Summary
The COVID-19 (coronavirus disease-2019) pandemic has presented unprecedented challenges to regulatory organizations, the biotech and pharmaceutical industry, and the publishing industry. This Translational Perspectives paper attempts to highlight some of the challenges and perils of moving extraordinarily fast in an effort to save human lives in the midst of a global pandemic. As with the development of all new therapeutic approaches, it will take time to assess the risks and benefits of developing new therapies at “warp speed”.
Key Words: accelerated approval, COVID-19, emergency use, hydroxychloroquine, operation warp speed, pandemic, vaccine approval
Abbreviations and Acronyms: BARDA, Biomedical Advanced Research and Development Authority; COVID-19, coronavirus disease-2019; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; OWSVI, Operation Warp Speed Vaccine Initiative
The coronavirus disease-2019 (COVID-19) pandemic has set off an extraordinary race for treatments and preventative vaccines, with hopeful announcements suggesting that a vaccine may be ready by the beginning of 2021, barely 12 months after recognition of onset of the pandemic. Such an achievement would represent a truly astounding acceleration over normal approval times. The proof of this lofty promise is yet a few months away, and whether it will be realized is in significant doubt. Nevertheless, the COVID pandemic has already provided some cautionary tales about “moving too fast.” Two stories include the use of hydroxychloroquine for COVID-19 infections, and the implementation of the Operation Warp Speed Vaccine Initiative (OWSVI).
Hydroxychloroquine: Moving Too Fast in Every Direction
Hydroxychloroquine was proposed as a treatment for COVID-19 prior to the pandemic’s arrival on U.S. shores, based on proven action against another coronavirus in vitro. It has long been U.S. Food and Drug Administration (FDA) approved as a treatment for malaria, rheumatoid arthritis, and systemic lupus. Although not approved at the time as a treatment for COVID-19, it could be legally used “off-label,” provided firm scientific rationale and sound medical evidence supported it. It has a complete supply chain in place and could be rapidly deployed. However, its toxicities include retinopathy, cardiomyopathy, seizures, liver failure, and fatal cardiac arrhythmias. Thus, it is a drug to be used with caution, particularly off-label, when risks are so significant and benefits are entirely unknown.”
On March 16, 2020, a controversial French researcher associated with a significant prior publication scandal (1) announced via an Internet video posting that he and his team had experienced success in treating 24 COVID-19 patients with a combination of hydroxychloroquine and azithromycin (2). A few days later, President Trump touted the drug as a virtually risk-free cure to COVID, asking rhetorically, “what do you have to lose?” (3). An almost immediate shortage of hydroxychloroquine resulted as the U.S. government stockpiled the drug, doctors began prescribing it off-label, and some patients began hoarding. Patients who needed hydroxychloroquine for its proven indications began to run out.
By March 28, hydroxychloroquine received conditional approval via the FDA’s Emergency Use Pathway for treatment of COVID-19 infections, and just 3 weeks later, the FDA approved a phase III clinical trial of hydroxychloroquine for COVID-19 treatment, sponsored by Novartis.
Results of clinical trials of hydroxychloroquine for COVID-19 were published quickly, sometimes in “preprint” form (pending complete peer review). The first studies failed to demonstrate efficacy of the drug. Then, a study involving an astounding 96,000 patients was published in The Lancet indicating that COVID-19 patients who received hydroxychloroquine had significantly higher mortality than did those who did not. The results were swift: funding agencies stopped or paused many of the 131 registered hydroxychloroquine COVID-19 trials that were underway, and the World Health Organization halted its own trial.
After global researchers raised serious doubts regarding the data in the new study, 3 of the authors requested retractions of that paper, as well as a of second paper they had published in the New England Journal of Medicine examining mortality in COVID-19 patients taking certain cardiovascular drugs. They cited questions concerning the data registry that both studies employed.
The database in question was from Surgisphere Corporation (Palatine, Illinois), for which one of the authors was the founder and CEO. Surgisphere—which has little online presence, and appeared to variously have between 3 and 11 employees who seemed to lack science or medical credentials (4)—refused to supply the studies’ datasets for independent review, and the authors declared that they could no longer vouch for the veracity of their primary data source. Both articles were retracted (5,6), and one of the authors has since lost his academic appointment (7). The World Health Organization announced that their trial would resume, and hours later a highly anticipated study was published from the University of Minnesota, which found that hydroxychloroquine failed to prevent COVID-19 (8).
As of this writing, shortages of hydroxychloroquine continue, although it has not been reliably shown to be safe or effective in preventing or treating COVID-19 infections. It continues to be prescribed off-label for COVID-19, with unknown, if any, benefits, despite its considerable and well-described risks. On June 15, the FDA revoked its Emergency Use Authorization for hydroxychloroquine due to lack of efficacy evidence.
A Vaccine at Warp Speed?
On May 15, 2020, President Trump announced a U.S. public-private partnership, the OWSVI (Operation Warp Speed Vaccine Initiative), to accelerate the development, manufacture, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. An explicit goal for the OWSVI is to make available hundreds of millions of doses of an effective vaccine—essentially enough to vaccinate the entire population of the United States—by January 2021 (9). Given that only 15 vaccines were approved in the Unites States between 1995 and 2014, only 1 in 15 vaccines that enter phase II trials is ever licensed, and the average development time for vaccines is usually measured in decades (Table 1) (10), this would indeed be a remarkable achievement.
Table 1.
Example Vaccine Development Times and Approval
| Disease | Time∗ | Year Approved |
|---|---|---|
| Varicella | 27.5 yrs | 1995 |
| Rotavirus | 15 yrs | 2006 |
| Human papillomavirus | 15 yrs | 2006 |
Times listed are the middle of the ranges listed in Douglas and Samant (16).
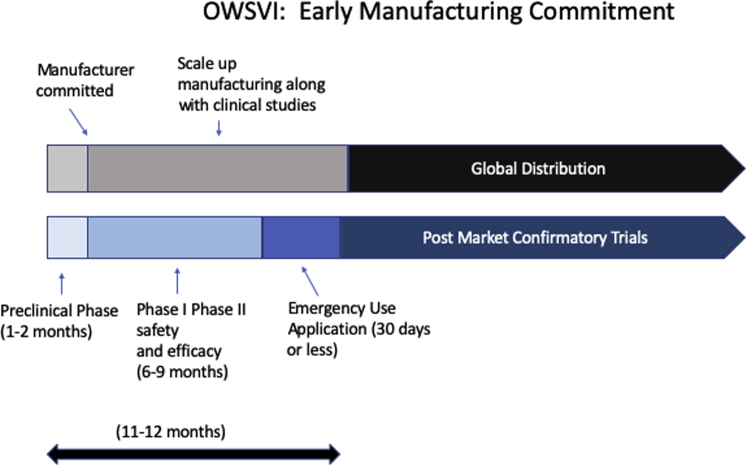
OWSVI partners with the Department of Health and Human Services, Centers for Disease Control and Prevention, FDA, National Institutes of Health, Biomedical Advanced Research and Development Authority (BARDA), Department of Defense, and various other agencies, as well as private firms. It will begin mass production of multiple vaccines based on preliminary evidence (i.e., prior to complete clinical trials and FDA approvals), so that once 1 or more of the vaccines proves clinically effective, distribution can begin immediately without a delay to gear up manufacturing facilities and distribution pathways (Figure 1). The program expects that some of these vaccines will not prove safe or effective. Indeed, the vast majority of vaccines initially tapped for the program will likely fail, and therefore the development of a COVID-19 vaccine will certainly be much costlier than a typical vaccine.
Figure 1.
OWSVI Phases With Early “All-In” Manufacturing Commitments
The manufacturer begins the process to scale up immediately after partnership commitment. Large-scale manufacturing begins during phase II, before clinical efficacy and safety are fully demonstrated, gambling that the vaccine will succeed. Emergency Use Authorization applications are submitted as soon as clinical efficacy has been demonstrated, which in this vaccine’s case will likely be determined by surrogate endpoints or biomarkers rather than by infection rates and mortality. Phase III can then be deferred to post-market clinical studies while global manufacturing and distribution proceed. OWSVI = Operation Warp Speed Vaccine Initiative.
OWSVI has an official budget of $10 billion, and can access additional funds through BARDA (9). Over $2 billion has already been allocated, and 5 vaccines, most in the preclinical phase, have been identified as promising enough to warrant OWSVI funding. AstraZeneca, in partnership with Oxford University, has been awarded over $1 billion in BARDA funding for the development, production, and delivery by next autumn of a vaccine that is currently recruiting for phase II/III trials while they complete phase I (11). Although there are many questions about OWSVI, including concerns over potentially significant commercial and political conflicts of interest within the program leadership, and questions of whether the program will stifle critical coordination and cooperation among other global entities trying to produce a vaccine, it is nevertheless enlightening to examine the strategies OWSVI is deploying to realize such a remarkable timeline (Figure 1).
COVID vaccine research has already benefitted from having been “jump-started” by the SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome) epidemics, which were also caused by coronaviruses (12). Much knowledge relevant to COVID-19 structure and function had already accumulated, and early tests for the virus—essential in assessing effectiveness of vaccines and treatments—appeared by mid-January.
OWSVI uses an “all-in” commercial investment strategy very early in development, and by multiple companies for multiple vaccines. This means that the investors, both government and private, incur very significant financial risks for these products, over 90% of which will almost certainly fail. Those risks involve not only research dollars, but also the proactive building and scaling up of manufacturing and distributing entities prior to completion of clinical studies. The Bill and Melinda Gates Foundation, for example, has committed to build factories for 7 global candidate vaccines. “Even though we’ll end up picking at most two of them, we’re going to fund factories for all seven, just so that we don’t waste time,” Bill Gates said during a recent interview (13). OWSVI plans to amass hundreds of millions of doses of vaccine prior to final safety and efficacy demonstration and regulatory approval, so that immediate deployment of vaccine can occur thereafter. It is virtually a given that developers will be granted accelerated regulatory pathways, allowing innovative and blended trial designs, rather than traditionally designed randomized controlled trials, as well as biomarkers and other novel endpoints to prove efficacy. Developers are also overlapping clinical trial phases in new ways, blending trials, or at the very least providing significant funding for overlapping recruitment for later-phase trials while earlier trials are ongoing, to save time.
COVID-19 was declared a public emergency by the Secretary of Health and Human Services on February 4, who also confirmed that circumstances existed to justify Emergency Use Authorizations (EUAs) for drugs and biological products that allow a product to proceed to clinical use once efficacy has been shown in phase II (14). Such EUAs have authorized the use of hydroxychloroquine and chloroquine, convalescent plasma, hyperimmune globulin, remdesivir, and Fresenius Propoven (propofol) 2% for treatment of COVID-19. EUAs can be issued by the FDA very quickly: automatic authorization occurs if the FDA does not object within 30 days of application, but the FDA can approve sooner, whenever review is complete. After the public health emergency ends, marketing of the drug or vaccine will require completion of a full investigational new drug application, if the drug is not already subject to one, and then a new drug application—although expanded access can be granted under a widespread expanded access treatment application for continued use until full approval is obtained. These accelerated programs have been available since 2012.
It is early in the pandemic, but research timelines have accelerated dramatically. Although the time from viral sequence selection to first human injection of a putative vaccine for SARS was 20 months, it was just 65 days for COVID-19 (12). A massive influx of investment dollars not only promoted rapid preclinical testing, but also is now allowing researchers to organize and recruit large numbers of subjects for human trials. The University of Oxford Virus Group (working with AstraZeneca) announced on May 22 that they were initiating recruitment of over 10,000 patients for what appears to be a type of blended phase II/III trial process while still completing phase I (15). Normally, commercial developers would wait to see full results of a phase I study and FDA review before investing in phases II or III, the most expensive phases of development, or before manufacturing more drug than is needed for such studies. Recruitment of large numbers of study subjects usually takes significant time. A more rapid recruitment of sufficient numbers of subjects for late-phase trials is likely to be facilitated by global public motivation to find a vaccine. Manufacturing is motivated by the promise of future government purchasing contracts, together with an immense, global target population. If a vaccine is developed and distributed for COVID-19 by January 2021, it will be as much due to unprecedented government and commercial financial investments and a highly motivated public as to new changes in FDA regulations.
Footnotes
Dr. Van Norman has received financial support from the Journal of the American College of Cardiology.
The author attests they are in compliance with human studies committees and animal welfare regulations of the author’s institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Mary C. Sound and fury in the microbiology lab. Science. 2012;335:1033–1035. doi: 10.1126/science.335.6072.1033. [DOI] [PubMed] [Google Scholar]
- 2.French researcher posts successful Covid-19 drug trial. The Connexion. https://www.connexionfrance.com/French-news/French-researcher-in-Marseille-posts-successful-Covid-19-coronavirus-drug-trial-results Available at:
- 3.‘What do you have to lose?’ How Trump has promoted malaria drug. The New York Times. https://www.nytimes.com/video/us/politics/100000007101599/trump-coronavirus-hydroxychloroquine.html Available at:
- 4.Servick K., Enserink M. A mysterious company’s coronavirus papers in top medical journals may be unraveling. Science. https://www.sciencemag.org/news/2020/06/mysterious-company-s-coronavirus-papers-top-medical-journals-may-be-unraveling Available at:
- 5.Mehra M.R., Ruschitzka F., Patel A.N. Retraction—hydroxychloroquine or chloroquine with or without macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 Jun 5 doi: 10.1016/S0140-6736(20)31324-6. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Retraction—cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020 Jun 4 doi: 10.1056/NEJMoa2007621. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Herper M., Sheridan K. Researcher involved in retracted Lancet study has faculty appointment terminated, as details in scandal emerge. STAT. https://www.statnews.com/2020/06/07/researcher-involved-in-retracted-lancet-study-has-faculty-appointment-terminated-as-details-in-scandal-emerge/ Available at:
- 8.Boulware D.R., Pullen M.F., Bangdiwala K.A. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020 Jun 3 doi: 10.1056/NEJMoa2016638. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services Trump administration announces framework and leadership for ‘Operation Warp Speed’. https://www.hhs.gov/about/news/2020/05/15/trump-administration-announces-framework-and-leadership-for-operation-warp-speed.html Available at:
- 10.Douglas R.G., Samant V.B. The vaccine industry. In: Plotkin S.A., Orenstein W.A., Offit P.A., Edwards K.M., editors. Plotkin’s Vaccines. 7th edition. Elsevier; Philadelphia, PA: 2018. pp. 41–50. [Google Scholar]
- 11.U.S. Department of Health and Human Services Trump administration’s Operation Warp Speed accelerates AstraZeneca COVID-19 vaccine to be available beginning in October. https://www.hhs.gov/about/news/2020/05/21/trump-administration-accelerates-astrazeneca-covid-19-vaccine-to-be-available-beginning-in-october.html Available at:
- 12.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N Eng J Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 13.Thompson S.A. How long will a vaccine really take? The New York Times. https://www.nytimes.com/interactive/2020/04/30/opinion/coronavirus-covid-vaccine.html Available at:
- 14.U.S. Food and Drug Administration Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization Available at:
- 15.Oxford University Virus Group. Oxford COVID-19 vaccine to begin phase II/III human trials. Oxford University, Oxford UK. May 22, 2020. Available at: http://www.ox.ac.uk/news/2020-05-22-oxford-covid-19-vaccine-begin-phase-iiiii-human-trials. Accessed June 26, 2020.
- 16.Douglas R.G., Samant V.B. The vaccine industry. In: Plotkin S.A., Orenstein W.A., Offit P.A., Edwards K.M., editors. Plotkin’s Vaccines. 7th Edition. Elsevier Inc.; Philadelphia PA: 2018. pp. 41–50. [Google Scholar]