Abstract
Tuna species: Skipjack (Katsuwonus pelamis), yellowfin (Thunnus albacares) and bigeye (Thunnus obesus) are mainly processed into canned products (loins, solid pack, flakes) either in water or oil, and pre-cooked frozen loins. The National Institute of Fisheries of Ecuador (ISO/IEC 17025 certified), which is the official control laboratory, samples and analyses production batches of companies exporting to the European Union in order to ensure the quality control of Ecuadorean tuna product. From 2009 to 2016, 2572 samples have been analysed (by standard methods) for mercury, cadmium, and lead. The averages were 0.24 ± 0.14; 0.03 ± 0.03 and 0.05 ± 0.05 mg kg−1 (wet weight) respectively; which are well below the norms; i.e., total mercury: 1 mg kg−1; Lead: 0.3 mg kg−1 and Cadmium: 0.1 mg kg−1 according to the EU maximum limits. Over time mercury levels in the sample seemed to decrease but for cadmium and lead no clear pattern was observed. Additionally; samples of tuna can products taken at random from local vendor stores gave concentrations of: Mercury: 0.043 ± 0.004 mg kg−1; Cadmium: 0.012 ± 0.002 mg kg−1; Lead: below detection limit (0.01 mg kg−1). There were a few cases (15 out of 2572: 0.58%) of samples with readings near or just over-limit concentrations; of these, 12 corresponded to Cd, two to Pb and one to Hg. Some of them can be considered statistical outliers as well as cross contamination during analytical procedures. Raw tuna samples have given similar or lower concentrations. No significant statistical correlation was found between Hg, Cd and Pb values, this would suggest that the bioaccumulation of each metal is independent of each other. Literature reports that surface dissolved Hg, Cd, and Pb in the eastern Pacific are in the range of 2–18 ng kg−1. Assuming suggested bioaccumulation of 2–6 times, the end concentration in the tuna would be 0.012–0.042; 0.036–0.108 and 0.010–0.027 μg kg−1 of Hg, Cd, and Pb respectively, that would be one order (or more) below the safe consumption limit. Most, if not all the tuna processed in Ecuador is captured in the eastern Pacific and within its EEZ. Ecuadorian canned tuna complies with stringent standards for presence of these metals; therefore, it can be considered safe to be consumed from the point of view of these metal concentrations. However, further studies should assess metal concentrations exclusively from Ecuadorian tuna captured close to coastal and insular areas.
Keywords: Food science, Food analysis, Environmental science, Ecuador, Mercury, Cadmium, Lead, Tuna, Eastern Pacific
Food science; Food analysis; Environmental science; Ecuador; Mercury; Cadmium; Lead; Tuna; Eastern Pacific.
1. Introduction
Dissolved metals in the water can be removed from the water by adsorption on suspended particulate matter, phytoplankton uptake and later deposition of particulate organic/inorganic matter to the deeper water column and finally to the seafloor, where it can be sequestrated or recycled back to the water column; for example, see: Mercury (Gworek et al., 2016), Cadmium (Van Urk and Marquenie, 1989; Cullen and Maldonado, 2013), Lead (Burnett et al., 1980; Cullen and McAlister, 2017). Also, they can be removed/recycled via the atmosphere in the gaseous phase, rain scavenging (Driscoll et al., 2013; Obrist et al., 2018) and snow (Planchon et al., 2002) or in the emulsion as a spray back to coastal areas. Removal from the oceans also occurs through captured fish species of the third and higher trophic level, especially as any type of edible predator fish such as tunas (Thunnus alalunga, T. obesus, T. albacares, Katsuwonus pelamis, etc.), swordfish (Xiphias gladius), mahi-mahi (Coriphaena hypurus, Adams 2009). A recent review (Gworek et al., 2016) evaluated the fate of Hg in coastal and oceanic waters finding that anthropogenic inputs are much larger than natural ones in some areas, especially in coastal waters from where the metals are redistributed and disseminated by tidal and littoral currents (e.g., Mart et al., 1982). Cadmium is a trace metal whose vertical and horizontal follow similar patterns as micronutrients, particularly as phosphate and nitrate (Ormaza-González, 1990); its concentrations are very low on the ocean surface due to phytoplankton uptake (Xu and Morel, 2013; Janssen et al., 2014). This metal has equally a biogeochemistry largely affected by anthropogenic activities since the industrial revolution due to fuel-burning and non-ferrous metal extraction (Cullen and Maldonado, 2013), like Hg (Gworek et al., 2016). Lead does not have any biological benefit. On the contrary, it is highly toxic, and its oceanographic content and biogeochemistry has been accelerated up by the industrial revolution (Cullen and McAlister, 2017).
Many elements, such as Hg, Cd, and Pb are bioaccumulated through the food chain (Squadrone et al., 2013) from the bottom (phytoplankton) to top levels (predator) of the trophic chain, even though there is no clear biological role (Mwashote, 2003; Xu and Morel, 2013). This bioaccumulation would depend basically on water body location where fish grow, age, species and tissue (muscles, liver, etc.) where there is higher bioaccumulation. For example, principally they tend to be in the muscles or fillets, which are the most edible and preferable part of fish. There are many possible health risk implications consuming some fish species with high concentrations of these and other metals (Castro-González and Méndez-Armenta, 2008; Bosch et al., 2016a; Ahmed et al., 2018; FAO, 2018). Many countries have alerts on these risks (Kumar, 2018); however, the population generally does not take into consideration this fact (Burger and Gochfeld, 2006; Ryder et al., 2014; FDA, 2018; EU, 2006; Galimberti et al., 2016). One of the sea fish species that can accumulate an important amount of these heavy metals are tunids, especially large ones, like albacore (T. alalunga), yellowfin (T. albacares), bigeye (T. obesus), blue fin (T. thynnus), in part because they filtrate an enormous amount of phytoplankton and predate lower levels of the trophic chain. The FDA (2018), one of the most informed seafood quality control agencies, reports Hg average content of samples from 1990 to 2012 content of a whole range of species. It reported the highest amount of Hg averaged 1.45 mg kg−1 for Tilefish (Mexico), followed by Shark and Swordfish (0.995 mg kg−1) whilst the lowest is reported for scallop/shrimp (0.001 mg kg−1). Tuna species (fresh, frozen, and canned) have registered Hg concentrations from 0.689 (bigeye) to 0.128 mg kg−1 (all species canned light); however, Chen et al. (2014) have reported concentrations up to 0.929 and 0.668 mg kg−1 for bigeye tuna. For Cadmium and Lead, 0.06 ± 0.01 and 0.32 ± 0.03 mg kg−1 respectively for yellowfin (El-Moselhy et al., 2014); and 0.02 ± .01 and 0.10 ± 0.03 mg kg−1 for blue fin (Thunnus thymus), in the same order (Storelli et al., 2005). More recently, Zuluaga et al. (2015) have reported a review on these metal concentrations in different for marine genera: Caranx, Scomberomorus, Epinephelus, Euthynnus, Lutjanus, and Megalops.
Recent evaluation of fish consumption finds that seafood is providing 20.5 kg/year per capita (FAO, 2018), of this roughly 55% comes from the sea (the rest from continental fishing and aquaculture/mariculture). Tuna is about 7% of the total fish capture and growing. Ecuador is the second most important country producing tuna products, especially in cans and pouches. A few years ago, Ecuadorian canning plants were processing around 200 thousand metric tons (mt) of raw tuna; nowadays this volume is well above 476 thousand mt (2015: 482 4489; 2016: 471 423 and 2017: 477 113 mt; IATTC, 2018) with a value of over 1.108 billion US dollars. Of this, nearly 66 % is exported to EU, 8.7 % to the USA and the rest to many countries and/or for national consumption (Anastacio, 2019). Therefore, the tuna canning industry including its fisheries is a real economic-social matter. Hence, for Ecuador, the tuna industry must be aligned to quality and food safety. The present work tries to research the concentrations of Hg, Cd, and Pb in canned tuna produced in Ecuador in the period 2009–2016, their behaviour over time and how they could interrelate between them. As well, it strives to explain such findings in terms of the oceanographic conditions of the eastern Pacific where the tuna processed in Ecuador is generally caught.
2. Materials and methods
2.1. Local sample collection and preparation
Most of the data analyzed in this study came from the quality and product safety certificates issued by INP laboratories (ISO/IEC 17025 certified) which included measures of Hg, Pb and Cd concentrations. The products registered in the certificates were canned fish (solid, chunks and flake form in water or oil), pre-cooked frozen loins and few samples of fresh fish. Two thousands five hundred seventy-two samples were collected and analysed in the period 2009–2016. The sampling was executed on batch production basis, which allows traceability of the tuna right down to where it was captured to the can and even to who it was sold. These samples included canned and pre-cooked tuna loins tuna. The samples were taken from the canneries and mainly included species such as skipjack (Katsuwonus pelamis), yellowfin (Thunnus albacares), and big eye (Thunnus obesus) tuna captured by Ecuadorian and other countries fleets that operate in the eastern Pacific international waters and within their EEZ. Ecuadorian fleet mainly captures Skipjack tuna and it makes up >50 % of the raw material, followed by yellow fin and big eye. Besides, canned tuna samples (9 samples in total, 5 main brands), were purchased at random from local store shelves located in Guayaquil (Ecuador), which is the city of Ecuador with one of highest tuna consumption rates. Before being taken to the laboratory, labels from cans were removed and they were coded. Then, samples were transported to laboratory and stored in a clean dry area. Before executing analysis for each sample, oil or water was drained from the canned tuna and submitted to analysis. All the samples contained only white muscular tissue (fresh loins); the muscles tend to accumulate the highest concentrations of these metals (Bosch et al., 2016b; Al-Najjar et al., 2018; Ruelas-Inzunza et al., 2018). Since no differences has been found between canned and fresh tuna regarding Cd (Flores et al., 2018), therefore, the study of canned and pre-cooked loins samples would represent fresh tuna loins as well.
2.2. Laboratory analysis
2.2.1. Mercury
It was determined by microwave digestion/cold vapor atomic absorption spectroscopy (Morgan et al., 1997; Evans et al., 2010). A standard calibration curve (0.0, 2.5, 5.0 and 10 μg [Hg] dm−3) was prepared in 10 cm3 of acid mixture (1.5% HNO3 and 1.5% H2SO4-). The sample preparation consisted in weighing 0.25 ± 0.05 g wet weight in the Teflon cups of the microwave, then 10 cm3 of 65% concentrated nitric acid were added. The stannous chloride dehydrate was used as the reducing solution. The wavelength used for Hg was 253.7 nm. Measurement was made by atomic absorption spectrophotometer (cold vapor) Varian SpectrAA (model 220z/220fs, see http://photos.labwrench.com/equipmentManuals/17060-6201.pdf). The SPECTRAA program was used for the calibration curve and gave results automatically.
2.2.2. Cadmium and lead
The determination method used was the AOAC (2002) Official Method 999.10. for Lead, Cadmium, Zinc, Copper, and Iron in food and determined by atomic absorption spectrophotometry, Pb and Cd were determined by graphite furnace (GFAAS), with appropriate background correction. Analyte range using GFAAS are ≥0.1 mg kg−1 for Pb and ≥0.01 mg kg−1 for Cd. Cd calibration plot (0, 1, 3, and 5 μg [Hg] dm−3) and Pb calibration plot (0, 50, 100 and 200 μg [Hg] dm−3) were prepared in 0.2% HNO3 and samples were diluted 1:4. It was weighed 0.2–0.5 g of wet material into digestion vessel. The digestion principle consisted on getting HNO3 and H2O2 under pressure in a closed vessel heated by microwaves. Then, the digestion vessels were taken out for cooling after the process. For dilution, H2O was used. The wavelengths used for Pb and Cd were 283.3 nm and 228.8 nm, respectively.
Calibration reference material used the homogenate fish standard from the IAEA Reference Products for Environment and Trade: IAEA-407 (https://nucleus.iaea.org/sites/ReferenceMaterials/Pages/IAEA-407.aspx) which is a standard made from mainly a pelagic fish as herring (Scombridae family), but the sample material also contained capelin and anchovy. Also LUST-1 and TORT2 were used. Reagent blanks, fortified (spiked) samples, recovery tests were in the order 100 ± 20%.
In general, when higher than allowed standard concentrations were obtained, stricter re-sampling process was made, all analytical parameters were reviewed, and analysis remade; thus, the initial value was validated or refused.
2.3. Statistical analyses
A database was built from the INP records of Quality Certificates, getting a record of 8 years (2009–2016) and input it to Excel software. Descriptive statistics (mean, standard deviation, range, correlation equations) were obtained using this software. Four different approaches were made: 1) scatter graphs plotting the heavy metals data per sample against time, 2) distribution graphs plotting the heavy metal data (transformed using normal probability density formula, not shown), 3) the mean concentration levels of heavy metals in tuna plotted (boxplot) per year of record, 4) correlations between Hg (assumed as independent variable) and Cd and Pb were researched year by year to analyze the possible relationship between them.
3. Results and discussion
Ecuador processes mostly light tuna skipjack followed by yellowfin for canned products, and bigeye and yellowfin for pre-cooked loins according to the records examined. The main products: canned tuna can be solid, chunks or flake form in water or oil. Pre-cooked loins of yellowfin and bigeye are also processed.
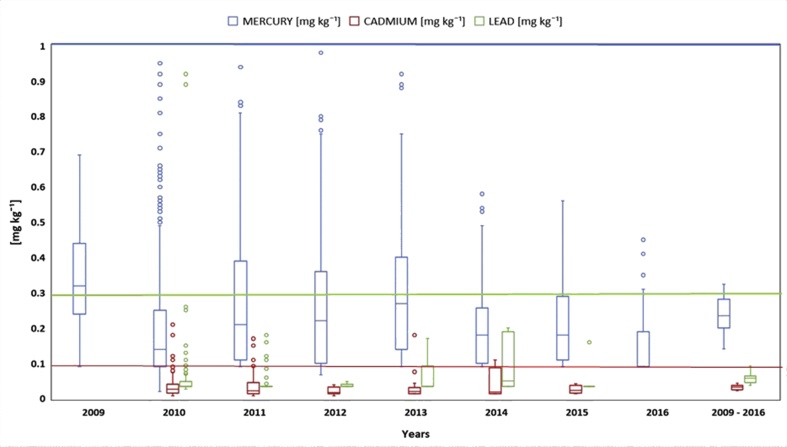
Mercury, Cadmium and Lead concentrations (wet weight basis) from 2009 to 2016 did not show a specific pattern in time, however, the tendency was to show a slight decline in that period (Figure 1). The range values were 1.490 - 0.021, 0.210 - 0.007 and 0.920–0.026 mg kg−1 respectively. On the other hand, the average concentrations for the whole studied period were 0.23 ± 0.14; 0.032 ± 0.027 and 0.058 ± 0.051 mg kg−1 in the same order (Table 1). The concentration tendency was to decrease in time for the studied period, it could be ascribed to the constant increase of skipjack tuna captures in the eastern Pacific, whose reported volumes in 2009 were 239 408 metric tons (mt) while in 2016 were 342 579 mt (IATTC, 2018), therefore an increased volume of this tuna species in Ecuadorian canneries. Skipjack tuna has been reported to have in general lower Hg than albacore (Thunnus alalunga) or white-style tuna (0.407 mg kg−1; Burger and Gochfeld, 2004); meanwhile Torres et al. (2016) reported that bigeye tuna (Thunnus obesus) has a higher concentration (0.139 mg kg−1) than skipjack (0.04 mg kg−1), because the latter feeds mainly on invertebrates whilst the former is piscivorous. Another plausible reason for the tendency is improvement over the years in the handling practices during initial refrigeration-freezing using brine on the boat. When the freezing process is better controlled the tuna is not permeated by the freezing brine, that could have up to 25% sea salts which include metals and other elements. It is accepted knowledge in the tuna industry that good handling practices on board the fishing boats (Carlson, 1948) maintains the tuna with almost with its natural content of NaCl of around 0.3%, whilst poor procedures (Mensah, 2013) could render salt a variable content up to 8 % in the tuna loins.
Figure 1.
Behavior of Hg, Cd, and Pb concentrations in tuna during the studied period. Norms for Mercury (1 mg kg−1), Cadmium (0.1 mg kg−1) and Lead (0.3 mg kg−1) are represented with thin red lines in accordance with: European Union, Maximum content in Heavy Metals in food products, EU (2006).
Table 1.
Summary of the concentration (mg kg−1) averages (±1SD) of Hg, Pb and Cd in canned tuna per every year of the studied period. Data source: INP.
| Year | Samples | Hg | Cd | Pb |
|---|---|---|---|---|
| 2009 | 34 | 0.30 ± 0.016 | ||
| 2010 | 759 | 0.20 ± 0.15 | 0.034 ± 0.031 | 0.064 ± 0.117 |
| 2011 | 562 | 0.27 ± 0.18 | 0.040 ± 0.030 | 0.046 ± 0.300 |
| 2012 | 377 | 0.26 ± 0.18 | 0.021 ± 0.011 | 0.038 ± 0.005 |
| 2013 | 377 | 0.25 ± 0.17 | 0.032 ± 0.037 | 0.060 ± 0.040 |
| 2014 | 294 | 0.20 ± 0.11 | 0.044 ± 0.039 | 0.093 ± 0.072 |
| 2015 | 89 | 0.21 ± 0.11 | 0.026 ± 0.011 | |
| 2016 | 80 | 0.14 ± 0.08 | ||
| 2009–2016 | 2572 | 0.23 ± 0.14 | 0.032 ± 0.027 | 0.058 ± 0.050 |
| Limits | 1.00 | 0.1 | 0.3 |
The bold values can be represents the plain text.
It was attempted to find the type of statistical distribution using normal probability density formula and correlation analysis for the whole period and individual years per metal. There were no distributions that fit the data, the curves found were mainly biased to the right without any consistency. The correlation analysis did not show any significant statistical pattern in time (per year).
Table 1 shows the average concentrations of the three metals studied in the period 2009–2016. Mercury showed consistently the highest levels and its averages were in the range of 0.14 (2016) to 0.30 (2009) mg kg−1, followed by Lead with 0.038 (2012) to 0.093 (2014) mg kg−1 and Cadmium: 0.021 (2012) – 0.044 (2014) mg kg−1. The box plot graphic depicted in Figure 2, shows around 50 outliers in eight-year period with a tendency to decrease in numbers in time, thus in 2010 from 15, 7 and 5 for Hg, Pb and Cd, in 2016 to only 3, 0 and 0 respectively. All outliers were skewed to the right, nonetheless all of them within the permissible limits, except 8 that belong to Cadmium. The declining number of outliers and skewness to the right of the data distribution would go along to what said above about the improvement of on boat freezing practices. It is also shown that the concentration order is Hg > Pb > Cd in every year, and they are statistically significant well below the limit of the norm. The sequence of concentrations has been generally reported for tuna (Khansari et al., 2005; Storelli et al., 2010; Abolghait and Garbaj, 2015). On the other hand, the samples obtained from the store shelves of different popular distribution points in Guayaquil-Ecuador rendered values of mercury: 0.043 ± 0.004 mg kg−1; cadmium: 0.012 ± 0.002 mg kg−1; lead < dl (0.01 mg kg−1), which were below 5.75, 2.6 and 5.8 times than the average of the archival data, and much less than allowable concentrations.
Figure 2.
Annual average concentration of Mercury, Lead and Cadmium in tuna products from Ecuador. The safety limits are indicated by the blue (Hg), green (Pb) and red (Cd) lines. Data source INP.
The concentrations reported here are similar to previously published papers; thus, for example, Khansari et al. (2005) reported for Hg: 0.043–0.253; Pb: 0.0366 mg kg−1, and Cd: 0.0046–0.0720 mg kg−1 in canned tuna from the Persian Gulf. In a similar study, Ikem and Egiebor (2005) registered ranges of 0.020–0.739, 0.000–0.050 and 0.00–0.030 mg kg−1 (ww) in the same order from samples collected in US supermarkets; where most of the canned tuna is imported from Thailand, Ecuador, Indonesia, and the Philippines (Brinson et al., 2015). Voegborlo et al. (1999) registered concentrations averaging 0.29, 0.18 and 0.40 for Hg, Cd, and Pb from tuna captured in Libya Mediterranean coasts, respectively. Also, Abolghait and Garbaj (2015) reported concentrations of Hg, Cd, and Pb in canned tuna (skipjack and yellowfin) sold in Tripoli market. These levels were well under permitted ones; thus, the lowest values for Hg were 0.163 ± 0.122; Cd: 0.027 ± 0.026, and Pb: 0.075 ± 0.071 mg kg−1. However, they reported a higher concentration for fresh little tunny (Euthynnus alletteratus), 1.185 ± 0.968 mg kg−1 of Hg; this tuna is from the Mediterranean Sea.
The averages reported here are around 4.4 (Hg), 5.17 (Pb) and 3.1 (Cd) times below the accepted limits for human consumption, which have been issued or promulgated by the Commission Regulation (EC) no. 1881/2006 (EU, 2006), or the FDA (USA). Also, the FDA (2018) reports that from 1993 to 2010, the average of Hg concentration for different types of tuna were: 0.350, 0.391, 0.358, 0.689, 0.144 and 0.354 (mg kg−1) for canned Albacore (Thunnus alalunga), frozen-fresh tuna, albacore, bigeye (Thunnus obesus), skipjack (Katsuwonus pelamis) and yellowfin (Thunnus albacares). The highest values found here is in canned tuna samples containing bigeye tuna, specie that made roughly 16 % of total catches in the same period. In a recent study, Houssard et al. (2019) in the central and western Pacific reported that bigeye had higher Hg content than in yellowfin and albacore, even sometimes above acceptable consuming limit. Also, Colman et al. (2015) informed that Pacific bluefin tuna (Thunnus orientalis) from the western Pacific has higher levels of Hg that their counterpart from the North-eastern Pacific. Similarly, Ruelas-Inzunza et al. (2018) have reported that skipjack tuna from the eastern Pacific is within the norms for safe consumption regarding Hg and As concentrations.
Fatty acids have been found to correlate with Hg content (Smith and Guentzel, 2010; Łuczyńska et al., 2017) thus less fat content implies less accumulation of these metals. In 2004, Burger and Gochfeld, documented also, that samples containing albacore presented higher the concentration of Hg (mean 0.407 mg kg−1) than those with skipjack (0.118 mg kg−1). The uppermost and lowermost concentrations (from FDA, 2018) were for the bigeye and skipjack tunas respectively, which in turn have the highest and lowest fat content; Mahaliyana et al. (2015) have reported a percentage of fat content of 2.06 ± 0.57 and 0.41 ± 0.56 (mg kg−1) respectively.
Very few values over the permissible limit were found. In 2009 no samples out of 509 tested; in 2010, 9 of 759 tested; in 2011, 3 of 562 tested; in 2012, none of 377 tested; in 2013, 1 of 377 tested; in 2014, 2 of 294 tested; and in 2015–2016, no cases from 463 samples tested. Mercury, Lead and Cadmium cases over permissible limits accounted for 0.04, 0.08, and 0.47 % respectively and the total cases: 0.58% of 2572 samples. Of the 15 instances over permissible levels, twelve corresponded to Cd, two to Pb and one to Hg. The sample with 1.49 mg[Hg] kg−1 occurred in 2010; the can contained bigeye tuna in oil. The lead was over limit with 0.92 and 0.89 mg[Pb] kg−1 in the same year, the cans also contained bigeye pre-cooked loins. Cadmium over-limits occurred eleven times in cans with skipjack and one in big-eye pre-cooked loins. Eight of the 15 values were statistically determined as outliers; i.e., Mercury (1), Cadmium (4) and Lead (3). Flores et al. (2018) has reported issues in some canned and fresh tuna samples with cadmium content in fresh tuna, but their sample universe was only 36 samples. When higher than standard limits are detected the INP labs proceed as referred in Material and methods section. In general, the total of cases above safe consumption limit can be considered very low comparing to others works (e.g., Ikem and Egiebor, 2005; Storelli et al., 2010; Russo et al., 2013; Alcala-Orozco et al., 2017). It was also found that concentrations of Hg, Cd, and Pb were not associated with the liquid of the cans (water, oil), similar results are reported by Burger and Gochfeld (2004).
In relation to the few samples with above limit Cadmium that were detected on skipjack products; it has recently been reported: 1) diet of the tuna is a driving factor on the type of element accumulation (Houssard et al., 2019), 2) Skipjack is merely an epipelagic species like Yellowfin, which has been found to feed on small fish, squids, phytoplankton, etc. (da Silva et al., 2019), 3) Phytoplankton up take Cadmium is similar to phosphate (Morel et al., 2020), and 4) Cadmium has a nutrient like vertical distribution that resembles much the dissolved organic/inorganic phosphate (Ormaza-González, 1990; de Baar et al., 1994) and correlate linearly with a high r2. It could be deemed that these facts could explain the few cases found, which only represent 0.47 % of the samples.
Assuming that Cd and Pb as well as Hg bio-accumulate at unknown rates, because 1) they would depend on many factors such as ingestion/assimilation/egestion processes apart from environmental, size, species, etc.; and, 2) knowing that these metals have no essential role on the living organisms (Egila and Daniel, 2011; Rajeshkumar and Li, 2018), statistical correlations were examined, taking Hg as the independent variable and Pb and Cd as dependent ones. This exercise failed to give a reasonable insight, although only in 2010 a linear correlation was found with a r2 of 0.128 (p < 0.003) with a linear regression: Pb (mg kg−1) = 0.424 Hg (mg kg−1) + 0.0062. Similar results occurred with Hg–Cd in the whole period. These results would confirm that the uptake and fixation of these metals in tuna fish are independent or unrelated to each other due to biological processes (through the trophic chain), tuna internal physiological processes, availability and speciation of metals in the water column, etc. Similar results have been reported on this in the reviewed literature.
It has been found that Hg content (and probably Cd, Pb, etc.) depend not only on the species, size, fat content, etc. but also on the water column where they live (Gworek et al., 2016). Recently, Houssard et al. (2019) have demonstrated that even though the fish size is the main explanation or reason for Hg content, oceanographic conditions like thermocline depth is an important fact that contributes, while the trophic level is of minor influence as well as the oceanic primary activity. There are vast differences between thermoclines depths between not only oceans but regions of them; for example, the thermocline depth of the Western Pacific Ocean is somewhere between 150–200 m, while the eastern Pacific it is 30–75 m deep depending on the season and regional phenomena like El Niño or rapid surface warming in the so-called area 1 + 2 that ranges from 0–10S to 90–100W (Ormaza-González and Cedeño, 2017; Lübbecke et al., 2019).
Around 44 % (2009–2016) of tuna captured in the eastern Pacific Ocean is skipjack (Ormaza-González et al., 2016; IATTC, 2018), particularly along the equatorial line (outside of marine reserves) that roughly covers from 80–150W, 10N–20S (Figure 3). In this area the biogeochemistry de Hg, Cd and Pb has not been determined yet, however, concentrations of some ocean regions can be found in the literature. For example, Gworek et al. (2016) reported dissolved total Hg of 0.006–0.018 with an average of 0.14 ± 0.06 ng kg−1 in the North Pacific; also, Bloom and Crecelius (1983) reported a higher range of 0.1–0.5 ng[Hg] kg−1 for the North-eastern (Sequin Bay, USA). One of the earliest works established a range concentration of 3–5 ng kg−1 and a media value of 5.3 ng kg−1 in Bering sea, North and South Pacific, Japan, South China and the Indian Ocean. Slightly higher concentrations were reported on the surface, as Hg main source is via aeolian dust, according to Nishimura et al. (1983); they reported 2–7 ng kg−1 for the equatorial line of the Pacific. Flegal and Patterson (1983) have registered dissolved Pb concentrations in the Central Pacific from 2.5 (20 South) to 11 (14 North) ng kg−1. More recently Boyle et al. (2014) reported 1.66–4.56 ng[Pb] kg−1 (8–20 pM[Pb]) from east to west along 12S latitude. Abe (2015) found Cadmium concentrations in the equatorial Pacific (160 W) surface water (0–150 m), ranged from 0.055 to 0.165 nM[Cd] (6–18 ng kg−1). Thus, the three dissolved metals are in the range of 2–18 ng kg−1, these concentrations are from open sea where anthropogenic sources perhaps do not play a strong role on them. However, higher concentration could be expected close to coastal areas anthropogenic Hg, Cd, and Pb are introduced to sea either by aeolian dust (Obrist et al., 2018), riverine water (through estuaries) and coastal erosion (Strode et al., 2007). Tuna habitat is generally open ocean; though sometimes important captures are reported close to coasts or islands.
Figure 3.
Distribution of tuna catching sets in the eastern Pacific from 2013 to 2017. Fishing sets DEL: Dolphin, NOA: Non-associate objects, OBJ: Floating aggregating devices, t: metric tonnes. Original graph from IATTC (2018). “Promedio” means average.
It has also been reported that background environmental concentration and bioavailability of Hg have biological (absorption/uptake) processes that are not well known and understood, and probably these processes are not only depending on the Hg concentrations (Gworek et al., 2016) but also on microbiological (Lehnherr et al., 2011), chemical and physical oceanographic conditions too, like the depth of the Dissolved Oxygen Minimum (DOM). Kumar (2018) reported that geographical origin of the tuna rendered important Hg variations and suggested long-term monitoring. Recently, Kumar (2018) and Houssard et al. (2019) are reporting a dependency on the depth of thermocline (that affects the depth of the DOM); and not only that but also the depth of the main fish habitat; for example, mesopelagic fish (living deeper >300 m) tend to have up to 4 times higher Hg than the epipelagic fish (<200 m deep). Further, Colman et al. (2015) using extensive archival tagging, bioaccumulation models, trophic investigations, and potential coastal sources of methylmercury, found that Hg bioaccumulation is likely higher in the western Pacific Ocean (East China Sea, Yellow Sea) than in the eastern (California Current).
Skipjack is an epipelagic species, whilst bigeye is mainly a mesopelagic fish (although it is facultative). Perhaps, for this reason, the latter generally tends to contain more Hg than skipjack. Also, the bigeye tuna tends to live around the thermocline bottom where the DOM and higher methyl-mercury is found (e.g. Blum et al., 2013). Around the DOM microbial community increase in both activity and volume and transform Hg into methyl Hg (Lehnherr et al., 2011). There has also been suggested possible bio-magnification from 2 to 6 times (Peterson et al., 1973) from the lowest trophic level to the third-fourth level. Nonetheless, Burger et al., 1992) said that Pb, Cd and Se environmental concentration did not replicate in some marine birds or even the waters are similar or higher than those in fauna specimens (Khoshnoud et al., 2011). Assuming the worst bioaccumulation scenario (6 times), tuna from the eastern Pacific could perhaps bio-accumulate: 0.012–0.042; 0.036–0.108 and 0.010–0.027 μg kg−1 of Hg, Cd and Pb respectively, which are one order lower than current concentrations found in this work and others mentioned here.
4. Conclusions
Low levels of mercury, lead, and cadmium were found in tuna canned products (2572 samples), which were processed in Ecuadorian canneries. The sampling covered a period for 8 years (2009–2016) and 2572 samples. Mercury showed consistently to have the highest levels and its averages where in the range of 0.14 (2016) to 0.30 (2009) mg kg−1, followed by lead with 0.038 (2012) to 0.093 (2014) mg kg−1 and cadmium: 0.021 (2012) – 0.044 (2014) mg kg−1. These levels are well below maximum allowed concentrations and those of the reported literature. Samples taken in 2018 from local vendors rendered concentrations of Hg: 0.043 ± 0.004 mg/kg; Cd: 0.012 ± 0.002 mg/kg and Pb < dl (0.01 mg/kg). These values are 5.75, 2.6 and 5.8 times below the average of the archival data, and again much less than allowable concentrations. The sequence of concentrations was Hg > Pb > Cd. During the studied period, a clear concentration pattern over time was not found. A slight decreasing concentration pattern was observed, particularly with Hg. It was found that skipjack tuna products tend to have Hg lower values than those from bigeye and yelowfin. The decreased of Hg concentration in time could plausible be ascribed to the fact that skipjack processed volume increased constantly during this period. This tuna species is mainly used by canneries, because availability and also because it is easier to can it. This species is epipelagic of short life span and its living habitat is basically the mixed layer (above the thermocline) where the methyl Hg is lower than below the thermocline depth. Overall, 15 (0.58%) samples of 2572 were found to have the metals above safe consumption. No important statistical correlation between Hg and Cd or Pb was found; this would suggest that the bioaccumulation of each metal is independent of each other. According to literature research, concentrations range found in the eastern Pacific for surface dissolved Hg, Cd, and Pb are in the range of 2–18 ng kg−1. Assuming a suggested bioaccumulation of 2–6 times, the end concentration in the tuna would be 0.012–0.042; 0.036–0.108 and 0.010–0.027 μg kg−1 of Hg, Cd, and Pb respectively; i.e., which are up to one order lower than safe limits. However, further studies should assess metal concentrations exclusively from Ecuadorian tuna captured close to coastal and insular (Galapagos Islands) allowed fishing areas, as Kumar (2018) has reported long term and wider geographical sampling is needed to have a region-specific data and information. It would be an important input to this kind of studies to add the spatial component; thus, to locate possible areas close or around to the Ecuadorian EEZ area, vertical habitats and bottom of the thermocline where Methyl-Hg is produced. Ideally, it would be recommendable to analyse the impact of brine (20–25%) used to freeze tuna fish in the fishing boats, when under certain conditions the first steps of freezing on board are deficient. It has been found that when this happens salts permeates through the skin and increases notably salt content of the loins. The hyper concentrated sea salt also could contain these metals.
Declarations
Author contribution statement
Franklin Isaac Ormaza-González: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gabriela Estefanía Ponce-Villao: Analyzed and interpreted the data; Wrote the paper.
Glenda Marlene Pin-Hidalgo: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
ESPOL - FIMCBOR faculty Dean and Sub-Dean. The National Institute of Fisheries for allowing GPV and GPH access to its laboratories and analysis archives. Daphne Vera-Mosquera and Nilanjana Das helped to improve the English. Three anonymous reviewers gave valuable comments.
References
- Abe K. Variation in the cadmium concentration related to phosphate in the surface layer of the equatorial Pacific. J. Oceanogr. 2015;61(200):783–788. [Google Scholar]
- Abolghait S., Garbaj A. Determination of cadmium, lead and mercury residual levels in meat of canned light tuna (Katsuwonus pelamis and Thunnus albacares) and fresh little tunny (Euthynnus alletteratus) in Libya. Open Vet. J. 2015;5(2):130–137. [PMC free article] [PubMed] [Google Scholar]
- Adams D. Consistently low mercury concentrations in dolphinfish, Coryphaena hippurus, an oceanic pelagic predator. Environ. Res. 2009;109(6):697–701. doi: 10.1016/j.envres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Ahmed Q., Levent B.A.T., Öztekin A., Ali Q.M. A review on studies of heavy metal determination in Mackerel and Tuna (Family-Scombridae) fishes. J. Anatolian Environ. Animal Sci. 2018;3(3):107–123. [Google Scholar]
- Al-Najjar T., Dahiyat N., Khalaf M., Sharari N., Wahsha M. LEVELS OF Pb AND Cd int three species of tuna (Katsuwonus pelamis, Auxis thazard and Euthynnu affinis) collected from the Jordanian coast of the Gulf of Aqaba, Red Sea. Fresenius Environ. Bull. 2018;27(6):4277–4284. [Google Scholar]
- Alcala-Orozco M., Morillo-Garcia Y., Caballero-Gallardo K., Olivero-Verbel J. Mercury in canned tuna marketed in Cartagena, Colombia, and estimation of human exposure. Food Addit. Contam. B. 2017;10(4):241–247. doi: 10.1080/19393210.2017.1323803. [DOI] [PubMed] [Google Scholar]
- Anastacio J. Poster session presented at: Cámara Nacional de Pesquería. 2019. Preparación y conservas de atún: Exportaciones.https://camaradepesqueria.ec/wp-content/uploads/2020/02/PREPARACIONES_CONSERVAS_REPORTE_EXPORTACIONES_2019-ene-dic.pdf [Google Scholar]
- AOAC Lead, cadmium, zinc, copper, and Iron in foods. Atomic absorption spectrophotometry after microwave digestion official method. 2002;999:10. [Google Scholar]
- Bloom N., Crecelius E. Determination of mercury in seawater at sub-nanogram per liter levels. Mar. Chem. 1983;14:49–59. [Google Scholar]
- Blum J.D., Popp B.N., Drazen J.C., Anela Choy C., Johnson M.W. Methylmercury production below the mixed layer in the North Pacific Ocean. Nat. Geosci. 2013;6(10):879–884. [Google Scholar]
- Bosch A.C., O'Neill B., Sigge G.O., Kerwath S.E., Hoffman L.C. Heavy metals in marine fish meat and consumer health: a review. J. Sci. Food Agric. 2016;96(1):32–48. doi: 10.1002/jsfa.7360. [DOI] [PubMed] [Google Scholar]
- Bosch A.C., O’Neill B., Sigge G.O., Kerwath S.E., Hoffman L.C. Mercury accumulation in Yellowfin tuna (Thunnus albacares) with regards to muscle type, muscle position and fish size. Food Chem. 2016;190:351–356. doi: 10.1016/j.foodchem.2015.05.109. [DOI] [PubMed] [Google Scholar]
- Boyle E., Lee J., Zhang J., Echegoyen Y. American Geophysical Union. Fall Meeting 2014; 2014. Dissolved lead in the Deep Southeast Pacific. Report No.: OS23E-1279. [Google Scholar]
- Brinson A., Cummings J., Dolinger L., Fabian J., Fenner J., Haverland T., Lewis M., Liddel M., Litwack A., Lowther A. NOAA: National Marine Fisheries Service; 2015. Seafood Health Facts. National Marine Fisheries Service.http://www.st.nmfs.noaa.gov/commercial-fisheries/fus/fus15/index NOAA FishWatch. [Google Scholar]
- Burger J., Gochfeld M. Mercury in canned tuna: white versus light and temporal variation. Environ. Res. 2004;96(3):239–249. doi: 10.1016/j.envres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Burger J., Schreiber E.A.E., Gochfeld M. Lead, cadmium, selenium and mercury in seabird feathers from the tropical mid-pacific. Environ. Toxicol. Chem. 1992;11:815–822. [Google Scholar]
- Burger J., Gochfeld M. Framework and information needs for the management of the risks from consumption of self-caught fish. Environ. Res. 2006;101(2):275–285. doi: 10.1016/j.envres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Burnett M., Settle A., Patterson C. Lead in the marine environment: impact of man on coastal marine ecosystems. In: Branica M., Konrad Z., editors. Pergamon; 1980. pp. 7–13. [Google Scholar]
- Carlson C.B. US Department of the Interior; 1948. Suggestions for Operations of Tuna Receiving Ships. Fisheries Leaflet 301. Fish and Wildlife Service; p. 15. [Google Scholar]
- Castro-González M., Méndez-Armenta M. Heavy metals: implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008;26(3):263–271. doi: 10.1016/j.etap.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Chen C., Lai C., Chen K., Hsu C., Hung C., Chen M. Total and organic mercury concentrations in the muscles of Pacific albacore (Thunnus alalunga) and bigeye tuna (Thunnus obesus) Mar. Pollut. Bull. Elsevier Ltd. 2014;85(2):606–612. doi: 10.1016/j.marpolbul.2014.01.039. [DOI] [PubMed] [Google Scholar]
- Colman J.A., Nogueira Jacob I., Pancorbo Oscar C., Batdorf Carol A., Block Barbara A. Mercury in Pacific bluefin tuna (Thunnus orientalis): bioaccumulation and trans-Pacific Ocean migration. Can. J. Fish. Aquat. Sci. 2015;72(7) 150310143352008. [Google Scholar]
- Cullen J., Maldonado M. Biogeochemistry of cadmium and its release to the environment. Cadmium: Toxicity Essential. 2013;11:31–62. doi: 10.1007/978-94-007-5179-8_2. [DOI] [PubMed] [Google Scholar]
- Cullen J., McAlister J. Lead: Its Effects on Environment and Health. De Gruyter; Berlin, Boston: 2017. Biogeochemistry of Lead. Its Release to the Environment and Chemical Speciation; pp. 21–48.https://www.degruyter.com/view/books/9783110434330/9783110434330-002/9783110434330-002.xml [DOI] [PubMed] [Google Scholar]
- da Silva G.B., Hazin H.G., Hazin F.H.V., Vaske-Jr T. Diet composition of bigeye tuna (Thunnus obesus) and yellowfin tuna (Thunnus albacares) caught on aggregated schools in the western equatorial Atlantic Ocean. J. Appl. Ichthyol. 2019;35:1111–1118. [Google Scholar]
- de Baar Hein J.W., Saager Paul M., Nolting Rob F., van der Meer Jaap. Cadmium versus phosphate in the world ocean. Mar. Chem. 1994;46(3):261–281. [Google Scholar]
- Driscoll C., Mason R., Chan H., Jacob D., Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environ. Sci. Technol. 2013;47(10):4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egila J.N., Daniel V.N. Trace metals accumulation in freshwater and sediment insects of Liberty Dam, Plateau State Nigeria. Int. J. Basic Appl. Sci. 2011;11:128–140. [Google Scholar]
- El-Moselhy K., Othman A., Abd El-Azem H., El-Metwally M. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt J. Basic Appl. Sci. Elsevier Ltd. 2014;1(2):1–9. [Google Scholar]
- EU Commission Regulation (EC) no. 1881/2006 (EU 2006) 2006. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF
- Evans S., Johnson M., Lea R. A Rapid, Automated Technique for Routine Analysis Varian, 1986. Agilent Technologies; USA: 2010. Determination of mercury in fish tissue.https://www.agilent.com/cs/library/applications/AA060.pdf [Google Scholar]
- FAO . CC BY-NC-SA 3.0 IGO; Roma, Licencia: 2018. El estado mundial de la pesca y la acuicultura 2018. Cumplir los objetivos de desarrollo sostenible. [Google Scholar]
- FDA (U.S FOOD & DRUG) 2018. What You Need to Know about Mercury in Fish and Shellfish.https://www.fda.gov/food/foodborneillnesscontaminants/metals/ucm351781.htm [Google Scholar]
- Flegal A., Patterson C. Vertical concentration profiles of lead in the Central Pacific at 15°N and 20°S. Earth Planet Sci. Lett. 1983;64(1):19–32. [Google Scholar]
- Flores E., Pozo W., Pernía B., Sánchez W. Niveles de cadmio en atún fresco y enlatado para consumo humano en Ecuador. Maskana. 2018;9(2):35–40. [Google Scholar]
- Galimberti C., Corti I., Cressoni M., Moretti V., Menotta S., Galli U., Cambiaghi D. Evaluation of mercury, cadmium and lead levels in fish and fishery products imported by air in North Italy from extra-European Union Countries. Food Contr. 2016;60:329–337. [Google Scholar]
- Gworek B., Bemowska-Kałabun O., Kijeńska M., Wrzosek-Jakubowska J. Mercury in marine and oceanic waters—a review. Water Air Soil Pollut. 2016;227:371. doi: 10.1007/s11270-016-3060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssard P., Point David, Tremblay-Boyer Laura, Allain Valérie, Pethybridge Heidi, Masbou Jeremy, Ferriss Bridget E., Baya Pascale A., Lagane Christelle, Menkes Christophe E., Letourneur Yves, Lorrain Anne. A model of mercury distribution in tuna from the western and central Pacific Ocean: influence of physiology, ecology and environmental factors. Environ. Sci. Technol. 2019;53(3):1422–1431. doi: 10.1021/acs.est.8b06058. [DOI] [PubMed] [Google Scholar]
- IATTC . 2018. 09/PDFs/Docs/_Spanish/SAC-09-03-ES-CORR-08-May-18_La-pesqueria-en-2017.pdf. [Google Scholar]
- Ikem A., Egiebor N. Assessment of trace elements in canned fishes (Mackerel, Tuna, Salmon, Sardines and Herrings) marketed in Georgia and Alabama (United States of America) J. Food Compos. Anal. 2005;18:771–787. [Google Scholar]
- Janssen D., Conway T., Johnb S., Christian J., Kramera D., Pedersena T., Cullena J. Undocumented water column sink for cadmium in open ocean oxygen-deficient zones. Proc. Natl. Acad. Sci. U. S. A. 2014;111(19):6888–6893. doi: 10.1073/pnas.1402388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari F., Ghazi-Khansari M., Abdollahi M. Heavy metals content of canned tuna fish. Food Chem. 2005;93:293–296. [Google Scholar]
- Khoshnoud M., Mobini K., Javidnia K., Hosseinkhezri P., Aeen Jamshid K. Heavy metals (Zn, Cu, Pb, Cd and Hg) contents and fatty acids ratios in two fish species (Scomberomorus commerson and Otolithes ruber) of the Persian Gulf. Iran. J. Pharm. Sci. 2011;7(3):191–196. [Google Scholar]
- Kumar Girish. Mercury concentrations in fresh and canned tuna: a review. Rev. Fisheries Sci. Aquacult. 2018;26(1):111–120. [Google Scholar]
- Lehnherr I., St Louis V.L., Hintelmann H., Kirk J.L. Methylation of inorganic mercury in polar marine waters. Nat. Geosci. 2011;4:298–302. [Google Scholar]
- Lübbecke J.F., Rudloff D., Stramma L. Stand-alone eastern Pacific coastal warming events. Geophys. Res. Lett. 2019;46:12360–12367. [Google Scholar]
- Mahaliyana A., Jinadasa B., Liyanage L., Jayasinghe G., Jayamanne S. Nutritional composition of skipjack tuna (Katsuwonus pelamis) caught from the oceanic waters around Sri Lankae. Am. J. Food Nutr. 2015;3(4):106–111. [Google Scholar]
- Mart L., Rützel P., Klahre L., Sipos U., Platzek P., Valenta H., Nürnberg Comparative studies on the distribution of heavy metals in the oceans and coastal waters. Sci. Total Environ. 1982;26:1–17. doi: 10.1016/0048-9697(82)90092-4. [DOI] [PubMed] [Google Scholar]
- Mensah M.E. North Carolina State University. MSc thesis; 2013. Desalination of Tuna after Excessive Salt Uptake from Brine Freezing; p. 82. [Google Scholar]
- Morel F.M.M., Lam P.J., Saito M.A. Trace metal substitution in marine phytoplankton. Annu. Rev. Earth Planet Sci. 2020;48:1. 2020. [Google Scholar]
- Morgan J., Berry M., Jr., Graves R. Poster session presented at: U. S. Environmental, Protection Agency, Environmental Monitoring Systems Laboratory, Quality Assurance Research Division, Development and Evaluation Branch. Cincinnati: Ohio. 1997. Determination of total mercury in raw and cooked fish tissue by microwave digestion/cold vapours atomic absorption spectroscopy. [Google Scholar]
- Mwashote B. Levels of cadmium and lead in water, sediments and selected fish species in Mombasa, Kenya. West. Indian Ocean J. Mar. Sci. 2003;2:25–34. [Google Scholar]
- Nishimura M., Konishi S., Matsunaga K., Hata K., Kosuga T. Mercury concentration in the ocean. J. Oceanogr. Soc. Jpn. 1983;39(6):295–300. [Google Scholar]
- Obrist D., Kirk J.L., Zhang L., Sunderland E.M., Jiskra M., Selin N.E. A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio. 2018;47(2):116–140. doi: 10.1007/s13280-017-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormaza-González F.I. Southampton University UK; 1990. Phosphorus in Estuarine and Oligotrophic Ocean Waters: Analytical and Biogeochemical Studies. PhD Thesis; p. 346. [Google Scholar]
- Ormaza-González F.I., Cedeño J. Coastal El Niño 2017 or simply: the carnival coastal warming event? MOJ Ecol. Environ. Sci. 2017;2(8) [Google Scholar]
- Ormaza-González F.I., Mora-Cervetto A., Bermúdez-Martínez R.M. Relationships between tuna catch and variable frequency oceanographic conditions. Adv. Geosci. 2016;42:83–90. www.adv-geosci.net/42/83/2016/ 2016. [Google Scholar]
- Peterson C., Klawe W., Sharp G. Mercury in tunas: a review. Fish. Bull. 1973;71(3):603–613. [Google Scholar]
- Planchon A., Boutron C., Barbante C., Cozzi G., Gaspari V., Woli E., Ferrari C., Cescon P. Changes in heavy metals in Antarctic snow from Coats Land since the mid-19th to the late-20th century. Earth Planet Sci. Lett. 2002;200:207–222. [Google Scholar]
- Rajeshkumar S., Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu lake, China. Toxicol. Rep. 2018;5:288–295. doi: 10.1016/j.toxrep.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelas-Inzunza J., Šlejkovec Z., Mazej D., Fajon V., Horvat M., Ramos-Osuna M. Bioaccumulation of As, Hg, and Se in tunas Thunnus albacares and Katsuwonus pelamis from the Eastern Pacific: tissue distribution and as speciation. Environ. Sci. Pollut. Control Ser. 2018;25(20):19499–19509. doi: 10.1007/s11356-018-2166-0. [DOI] [PubMed] [Google Scholar]
- Russo R., Lo Voi A., De Simone A., Serpe F., Anastasio A., Pepe T., Cacace D., Severino L. Heavy metals in canned tuna from Italian markets. J. Food Protect. 2013;76(2):355–359. doi: 10.4315/0362-028X.JFP-12-346. [DOI] [PubMed] [Google Scholar]
- Ryder J., Iddya K., Ababouch L. FAO; 2014. Assessment and Management of Seafood Safety and Quality Current Practices and Emerging Issues. Report No.: Doc 574. [Google Scholar]
- Smith K., Guentzel J. 2010. Mercury Concentrations and omega-3 Fatty Acids in Fish and Shrimp: Preferential Consumption for Maximum Health Benefits. [DOI] [PubMed] [Google Scholar]
- Squadrone S., Prearo M., Brizio P., Gavinelli S., Pellegrino M., Scanzio T., Guarise S., Benedetto A., Abete M. Heavy metals distribution in muscle, liver, kidney and gill of European catfish (Silurus glanis) from Italian Rivers. Chemosphere. 2013;90:358–365. doi: 10.1016/j.chemosphere.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Storelli M., Storelli A., Marcotrigiano G. Accumulation of mercury, cadmium, lead and arsenic in sword sh and blue n tuna from the Mediterranean Sea: a comparative study. Mar. Pollut. Bull. 2005;50:1004–1007. doi: 10.1016/j.marpolbul.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Storelli M., Barone G., Cuttone G., Giungato D., Garofalo R. Occurrence of toxic metals (Hg, Cd and Pb) in fresh and canned tuna: public health implications. Food Chem. Toxicol. 2010;48(11):3167–3170. doi: 10.1016/j.fct.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Strode S., Jaegle L., Selin N., Jacob D., Park R., Yantosca R., Mason R., Slemr F. Air-sea exchange in the global mercury cycle. Global Biogeochem. Cycles. 2007;21:GB1017. [Google Scholar]
- Torres P., Rodrigues A., Soares L., Garcia P. Metal concentrations in two commercial tuna species from an active volcanic region in the Mid-Atlantic Ocean. Arch. Environ. Contam. Toxicol. 2016;70(2):341–347. doi: 10.1007/s00244-015-0249-1. [DOI] [PubMed] [Google Scholar]
- Van Urk G., Marquenie J. Environmental behaviour of cadmium: WHO are at risk and Why. Heavy Metals Environ. 1989;11:456–459. [Google Scholar]
- Voegborlo R., El-Methnani A., Abedin M. Mercury, cadmium and lead content of canned tuna fish. Food Chem. 1999;67(4):341–345. [Google Scholar]
- Xu Y., Morel F. Cadmium in marine phytoplankton. Met. Ions Life Sci. 2013;11:509–528. doi: 10.1007/978-94-007-5179-8_16. [DOI] [PubMed] [Google Scholar]
- Zuluaga J., Gallego S., Ramírez C. Content of Hg, Cd, Pb and as in fish species: a review. Vitae. 2015;22(2):148–149. [Google Scholar]
- Łuczyńska J., Paszczyk B., Nowosad J., Łuczyńska M. Mercury, fatty acids content and Lipid quality indexes in muscles of freshwater and marine fish on the polish market. Risk assessment of fish consumption. Int. J. Environ. Res. Publ. Health. 2017;14(1120):2–17. doi: 10.3390/ijerph14101120. [DOI] [PMC free article] [PubMed] [Google Scholar]