Visual Abstract
Key Words: acute myocardial infarction, adjunctive therapy, large animal model, mitochondrial-derived peptide, myocardial ischemia-reperfusion injury
Abbreviations and Acronyms: AAR, area-at-risk; Bax, Bcl-2–associated X protein; cTnI, cardiac troponin I; DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunoadsorbent assay; h-FABP, heart fatty acid–binding protein; HNG, S14G-humanin analogue; IGFBP3, insulin-like growth factor–binding protein-3; IV, intravenously; LAD, left anterior coronary artery; LV, left ventricular; MDP, mitochondrial-derived peptide; MI, myocardial infarction; MI/R, myocardial ischemia/reperfusion; NIZ, nonischemic zone; RMBF, regional myocardial blood flow; STAT, signal transducer and activator of transcription; TBARS, thiobarbituric acid–reactive substances; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling
Highlights
-
•
A mitochondrial-derived peptide therapy, HNG, was safe and was delivered as adjunctive therapy with standard-of-care reperfusion in a translational large animal model of myocardial ischemia/reperfusion injury.
-
•
HNG reduced infarct size per area-at-risk by 41% with an ischemic time of 60 min followed by 48 h of reperfusion.
-
•
The infarct-sparing effects of HNG were abolished when the ischemic time was increased to 75 min followed by 48 h of reperfusion.
-
•
The use of rigorous translational large animal models that account for clinically relevant variables is a prerequisite to better predict the clinical efficacy and outcomes of novel therapeutic strategies.
Summary
With the complexities that surround myocardial ischemia/reperfusion (MI/R) injury, therapies adjunctive to reperfusion that elicit beneficial pleiotropic effects and do not overlap with standard of care are necessary. This study found that the mitochondrial-derived peptide S14G-humanin (HNG) (2 mg/kg), an analogue of humanin, reduced infarct size in a large animal model of MI/R. However, when ischemic time was increased, the infarct-sparing effects were abolished with the same dose of HNG. Thus, although the 60-min MI/R study showed that HNG cardioprotection translates beyond small animal models, further studies are needed to optimize HNG therapy for longer, more patient-relevant periods of cardiac ischemia.
In the United States, coronary heart disease afflicts >16.5 million individuals, with 48% of those having experienced an acute myocardial infarction (MI) (1). The single most impactful therapeutic intervention developed for MI is reperfusion therapy using percutaneous coronary intervention. Early reperfusion can significantly reduce infarct size and mortality (2). Despite the success of reperfusion therapy, a large portion of these patients still have large infarcts and ultimately develop heart failure. The high incidence of developing heart failure and re-hospitalization represents a major health care issue. Over the last 5 decades, numerous novel therapeutic strategies have shown promise in preclinical studies but have failed in clinical trials (3). Despite the lack of success, it is necessary to develop and test novel adjunctive therapy that can further reduce infarct size, improve survival, and reduce re-hospitalization rates because of the prevalence and socioeconomic burden of coronary heart disease.
Therapeutic agents that inhibit cell death, attenuate oxidative stress, and modulate the inflammatory response have been at the forefront of basic and clinical investigations to salvage the myocardium (4). The mitochondria are a central regulator of cellular energetics and redox signaling that influence many cellular processes, most importantly cell survival and oxidative stress. This has made mitochondria a top target in attempts to mitigate ischemia/reperfusion injury. A wide variety of mitochondrial-targeted therapeutic agents have been tested for efficacy in MI patients (4). Direct reactive oxygen species scavengers, such as triphenylphosphonium or coenzyme Q10 (5,6), metabolic energetic modulators (7), and mitochondrial permeability transition pore inhibitors (i.e., cyclosporin A) (8,9), have all been tested in clinical trials. With neutral results, none is currently approved by the U.S. Food and Drug Administration.
Investigation into small open reading frames within mitochondrial DNA led to the discovery of humanin, a mitochondrial-derived peptide (MDP) (10). Comprising only 24 amino acids, humanin was serendipitously discovered to protect against neurotoxicity caused by formation of amyloid-beta plaque. This MDP is widely conserved across species and is an intracellular and endocrine-signaling molecule (11,12). Humanin was initially shown to be cytoprotective through interaction with insulin-like growth factor–binding protein-3 (IGFBP3) (13) and inhibition of Bcl-2–associated X protein (Bax) activity (11). Ikonen et al. (13) first described the interaction between humanin and IGFBP3 as being cooperative and antagonistic depending on cell subtype and tissue. They also described the presence of humanin in regions of healthy neuronal tissue in the brains of people with Alzheimer disease protecting against IGFBP3-induced cell death. Guo et al. (11), in an in vitro staurosporine-induced cell death model, reported the specificity of humanin to bind Bax in its inactive form, thereby inhibiting the conformational change necessary for Bax translocation into the mitochondria. This lack of translocation leads to a suppression of cytochrome C release, an initiating step for apoptosis.
Humanin has been shown to be a key regulator in mitochondrial homeostasis and other regulatory pathways within the cell that confer cytoprotection, specifically to stress in multiple organs (14, 15, 16, 17, 18, 19, 20, 21, 22). Humanin is an intracellular signaling peptide that is also found in circulation, suggesting that it may also possess endocrine properties. Due to the small size of humanin, a thorough investigation into the functional characteristics of each amino acid has been performed (14). It was determined that the point mutation at serine 14 to glycine (i.e., S14G-humanin [HNG]) increased humanin’s potency to be neuroprotective in the face of gene mutations that lead to early-onset familial Alzheimer disease (10). Several basic research studies have now reported on the ability of HNG to modulate oxidative stress (23,24), regulate insulin sensitivity (25) and cell survival, and inhibit canonical apoptotic signaling (11,26). The administration of HNG in aged mice induces Janus kinase/signal transducer and activator of transcription (STAT) signaling and increases protein kinase B and extracellular signal-regulated kinase 1/2 phosphorylation with downstream effects on mitochondrial respiration and cytoprotection through bcl-2/BAX (16). This scenario has been corroborated by us and others. Exogenous humanin administration leads to activation of STAT3 activation and the extracellular signal-regulated kinase 1/2 pathway triggering cytoprotection against oxygen-glucose deprivation (27) and oxidative stress (21), and improves glucose tolerance and delays onset of diabetes (28).
In addition to their involvement in Alzheimer disease, humanin and MDPs have been implicated in other diseases, including type 2 diabetes (25), obesity (29,30), atherosclerosis (17), and age-related cardiac fibrosis (18). Previously, we showed the importance of humanin-mediated STAT3 activation in the context of insulin sensitivity (25). This study also showed that the circulating levels of humanin decrease with age in rodents and humans. Reduction in endogenous humanin with age correlates with increased age-related oxidative stress burden and cellular damage. Apolipoprotein E–deficient mice, fed a high-cholesterol diet and treated with the humanin analogue (HNGF6A), were protected against endothelial dysfunction and vascular plaque formation via reduction of oxidative and nitrosative stress as measured by reduced nitrotyrosine immunoreactivity, preserved endothelial nitric oxide synthase, and reduced terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (17). A more recent study showed that age-related cardiac fibrosis in C57BL/6N mice was attenuated through the exogenous administration of HNG (18) and corroborated previous findings by which humanin activates canonical antiapoptotic pathways. Continued research, over a wide variety of age-related diseases, has shown that loss of humanin is deleterious and confirmed several signaling cascades that are altered by exogenous humanin during cellular stress, cardiovascular-related morbidities, and other age-related pathologies.
Therapeutic efficacy testing of humanin analogues have shown effects on metabolism, oxidative stress, insulin sensitivity, body weight gain, and cell survival, which are all part of the pathophysiological responses observed during and after an MI (13,16,17,25,28,29,31). Furthermore, we (31) and others (11) have reported on the antiapoptotic effects of a potent humanin analogue (HNG) through inhibition of canonical cell death–signaling pathways. Previously, this humanin analogue reduced infarct size in a dose-dependent manner in a mouse model of myocardial ischemia/reperfusion (MI/R) injury (31). Furthermore, administration of HNG led to improved left ventricular (LV) function 1 week after MI/R injury, suggesting that the acute administration of humanin was cardioprotective and led to sustained improvements in cardiac structure and function after MI/R injury.
Although rodent models are ideal for the initial screening of novel therapeutic agents, it is necessary to use rigorous translational large animal models that better recapitulate the anatomy and pathological responses to injury observed in patients who experience an MI (32). Given our previous work on the potent cardioprotective effects of HNG in rodent models of MI/R injury, we sought to determine the effects of HNG in a more clinically relevant preclinical model of MI/R injury. The current study tested the cardioprotective and translational potential of acute intravenous infusion of a potent humanin analogue just before reperfusion in a porcine model of MI/R injury.
Methods
Animals and study design
Thirty-nine female Yucatan minipigs (S and S Farms, Ramona, California) at 6 to 8 months of age and weighing 30 to 35 kg were subjected to percutaneous MI/R injury. Figures 1A and 1B describe the experimental protocols for the 60 min (n = 16) and 75 min (n = 23) of myocardial ischemia followed by 48 h of reperfusion. Animals were acclimated and maintained on a standard commercial diet (Teklad Miniswine Diet, 8753, Harlan Laboratories, Indianapolis, Indiana). Animals that were excluded or died prematurely are not represented in the study numbers in Figure 1. All animal procedures were approved by the Institute for Animal Care and Use Committee at Louisiana State University Health Sciences Center and handled in compliance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health.
Figure 1.
Experimental Protocols
Female Yucatan minipigs were subjected to either 60 min (A) or 75 min (B) of myocardial ischemia by occluding the left anterior descending (LAD) coronary artery via balloon catheter placement and 48 h of reperfusion. A humanin analogue, S14G-humanin (HNG) (2 mg/kg, IV), or vehicle (saline) was infused 10 min before reperfusion. At baseline, 45 min, or 60 min of LAD occlusion, microspheres labeled with samarium or europium were injected to measure regional myocardial blood flow (RMBF). At baseline, during ischemia, at 15 min, and at 2, 4, 6, 24, and 48 h, blood samples were collected for measurement of cardiac troponin I and heart fatty acid–binding protein. At 48 h of reperfusion, the heart was collected for infarct size determination and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. TTC = triphenyltetrazolium chloride.
MI/R protocol
MI/R injury was performed as previously described using the CAESAR (Consortium for Preclinical Assessment of Cardioprotective Therapies) model with minor modification (33,34). Swine received aspirin (81 mg orally) 1 day before the MI/R procedure. They were sedated with ketamine/xylazine (15:1 mg/kg intramuscularly), administered diazepam (0.5 mg/kg intravenously [IV]) to aid with intubation, mechanically ventilated, and anesthetized with methohexital sodium (Brevital, Par Pharmaceutical, Inc., Chestnut Ridge, New York; 7 to 8 mg/kg/h IV). Pigs were given aspirin (300 mg IV) and an antibiotic (ceftiofur sodium [Naxcel, Zoetis Inc., Parsippany, New Jersey]; 3 mg/kg intramuscularly); electrocardiography, heart rate, respiration, oxygen saturation, arterial blood pressure, and body temperature were monitored continuously. Using a standard sterile technique, an indwelling 7-F polyurethane catheter (HICKMAN, BARD Medical, Covington, Georgia) was placed in the right jugular vein for vehicle or drug infusion and serial blood draws at multiple postoperative time points. Appropriately sized sheath introducers were placed percutaneously in the femoral arteries. An amiodarone (4 mg/kg) and lidocaine (2 mg/kg) bolus were given IV in the perioperative time frame before left anterior descending (LAD) coronary artery occlusion. Maintenance amiodarone (0.04 mg/kg/min IV) and lidocaine (0.05 mg/kg/min IV) were given throughout the occlusion procedure until the 15-min reperfusion time point to reduce the likelihood of arrhythmogenic events occurring during the MI/R procedure. Heparin (300 U/kg IV) was administered and activate clotting time maintained >250 s. Under fluoroscopic guidance (Optima CL323i, GE Healthcare, Chicago, Illinois), a 5-F pigtail catheter (Cordis, Santa Clara, California) was introduced into the left ventricle and was used for microsphere injections, and a 6-F hockey stick catheter (Cordis) was placed in the left coronary ostia for coronary angiography and angioplasty balloon placement. Myocardial ischemia was induced by angioplasty balloon occlusion (2.5 to 3.0 × 6 mm, EMPIRA RX percutaneous transluminal coronary angioplasty; Cordis) of the proximal LAD coronary artery for 60 min (n = 14) or 75 min (n = 19), followed by balloon deflation and confirmed reperfusion via coronary angiography demonstrating complete LAD patency. Buprenorphine (0.025 mg/kg intramuscularly) was administered for post-operative analgesia, and the pigs recovered.
Intravenous administration of potent humanin analogue
The humanin analogue (HNG, GenScript, Piscataway, New Jersey) administered in this study consists of a point mutation at amino acid 14 from a serine to glycine and has been shown to be highly potent toward inhibition of canonical apoptotic signaling (10,31). Pigs were randomized to 60 min of MI and received vehicle (saline; n = 7) or HNG (2 mg/kg; n = 7) or 75 min of MI and received vehicle (saline; n = 9) or HNG (2 mg/kg; n = 10). Ten minutes before reperfusion, 10 ml of vehicle or the HNG was infused via a Hickman catheter at a rate of 2 ml/min over a period of 5 min. HNG or saline was administered in a blinded manner, and all study investigators remained blinded as to the treatment until all data were fully analyzed.
Terminal procedure and euthanasia
After 48 h of reperfusion, the pigs were sedated, ventilated as previously described, anesthetized (1% to 3% isoflurane in oxygen), and administered heparin (300 U/kg IV). A right carotid cutdown for LV catheter microsphere injections was performed as described earlier. Final systemic invasive hemodynamic variables and blood draw were performed, and the animals were subsequently euthanized with potassium chloride (40 mEq IV). The heart was explanted for assessment of infarct size and histological TUNEL staining.
Myocardial area-at-risk and infarct size determination
The procedure was performed as previously described, with minor modifications (34). Briefly, hearts were explanted and rinsed in cold Krebs-Henseleit buffer. The pig hearts were mounted onto a dual perfusion system and maintained at ∼80 mm Hg. The LAD was cannulated at the previous in vivo occlusion site to perfuse the ischemic/reperfused myocardium (area-at-risk [AAR]) with 1% triphenyltetrazolium chloride buffer in phosphate-buffered saline at 37oC and the aortic root for retrograde perfusion with 5% solution of Phthalo blue in saline at 37oC to delineate the infarct and nonischemic zone (NIZ), respectively. The heart was then sectioned from apex to base into 9 to 10 transverse slices. All pig heart slices were photographed (basal and apical sides), weighed (after the right ventricle was removed), and then analyzed for determination of AAR/left ventricle area, infarct/AAR, and infarct/left ventricle area by using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Measurement of regional myocardial blood flow
Regional myocardial blood flow (RMBF) was measured in pig myocardial tissue and blood samples by using stable-isotope neutron-activated microspheres (BioPhysics Assay Laboratory, Inc., Worcester, Massachusetts) as previously described (34). Microspheres were injected at 2 procedural time points: baseline (time 0 min ischemia) and during ischemia (either 45 min or 60 min of ischemia). At each time point, a total of 5 × 106 microspheres (2 ml) labeled with samarium or europium were injected into the LV cavity through the pigtail catheter. A reference blood sample was drawn from the side arm of the arterial sheath catheter using a withdrawal pump at 7 ml/min for 90 s. Transmural LV blocks (≈1 g) were obtained from both the ischemic zone and the NIZ and divided into endocardial and epicardial halves. Tissue and blood samples were processed according to manufacturer instructions and sent for analysis. Absolute RMBF (in milliliters per minute per gram) was calculated by using the following formula:
RMBF = (counts in tissue sample × reference blood sample withdrawal rate)/(counts in the reference blood sample × tissue weight in grams)
Measurement of circulating biomarkers of cardiac injury and oxidative stress
Serial blood samples (∼4.0 ml each) were collected from the indwelling jugular vein catheter in heparinized tubes and centrifuged at 2,100 rpm for 15 min at 4°C to separate plasma. Blood was obtained at pre–MI/R (time point 0), 45 or 60 min of ischemia, 15 min post-reperfusion, and at 2, 4, 6, 24, and 48 h after reperfusion (Figures 1A and 1B). Plasma samples were assessed for levels of cardiac troponin I (cTnI) (Antech Diagnostics or Life Diagnostics, Inc., West Chester, Pennsylvania [60-min study] or by an enzyme-linked immunoadsorbent assay [ELISA] kit [Life Diagnostics, Inc.] [75-min study]) and for heart fatty acid–binding protein (h-FABP) by ELISA (Life Diagnostics, Inc.) as biomarkers of acute cardiac injury. Circulating thiobarbituric acid–reactive substances (TBARS) were measured in the serum by using a commercially available kit (Cayman Chemical, Ann Arbor, Michigan) as an indirect marker of lipid peroxidation induced by oxidative stress.
Histological assessment of apoptosis
Histological assessment of apoptosis was performed on viable myocardium by TUNEL staining. The heart tissue sections from the NIZ were stained by using an in situ cell death detection kit, with fluorescein-labeled dUTP (TMR red, MilliporeSigma, Darmstadt, Germany) according to the manufacturer's instructions. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Images were taken by using Nikon Eclipse E600 fluorescent microscopy (Nikon, Tokyo, Japan). The apoptotic cells were visualized in purple by co-localization of the TUNEL and DAPI. The quantification was conducted by using ImageJ software by counting the purple (TUNEL-positive) cells and blue cells (total nuclei). The percentage of apoptosis was calculated as the TUNEL-positive cells divided by the total number of DAPI-positive cells.
Statistical analyses
All data are expressed as the mean ± SEM. Data were statistically analyzed by using Prism 6 (GraphPad Software, San Diego, California) with a Student’s unpaired, 2-tailed, t-test when comparing 2 groups at a single time point and a repeated 2-way analysis of variance with a Bonferroni post hoc test when performing multiple pairwise comparisons between 2 groups or within a group at multiple time points. A p value of <0.05 were considered statistically significant.
Results
A total of 39 animals were enrolled in the studies (60 min, n = 16; 75 min, n = 23). In the 60-min study, 14 animals completed the protocol (Figure 1A). The survival rate was 87.5% (14 of 16). One animal died during the 60-min myocardial ischemia procedure; this animal did not receive vehicle or HNG. Another died within 24 h after the operation, with no confirmed cause of death. The animal had been randomized to the HNG-treated group; however, this premature death was most likely due to a lethal arrhythmia and not HNG infusion. In the 75-min study, of the 23 animals enrolled, 1 animal was excluded due to repositioning of the angioplasty balloon and another due to lack of complete LAD occlusion (total n = 21). Two animals died before the 48-h time point: 1 died during myocardial ischemia, and another died in recovery. In total, 19 animals completed the 75-min experimental protocol (Figure 1B). The survival rate in the 75-min study was 90.4% (19 of 21). Representative coronary angiography imaging at baseline, during occlusion, and after reperfusion in the 60- and 75-min studies is shown in Supplemental Figure 1.
Regional myocardial blood flow
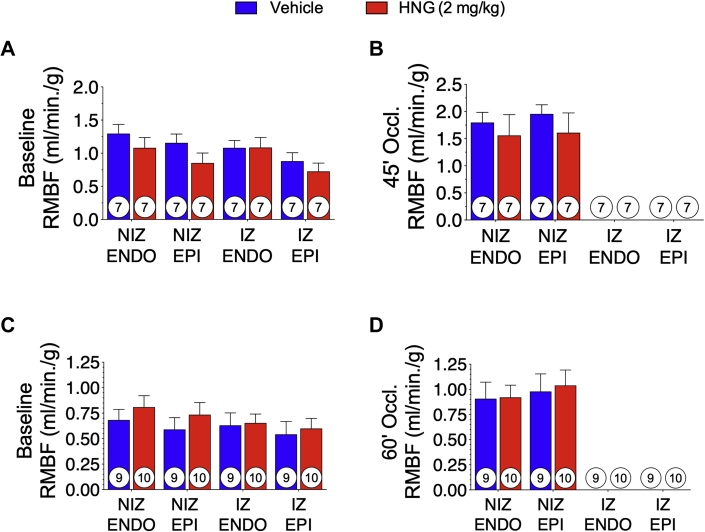
The nonradioactive microspheres were used to assess RMBF at baseline and during balloon occlusion of the LAD coronary artery to confirm ischemia in all animals in these studies. Microspheres were injected at baseline and at 15 min before reperfusion. At baseline, normal blood flow in the epicardium and endocardium of the NIZ and ischemic zone was observed (Figures 2A and 2C). At 15 min before reperfusion, there were no measurable microspheres in the epicardium and endocardium of the ischemic zone in any of the animals included in the study (Figures 2B and 2D). These data indicate the absence of blood flow to the LAD, confirming complete coronary balloon inflation with severe ischemia and lack of collateral coronary circulation within the LAD-perfused region of the heart.
Figure 2.
Regional Myocardial Blood Flow
RMBF was assessed by microsphere quantification and blood flow analysis at baseline and 15 min before reperfusion. In the 60-min study (A) and the 75-min study (B), blood flow was calculated in the endocardium and epicardium of the nonischemic and ischemic regions of the myocardium. Results are shown as mean ± SEM. No statistically significant difference was observed between groups within any region at any time point. Number in circle = number of animals analyzed. ENDO = endocardium; EPI = epicardium; IZ = ischemic zone; NIZ = nonischemic zone; other abbreviations as in Figure 1.
HNG reduced infarct size after 60 min but not 75 min of myocardial ischemia
Ten minutes before reperfusion, vehicle or HNG was administered IV at a dose of 2 mg/kg. Table 1 contains systemic invasive hemodynamics data at baseline, 15 min before and after reperfusion, and at 48 h reperfusion. There was no significant difference in arterial blood pressure during or after the MI/R procedure. Figures 3A and 4A illustrate representative photomicrographs at 48 h post–MI/R of myocardial cross-sections of vehicle- and HNG-treated hearts. Full sets of photomicrographs, from base to apex, that were used for analysis are presented in Supplemental Figures 2 to 5. All animals, in both studies and treatment groups, had a total AAR/left ventricle area of ∼45% (60 min study; p = 0.699; 75 min study; p = 0.179) (Figures 3B and 4B). In the 60-min occlusion study, the infarct area in animals administered vehicle was 56.8% of the AAR and 25.3% of the left ventricle. Myocardial infarct size was significantly (p = 0.017) reduced in the HNG-treated animals compared with vehicle treatment: 56.8% compared with 33.7% infarct/AAR. In contrast, we observed no significant reductions in infarct/AAR (p = 0.541) or infarct/left ventricle area (p = 0.293) in animals subjected to 75 min of myocardial ischemia. These data indicate that acute treatment with the HNG peptide significantly attenuates myocardial cell death after MI/R injury with shorter durations of ischemia but fails to protect the heart with more prolonged ischemic times.
Table 1.
Systemic Invasive Hemodynamics Data
| 60 Min of Ischemia |
75 Min of Ischemia |
|||||
|---|---|---|---|---|---|---|
| Vehicle (n = 7) | HNG (2 mg/kg) (n = 7) | p Value | Vehicle (n = 9) | HNG (2 mg/kg) (n = 10) | p Value | |
| Baseline | ||||||
| Systolic blood pressure (mm Hg) | 129 ± 5.3 | 116.0 ± 4.9 | 0.096 | 126 ± 6.3 | 128 ± 5.4 | 0.831 |
| Diastolic blood pressure (mm Hg) | 96 ± 4.8 | 79.0 ± 2.3 | 0.008 | 91 ± 6.4 | 91.4 ± 4.2 | 0.981 |
| Mean arterial blood pressure (mm Hg) | 112 ± 4.9 | 96.7 ± 3.3 | 0.022 | 109 ± 6.3 | 108 ± 4.7 | 0.984 |
| 45-min occlusion | ||||||
| Systolic blood pressure (mm Hg) | 123 ± 9.9 | 101.0 ± 9.9 | 0.085 | – | – | – |
| Diastolic blood pressure (mm Hg) | 92 ± 8.3 | 81.0 ± 7.7 | 0.291 | – | – | – |
| Mean arterial blood pressure (mm Hg) | 107 ± 6.2 | 84.0 ± 11.3 | 0.088 | – | – | – |
| 60-min occlusion | ||||||
| Systolic blood pressure (mm Hg) | – | – | – | 109 ± 8.6 | 111 ± 6.4 | 0.869 |
| Diastolic blood pressure (mm Hg) | – | – | – | 86 ± 5.7 | 92 ± 6.5 | 0.514 |
| Mean arterial blood pressure (mm Hg) | – | – | – | 96 ± 6.2 | 100 ± 6.8 | 0.687 |
| 15-min reperfusion | ||||||
| Systolic blood pressure (mm Hg) | 111 ± 8.7 | 108.0 ± 10.3 | 0.808 | 98 ± 8.7 | 100 ± 6.0 | 0.837 |
| Diastolic blood pressure (mm Hg) | 79 ± 8.3 | 76.0 ± 8.7 | 0.741 | 78 ± 7.5 | 78 ± 5.4 | 0.916 |
| Mean arterial blood pressure (mm Hg) | 95 ± 5.9 | 91.0 ± 9.5 | 0.740 | 87 ± 8.2 | 88 ± 6.4 | 0.907 |
| 48-h reperfusion | ||||||
| Systolic blood pressure (mm Hg) | 106 ± 5.9 | 108.0 ± 7.0 | 0.809 | 84 ± 6.6 | 93 ± 4.3 | 0.328 |
| Diastolic blood pressure (mm Hg) | 70 ± 7.6 | 69.0 ± 5.0 | 0.903 | 56 ± 5.3 | 66 ± 3.7 | 0.131 |
| Mean arterial blood pressure (mm Hg) | 86 ± 4.1 | 85.0 ± 5.8 | 0.912 | 71 ± 6.4 | 80 ± 4.3 | 0.284 |
Values are mean ± SD. The p value is between groups at time point for given measurement.
HNG = S14G-humanin.
Figure 3.
HNG Administration Reduces Infarct Size in 60-Min Study
(A) Representative cross-sectional photomicrographs of infarct size analysis in a vehicle- and S14G-humanin (HNG)-treated heart. (B) Quantification of percentage of area-at-risk (AAR) to the area of the left ventricle (LV), infarct area (INF) to AAR, and INF to LV. Results are shown as mean ± SEM. Scale bar = 1 cm. Number in circle = number of animals analyzed.
Figure 4.
Infarct Size Analysis in 75-Min Study
(A) Representative cross-sectional photomicrographs of infarct size analysis in a vehicle- and HNG-treated heart. (B) Quantification of percentage of AAR to LV, INF to AAR, and INF to LV. Results are shown as mean ± SEM. Scale bar = 1 cm. No statistically significant difference was observed between groups within any region. Number in circle = number of animals analyzed. Abbreviations as in Figure 3.
HNG administration did not alter circulating biomarkers of myocardial cell injury
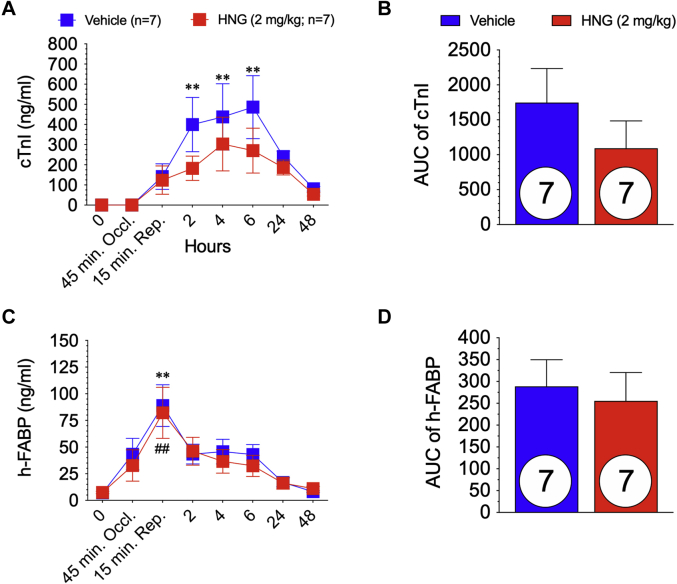
Plasma was collected at baseline, 15 min before reperfusion, 15 min after reperfusion, and 2, 4, 6, 24, and 48 h post–MI/R. Circulating cTnI was measured in all animals to confirm myocardial cell death. In the 60-min ischemia study, cTnI measurements were performed in a blinded manner by an independent laboratory (Antech Diagnostics, Irvine, California). There was a significant increase in circulating cTnI at 2, 4, and 6 h post–MI/R in the vehicle-treated group compared with baseline (p = 0.002; p = 0.001; and p < 0.001, respectively) (Figure 5A). In the HNG-treated animals, there was elevated cTnI post–MI/R but no significant change compared with baseline (p = 0.839; p = 0.066; and p = 0.168, respectively). However, there was no significant difference in cTnI between vehicle and HNG groups at any time point during the experimental protocol, and no difference in the area under the curve (p = 0.32) (Figures 5A and 5B). h-FABP is a highly sensitive and early biomarker of myocardial cell injury (35) and is diagnostic for acute MI (36,37). In the 60-min study, both treatment groups at 15 min of reperfusion had a significantly (p ≤ 0.001) elevated h-FABP (Figure 5C), indicating myocardial injury after MI/R. There was no significant difference in h-FABP between the vehicle and HNG groups at any time point during the 48-h experimental protocol. Furthermore, the area under the curve for h-FABP was also not statistically significantly different between groups (p = 0.714) (Figure 5D).
Figure 5.
Circulating Levels of Biomarkers of Myocardial Infarction in 60-Min Study
(A) Plasma levels of cardiac troponin I (cTnI) at baseline through 48 h post-reperfusion in vehicle- and S14G-humanin (HNG)-treated animals were measured. (B) area under the curve (AUC) of circulating cTnI. (C) Plasma levels of heart fatty acid–binding protein (h-FABP) at baseline through 48 h post-reperfusion in vehicle and HNG-treated animals. (D) AUC of circulating h-FABP. ∗∗p < 0.01, baseline versus time point in vehicle-treated group. ##p < 0.01, baseline versus time point in HNG-treated group. Number in circle = number of animals analyzed.
There was no significant difference in circulating cTnI at any time point throughout the 75-min experimental protocol between groups as measured by using an in-house ELISA kit (Figure 6A). Moreover, there was no significant difference (p = 0.837) in the area under the curve as shown in Figure 6B. As shown in Figures 6C and 6D, h-FABP in circulation was not statistically different between groups throughout the study protocol. Despite a significant reduction in myocardial infarct size (measured by using triphenyltetrazolium chloride) with 60 min of ischemia followed by 48 h of reperfusion, we failed to observe any significant reductions in 2 circulating biomarkers of myocardial injury in either the 60- and 75-min MI/R experimental protocols. HNG failed to protect the porcine heart following 75 min of coronary artery occlusion.
Figure 6.
Circulating Levels of Biomarkers of Myocardial Infarction in 75-Min Study
(A) Plasma levels of cTnI at baseline through 48 h post-reperfusion in vehicle- and HNG-treated animals. (B) AUC of circulating cTnI. (C) Plasma levels of h-FABP at baseline through 48- h post-reperfusion in vehicle-treated and HNG-treated animals. (D) AUC of circulating h-FABP. ∗p < 0.05, baseline versus time point in the vehicle-treated group. ∗∗p < 0.01, baseline versus time point in the vehicle-treated group. #p < 0.05, baseline versus time point in the HNG-treated group. ##p < 0.01, baseline versus time point in the HNG-treated group. Number in circle = number of animals analyzed. Abbreviations as in Figure 5.
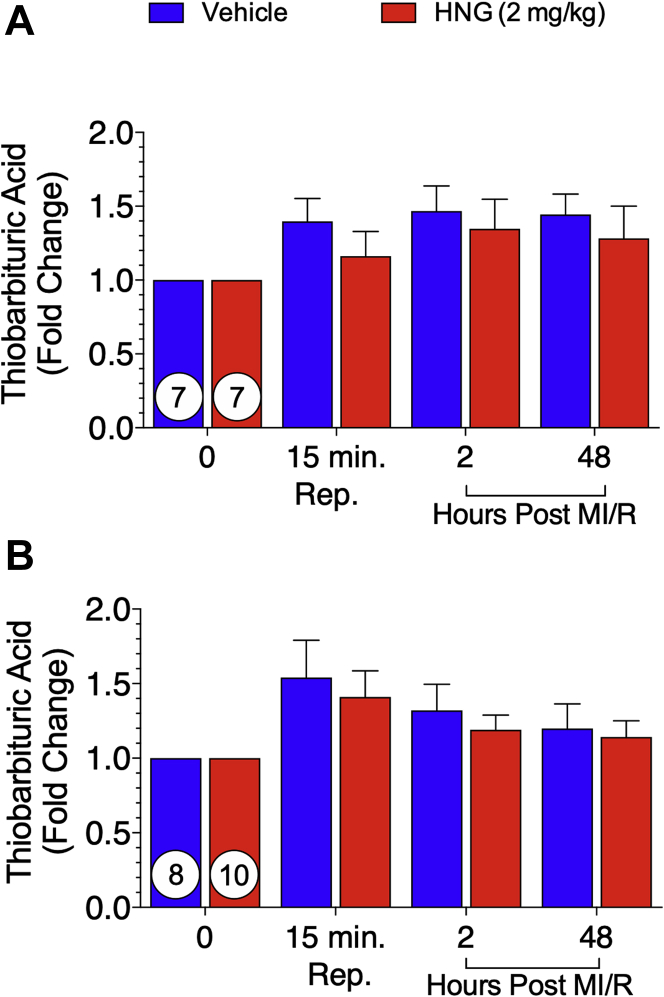
Effects of HNG therapy on global oxidative stress
Increased oxidative stress in the myocardium has been implicated in the pathogenesis of MI/R (31). We and others have shown that HNG mitigates oxidative stress (17,18,31). To study differences in oxidative markers, we assessed TBARS levels in serum (Figure 7), a method for monitoring lipid peroxidation and oxidative stress. There was no difference between groups at baseline. No significant difference was observed in circulating TBARS at 15 min of reperfusion or at 2 and 48 h post–MI/R (Figure 7A). In the 75-min MI/R experimental protocol, no significant difference in circulating TBARS was noted at any time point throughout the study (Figure 7B).
Figure 7.
Measurement of Circulating Thiobarbituric Acid–Reactive Substances
Quantification of thiobarbituric acid–reactive substances in circulation of vehicle- and S14G-humanin (HNG)-treated animals as a fold change from baseline, at 15 min of reperfusion, and 2 h and 48 h post-reperfusion in the 60-min study (A) and the 75-min study (B). In the 75-min study, thiobarbituric acid–reactive substance analysis at 48 h in the vehicle-treated group, N = 7, and in the HNG-Treated group, N = 8. No statistically significant difference was observed between groups at either time point. Number In circle = number of animals analyzed. MR/R = myocardial ischemia/reperfusion.
HNG administration reduced apoptosis in the viable myocardium at 60 min of myocardial ischemia
This study regarded the potent antiapoptotic effects of HNG as a key mechanism of its potential cardioprotective characteristics observed in the 60-min study. To study differences in cytoprotection, we performed histological assessment of apoptosis in the NIZ by TUNEL staining in vehicle-treated and HNG-treated animals after 60-min MI followed by 48 h of reperfusion (Figure 8). Representative photomicrographs show a reduced co-localization of the red (TUNEL stain) and blue (DAPI nuclear stain) channels in the HNG-treated animal (Figure 8A). This finding was quantified by counting the total number of TUNEL-positive cells as a percentage of the total number of DAPI-positive cells. We observed a significant (p = 0.019) 50% reduction in TUNEL-positive cells in the HNG group compared with the vehicle-treated groupe (Figure 8B). These data suggest that inhibition of apoptosis is 1 potential mechanism by which HNG conferred a cardioprotective effect in the 60-min study.
Figure 8.
HNG Reduces Apoptosis in 60-Min Study
(A) Representative photomicrographs of TUNEL staining in vehicle and HNG-treated hearts in the 60-min MI/R study. (B) The percentage of TUNEL-positive nuclei in the nonischemic zone was assessed by quantification of the total number of TUNEL-positive nuclei (purple nuclei) over the total nuclei (blue). Abbreviations as in Figures 1 and 7.
Discussion
For the >800,000 patients who experience a new or recurrent MI each year (1), novel therapeutic strategies to reduce myocardial cell death would have profound effects on their long-term prognosis. In recent years, treatment strategies for patients experiencing acute MI have largely focused on reducing the duration of myocardial ischemia to improve outcomes (38, 39, 40). This approach, commonly referred to as “door-to-balloon-time,” involves improving the diagnosis of acute MI and reducing the time required to perform coronary interventions that restore myocardial blood flow to ischemic regions (39,41). Despite significant reductions in hospital door-to-balloon times, myocardial reperfusion injury after an acute MI remains a significant clinical issue (24,42). It has been reported (23) that despite a shortened time frame from hospital admission to percutaneous coronary intervention (on average, 90 min), there has not been a reduction in mortality. This suggests that myocardial injury after reperfusion may have a more profound impact on overall survival than previously appreciated. The development of adjunctive therapeutic strategies that can be administered in the peri-reperfusion time frame to mitigate infarct size and protect the reperfused myocardium is warranted.
For >40 years, basic and clinical research has failed to deliver an efficacious strategy to limit reperfusion injury as blood flow is restored to the ischemic myocardium. Numerous therapeutic strategies including mechanical, pharmaceutical, and biological approaches have been shown to reduce myocardial infarct size in preclinical animal models but have proved to be ineffective in clinical trials. In rodent and large animal models, strategies of ischemic preconditioning (26), remote ischemic preconditioning (43,44), cardiac hypothermia (45,46), and beta-blockers (47) have been efficacious as adjunctive therapies in limiting infarct size. However, clinical translation and application of these and many other interventions have uniformly failed to reduce infarct size and circulating myocardial injury biomarkers, improve myocardial function, or outcomes in patients with acute MI.
The current study was based on our earlier studies of humanin in rodent models of MI/R injury. Previously, we investigated the infarct-sparing effects of the humanin analogue HNG in a well-characterized murine model of MI/R (31) with 45 min of myocardial ischemia and 24 h or 1 week of reperfusion. In this study, we showed that HNG (2 mg/kg) reduced infarct size per AAR by a maximum of 48% and in a dose-dependent manner. Furthermore, this led to preservation of LV function 1-week post–MI/R with a 42% increase in LV ejection fraction compared with vehicle-treated animals. We also showed that HNG administration activated endothelial nitric oxide synthase and antiapoptotic signaling cascades as the mechanism of cardioprotection. This work provided the impetus for the current study, which investigated the infarct-sparing effect of acute intravenous administration of HNG in the peri-reperfusion period.
The primary purpose of the current study was to assess the potential clinical applicability of humanin to treat acute MI in patients. We hypothesized that humanin, an MDP, would significantly attenuate MI/R infarct size. This study was performed in a highly rigorous manner that involved blinding of all investigators, and the MI/R protocol was directly modeled from the National Institutes of Health CAESAR Consortium porcine MI model (34) without the offsite core laboratories to provide drug and to perform assays and data analysis. We performed this study to mimic the clinical scenario by targeting the “acute phase” with a single administration of HNG given at time of reperfusion under different ischemic times. Our study evaluated varying times of coronary artery occlusion, given the significance of ischemic duration as a major determinant of infarct size (48).
With a 41% reduction in myocardial infarct size to the AAR in the 60-min study, we validated the ability of HNG to reduce infarct size using a large animal swine model of MI/R injury. Furthermore, using the 2 mg/kg dose, the reduction in infarct size observed in the pigs is comparable to the 48% reduction in infarct size we reported in our previous murine MI/R study (31). However, with no significant reductions in the clinically relevant circulating biomarkers (cTnI and h-FABP) used to assess infarct size, these data suggest that HNG provided no benefit in reducing the acute-phase reperfusion injury. Nonetheless, HNG may have attenuated later myocardial cell death over the course of 48 h post–MI/R via inhibition of apoptosis. In this study and as previously described (31), HNG exerted a cytoprotective effect in the presence of oxidative stress and a pro-apoptotic milieu to MI/R injury as measured by reduced TUNEL staining in the NIZ. Although apoptosis occurs throughout the ischemic time frame, reperfusion can accelerate the rate of apoptosis (49). The moderate ischemic time of 60 min coupled with the cytoprotective characteristics of HNG may have affected the rate of apoptosis, thereby leading to less total apoptosis observed at the 48 h time point. Although we did not study the temporal changes in apoptosis, these data suggest a preservation of the myocardium compared with vehicle-treated animals after 48 h of reperfusion. However, when we increased the ischemic time to 75 min, the reduction in myocardial infarct size was lost, and the potential cardioprotective effects of HNG (2 mg/kg) were abolished. Further studies investigating the use of varying doses of HNG, as opposed to a single dose, at various ischemic times are warranted. One could hypothesize that an increased dose was necessary to show the beneficial effects in the 75-min MI/R study. In the current study, we did not perform pharmacokinetic studies to determine the circulating HNG concentration over time, nor did we corroborate the molecular changes in canonical apoptotic signaling or endothelial nitric oxide synthase expression as previously described in our murine model of MI/R (31).
Study limitations
There are several factors that have led to preclinical success being lost in clinical translation. First, animal models have well-established limitations; after all, they are models. Research models do not fully recapitulate the pathological state occurring in human disease, and most, if not all, do not incorporate frequently coinciding comorbidities (i.e., long-standing obesity, type 2 diabetes, hypertension, coronary atherosclerosis). In addition, patients who present with acute MI may be medicated with multiple pharmacotherapies that overlap the target of a novel therapeutic being tested (50,51). Second, the pathophysiological complexity of MI/R must not be overlooked. MI/R involves an intricate set of processes that are interdependent on numerous circumstances (i.e., duration of ischemia, comorbidities, regional access to standard of care). Although experimental approaches typically target a single downstream signaling cascade or gene, it would be advantageous to have strategies that confer pleiotropic effects that are antioxidative, antiapoptotic, mitoprotective, and/or immunomodulatory. Third, in the laboratory models of MI/R, the occlusion of coronary blood flow is acute and total. This is significantly different from the more gradual progression and varying degrees of coronary occlusion in humans that allow establishment of coronary collateral vessels.
The current study data further confirm that the duration of myocardial ischemia is a critical determinant of the cardioprotective efficacy of any novel therapeutic strategy. Although this paradigm is well established (40,52, 53), it is important to take ischemic time into consideration when developing models for testing novel therapeutic agents as adjunctive therapy to reperfusion. Also, the temporal nature of cellular-specific (e.g., cardiomyocytes, endothelial and inflammatory cells) pathophysiological responses (i.e., calcium overload, oxidative stress, mitochondrial dysfunction, apoptosis) to MI/R injury is complex, such that cell death and cell survival signaling pathways occur simultaneously, and the timing of these events is still not fully understood (54). Other variables to consider are adjunctive therapies that are used in standard of care (i.e., antiplatelet therapies, beta-blockade) and the addition of comorbidities (i.e., type 2 diabetes, obesity, hypertension), which are usually lacking in large animal models of MI/R. Sex differences (the current study used female pigs) also play a key role in determining the responses to pathophysiological stimuli (55). Although the current study used an intravenous route of administration, alternative routes should be explored (i.e., intracoronary, intramuscular) to optimize target tissue exposure to HNG. Although these limitations exist, studies in preclinical animal models are more complex, leading to an inability to tease out mechanistic insight but inevitably give us better strength in garnering a more predictable outcome when brought to the clinic.
Conclusions
This study is the first report of the use of humanin in a clinically relevant porcine model of coronary artery occlusion and reperfusion; however, given the plethora of previous studies demonstrating potent cytoprotective effects of humanin in both cell and animal models of injury, we failed to observe cardioprotective actions with a more prolonged period of myocardial ischemia. This study does not detract from the previously described therapeutic potential of humanin and other MDPs in age-related diseases such as diabetes and neurological disorders. Humanin has consistently shown beneficial effects toward type 2 diabetes, obesity, and other cardiovascular diseases. Humanin as a targeted therapeutic which attenuates comorbidities that are risk factors for coronary heart disease may aid in limiting the incidence of acute MI. Alternatively, in progressive heart failure, increased oxidative stress, energy dysregulation, and cell death are therapeutic areas with unmet needs. Humanin might represent a powerful therapeutic to treat heart failure given its capacity to regulate mitochondrial respiration, insulin sensitivity, attenuate oxidative stress, and inhibit apoptosis. Notwithstanding the lack of cardioprotection observed in this study with prolonged ischemia, humanin and other MDPs should be further tested in varying conditions of MI/R and other translational large animal models of the aforementioned metabolic- and cardiovascular-related diseases.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Despite better diagnostic tools and a significant reduction in door-to-balloon time, a recent study shows a lack of corresponding reduction in mortality in patients who experience an acute MI. These data would suggest that myocardial injury after reperfusion remains a clinically relevant unmet therapeutic target. Adjunctive therapies to reperfusion that have the capacity to salvage myocardium through inhibition of apoptotic signaling provide antioxidative capabilities while protecting mitochondrial function would be advantageous. Previously, humanin, an MDP, has been shown to incorporate these pleiotropic effects in several disease models. Here, under varying ischemic times, we administered a single dose of HNG, a potent humanin analogue, as adjunctive therapy to reperfusion. Under a shorter time period of ischemia (60 min), HNG reduced infarct size. When the ischemic time was prolonged, the infarct-sparing effects were lost. Although there was a lack of cardioprotection in this large animal model of MI/R, HNG and other MDPs warrant further investigation into their therapeutic potential for MI/R injury and other co-morbidities that drive the pathogenesis of cardiovascular diseases leading to heart failure.
TRANSLATIONAL OUTLOOK: HNG has never been tested clinically. There are several small animal models that have reported the antiapoptotic, antioxidative, and metabolic homoeostatic regulatory properties of this MDP. Here, for the first time, we performed a study investigating the efficacy of HNG to reduce infarct size in a clinically relevant large animal model of MI/R. Despite positive outcomes in small animal models, this initial large animal study showed that the efficacy of HNG is largely determined by ischemic time. Further studies that vary HNG dose, ischemic times, and comorbidities (i.e., obesity, hypertension, diabetes) and incorporate standard of care for these comorbidities are warranted.
Footnotes
This study was funded by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (1R56HL137711) to Drs. Muzumdar and Lefer. Other National Institutes of Health funding for Dr. Lefer included grants 1R44 HL139161, 1R44 HL139195, and 1R01 HL116571. Drs. Muzumdar and Lefer have a U.S. patent on the use of humanin and humanin analogues to treat acute myocardial infarction. Dr. Sharp is funded through a grant from the American Heart Association 18POST34020143. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental figures, please see the online version of this article.
Contributor Information
Radhika H. Muzumdar, Email: radhika.muzumdar@chp.edu.
Traci T. Goodchild, Email: tgoodc@lsuhsc.edu.
Appendix
References
- 1.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 3.Lefer D.J., Marban E. Is cardioprotection dead? Circulation. 2017;136:98–109. doi: 10.1161/CIRCULATIONAHA.116.027039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloner R.A., Brown D.A., Csete M. New and revisited approaches to preserving the reperfused myocardium. Nat Rev Cardiol. 2017;14:679–693. doi: 10.1038/nrcardio.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni R., Cao T., Xiong S. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maulik N., Yoshida T., Engelman R.M., Bagchi D., Otani H., Das D.K. Dietary coenzyme Q(10) supplement renders swine hearts resistant to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;278:H1084–H1090. doi: 10.1152/ajpheart.2000.278.4.H1084. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q., Camara A.K., Stowe D.F., Hoppel C.L., Lesnefsky E.J. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 8.Piot C., Croisille P., Staat P. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 9.Cung T.T., Morel O., Cayla G. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto Y., Niikura T., Tajima H. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo B., Zhai D., Cabezas E. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Y., Kurita M., Aiso S., Nishimoto I., Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikonen M., Liu B., Hashimoto Y. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen K., Lee C., Mehta H., Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50:R11–R19. doi: 10.1530/JME-12-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuliawat R., Klein L., Gong Z. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the beta cell. FASEB J. 2013;27:4890–4898. doi: 10.1096/fj.13-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S.J., Guerrero N., Wassef G. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget. 2016;7:46899–46912. doi: 10.18632/oncotarget.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh Y.K., Bachar A.R., Zacharias D.G. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219:65–73. doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Q., Mehta H., Yen K. Chronic treatment with the mitochondrial peptide humanin prevents age-related myocardial fibrosis in mice. Am J Physiol Heart Circ Physiol. 2018;315:H1127–H1136. doi: 10.1152/ajpheart.00685.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X., Urbieta-Caceres V.H., Eirin A. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci. 2012;91:199–206. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobb L.J., Lee C., Xiao J. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 2016;8:796–809. doi: 10.18632/aging.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein L.E., Cui L., Gong Z., Su K., Muzumdar R. A humanin analog decreases oxidative stress and preserves mitochondrial integrity in cardiac myoblasts. Biochem Biophys Res Commun. 2013;440:197–203. doi: 10.1016/j.bbrc.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nashine S., Cohen P., Chwa M. Humanin G (HNG) protects age-related macular degeneration (AMD) transmitochondrial ARPE-19 cybrids from mitochondrial and cellular damage. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menees D.S., Peterson E.D., Wang Y. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. doi: 10.1056/NEJMoa1208200. [DOI] [PubMed] [Google Scholar]
- 24.Hausenloy D.J., Botker H.E., Engstrom T. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2016;38:935–941. doi: 10.1093/eurheartj/ehw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzumdar R.H., Huffman D.M., Atzmon G. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.W., Whittaker P., Kloner R.A. The transient nature of the effect of ischemic preconditioning on myocardial infarct size and ventricular arrhythmia. Am Heart J. 1992;123:346–353. doi: 10.1016/0002-8703(92)90645-c. [DOI] [PubMed] [Google Scholar]
- 27.Gao G.S., Li Y., Zhai H. Humanin analogue, S14G-humanin, has neuroprotective effects against oxygen glucose deprivation/reoxygenation by reactivating Jak2/Stat3 signaling through the PI3K/AKT pathway. Exp Ther Med. 2017;14:3926–3934. doi: 10.3892/etm.2017.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang P.T., Park P., Cobb L.J. The neurosurvival factor humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta H.H., Xiao J., Ramirez R. Metabolomic profile of diet-induced obesity mice in response to humanin and small humanin-like peptide 2 treatment. Metabolomics. 2019;15:88. doi: 10.1007/s11306-019-1549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Z., Su K., Cui L. Central effects of humanin on hepatic triglyceride secretion. Am J Physiol Endocrinol Metab. 2015;309:E283–E292. doi: 10.1152/ajpendo.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzumdar R.H., Huffman D.M., Calvert J.W. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30 doi: 10.1161/ATVBAHA.110.205997. 1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houser S.R., Margulies K.B., Murphy A.M. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 33.Sharp T.E., Polhemus D.J., Li Z. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J Am Coll Cardiol. 2018;72:2609–2621. doi: 10.1016/j.jacc.2018.08.2186. [DOI] [PubMed] [Google Scholar]
- 34.Jones S.P., Tang X.L., Guo Y. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res. 2015;116:572–586. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii J., Ozaki Y., Lu J. Prognostic value of serum concentration of heart-type fatty acid–binding protein relative to cardiac troponin T on admission in the early hours of acute coronary syndrome. Clinical Chemistry. 2005;51:1397–1404. doi: 10.1373/clinchem.2004.047662. [DOI] [PubMed] [Google Scholar]
- 36.Kilcullen N., Viswanathan K., Das R. Heart-type fatty acid-binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007;50:2061–2067. doi: 10.1016/j.jacc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Haltern G., Peiniger S., Bufe A., Reiss G., Gülker H., Scheffold T. Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol. 2010;105:1–9. doi: 10.1016/j.amjcard.2009.08.645. [DOI] [PubMed] [Google Scholar]
- 38.Greulich S., Mayr A., Gloekler S. Time-dependent myocardial necrosis in patients with ST-segment-elevation myocardial infarction without angiographic collateral flow visualized by cardiac magnetic resonance imaging: results from the multicenter STEMI-SCAR Project. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradley E.H., Herrin J., Wang Y. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med. 2006;355:2308–2320. doi: 10.1056/NEJMsa063117. [DOI] [PubMed] [Google Scholar]
- 40.Francone M., Bucciarelli-Ducci C., Carbone I. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:2145–2153. doi: 10.1016/j.jacc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Bradley E.H., Roumanis S.A., Radford M.J. Achieving door-to-balloon times that meet quality guidelines: how do successful hospitals do it? J Am Coll Cardiol. 2005;46:1236–1241. doi: 10.1016/j.jacc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Kloner R.A., Hale S.L., Dai W., Shi J. Cardioprotection: where to from here? Cardiovasc Drugs Ther. 2017;31:53–61. doi: 10.1007/s10557-016-6691-0. [DOI] [PubMed] [Google Scholar]
- 43.Przyklenk K., Bauer B., Ovize M., Kloner R.A., Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 44.Birnbaum Y., Hale S.L., Kloner R.A. Ischemic preconditioning at a distance. Circulation. 1997;96:1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 45.Dai W., Herring M.J., Hale S.L., Kloner R.A. Rapid surface cooling by ThermoSuit system dramatically reduces scar size, prevents post–infarction adverse left ventricular remodeling, and improves cardiac function in rats. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hale S.L., Kloner R.A. Myocardial hypothermia. J Cardiovasc Electrophysiol. 1999;10:405–413. doi: 10.1111/j.1540-8167.1999.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 47.Hammerman H., Kloner R.A., Briggs L.L., Braunwald E. Enhancement of salvage of reperfused myocardium by early beta-adrenergic blockade (timolol) J Am Coll Cardiol. 1984;3:1438–1443. doi: 10.1016/s0735-1097(84)80282-x. [DOI] [PubMed] [Google Scholar]
- 48.Reimer K.A., Lowe J.E., Rasmussen M.M., Jennings R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 49.Eefting F., Rensing B., Wigman J. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Cohen M.V., Downey J.M. Signalling pathways and mechanisms of protection in pre- and postconditioning: historical perspective and lessons for the future. Br J Pharmacol. 2015;172:1913–1932. doi: 10.1111/bph.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye Y., Birnbaum G.D., Perez-Polo J.R., Nanhwan M.K., Nylander S., Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35:1805–1814. doi: 10.1161/ATVBAHA.115.305655. [DOI] [PubMed] [Google Scholar]
- 52.Hochman J.S., Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 1987;75:299–306. doi: 10.1161/01.cir.75.1.299. [DOI] [PubMed] [Google Scholar]
- 53.Brodie B.R., Webb J., Cox D.A. Impact of time to treatment on myocardial reperfusion and infarct size with primary percutaneous coronary intervention for acute myocardial infarction (from the EMERALD Trial) Am J Cardiol. 2007;99:1680–1686. doi: 10.1016/j.amjcard.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 54.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beale A.L., Meyer P., Marwick T.H., Lam C.S.P., Kaye D.M. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138:198–205. doi: 10.1161/CIRCULATIONAHA.118.034271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.