSH ratio) levels and ovarian histomorphological results, the remaining DHEA treated mice were organized into 5 groups of 8 mice each. The groups are Negative control (NC), MitoQ10 (MQ), Vitamin D3 (VD), Vitamin D3 vehicle (VDV) and Vitamin D3 plus MitoQ10 (V+M). The NC group continued to receive subcutaneous injection of injection of DHEA (6mg/100g body weight dissolved in 95% ethanol and mixed with 0.09ml sesame oil) for 20 days. The VD group, received daily intraperitoneal (IP) injection of Vitamin D3 (Iran Hormone and Pharmaceutical Co. Iran) of 1 mg/kg body weight (as previously reported [15]) dissolved in 98% ethanol and distilled water and at a dose of 100μl. In the VDV group, 98% ethanol mixed distilled water at a dose of 100μl was injected daily via IP for 20 days. The MQ group mice were given 250μM (Sigma Aldrich, USA) of MitoQ10 as the only source of drinking water for 20 days as previously stated [16]. Mice in V+M group received daily IP injection of Vitamin D3 (1 mg/kg body weight) dissolved in 98% ethanol and distilled water at a dose of 100 μl for 20 days while concurrently given 250μM of MitoQ10 as the only source of drinking water. The remaining 8 mice in the Control group during induction of PCOS model were then labeled as Positive control (PC) and received no treatment. The animals were continuously kept in the facility with the same conditions as stated earlier. In order to keep tract, each mouse was given unique permeant identification mark. Daily weighing assessment and virginal smear were continuously performed.
After 20 days of Vitamin D3 and MitoQ10 treatments, all the 8 mice (70 days old) in each group were anesthetized and sacrificed by cervical dislocation. Blood serum and ovarian samples were collected and stored at -80 °C until further analysis. The collected sera were later analyzed to determine the amount of LH, FSH, estradiol, and progesterone by using specific CLIA kit according to the manufacture's instruction as stated earlier. In addition, the blood sera of all the 8 mice in each group were also subjected to biochemical analysis to determine the level of MDA SOD and protein concentration by using specific kits (Pazhouhan Razi, Iran).
To assess histomorphological features, 5 ovaries from each group were fixed in 10% formalin and later subjected to routine histological preparation as already described. Follicles were identified and counted based on their mean diameters, which were determined by two perpendicular diameters in the histological sections in which the nucleolus of the oocyte is present. Follicle with diameter less than 100μm were counted as pre antral follicles while those with diameter above 100μm with antrum were considered as antral follicles. Follicles with lose and thin granulosa cell layer and degenerative oocyte were identified as atretic. Follicular cysts were characterized with thin wall lined with flattened or cuboidal granulosa cells, absence of oocyte and large amount of follicular space (fluid). The remaining ovaries in each group were quickly cleared up in HBSS culture medium, washed in ice cold PBS and kept at -80 °C for western blotting, real time PCR and biochemical analysis.
To assess mRNA expressing of steroidogenic genes (Cyp 11a1, Cyp 19a1, 3β-HSD and StAR), the Control, Vehicle, Dehydroepiandrosterone, NC, VDV, VD, MQ, V+M as well as PC groups, RNA extraction (from whole ovarian tissue), cDNA synthesis, and RT-PCR were carried out. In addition, the expression of mRNA of Keap 1, Nrf2 and HO-1 in ovarian tissue were determined in NC, VDV, VD, MQ, V+M and PC groups. Moreover, western blotting analysis was also carried out to determine the rate of enzyme expression of Cyp 19a1 and 3β-HSD genes in the ovaries of Control, Vehicle, Dehydroepiandrosterone, NC, VDV, VD, MQ, V+M well as PC groups while the ovarian enzyme expression of HO-1 and Nrf2 were determined only in the NC, VDV, VD, MQ, V+M and PC groups.
2.2.1. RNA extraction, cDNA synthesis and real-time PCR
Five ovaries from each group (Control, Vehicle, Dehydroepiandrosterone, NC, VDV, VD, MQ, V+M well as PC) were used for RNA extraction. The RNA extraction was carried out by using BioFact RNA extraction kit according to the manufacture's instruction. Briefly: The ovary tissues were grinded in liquid nitrogen using sterilized mortar and pistil and transferred into 1.5ml tube. Nine hundred microliters of lysis buffer was added to the sample and vortex for 20sec followed by 8 min of incubation at room temperature. Four hundred microliter of chloroform was added, vortexed for 10s incubated at room temperature for 2 min followed by 5 min of centrifugation at 1300rpm. About 500 μl of the upper phase was transferred to new tube and 250 μl of isopropanol was added. The solution was then subjected to series of centrifugation and addition of RW and the column was transferred to 1.5μl tube. Forty microliters of diethylpyrocarbonate (DEPC)-treated water was added, incubated for 3 min at room temperature and centrifuged at 11000rpm for 1min. The extracted RNA was analyzed for its concentration and purity using spectrophotometer and stored at -80 °C until further analysis.
The extracted RNA was treated with DNase to get rid of any DNA that might be still present. cDNA was synthesized using BioFact cDNA synthesis kit strictly according to the manufacturer's instructions. Briefly: 500 ng/μl RNA (amount based on extracted RNA concentration), 1 μl of oligo dt primer, 10 μl of 2x RT pre-mix were mixed together. The total volume of the mixture was amounted to 20 μl by addition of diethylpyrocarbonate (DEPC)-treated water. The cDNA was synthesized by conventional PCR machine (Corbett Research CG1-96, Australia) programmed to run with incubations of 5 min room temperature, heating the mixture for 30 min at 50 °C and inactivation of RTase at 95 °C for 5 min. For quantitative PCR, cDNA of Cyp19a1, 3β-HSD, Cyp11a1, StAR, Keap 1, Nrf2, NO-1 and β-actin genes, were mixed with SYBR Premix EX TaqTM, primers of each gene and ROXTM Reference Dye, and amplified using a StepOne TM Real-Time PCR System. The primers were designed using the allele ID software and NCBI gene database. Primers were verified and optimized to ensure their high level of accuracy and specificity before using in RT-PCR. Details of the primers are given in Table 1. The Real-Time PCR results were analyzed using delta-CT values to estimate the amount of mRNA.
Table 1.
Primers used for real-time PCR analysis.
| Gene | Forward sequence (5′-3′) | Reverse sequence (5′-3′) | Tm (°C) |
|---|---|---|---|
| CYP 11A1 | GCGGTTCATCAATGCTGTCTAC | AGGTCTTAGTTCTGAGGAGTCG | 73.9 |
| CYP 19A1 | TGGACGAAAGTGCTATTGTGAAG | AGATGTTTGGTTTGATGAGGAGAG | 69.1 |
| 3β-ΒHSD2 | AGGGAGACACTGGACACAAAG | TTGCTGAAGGACTTGATTACACC | 72.3 |
| StAR | GACTAAACTCACTTGGCTGCTC | TGGTTGGCGAACTCTATCTGG | 74.6 |
| Nrf2 | GAGGTCACCACAACACGAAC | ATCTCATAAGGCCCCACCTC | 60.5 |
| Keap1 | CTGCCCAATTCATGGCTCACA | CTTAGGGTGGATGCCTTCGAT | 61.3 |
| HO-1 | CACGCATATACCCGCTACCT | CCAGAGTGTTCATTCGAGC | 53.3 |
| β-actin | CAGCCTTCCTTCTTGGGTATGG | AGAGGTCTTTACGGATGTCAACG | 56.0 |
2.2.2. Western blot
Protocol for western blotting was according to the method previously described by Bakshilazedeh et al. [14] Briefly: protein was extracted from 4 ovaries of mice in all the groups. The protein extraction was done by using 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, lysis buffer (50 mM Tris–HCl [pH 7.4]), 150 mMNaCl, 1 mM PMSF, 1 mM sodium fluoride, 1% NP-40, 1mM EDTA and 1 mM sodium orthovanadate (Na3VO4) supplemented with phosphatase and protease inhibitor cocktail. The protein concentration was determined by using Bio-Rad Protein Assay. Twenty micrograms (20-μg) of protein was loaded into each lane to be separated electrophoretically by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto polyvinylidene difluoride (PVDF). Five percent non-fat dried milk was used block the membrane for 1 h at room temperature to enhance specific binding. PVDF membrane was incubated with primary antibodies (Santa Cruz Biotechnology, Inc, USA), a mouse monoclonal antibodies (at final dilution of 1:1000) to Cyp19a1, 3β-HSD, HO-1and Nrf2 in TBST (containing 5% non-fat dried milk powder) for overnight at 4 °C. After three times of 20 min washing with TBST, the membrane was incubated with secondary antibody (Santa Cruz Biotechnology, Inc, USA), a horseradish peroxidase [HRP]-conjugated anti-mouse antibodies (at final dilution of 1:500) in TBST with 5% non-fat milk powder in room temperature for 1 h. Subsequently, the incubated membrane was washed with TBST (three times for 20 min) and incubated with substrate (ABTS). Immunoreactivity and signals were monitored by chemiluminescence autoradiography. Quantification of protein bands was finally carried out using NIH image J Software.
2.3. Statistical analysis
Statistical analyses were performed using SPSS software (version 22.0 for Windows; SPSS Inc. Chicago, IL, USA) and Microsoft Excel for windows (version 2016). Graphs were plotted using GraphPad Prism (version 8.0.1, La Jolla, CA, USA). For evaluating the statistical significance of differences between reference group and other groups, Student's t test or one-way analysis of variance (ANOVA) with Tukey's post hoc test were used. Unless otherwise indicated, Control (CONT) and Negative Control (NC) were selected as reference groups in the model induction and treatment groups respectively. Data are expressed as mean ± SD. P value less than 0.05 was considered statistically significant.
3. Results
3.1. Determination of estrus cycle
Daily assessment of estrous cycle (proestrous, estrous, metestrus and diestrus) were made by staining vaginal smears with crystal violet and observed under camera-equipped microscope. Many nucleated epithelia cells and cornified epithelia cells respectively characterized the proestrus and estrous stages. In the metestrus stage, the nucleated epithelia cells were relatively in smaller amount as compared with the cornified epithelia cells and leucocytes. However, in the diestrus stage, predominant cells were leukocytes. All the four stages of estrus cycles were observed in the Control and Vehicle groups while in DHEA treated group, the cycle was halted at estrus stage (Figure 1). The estrus cycle assessment continued during period of Vitamin D3 and MitoQ10 administration. It was observed that, the mice in NC and VDV groups continued to have estrous cycle arrested at estrous stage while in VD, MQ, V+M and PC groups, all the four stages were observed (Figure 2). Interestingly, 75% and 87.5 % of mice in MQ and VD groups respectively had a complete recovery of estrus cycle. However, in V+Q group, all the mice had complete estrus cycle recovery (Table 2).
Figure 1.
Vaginal smear of DHEA, Control and Vehicle groups showing A (proestrus), B (estrus), C (metestrus) and D (diestrus). Nucleated epithelia cells (white arrow), cornified epithelia cells (red arrow) and leukocytes (yellow arrow) were observed (crystal violet stain, X100).
Figure 2.
Vaginal smear of Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups showing A (proestrus), B (estrus), C (metestrus) and D (diestrus). Nucleated epithelia cells (white arrow), cornified epithelia cells (red arrow) and leukocytes (yellow arrow) were observed (crystal violet stain, X100).
Table 2.
Percentage of mice that restored normal estrus cycle among Negative control (NC), MitoQ10 (MQ), Vitamin D3 (VD), Vitamin D3 vehicle (VDV), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups.
| Groups | Total number of mice | Total number of mice that restored estrous cycle | Percentage of estrous cycle restoration |
|---|---|---|---|
| Negative Control (NC) | 8 | 0 | 0.0% |
| Vitamin D3 Vehicle (VDV) | 8 | 0 | 0.0% |
| Vitamin D3 (VD) | 8 | 7 | 85.5% |
| MitoQ10 (MQ) | 8 | 6 | 75.0% |
| Vitamin D3 and MitoQ10 (V+M) | 8 | 8 | 100.0% |
| Positive Control (PC) | 8 | 8 | 100.0% |
3.2. Hormonal assay
Blood sera collected from mice in DHEA, Control and Vehicle groups were analyzed using specific CLIA kits to determine the level of Estradiol, Progesterone, LH and FSH. The amount of these hormones were higher in DHEA when compared with control. However, between the control and vehicle groups, no significant different level of these hormones was observed (Table 3). After 20 days of Vitamin D3 and MitoQ10 administration to DHEA induced PCOS mice, the sera of the mice of NC, VDV, VD, MQ, V+M and PC groups were also analyzed with specific CLIA kits to quantify the levels of Estradiol, Progesterone, LH and FSH. The amount of all of these hormones were significantly higher in the NC as compared with all the remaining groups except with the VDV where no significant difference was recorded (Table 4).
Table 3.
Serum hormonal levels of DHEA, Control and Vehicle groups.
| Hormone | Control (Mean ± SD) | Vehicle (Mean ± SD) | DHEA (Mean ± SD) |
|---|---|---|---|
| Estradiol (pg/ml) | 24.9 ± 1.3 | 26.0 ± 1.2# | 36 ± 1.8∗∗ |
| Progesterone (ng/ml) | 1.6 ± 0.1 | 1.7 ± 0.1# | 5.1 ± 0.4∗∗ |
| LH (IU/L) | 2.2 ± 0.5 | 2.0 ± 0.1# | 8.1 ± 0.7∗∗ |
| FHS (IU/L) | 1.0 ± 0.2 | 0.9 ± 0.2# | 2.4 ± 0.2∗∗ |
| LH/FSH | 2.1 | 2.3# | 3.4∗∗ |
#indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when compared with control group.
Table 4.
Serum hormonal levels of Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups.
| Hormone | NC (Mean ± SD) | VDV (Mean ± SD) | VD (Mean ± SD) | MQ (Mean ± SD) | V+M (Mean ± SD) | PC (Mean ± SD) |
|---|---|---|---|---|---|---|
| Estradiol (pg/ml) | 77.3 ± 6.4 | 81.3 ± 4.7# | 36.7 ± 1.2∗∗ | 42.3 ± 1.5∗ | 33.3 ± 1.3∗∗ | 31 ± 1.5∗∗ |
| Progesterone (ng/ml) | 7.2 ± 0.2 | 7.1 ± 0.2# | 4.1 ± 0.4∗ | 4.5 ± 0.3∗ | 3.3 ± 0.1∗∗ | 3.4 ± 0.1∗∗ |
| LH (IU/L) | 18.8 ± 1.1 | 18.5 ± 1.2# | 8.2 ± 0.3∗∗ | 8.8 ± 0.2∗∗ | 6.3 ± 0.1∗∗ | 6.7 ± 0.2∗∗ |
| FHS (IU/L) | 5.1 ± 0.1 | 5.4 ± 0.2# | 4.0 ± 0.1∗∗ | 3.8 ± 0.1∗∗ | 3.2 ± 0.1∗∗ | 3.2 ± 0.1∗∗ |
| LH/FSH | 3.7 ± 0.3 | 3.4 ± 0.1# | 2.1 ± 0.1∗∗ | 2.3 ± 0.1∗ | 2.0 ± 0.0∗∗ | 2.1 ± 0.1∗∗ |
# indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when compared with Negative control (NC) group.
3.3. Histomorphological assessment of ovaries
Hematoxylin and eosin stained section of ovaries from all the groups were examined under microscope. The ovaries from Control and Vehicle groups were observed to have normal cortical and medullar histological features. Follicles at various stages of development such as pre antral, antral, atretic as well as corpora lutea were also observed. In contrast, the ovaries from the DHEA treated group appeared to have abnormal histomorphology with many atretic follicles, follicular cyst and no corpus luteum. The appearance was typical of PCOM with cyst having compacted small sized granular cells and very thin theca cells (Figure 3). The PC and M+Q groups ovarian histomorphological features were very similar to that of the Vehicle and Control groups while that of NC and VDV also appeared to be very similar to that of DHEA treated group. The histomorphological features of ovaries from VD and MQ group were improved but substantial number of atretic follicles and follicular cysts were still present (Figure 4). Counting was done to estimate and compare the mean number of follicular structures in the histological section from the various groups. Intriguingly, significant differences in the average number of, antral follicles, atretic follicles, follicular cysts and corpora lutea were observed between the NC group and the all the other groups except the VDV group (Table 5).
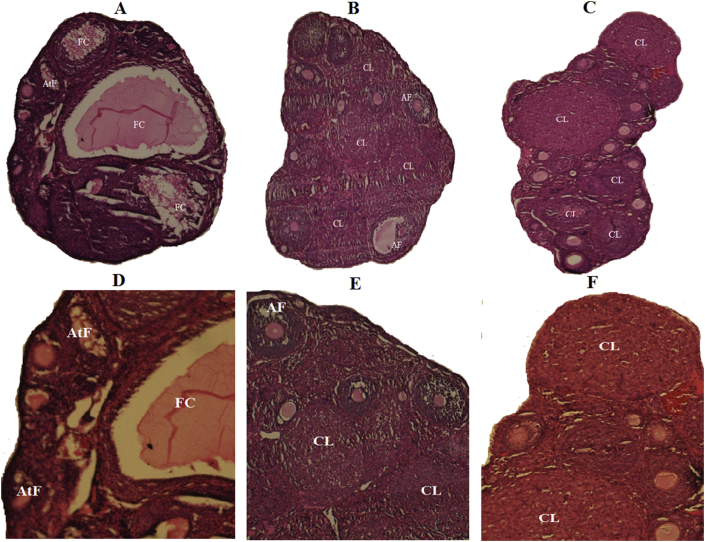
Figure 3.
Histomorphological sections of ovaries from DHEA: A&D, Vehicle: B&E and Control: C&F groups. In A and D, many follicular cyst (FC) and atretic follicle (AtF) but no corpus luteum (CL) was observed. In B, E, C and C various stages of follicular development such as pre-antral follicle, antral follicle (AF), and corpus luteum (CL) were observed (H&E, A B C: × 40, D E F: ×100).
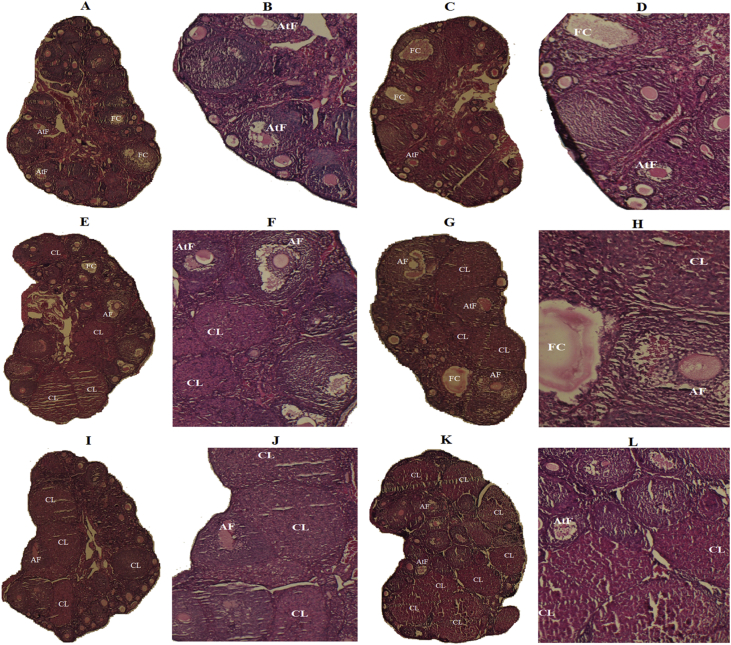
Figure 4.
Histomorphological sections of ovaries from: Negative control (NC): A&B, Vitamin D3 vehicle (VDV): C&D, MitoQ10 (MQ): E&F, Vitamin D3 (VD): G&H, Vitamin D3 plus MitoQ10 (V+M): I&J and Positive control (PC): K&L groups. In A, B, C and D sections, many follicular cyst (FC) and atretic follicle (AtF) but no corpus luteum (CL) were observed. E, F, G, H, I, J, K and L sections show various stages of follicular development such as pre-antral follicle, antral follicle (AF), few atretic follicle (AtF) and many corpora lutea (CL) (H&E, A C E G I K: × 40, B D F H J L: × 100).
Table 5.
The mean number of follicles in the ovaries of mice from Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups.
| Follicles | NC (Mean ± SD) | VDV (Mean ± SD) | VD (Mean ± SD) | MQ (Mean ± SD) | V + M (Mean ± SD) | PC (Mean ± SD) |
|---|---|---|---|---|---|---|
| Pre antral | 7.8 ± 0.5 | 8.1 ± 0.2# | 8.4 ± 0.4# | 8.2 ± 0.3 | 8.5 ± 0.5# | 8.4 ± 0.6# |
| Antral follicle | 1.8 ± 0.2 | 3.3 ± 0.3∗ | 5.8 ± 0.3∗∗ | 6.2 ± 0.1∗∗ | 8.4 ± 0.4∗∗ | 8.5 ± 0.5∗∗ |
| Atretic follicle | 8.8 ± 0.3 | 9.1 ± 0.4# | 5.1 ± 0.5∗∗ | 6.4 ± 0.4∗∗ | 3.4 ± 0.3∗∗ | 3.0 ± 0.0∗∗ |
| Follicular cyst | 7.2 ± 0.2 | 6.9 ± 0.5# | 4.4 ± 0.2∗∗ | 5.0 ± 0.0∗∗ | 0∗∗ | 0∗∗ |
| Corpus luteum | 0 | 0# | 6.2 ± 0.3∗∗ | 5.9 ± 0.1∗∗ | 8.3 ± 0.4∗∗ | 7.8 ± 0.3∗∗ |
# indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when compared with Negative control (NC) group.
3.4. Expression of steroidogenic genes and enzymes
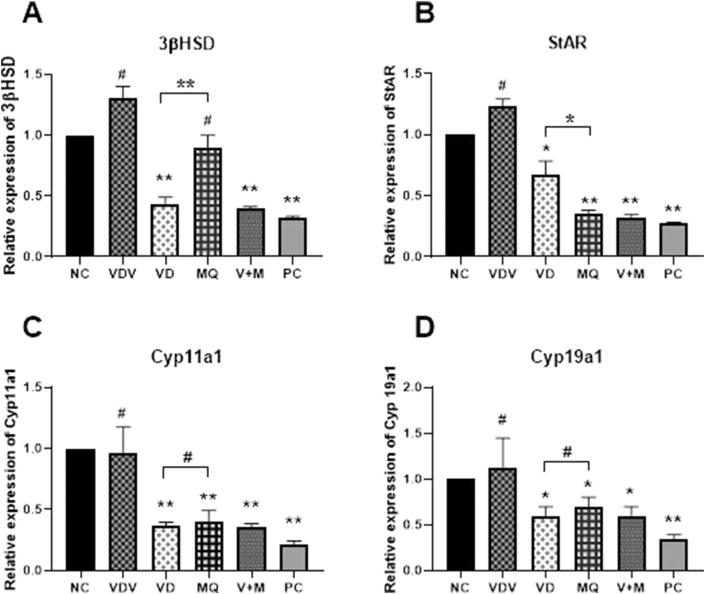
To confirm successful induction of PCOS mice model, steroidogenic genes (StAR, Cyp 11a1, 3βHSD and Cyp19a1) mRNA expression levels were compared among the Control, Vehicle, and DHEA group. It was observed that the expression rate of StAR, Cyp 11a1, 3β-HSD and Cyp19a1 were at least, significantly higher in DHEA group when compared with the Control (P < 0.05 or 0.001). There was no significant difference in the rate of expression of mRNAs of these genes in Vehicle group as compared with the Control group (Figure 5). Moreover, western blotting analysis also revealed high expression of 3β-HSD and Cyp19a1 proteins in DHEA treated mice as compared to those of Control or Vehicle group (Figure 6). In assessing the effect of Vitamin D3 and MitoQ10 on the DHEA treated mice, the expression rate of the mRNA of StAR, Cyp 11a1, 3β-HSD and Cyp19a1 were compared between NC and VDV, VD, MQ, V+M as well as PC groups. The results showed that, the mRNA expression level of Cyp 11a1, Star and Cyp19a1 in VD, MQ, V+M and PC groups were at least significantly lower as compared with the NC group (P < 0.05 or 0.001).
Figure 5.
Relative expression of mRNAs of A: 3β-hydroxysteroid dehydrogenase (3β-HSD), B: steroidogenic acute regulatory (StAR), C: cytochrome P450 family 11 subfamily member 1 (Cyp11a1), D: cytochrome P450 aromatase (Cyp19a1) by ovaries of mice in Control (CONT), Vehicle (VEHI) and dehydroepiandrosterone (DHEA) treated groups. # indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when rate of expression was compared with the CONT group.
Figure 6.
Expression of A: 3β-hydroxysteroid dehydrogenase (3β-HSD) and B: cytochrome P450 aromatase (Cyp19a1) proteins by ovaries of mice in Control (CONT), Vehicle (VEHI) and dehydroepiandrosterone (DHEA) treated groups. # indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when rate of expression was compared with the CONT group. (Full-unadjusted images are shown in supplementary material 1, 2 and 5).
However VD, V+M and PC groups showed at least significantly lower rate of expression level of 3β-HSD mRNA as compared to NC (P < 0.05 or 0.001) group while no significant difference in expression rate of 3β-HSD mRNA was observed between the MQ and NC groups. Moreover, between VD and MQ group, significant difference of 3β-HSD and StAR mRNA expression was observed (p < 0.05 or 0.001) but no significant difference was observed in Cyp19a1 and Cyp11a1 mRNA expression (p > 0.05). The expression rate of mRNA of all the steroidogenic genes showed no significant difference between NC and VDV groups (Figure 7). Correspondingly, the western blotting results confirmed the RT-PCR results. The expression of 3β-HSD and Cyp19a1 proteins were higher in NC and VDV. Comparatively, moderately expression of 3β-HSD and Cyp19a1 proteins was observed in MQ and VD groups while in V+M and PC groups the amount the proteins expressed were very low. Between MQ and VD groups, no significant difference in amount of Cyp19a1 and 3β-HSD proteins was observed (p > 0.05) (Figure 8).
Figure 7.
Relative expression of mRNAs of A: 3β-hydroxysteroid dehydrogenase (3β-HSD), B: steroidogenic acute regulatory (StAR), C: cytochrome P450 family 11 subfamily member 1 (Cyp11a1), D: cytochrome P450 aromatase (Cyp19a1) by ovaries of mice in Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups. # indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when the rate of expression of the other groups was compared with that of NC group.
Figure 8.
Expression of A: 3β-hydroxysteroid dehydrogenase (3β-HSD) and B: cytochrome P450 aromatase (Cyp19a1) proteins by ovaries of mice in Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups. # indicate p > 0.05 ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when the mean level of the other groups was compared with that of NC group. (Full-unadjusted images are shown in supplementary material 1, 2 and 5).
3.5. Expression of OS genes and proteins
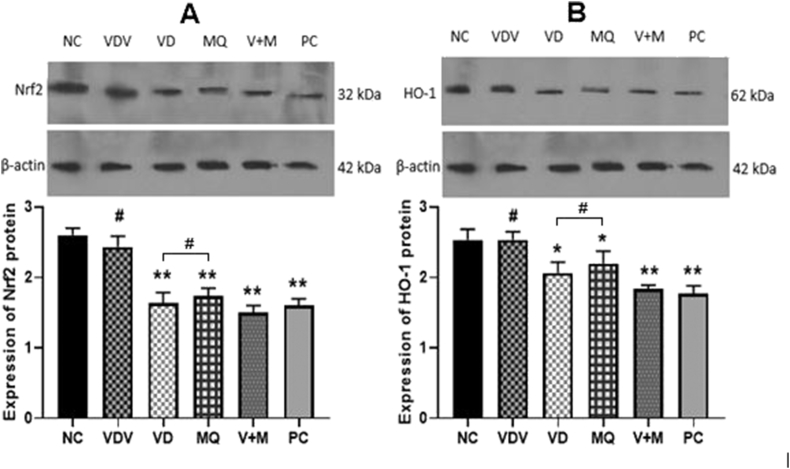
The mRNA expression level of Keap 1, HO-1 and Nrf2 genes were compared between NC and VDV, VD, MQ, V+M as well as PC groups in order to assess the effect of MitoQ10 and Vitamin D3 on ovarian OS of DHEA treated mice. The RT- PCR results showed that, the expression level of Keap 1, HO-1 and Nrf2 genes mRNA were significantly lower in MQ, V+M and PC when compared with that of NC group (P < 0.05 or 0.001). Between the NC and VDV groups, no significant difference was observed in the mRNA expression level of Keap 1, HO-1 and Nrf2 genes. (Figure 9). However, when NC was compared with VD group, it was observed that mRNA expression of HO-1 and Nrf2 (P < 0.05) but not Keap1 (P > 0.05), were significantly lower. Moreover, significant difference of mRNA expression of Keap1, HO-1 and Nrf2 were observed, when VD and MQ were compared (p < 0.05) (Figure 9). In accordance, the western blotting results showed similar observations. There were high, moderate and low expression of Nrf2 and HO-1 proteins by PC and VDV, MQ and VD and V+M and PC groups respectively. Between MQ and VD groups no significant difference were observed in the amount Nrf2 and HO-1 proteins (p > 0.05) (Figure 10).
Figure 9.
Relative expression of mRNAs of A: Heme oxygenase-1 (HO-1),B: Nuclear factor erythroid-2-related factor 2 (Nrf2) and C: Kelch-like ECH-associated protein 1 (Keap1) by ovaries of mice in Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups. # indicate p > 0.05, ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when the rate of expression of the other groups was compared with that of NC group.
Figure 10.
Expression of A: Heme oxygenase-1 (HO-1) and B: Nuclear factor erythroid-2-related factor 2 (Nrf2) proteins by ovaries of mice in Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V+M) and Positive Control (PC) groups. # indicate p > 0.05 ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when the mean level of the other groups was compared with that of NC group. (Full-unadjusted images are shown in supplementary material 3, 4 and 5).
3.6. OS markers analysis
Oxidative stress (OS) status was verified by assessing the levels of MDA (in sera) and SOD (in both sera and ovarian tissue) after administering MitoQ10 and Vitamin D3 in the DHEA treated mice. The serum level of MDA and SOD were observed to be significantly lower in VD, MQ, V+M and PC groups when compared with NC group (P < 0.05 or 0.001). Interestingly, the amount of ovarian levels of SOD was lower in NC group when compared with MQ, VD, PC and M+Q groups (P < 0.05 or 0.001). Between MQ and VD groups, significant difference of serum level of SOD and MDA but not ovarian level of SOD were noted (P < 0.05). Moreover, between NC and VDV groups, no significant difference was observed in terms of sera level of MDA and SOD or ovarian tissue level of SOD (Figure 11).
Figure 11.
Amount of A: serum Malondialldehyde (MDA), B: serum superoxide dismutase (SOD) activities and C: ovarian tissue superoxide dismutase (SOD) activities of mice in Negative control (NC), Vitamin D3 vehicle (VDV), Vitamin D3 (VD), MitoQ10 (MQ), Vitamin D3 plus MitoQ10 (V + M) and Positive Control (PC) groups. # indicate p > 0.05 ∗ indicates p < 0.05 and ∗∗ indicates p < 0.01 when the mean level of the other groups was compared with that of NC group.
4. Discussion
In this present study, the PCOS model was induced by subcutaneous injection of DHEA in pre-pubertal mice. When the biochemical data were compared, estradiol, progesterone, LH, FSH and LH/FSH levels were significantly higher in DHEA treated group. The DHEA treated group also had persistence estrus stage. The ovarian histomorphological sections of DHEA treated group revealed many atretic and follicular cyst with no corpus luteum. High rate of expression of steroidogenic enzymes (3β-HSD, Cyp19a1, Cyp11a1and StAR) was also observed in DHEA treated group. In a comparative study Motta et al. reported that DHEA-induced PCOS mouse model exhibited most of the features seen in Human PCOS patients including abnormal steroidogenesis, hyperandrogenism, insulin resistance, and abnormal follicular development leading to anovulation and infertility [17]. Arrested estrus cycle, high LH/FSH ratio and the presence of many atretic and follicular in ovary after treatment with DHEA have been reported by many researchers [14, 18, 19, 20], In addition high rate of expression mRNA and proteins of 3βHSD, Cyp19a1, Cyp11a1and StAR has been observed in patients with PCOS when compares with their normal counterparts [21, 22].
High level of androgens directly causes hypothalamic - pituitary axis to produce more LH or cause insulin resistance and its consequence of hyperinsulinemia [23]. High level of insulin further disturb hypothalamic-pituitary axis leading to high amount of LH [24, 25]. Hyperandrogenism also causes hyper activation of steroidogenic enzymes leading to high level of estradiol which inhibit FHS production by the hypothalamic - pituitary axis [26, 27]. High level of LH and low level of FSH results increase in LH/FSH, which consequently causes follicular arrest, atretic follicles and follicular cyst formation, anovulation and disturbed estrus cycle seen in PCOS patience [13, 28].
Following confirmation of PCOS, the mice were divided into five groups and administered with DHEA (NC), Ethanol dissolved with distilled water (VDV), Vitamin D3 (VD), MitoQ10 (MQ), and both MitoQ10 and Vitamin D3 (V+M). To avoid injury to the esophagus due to long time gavage, Vitamin D3 and MitoQ10 were administered intraperitoneal and orally respectively.
As a result of abnormal histomorphology, many atretic follicles and follicular cyst as well as absence corpus luteum observed in DHEA treated group, Vitamin D3 and MitoQ10 were administered for 20 days to allow enough time for oogenesis which takes at least three weeks to produce antral follicles in mice [29].
To rule out the possibility of self-recovery, the VDV group was included in the study where DHEA treatment was discontinued and neither Vitamin D3 nor MitoQ10 was administered. The results from NC and VDV showed similar serum estradiol, progesterone, LH, FSH, LH/FSH SOD and MDA, and ovarian SOD level. Moreover, there was no significant difference in the expression of 3β-HSD, Cyp19a1, Cyp11a1, StAR, Keap1, HO-1 and Nrf2. Histomorphological observations of ovary sections from these groups were very similar, characterized with many atretic follicles and follicular cyst but no corpus luteum. In comparative reports, low ovarian level of SOD, high serum level of MDA and SOD have been reported in PCOS women [30, 31]. Increased Cyp11a1 and Cyp17a1 mRNA expression, as well as increased 3βHSD and 17βHSD enzyme activity by ovarian cells and consequently increased production of progesterone, and estradiol are stable properties PCOS [32].
Oxidative stress (OS) is closely link with low-grade inflammation in the ovarian tissues, which may leads to hyperandrogenism [33, 34, 35] while hyperandrogenism results OS through insulin resistance. Hyperandrogenism is characterized by high level of androgens such as testosterone, Dihydrotestosterone and androstenedione. Even though this study did not include protocols for assessing the levels of androgens but hyperandrogenism is indirectly confirmed by high level of estradiol that might be due to conversion of androgens such as testosterone and androstenedione by 17β-HSD2 and Cyp19a1 enzymes respectively. Hurrle and Hsu reported that, OS influences insulin resistance and vice versa [36]. This suggest that the pathophysiological circle of PCOS involving OS, hyperandrogenemia and insulin resistance may still continue, resulting chronic PCOS even if the initial exposure of androgen that initiated the PCOS is absent. Subcutaneous injection of DHEA in pre-pubertal mice for 20 consecutive day induces chronic PCOS phenotype very similar to that of humans [37]. Thus, mice in VDV groups continued to exhibits PCOS features despite discontinued injection of DHEA.
MitoQ10 was very effective in reducing ovarian oxidative stress. In MQ group, significant downregulation of HO -1, Keap1 and Nrf2 was observed. In addition, serum MDA and SOD levels were significantly lower while ovarian tissue level of SOD significantly improved. Our results is in agreement with earlier study by Ding et al, where they observed significantly low serum amount of SOD and MDA in MitoQ10 treated group as compared with PCOS-IR group [16]. Several researchers have also reported antioxidant capabilities of MitoQ10. Zhou et al. reported MitoQ10 administration increased the activity of SOD and decreased MDA level in mice [38]. MitoQ10 replenished mitochondrial SOD, an important agent for dampening superoxide anions and subsequent lipid peroxidation [39]. Moreover, MitoQ10 increased mitochondrial glutathiones and GPx [40, 41]. A previous study indicated that, MitoQ10 prevented the inhibition of SOD and glutathiones from damage by free radicals in cardiac tissue [42]. Hu et al. concluded that, MitoQ10 prevented intestinal cells damage by prevention OS through Nrf2/Keap 1 antioxidant related enzymes (ARE) pathway [43]. Furthermore, MitoQ10 ameliorates testis injury from OS attack by repairing mitochondria and promoting the Nrf2/Keap1 pathway [44].
Keap1codes for repressor protein that binds to Nrf2 protein. Under OS condition, keap1 protein dissociate from Nrf2 causing it to be activated. The activated Nrf2 moves into the nucleus and promote transcription of antioxidant related enzymes (ARE) such as HO-1, which leads production of antioxidants including SOD, GPx and CAT. Thus, cell under OS therefore expresses high level of Nrf2, HO-1 and Keap1 leading to elevated level of natural antioxidants. MDA, and SOD are commonly used as markers to evaluate OS levels in PCOS. Of these, MDA is the product of lipid oxidation, and high level indicate high amount of ROS. SOD is an enzyme, which can catalyze dismutation of superoxide radicals into ordinary molecular oxygen or hydrogen peroxide [45]. High amount of SOD is an indication of low OS [38]. Low serum level of MDA and high Level of ovarian tissue SOD coupling with low expression of Nrf2, Keap1 and HO-1 observed in MQ group suggest that MitoQ10 was effective in reducing ROS in the ovary of DHEA induced PCOS model.
In the same MQ group in this study, downregulation of steroidogenic enzymes including Cyp19a1, Cyp11a1 and StAR was observed. However, MitoQ10 failed to downregulate the expression of 3β-HSD though, significantly low amount of estrogen, progesterone, LH, FSH and LH/FSH were observed. This present findings is in some way contrary to previous study executed by Ibrahim et al [46] where they concluded that MitoQ10 modulated testicular steroidogenesis by regulating the expression of 3βHSD, 17β-HSD and StAR, genes. This present study used female mice of which the reproductive system and expression of pattern of steroidogenic genes may be different from that of male counterpart. As a result, steroidogenic genes expression response to MitoQ10 may be different between the two sexes.
Moreover, histomorphological section of ovaries from mice in the MQ group in this present study showed partial restoration of ovarian morphology. Even though follicles at various stages of development were observed but many follicular cyst and atretic follicle were still present. As far as we know, studies that have assessed the effect of MitoQ10 on ovarian morphology in PCOS is very limited, in spite of the powerful antioxidant capacity of MitoQ10. However, similar to our outcome, Ding et al 2019 pointed out that, MitoQ10 improved the morphology of ovarian follicles and increases the granulosa cells of the PCOS-IR rat model [16].
Hyperandrogenism in PCOS is strongly linked with OS and many authors have concluded that, the pathogenesis progression is closely associated with ROS [47, 48]. Accumulation of ROS in mitochondrion causes up regulation of steroidogenic enzymes resulting in hyperandrogenism.
Though there are natural antioxidants, such as Vitamin C and Vitamin E and SOD but they are less efficient in scavenging ROS in mitochondrion. MitoQ10 is a modified ubiquinone moieties by conjugating with TPP lipophilic cations, making it easy to move to mitochondrion matrix through phospholipid bilayers to combat with ROS [49, 50]. Thus, our results suggests that MitoQ10 reduced ovarian OS. The reduced OS paved way for downregulation of steroidogenic enzymes resulting in partial improvement of ovarian histomorphology of DHEA induced PCOS mice.
In the VD group, it was observed that, HO-1 and Nrf2 genes and the corresponding proteins were substantially downregulated. However, Keap1 failed to be downregulated in this group. Nevertheless, serum MDA and SOD levels were significantly lower while ovarian tissue level of SOD was significantly increased. In a study that compared serum level of Vitamin D3 with amount of ROS in follicular, Masjedi et al concluded that the amount of Vitamin D3 negatively correlated with amount of ROS [51]. Moreover, several authors have attested the antioxidant capability of Vitamin D3. In a study conducted on rats, antioxidant effects of Vitamin D3 was better than vitamin E in inhibiting lipid peroxidation and improving SOD activity [52]. Vitamin D3 supplement decreased serum MDA and SOD [53, 54, 55, 56, 57]. Wiseman reported antioxidant nature of Vitamin D3 as it inhibited iron-dependent lipid peroxidation in liposomes which is major source of MDA [58].
Steroidogenesis was modulated in the Vitamin D3 treated group by significantly down regulating the expression of 3β-HSD, Cyp19a1, Cyp11a1 and StAR, In addition, significant reduction of LH, FSH, LH/FSH ratio, estradiol and progesterone were observed in this group. Bakhshalizadeh et al demonstrated that Vitamin D3 reduced the hyper expression of 3β-HSD, Cyp19a1, Cyp11a1, StAR by granulosa cells of PCOS (induced by DHEA) mouse model [14]. In another related studies, it was reported that Vitamin D3 decreased Cyp19a1 and 3β-HSD expression, which led to decreased in the serum amount of estradiol and progesterone [59]. Our observation clearly supports the fact that Vitamin D3 has ability to downregulate steroidogenic enzymes.
The histomorphological sections from Vitamin D3 treated group showed significant low number of follicular cyst and atretic follicle. In addition, follicles at various stages of development including pre antral and antral follicles as well as corpora lutea were observed. In the related studies, Celik et al reported that Vitamin D3 prevented the formation of PCOM induced by DHEA in rats [60]. Behmanesh et al also observed that morphology of follicles at various stages of development and corpora lutea were normal when PCOS rats were treated with Vitamin D3 [15]. The mechanism through which Vitamin D improves PCOM is not fully understood. However Vitamin D receptors (VDRs) have been expressed by placenta, endometrium and ovary [61] and low level of Vitamin D is associated with calcium dysregulation, resulting in development of follicular arrest as well as menstrual and fertility dysfunction in women with PCOS [62]. Moreover, Vitamin D also plays a physiological role in reproduction such as ovarian follicular development and luteinization through anti-müllerian hormone (AMH) signaling, FSH sensitivity and progesterone production in human granulosa cells [63, 64]. Vitamin D levels in follicular fluid negatively correlated with AMH and AMH receptor (AMHR)-II mRNA levels in cumulus granulosa cells of small follicles. This suggest that Vitamin D alters AMH signaling and steroidogenesis in human cumulus granulosa cells, possibly reflecting a state of granulosa cells luteinization potentiation [65]. According to Jones et al, Vitamin D regulates steroidogenic gene transcription through nuclear Vitamin D receptors (VDRs) that are found in ovaries [66].
Data from VD group suggest that Vitamin D3 moderately reduced ovarian OS. Reduced OS enhanced Vitamin D3 steroidogenesis signaling pathways causing reduction of estradiol, progesterone, LH, FSH and LH/FSH ratio and consequently improving folliculogenesis and ovarian tissue histomorphology in DHEA induced PCOS mouse model.
In this study, comparison of effectiveness between MitoQ10 and Vitamin D3 was made. It was observed that, while Vitamin D3 was better in modulating steroidogenic enzymes, MitoQ10 was very effective in reducing OS. The data confirms antioxidant capability as reported in the literature but unlike MitoQ10, Vitamin D3 lack the ability to effectively scavenge ROS in mitochondria matrix that might have resulted from hyper expression of steroidogenic enzymes. MitoQ10 may combat with OS in both cytoplasm and mitochondrion hence it offer better capability as antioxidant in reducing ovarian OS in DHEA induced PCOS model than VitaminD3.
The combination of Vitamin D3 and MitoQ10 had great ability in reducing OS. The serum MDA and SOD levels were significantly reduced while ovarian tissue SOD level increased in V+Q group. The mRNA expression level of Keap1, HO-1 and Nrf2 were significantly low.
Data from this group also showed that, combination of Vitamin D3 and MitoQ10 had a profound effect on DHEA induced PCOS model ovarian steroidogenesis. In this group, the mRNA expression levels of 3β-HSD, Cyp19a1, Cyp11a1 and StAR were significantly reduced when compared with that of NC group. In addition, significantly low serum levels of progesterone, estradiol, LH, FSH, LH/FSH were observed.
Ovarian histomorphological sections of mice from the very same group revealed very healthy ovary characterized with significantly higher number of follicles at various stages of development as well as many corpora lutea. Most interestingly, no follicular cyst was observed in any of the histomorphological sections from this group. Moreover, there was complete restoration of estrus cycle of all the mice in this group. More importantly, all the observations made on V+M group were very similar to that of the PC group, indicating that combination of Vitamin D3 and MitoQ10 had significantly positive effect on DHEA PCOS mouse model.
Literature on the study that has assessed the combine effect of MitoQ 10 and Vitamin D3 on PCOS is lacking. However, the efficacy of Vitamin D and metformin in management of infertility in PCOS have been attested [67, 68]. Abdelaziz et al. reported combination of CoQ10 (an analogue of MitoQ10) and clomiphene citrate was effective in induction of ovulation in PCOS patients [69]. Though Vitamin D3 has been used to manage PCOS but the results are inconsistent and whether Vitamin D deficiency is etiologic agent or as a symptom of PCOS still remain enigmatic [65]. It is believed that, Vitamin D deficiency effect on PCOS might be synergistic or depends on other factors. Therefore this study was intended to investigate the link between Vitamin D deficiency and OS in PCOS hence for the first time, the combine effect of Vitamin D3 and MitoQ10 on DHEA induced PCOS mice has been assessed.
The fact that the parameters of the V+M group were very similar to that of the PC group suggests that the combine effect of Vitamin D3 and MitoQ10 on DHEA induced PCOS mouse model was outstanding. The mechanism through which Vitamin D3 and MitoQ10 attenuates PCOS has not been covered by this present study. However based on the results of the present study and the available literature, we propose that both Vitamin D3 and MitoQ10 significantly reduced ovarian OS. Reduction of OS coupling with Vitamin D3 signaling effect on steroidogenesis consequently ameliorated hyperandrogenism thereby reducing LH/FSH ratio. Once LH/FSH ratio is normal, hapothalomo-pituitary-gonadal axis was restored thereby improving folliculogenesis and healthy ovarian morphology.
5. Conclusion
In conclusion, the findings of this present study support the idea that, OS and Vitamin D3 deficiency play a significant role in the pathogenesis of PCOS. Thus, Vitamin D3 and antioxidant (MitoQ10) supplement may be important in the management of PCOS. More similar studies on other subjects is warranted.
Declarations
Author contribution statement
G. Kyei: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
F. Amidi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Sobhani and F. Ebrahimi: Performed the experiments.
S. Nekonam: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
M. Shabani, M. Qasemi and E. Salahi: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the International Campus of Tehran University of Medical Sciences.
Competing interest statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2020.e04279.
Acknowledgements
The authors would like to acknowledge the help of Ms. Soudabeh Ghasemi and all technical staff at the Department of Anatomy, Tehran University of Medical Sciences.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
suplementary material 1.
suplementary material 2.
suplementary material 3.
suplementry material 4.
suplementary material 5.
suplemantary material 6.
References
- 1.March W.A., Moore V.M., Willson K.J., Phillips D.I., Norman R.J., Davies M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2009;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Yeon Lee J., Baw C.-K., Gupta S., Aziz N., Agarwal A. Role of oxidative stress in polycystic ovary syndrome. Curr. Womens Health Rev. 2010;6(2):96–107. [Google Scholar]
- 3.Zuo T., Zhu M., Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid. Med. Cell. Longev. 2016;2016:1–14. doi: 10.1155/2016/8589318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int. J. Prev. Med. 2019;10:86–101. doi: 10.4103/ijpvm.IJPVM_576_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn S., Haselhorst U., Tan S., Quadbeck B., Schmidt M., Roesler S., Kimmig R., Mann K., Janssen O.E. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes. 2006;114(10):577–583. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 6.Wehr E., Pilz S., Schweighofer N., Giuliani A., Kopera D., Pieber T., Wang Z. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur. J. Endocrinol. 2009;161(4):575–582. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 7.Yildizhan R., Kurdoglu M., Adali E., Kolusari A., Yildizhan B., Sahin H.G., Kamaci M. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2009;280(4):559–568. doi: 10.1007/s00404-009-0958-7. [DOI] [PubMed] [Google Scholar]
- 8.Selimoglu H., Duran C., Kiyici S., Ersoy C., Guclu M., Ozkaya G., Tuncel E., Erturk E., Imamoglu S. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J. Endocrinol. Invest. 2010;33(4):234–238. doi: 10.1007/BF03345785. [DOI] [PubMed] [Google Scholar]
- 9.Raja-Khan N., Shah J., Stetter C.M., Lott M.E., Kunselman A.R., Dodson W.C., Legro R.S. High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil. Steril. 2014;101(6):1740–1746. doi: 10.1016/j.fertnstert.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy M.P., Smith R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 11.Magwere T., West M., Riyahi K., Murphy M.P., Smith R.A., Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2006;127(4):356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Walters K.A., Allan C.M., Handelsman D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012;86(5):149. doi: 10.1095/biolreprod.111.097808. 1-12. [DOI] [PubMed] [Google Scholar]
- 13.Lee M.T., Anderson E., Lee G.Y. Changes in ovarian morphology and serum hormones in the rat after treatment with dehydroepiandrosterone. Anat. Rec. 1991;231(2):185–192. doi: 10.1002/ar.1092310206. [DOI] [PubMed] [Google Scholar]
- 14.Bakhshalizadeh S., Amidi F., Alleyassin A., Soleimani M., Shirazi R., Shabani Nashtaei M. Modulation of steroidogenesis by vitamin D3 in granulosa cells of the mouse model of polycystic ovarian syndrome. Syst. Biol. Reprod. Med. 2017;63(3):150–161. doi: 10.1080/19396368.2017.1296046. [DOI] [PubMed] [Google Scholar]
- 15.Behmanesh N., Abedelahi A., Charoudeh H.N., Alihemmati A. Effects of vitamin D supplementation on follicular development, gonadotropins and sex hormone concentrations, and insulin resistance in induced polycystic ovary syndrome. Turk. J. Obstet. Gynecol. 2019;16(3):143. doi: 10.4274/tjod.galenos.2019.46244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y., Jiang Z., Xia B., Zhang L., Zhang C., Leng J. Mitochondria-targeted antioxidant therapy for an animal model of PCOS-IR. Int. J. Mol. Med. 2019;43(1):316–324. doi: 10.3892/ijmm.2018.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motta A.B. Dehydroepiandrosterone to induce murine models for the study of polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2010;119(3-5):105–111. doi: 10.1016/j.jsbmb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Wang F., Zhang Z., Wang Z., Xiao K., Wang Q., Su J., Wang Z. Expression and clinical significance of the HIF-1a/ET-2 signaling pathway during the development and treatment of polycystic ovary syndrome. J. Mol. Histol. 2015;46(2):173–181. doi: 10.1007/s10735-015-9609-4. [DOI] [PubMed] [Google Scholar]
- 19.Wu C., Lin F., Qiu S., Jiang Z. The characterization of obese polycystic ovary syndrome rat model suitable for exercise intervention. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0099155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Zhang C., Shen S., Xia Yj, Yi L., Gao Q., Wang R. Dehydroepiandrosterone induces ovarian and uterine hyperfibrosis in female rats. Hum. Reprod. 2013;28(11):3074–3085. doi: 10.1093/humrep/det341. [DOI] [PubMed] [Google Scholar]
- 21.Kozlowska A., Majewski M., Jana B. Expression of steroidogenic enzymes in porcine polycystic ovaries. Folia Histochem. Cytobiol. 2009;47(2):257–264. doi: 10.2478/v10042-009-0043-x. [DOI] [PubMed] [Google Scholar]
- 22.Tosca L., Chabrolle C., Uzbekova S., Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK)1. Biol. Reprod. 2007;76(3):368–378. doi: 10.1095/biolreprod.106.055749. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfield R.L., Ehrmann D.A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016;37(5):467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta R.V., Patel K.S., Coffler M.S., Dahan M.H., Yoo R.Y., Archer J.S., Malcom P.J., Chang R.J. Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. J. Clin. Endocrinol. Metab. 2005;90(4):2136–2141. doi: 10.1210/jc.2004-1040. [DOI] [PubMed] [Google Scholar]
- 25.Poretsky L., Piper B. Insulin resistance, hypersecretion of LH, and a dual-defect hypothesis for the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. 1994;84(4):613–621. [PubMed] [Google Scholar]
- 26.Fuller P.J., Young M.J. Elsevier; 2016. Aldosterone Secretion and Action. Endocrinology: Adult & Pediatric; pp. 1756–1762. [Google Scholar]
- 27.Kaiser U., Ho K.K. Elsevier; 2016. Pituitary Physiology and Diagnostic Evaluation. Williams Textbook of Endocrinology; pp. 176–231. [Google Scholar]
- 28.Luchetti C.G., Solano M.E., Sander V., Arcos M.L., Gonzalez C., Di Girolamo G., Chiocchio S., Cremaschi G., Motta A.B. Effects of dehydroepiandrosterone on ovarian cystogenesis and immune function. J. Reprod. Immunol. 2004;64(1-2):59–74. doi: 10.1016/j.jri.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Wang J.-J., Ge W., Liu J.-C., Klinger F.G., Dyce P.W., De Felici M. Complete in vitro oogenesis: retrospects and prospects. Cell Death Differ. 2017;24(11):1845–1852. doi: 10.1038/cdd.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuşçu N.K., Var A. Oxidative stress but not endothelial dysfunction exists in non-obese, young group of patients with polycystic ovary syndrome. Acta Obstet. Gynecol. Scand. 2009;88(5):612–617. doi: 10.1080/00016340902859315. [DOI] [PubMed] [Google Scholar]
- 31.Sumithra N.U.C., Lakshmi R.L., Menon N.L., Subhakumari K., Sheejamol V. Evaluation of oxidative stress and hsCRP in polycystic ovarian syndrome in a tertiary care hospital. Indian J. Clin. Biochem. 2015;30(2):161–166. doi: 10.1007/s12291-014-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson V.L., Legro R.S., Strauss J.F., III, McAllister J.M. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol. Endocrinol. 1999;13(6):946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 33.González F., Minium J., Rote N.S., Kirwan J.P. Hyperglycemia alters tumor necrosis factor-α release from mononuclear cells in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005;90(9):5336–5342. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y., Qiao J., Li R., Li M.-Z. Is interleukin-18 associated with polycystic ovary syndrome? Reprod. Biol. Endocrinol. 2011;9(1):7–12. doi: 10.1186/1477-7827-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yilmaz M., Bukan N., Ayvaz Gk, Karakoç A., Toruner F., Çakir N., Aslan M. The effects of rosiglitazone and metformin on oxidative stress and homocysteine levels in lean patients with polycystic ovary syndrome. Hum. Reprod. 2005;20(12):3333–3340. doi: 10.1093/humrep/dei258. [DOI] [PubMed] [Google Scholar]
- 36.Hurrle S., Hsu W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017;40(5):257–262. doi: 10.1016/j.bj.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakley O., Lin P.-C., Bridges P., Ko C. Animal models for the study of polycystic ovarian syndrome. Endocrinol. Metab. 2011;26(3):193–202. [Google Scholar]
- 38.Zhou J., Wang H., Shen R., Fang J., Yang Y., Dai W. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018;10(6):1887–1899. [PMC free article] [PubMed] [Google Scholar]
- 39.Epperly M.W., Gretton J.E., Sikora C.A., Jefferson M., Bernarding M., Nie S. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat. Res. 2003;160(5):568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 40.Guerriero G., Trocchia S., Abdel-Gawad F.K., Ciarcia G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 2014;5:56–60. doi: 10.3389/fendo.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneko T., Iuchi Y., Kobayashi T., Fujii T., Saito H., Kurachi H., Fujii J. The expression of glutathione reductase in the male reproductive system of rats supports the enzymatic basis of glutathione function in spermatogenesis. Eur. J. Biochem. 2002;269(5):1570–1578. doi: 10.1046/j.1432-1033.2002.02809.x. [DOI] [PubMed] [Google Scholar]
- 42.Vergeade A., Mulder P., Vendeville-Dehaudt C., Estour F., Fortin D., Ventura-Clapier R. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: prevention by the targeted antioxidant MitoQ. Free Radic. Biol. Med. 2010;49(5):748–756. doi: 10.1016/j.freeradbiomed.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Hu Q., Ren J., Li G., Wu J., Wu X., Wang G., Gu G., Ren H., Hong Z., Li J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9(3):1–15. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J., Bao X., Zhang M., Zhu Z., Zhou L., Chen Q., Zhang Q., Ma B. MitoQ ameliorates testis injury from oxidative attack by repairing mitochondria and promoting the Keap1-Nrf2 pathway. Toxicol. Appl. Pharmacol. 2019;370:78–92. doi: 10.1016/j.taap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Yasui K., Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006;55(9):359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- 46.Ibrahim A.A., Karam H.M., Shaaban E.A., Safar M.M., El-Yamany M.F. MitoQ ameliorates testicular damage induced by gamma irradiation in rats: modulation of mitochondrial apoptosis and steroidogenesis. Life Sci. 2019;232:116655–116664. doi: 10.1016/j.lfs.2019.116655. [DOI] [PubMed] [Google Scholar]
- 47.Mujica L.S., Bridi A., Della Méa R., Rissi V.B., Guarda N., Moresco R.N., Premaor M.O., Antoniazzi A.G., Goncalves P.B.D., Comim F.V. Oxidative stress and metabolic markers in pre-and postnatal polycystic ovary syndrome rat protocols. J. Inflamm. Res. 2018;11:193–202. doi: 10.2147/JIR.S160264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Victor V.M., Rovira-Llopis S., Bañuls C., Diaz-Morales N., de Marañon A.M., Rios-Navarro C. Insulin resistance in PCOS patients enhances oxidative stress and leukocyte adhesion: role of myeloperoxidase. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Firsov A.M., Kotova E.A., Orlov V.N., Antonenko Y.N., Skulachev V.P. A mitochondria-targeted antioxidant can inhibit peroxidase activity of cytochrome c by detachment of the protein from liposomes. FEBS Lett. 2016;590(17):2836–2843. doi: 10.1002/1873-3468.12319. [DOI] [PubMed] [Google Scholar]
- 50.Ross M.F., Kelso G., Blaikie F.H., James A.M., Cocheme H.M., Filipovska A., Da Ros T., Hurd T.R., Smith R.A., Murphy M.P. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochem. (Mosc) 2005;70(2):222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 51.Masjedi F., Keshtgar S., Agah F., Karbalaei N. Association between sex steroids and oxidative status with vitamin D levels in follicular fluid of non-obese PCOS and healthy women. J. Reproduction Infertil. 2019;20(3):132–142. [PMC free article] [PubMed] [Google Scholar]
- 52.Sardar S., Chakraborty A., Chatterjee M. Comparative effectiveness of vitamin D3 and dietary vitamin E on peroxidation of lipids and enzymes of the hepatic antioxidant system in Sprague--Dawley rats. Int. J. Vitam. Nutr. Res. 1996;66(1):39–45. [PubMed] [Google Scholar]
- 53.Asemi Z., Hashemi T., Karamali M., Samimi M., Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am. J. Clin. Nutr. 2013;98(6):1425–1432. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- 54.Nasri K., Akrami S., Rahimi M., Taghizadeh M., Behfar M., Mazandaranian M.R., Kheiry A., Memarzadeh M.R., Asemi Z. The effects of vitamin D and evening primrose oil co-supplementation on lipid profiles and biomarkers of oxidative stress in vitamin D-deficient women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Endocr. Res. 2018;43(1):1–10. doi: 10.1080/07435800.2017.1346661. [DOI] [PubMed] [Google Scholar]
- 55.Nikooyeh B., Neyestani T., Tayebinejad N., Alavi-Majd H., Shariatzadeh N., Kalayi A. Daily intake of vitamin D-or calcium-vitamin D-fortified Persian yogurt drink (doogh) attenuates diabetes-induced oxidative stress: evidence for antioxidative properties of vitamin D. J. Hum. Nutr. Diet. 2014;27:276–283. doi: 10.1111/jhn.12142. [DOI] [PubMed] [Google Scholar]
- 56.Shab-Bidar S., Neyestani T., Djazayery A. The interactive effect of improvement of vitamin D status and VDR Fok I variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial. Eur. J. Clin. Nutr. 2015;69(2):216–222. doi: 10.1038/ejcn.2014.240. [DOI] [PubMed] [Google Scholar]
- 57.Sharifi N., Amani R., Hajiani E., Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47(1):70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 58.Wiseman H. Vitamin D is a membrane antioxidant Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326(1-3):285–288. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- 59.Buckler H., Phillips S., Cameron I., Healy D., Burger H. Vaginal progesterone administration before ovulation induction with exogenous gonadotropins in polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 1988;67(2):300–306. doi: 10.1210/jcem-67-2-300. [DOI] [PubMed] [Google Scholar]
- 60.Çelik L.S., Kuyucu Y., Yenilmez E.D., Tuli A., Dağlıoğlu K., Mete U.Ö. Effects of vitamin D on ovary in DHEA-treated PCOS rat model: a light and electron microscopic study. Ultrastruct. Pathol. 2018;42(1):55–64. doi: 10.1080/01913123.2017.1385668. [DOI] [PubMed] [Google Scholar]
- 61.Stumpf W.E., Denny M.E. Vitamin D (soltriol), light, and reproduction. Am. J. Obstet. Gynecol. 1989;161(5):1375–1384. doi: 10.1016/0002-9378(89)90699-6. [DOI] [PubMed] [Google Scholar]
- 62.Thys-Jacobs S., Donovan D., Papadopoulos A., Sarrel P., Bilezikian J.P. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64(6):430–435. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 63.Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F., Lieben L., Mathieu C., Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 2008;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irani M., Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil. Steril. 2014;102(2):460–468. doi: 10.1016/j.fertnstert.2014.04.046. e3. [DOI] [PubMed] [Google Scholar]
- 65.Merhi Z., Doswell A., Krebs K., Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J. Clin. Endocrinol. Metab. 2014;99(6):E1137–E1145. doi: 10.1210/jc.2013-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones G., Strugnell S.A., DeLuca H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 67.dehghani Firouzabadi R., Aflatoonian A., Modarresi S., Sekhavat L., MohammadTaheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Clin. Pract. 2012;18(2):85–88. doi: 10.1016/j.ctcp.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Garg G., Kachhawa G., Ramot R., Khadgawat R., Tandon N., Sreenivas V., Alka K., Gupta N. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: a pilot study. Endocr. Connect. 2015;4(2):108–116. doi: 10.1530/EC-15-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El Refaeey A., Selem A., Badawy A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reprod. Biomed. Online. 2014;29(1):119–124. doi: 10.1016/j.rbmo.2014.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.