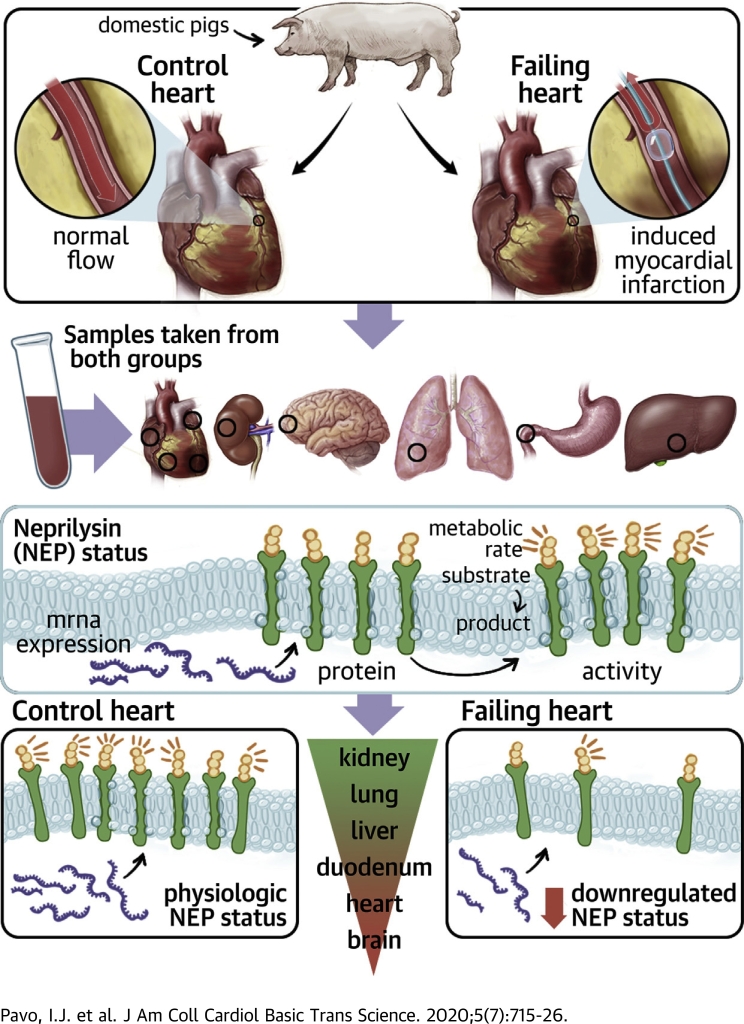
Visual Abstract
Key Words: ARNI, biomarker, gene expression, metalloproteinase, neprilysin, translational model of heart failure
Abbreviations and Acronyms: ANP, atrial natriuretic peptide; ARNI, angiotensin-receptor neprilysin-inhibitor; BNP, B-type natriuretic peptide; CMRI+LE, cardiac magnetic resonance and late enhancement; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; Q1 to Q3, 25th to 75th percentile; left atrial, left atrial; LV, left ventricular; mRNA, messenger RNA; NEP, neprilysin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; qPCR, real-time polymerase chain reaction; RA, right atrial; RV, right ventricular
Highlights
-
•
The kidneys might play a crucial role in regulating systemic NEP actions based on 20 to 100 higher NEP content and activity of the kidneys compared with any other organ.
-
•
Tissue NEP expression seems to be downregulated and translates into reduced tissue protein concentrations and activity in HF.
-
•
Neither plasma or liquor NEP concentrations and activities reflect tissue NEP regulation; therefore, using NEP as a circulating biomarker seems to be questionable.
Summary
Based on the investigation of neprilysin (NEP) regulation in a translational porcine model of chronic heart failure (HF), this study concluded: 1) that kidneys might play a crucial part in systemic NEP regulation based on 20 to 100 higher NEP content and/or activity compared with any other organ; 2) NEP seems to be downregulated under HF conditions; and 3) that the value of plasma NEP concentrations and activity as biomarkers is questionable. For the first time, these data provide basic knowledge on HF-related pathophysiological alterations of the NEP system and contribute to understanding the mechanism of action of angiotensin-receptor neprilysin-inhibitors, which remains elusive despite broad clinical applications.
Recently, established pharmacological therapy for heart failure (HF) with reduced ejection fraction (HFrEF) has been extended by the first-in-class angiotensin-receptor and neprilysin inhibitor (ARNI) that has introduced neprilysin (NEP) inhibition as a novel mechanism in the combat against HF (1,2). NEP is a ubiquitous enzyme with pleiotropic effects that involve the breakdown of vasoactive and natriuretic peptides as well as breakdown of the angiotensins (3,4). Although the main molecular mechanism of NEP inhibition responsible for the marked clinical benefit of ARNI over angiotensin-converting enzyme inhibition remains a subject of debate, NEP has developed a growing interest as cardiovascular biomarker (4).
NEP is a membrane-bound, zinc-dependent metallo-endopeptidase with a wide tissue expression that has high concentrations in kidney tissue, adipose tissue, and lungs (5,6). The presence of a nonmembrane-associated or soluble form has also been reported in blood, urine, and cerebrospinal fluid (7,8). NEP in the blood stream apparently retains its catalytic activity, which is supported by several studies that reported NEP activity in plasma samples (7, 8, 9, 10). Alterations of circulating NEP concentrations and NEP activity have been found to be associated with various diseases, such as metabolic syndrome, lung disease, chronic rheumatoid disease, and Alzheimer’s disease (11, 12, 13, 14, 15, 16). In HF, plasma concentrations of NEP were found to be a risk factor for cardiovascular death in stable patients (17) and were likewise adversely associated with outcome in acute HF (18). In contrast, no association with outcome was proven for patients with HF and preserved ejection fraction (19). One study reported measurable circulating plasma NEP activity determined by a fluorometric method, with modest correlation between plasma NEP concentration and activity in stable HFrEF (20). However, in a mixed cohort of patients with stable and acute HF, plasma NEP activity, but not concentration, was found to be inversely related to B-type natriuretic peptide (BNP) levels (9).
It can be assumed that circulating NEP levels and NEP activity are indicators of the systemic activation and/or inhibition of the NEP system. Yet, circulating NEP concentrations or activities are not convincing as biomarkers in HFrEF. There are no data regarding either NEP regulation in the pathophysiological condition of HF or the relationship among tissue NEP expression, concentrations, and activity with circulating NEP concentrations and activity. Understanding the regulation of circulating NEP and its usefulness as a biomarker is of great importance because LCZ696 (sacubitril/valsartan [ARNI]) is already widely used and its use will be further increased in patients with HFrEF. The aim of this study was to investigate differential NEP expression (i.e., mRNA levels, NEP content [protein concentrations]) and enzymatic NEP activity of various tissues, as well as the relationship between tissue NEP concentrations and activity with circulating NEP status in a translational model of chronic HF.
Methods
Ethical statement
Animal investigations were carried out in accordance with the “Position of the American Heart Association on Research Animal Use,” as adopted by the American Heart Association on November 11, 1984. The study was approved by the Ethics Committee on Animal Experimentation at the University of Kaposvar, Hungary. The study corresponds to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting animal research (21).
Porcine model of chronic HF
Domestic pigs (male; 15 kg; n = 5) at the age of 3 months fasted overnight and then were sedated with 12 mg/kg ketamine hydrochloride, 0.04 mg/kg atropine, and 1.0 mg/kg xylazine, followed by intratracheal intubation. Anesthesia was then continued with 1.5 to 2.5 vol% isofluran, 1.6 to 1.8 vol% oxygen, and 0.5 vol% nitric oxide. During anesthesia, continuous monitoring of oxygen saturation and electrocardiography were performed. After surgical preparation of the right femoral artery, a 6-F introduction sheath (Medtronic, Inc., Minneapolis, Minnesota) was placed into the right femoral artery followed by intra-arterial administration of unfractionated heparin (200 IU/kg). A 6-F coronary catheter (Medtronic, Inc.) was placed into the ascending aorta, and selective angiography of the left coronary arteries was performed. A guidewire (Medtronic, Inc.) and then a coronary balloon dilation catheter (2.75-mm diameter, 12-mm length; Medtronic, Inc.) were placed into the left anterior descending coronary artery below the origin of the second diagonal branch. Coronary occlusion was performed for 90 min with 6-atm inflation pressure. Subsequent balloon deflation resulted in reperfusion. Coronary angiography was done by injecting nonionic contrast media (Takeda, Zürich, Switzerland) to monitor occlusion and reperfusion of the left anterior descending artery.
Follow-up investigations and sampling
Cardiac magnetic resonance imaging (CMRI) with late enhancement (CMRI+LE) was performed at 3 days and 6 months. At 6 months, follow-up animals were sacrificed, and plasma, liquor, and tissue samples were obtained. Plasma and liquor samples were immediately frozen and stored at −70 °C. Tissue samples of different myocardial areas including the left ventricular (LV) apex (i.e., infarcted region), LV septum, and LV lateral wall, as well as samples from the right ventricle (RV), left atrium (LA), and right atrium (RA) were obtained. Tissue samples of the liver, lung, kidneys, duodenum, and the frontal cortex of the brain were harvested and were shock-frozen for the assessment of NEP activity, the measurement of NEP concentrations, and immunohistology. Additional tissue samples were stored in RNA later for the quantification of NEP expression. Healthy domestic pigs (male; 75 kg; n = 5) at the age of 9 months served as control animals for the CMRI+LE parameters and samples.
Characterization of the HF model
LV and RV systolic function and myocardial infarct size were determined by CMRI at day 3. At the final follow-up at 6 months, CMRI analysis was performed, measuring end-systolic and end-diastolic dimensions and to confirm an impaired LV ejection fraction (LVEF). From the LV volume curves, the maximum and minimum amplitudes of the flow signals (dV/dt) referred to as peak ejection rate (during systole; min dV/dt) and peak filling rate (during diastole; max [dV/dt]), expressed as ml/s, were calculated. The plasma biomarkers N-terminal (NT)−proBNP, atrial natriuretic peptide (ANP), renin, and creatinine were determined by specific immunoassays at baseline, directly post-myocardial infarction, and at final follow-up (NT-proBNP: SEA485Po by Cloud-Clone Corp., Houston, Texas; ANP: SEA225PO by Cloud-Clone Corp.; renin: LS-F32145 by LSBio, Seattle, Washington; and creatinine: LS-F13025 by LSBio). To assess the activation of the natriuretic peptide system, ANP and BNP expression analysis was performed by real-time polymerase chain reaction (qPCR) using gene-specific primers (ANP forward: CAG GAG GGG GAA ACC AGA AAG, reverse: CAG CAA ATT CTT GAA ATC CAT CAG G; BNP forward: CGC AGT AGC ATC TTC CAA GTC, reverse: ACC TCC TGA GCA CAT TGC AG). Peptide concentrations were measured by specific immunoassays (ANP: LS-F14047 by LSBio, NT-proBNP: SEA485Po by Cloud-Clone Corp.).
NEP expression
Tissue samples of approximately 25 mg stored in RNALater were cut into small pieces, transferred to a 700 μl Qiazol solution, and homogenized using a Precellys system (Bertin Instruments, Montigny-le-Bretonneux, France). Total RNA, including small RNA, was extracted using the miRNeasy Mini Kit (Qiagen GmbH, Düsseldorf, Germany) on a Qiacube according to the manufacturer’s instruction. RNA quantities were assessed on Nanodrop 1000 (Thermo Fisher, Waltham, Massachusetts). RNA quality was checked on RNA Nano chips on the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California). All samples resulted in RNA integrity number (RIN) values >7 and intact 18S and 28S bands. RNA was then transcribed to cDNA using the Qiagen QuantiTect Kit according to the manufacturer’s instructions. For NEP mRNA quantification, duplex qPCR was performed using the Qiagen QuantiNova Probe PCR Kit with gene specific primers (NEP forward: GGTGTACCAGTATGGAAACTTCT, reverse: TGGCCAATACCACCGTTATC, probe: 5′-FAM-AGCAGGTGGACAGCATCTCAATGG-BHQ1-3′ beta-Actin forward: GGTCGGAGTGAACGGATTT, reverse: ATGTAGTGGAGGTCAATGAAGG, probe: 5′-YY-TGATGGCGACAATGTCCACTTTGC-BHQ1-3′) on an Applied Biosystems QuantStudio 5 system (Thermo Fisher). A standard curve was prepared and run to correct values for qPCR efficiency. Expression quantities were calculated with Thermo Fisher software and expressed relative to beta-Actin.
NEP concentrations
NEP concentrations were measured using a specific sandwich immunoassay (DY1182, R and D Systems, Minneapolis, Minnesota) according to the manufactureŕs protocol. Plasma and liquor samples were diluted 1:2 or 1:20 in phosphate-buffered saline (D8537 Sigma-Aldrich, Germany), and final extinction was assessed using the Victor3 multilabel plate reader (PerkinElmer, Waltham, Massachusetts) at 450 nm. Snap-frozen tissue samples were cut to small pieces and homogenized in chilled 400 μl of 100 mM Tris/150 mM sodium chloride buffer, pH 7.4 using a Precellys system. Homogenates were centrifuged at 4°C at 11,000g for 15 min, and the supernatants were collected for an enzyme-linked immunosorbent assay. Results were normalized to protein concentration determined by Bradford assay.
NEP activity
A noncommercial fluorimetric peptide cleavage assay was performed for the measurement of NEP activity, as previously described (8). Briefly, plasma and liquor samples were diluted 1:20, tissue homogenates were diluted 1:10 (kidney 1:100) in phosphate-buffered saline. Forty microliters of diluted samples and 20 μl of 0.1 M Tris/HCl buffer (for active wells) or 20 μl 0.1 mM phosphoramidon solution (for reference wells) were mixed, and reaction was started by adding 100 μl of 1 mM fluorogenic NEP substrate (Glutaryl-Ala-Ala-Phe-AMC) solution. After 60 min of incubation at 37°C, 20 μl of 0.1 mM phosphoramidon and 20 μl of aminopeptidase M solution (4 U/well) were added. Fluorescence was analyzed after another 60 min of incubation at 37°C at 460 nm, with an excitation at 355 nm on a Victor3 multilabel plate reader (PerkinElmer, Waltham, Massachusetts). Serial dilutions of 4-methylcoumaryl- 7-amide (AMC) were used for preparation of a calibration curve. Enzymatic activities were calculated by determining cleaved AMC (difference between samples with and without phosphoramidon inhibition) per minute and milligram protein (tissue) or milliliter (plasma and liquor).
Statistical analysis
Continuous data are presented as median and 25th and 75th percentiles (Q1 to Q3), and categorical data are presented as counts and percentages. Continuous variables were compared using the Mann-Whitney U test, and categorical data were compared by Fisheŕs exact test. For the comparison of NEP expression, NEP concentrations, and NEP activity between the 2 groups, relative values of all tissues and animals were pooled, and the groups were compared by the Mann-Whitney U test. In this analysis, variables were normalized to the respective tissue measures of the control animals. Correlation between NEP expression, concentration, and activities were analyzed by calculating Pearsońs and Spearmańs correlation coefficients, which are indicated as rp and rs, respectively. In addition, linear regression curves were built by Graph Pad Prism 6.0 (GraphPad, San Diego, California) considering each Y value as individual point without constrains. SPSS 26.0 (IBM, Armonk, New York) was used for analysis of all other data. For all tests, 2-sided p values <0.05 were considered statistically significant.
Results
Translational model of HF
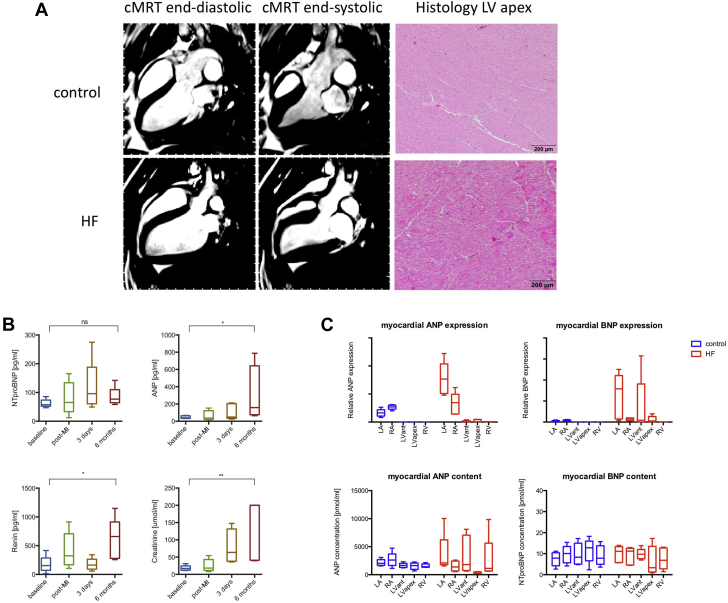
At day 3, HF animals showed an LVEF of 35.7% (Q1 to Q3: 32.0% to 36.2%), a cardiac output of 1.6 l/min (Q1 to Q3: 1.4 to 1.8 l/min), and an infarction size of 21.5% (Q1 to Q3: 20.2% to 22.4%) of the total LV. The CMRI+LE results at 6 months are displayed in Table 1. Representative images of the CMRI+LE, as well as histology of the ischemia-affected region and control animals are shown in Figure 1A; neurohumoral biomarkers and myocardial natriuretic peptide expression and content are displayed in Figures 1B and 1C. At the 6-month follow-up, animals with HF had a higher LV end-diastolic volume (100.8 ml; Q1 to Q3: 95.2 to 110.2 ml vs. 79.0 ml; Q1 to Q3: 78.9 to 82.9 ml; p = 0.016) and a lower LVEF (41.8%; Q1 to Q3: 41.3% to 44.1% vs. 53.0%; Q1 to Q3: 51.8% to 55.0%; p = 0.008) compared with the control group. Both the peak ejection rate and peak filling rate decreased in the HF animals. Plasma ANP, renin, and creatinine levels were elevated at 6 months follow-up compared with baseline. Myocardial ANP and BNP expressions were elevated in atria and ventricles of the HF group.
Table 1.
Results of the CMRI+LE Examinations of the HF and Control Animals at 6- Months Follow-Up
| CMRI Parameters | HF Group | Control Group | p Value |
|---|---|---|---|
| HR, beats/min | 102 (73−110) | 89 (86−91) | NS |
| LVM, g | 98 (95−105) | 63 (61−64) | 0.008 |
| LVEDV, ml | 101 (95−110) | 79 (79−83) | 0.016 |
| LVESV, ml | 56 (56−64) | 39 (36−40) | 0.008 |
| LVEF, % | 42 (41−44) | 53 (52−55) | 0.008 |
| CI, l/min | 4.33 (3.38−4.64) | 3.90 (3.66−4.30) | NS |
| PER, ml/s | 142 (117−188) | 239 (184−296) | 0.018 |
| PFR, ml/s | 163 (152−177) | 248 (210−285) | 0.001 |
Values are median (25th to 75th percentiles).
CI = cardiac index; CMRI = cardiac magnetic resonance imaging; HF = heart failure; HR = heart rate; LE = late enhancement; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; LVM = left ventricular mass; PER = peak ejection rate, PFR = peak filling rate.
Figure 1.
HF Model
Representative cardiac magnetic resonance images (cMRIs) and apex histology for control animals and animals with heart failure (HF) as well plasma neurohumoral markers and myocardial atrial natriuretic peptide/B-type natriuretic peptide (ANP/BNP) expression and content. (A) Left ventricular (LV) end-diastolic and end-systolic contour and corresponding histological sections of myocardium from the apical region in (upper panel) control animals and (bottom panel) animals with HF, respectively. (B) Plasma neurohumoral markers (i.e., ANP, N-terminal proBNP [NT-proBNP], renin, and creatinine), following myocardial infarction (MI) of the HF group. (C) Myocardial ANP/BNP expression patterns and content of the control animals and animals with HF according to different myocardial regions. Relative expression of ANP and BNP and peptide concentrations are displayed as Tukey boxplots. LA = left atrium; MI = myocardial infarction; RA = right atrium; RV = right ventricle.
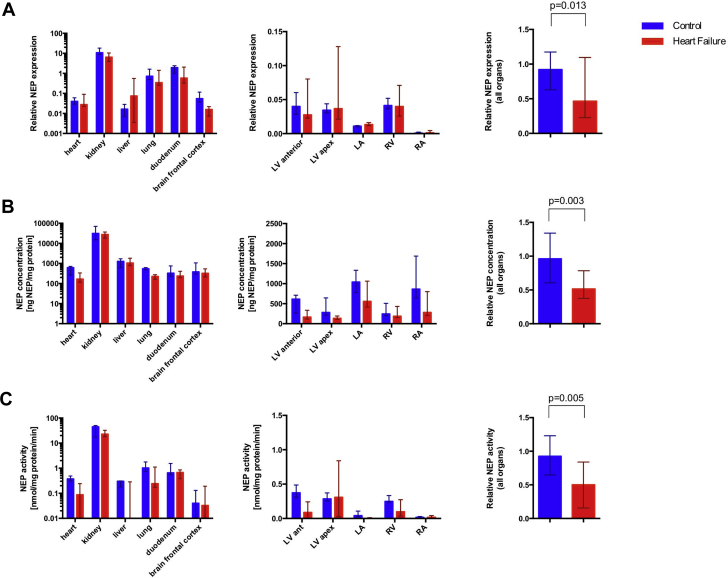
HF is characterized by a systemic NEP down-regulation. The results of tissue NEP expression, concentrations and activity are displayed in Figure 2. Corresponding numerical values are listed in Table 2.
Figure 2.
Nep Expression, Concentrations, Activity
(A) Neprilysin (NEP) expression, (B) NEP concentrations, and (C) NEP activity of different tissues in animals with HF compared with control animals (n = 5 for both groups). (A) NEP mRNA expression relative to glyceraldehyde 3-phosphate dehydrogenase measured by duplex real-time polymerase chain reaction, (B) protein concentrations measured by enzyme-linked immunosorbent assay, and (C) NEP activity assessed by a fluorimetric peptide cleavage assay are shown for different organs and cardiac regions. The region LV anterior represents primarily nonaffected myocardium of the LV, whereas the sample LV apex originates from the infarcted area in the HF group and healthy apical tissue in the control group, respectively. Results are shown as median and 25th to 75th percentile. Pooled samples were compared by the Mann-Whitney U test. Abbreviations as in Figure 1.
Table 2.
Numerical Values of NEP Expression, NEP Concentrations, and NEP activity in HF and Control Animals
| NEP Expression (Relative Expression) |
NEP Content (ng NEP/mg Protein) |
NEP Activity (nmol/mg Protein/min) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HF Animals | Control Animals | p Value∗ | HF Animals | Control Animals | p Value∗ | HF Animals | Control Animals | p Value∗ | ||
| LV myocardium | 0.03 (0.03−0.07) | '0.04 (0.03-0.05) | 0.013 | 166 (154−235) | 613 (337−676) | 0.003 | 0.09 (0.00−0.18) | 0.37 (0.35−0.40) | 0.005 | |
| Kidney | 6.40 (6.19−8.11) | 10.79 (9.31−15.12) | 27,419 (18,881−27,510) | 31,192 (16,787−51,268) | 22.60 (18.35−27.54) | 44.84 (23.42−46.69) | ||||
| Lung | 0.34 (0.31−0.67) | 0.71 (0.62−0.84) | 225 (198−271) | 542 (541−591) | 0.24 (0.20−1.02) | 1.02 (0.78−1.41) | ||||
| Liver | 0.07 (0.00−0.19) | 0.02 (0.01−0.02) | 1,039 (889−1,330) | 1.264 (1,111−1,689) | 0.00 (0.00−0.21) | 0.30 (0.25−0.30) | ||||
| Duodenum | 0.58 (0.36−1.62) | 1.93 (1.75−2.07) | 263 (233−263) | 327 (293−412) | 0.67 (0.47−0.72) | 0.65 (0.64−0.73) | ||||
| Brain | 0.02 (0.01−0.02) | 0.05 (0.04−0.10) | 320 (238−470) | 380 (366−869) | 0.03 (0.02−0.14) | 0.04 (0.00-0.07) | ||||
Values are median (25th to 75th percentile).
Pooled values were compared using the Mann-Whitney U test.
NEP = neprilysin; other abbreviations as in Table 1.
The p value was calculated for the comparison of pooled samples between HF and control animals
NEP expression was detected in all investigated organs. However, myocardium, liver, and brain samples showed much lower expression compared with the lungs, duodenum, and kidneys. Kidneys showed the highest NEP expression values, which were >100-fold higher than in myocardial samples of the nonischemic and control LVs (HF: myocardium 0.03; Q1 to Q3: 0.03 to 0.07 vs. kidney: 6.40; Q1 to Q3: 6.19 to 8.11; p = 0.043; and control animals: 0.04; Q1 to Q3: 0.03 to 0.05 vs. 10.79; Q1 to Q3: 9.31 to 15.12; p = 0.043). A trend toward reduced renal expression in animals with HF was found compared with control animals (6.40; Q1 to Q3: 6.19 to 8.11 vs. 10.79; Q1 to Q3: 9.31 to 15.12; p = 0.056).
In contrast to high variations in NEP expression, the NEP protein concentration of the myocardium, brain tissue, lungs, and duodenum were in a similar range for healthy animals and animals with HF, whereas NEP concentrations of the kidneys were roughly 25- to 100-fold higher than those of other organs, especially compared with the LV ischemia nonaffected myocardium (HF: 27,419 ng NEP/mg protein; Q1 to Q3: 18,881 to 27,510 vs. 166 ng NEP/mg protein; Q1 to Q3: 154 to 235; p = 0.043 and control animals: 31,192 ng NEP/mg protein; Q1 to Q3: 167,87 to 51,268 vs. 613 ng NEP/mg protein; Q1 to Q3: 337 to 676; p = 0.043).
NEP activity was highest in the kidneys, followed by the lungs and duodenum. NEP activity in the kidneys was 20- to 100-fold compared with all other organs, including the myocardium (HF: 22.60 nmol/mg protein/min; Q1 to Q3: 18.35 to 27.54 vs. 0.09 nmol/mg protein/min; Q1 to Q3: 0.00 to 0.18; p = 0.043 and control animals: 44.84 nmol/mg protein/min; Q1 to Q3: 23.42 to 46.69 vs. 0.37 nmol/mg protein/min; Q1 to Q3: 0.35 to 0.40; p = 0.043).
NEP expression, NEP content, and NEP activity were significantly reduced in animals with HF compared with controls in the pooled analysis (p = 0.013; p = 0.003, and p = 0.005). A relationship between cardiac natriuretic peptide regulation, plasma neurohormones, or renal function or kidney NEP was not observed (Supplemental Figures 1 and 2).
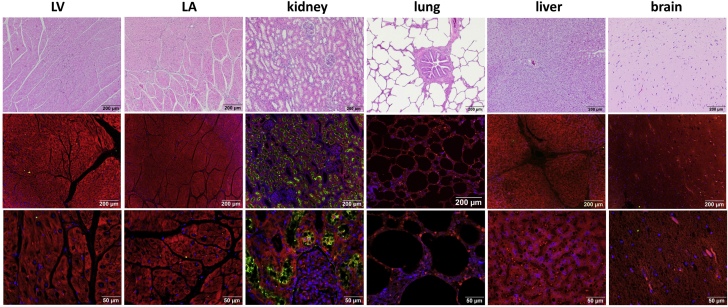
Representative immunostaining of NEP in different tissue samples as well as myocardial regions are shown in Figure 3. A strong NEP staining was only recognizable in the kidneys according to the highest measured tissue NEP content of this organ.
Figure 3.
HE and Immunostaining of Main Organs
(Top row) Hematoxylin eosin staining and (bottom rows) immunohistochemistry images at different scales for the organs in the top rows. Immunostaining was performed with DAPI (blue), phalloidin (red), and NEP as primary antibody (R and D) (green). Abbreviations as in Figures 1 and 2.
Cardiac regions showed unique myocardial tissue NEP patterns. Regarding different myocardial regions, NEP mRNA, protein content, and activity were detectable in both the normal LV and ischemia-affected LV samples as well as the RV, LA and RA. NEP expression and activity were significantly higher in the ventricular myocardium than that in the atrium (animals with HF: 0.05; Q1 to Q3: 0.03 to 0.07 vs. 0.01; Q1 to Q3: 0.00 to 0.01; p = 0.005 and control animals: 0.04; Q1 to Q3: 0.03 to 0.05 vs. 0.01; Q1 to Q3: 0.00 to 0.01; p = 0.005 for NEP expression and HF: 0.09 nmol/mg protein/min; Q1 to Q3: 0.00 to 0.27 vs. 0.00 nmol/mg protein/min; Q1 to Q3: 0.00 to 0.02; p = 0.018 and control animals: 0.32 nmol/mg protein/min; Q1 to Q3: 0.25 to 0.38 vs. 0.03 nmol/mg protein/min; Q1 to Q3: 0.02 to 0.04; p = 0.005 for NEP activity), whereas LV and RV samples showed similar values (p = 0.273 for animals with HF and p = 0.080 for control animals).
Plasma and liquor NEP concentrations and activities were unaffected in the condition of HF. In contrast to down-regulation of NEP in the tissue of animals with HF, changes in plasma and liquor NEP were not detected (613 ng NEP/mg protein; Q1 to Q3: 308 to 692 vs. 671 ng NEP/mg protein; Q1 to Q3: 408 to 1,813 and 0.11 nmol/mg protein/min; Q1 to Q3: 0.10 to 0.12 vs. 0.12 nmol/mg protein/min; Q1 to Q3: 0.10 to 0.19); for plasma NEP concentrations and activity: 394 ng NEP/mg protein; Q1 to Q3: 34 to 1,043) vs. 79 ng NEP/mg protein; Q1 to Q3: 0 to 291 and 0.01 nmol/mg protein/min; Q1 to Q3: 0.00 to 0.05 vs. 0.09 nmol/mg protein/min; Q1 to Q3: 0.00 to 0.10 for liquor NEP concentrations and activity; animals with HF vs. control animals: p = 0.690 for all). The results of the measurements of NEP concentration and enzymatic activity in plasma and liquor samples are shown in Supplemental Figure 1.
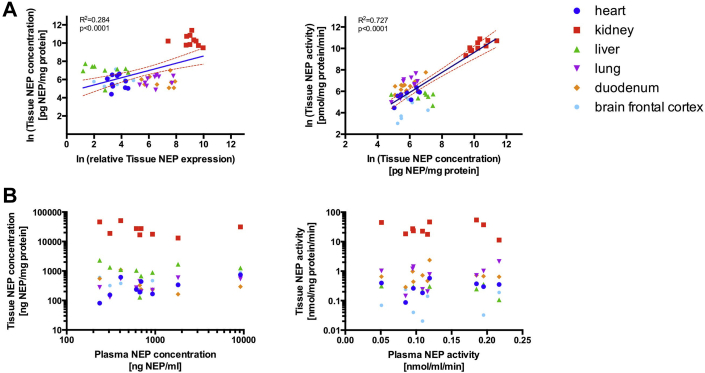
Circulating NEP measures did not reflect tissue NEP regulation. Scatterplots and linear regression analysis of tissue NEP expression, protein content, and enzymatic activity for all animals are shown in Figure 4. NEP expression correlated well with NEP concentrations (rp = 0.53; p < 0.001) across all tissues. NEP activity was strongly related to both NEP expression (rp = 0.79; p < 0.001) and protein concentrations (rp = 0.85; p < 0.001). When using rank-correlation, circumventing the potentially strong influence of the kidneys with much higher values, the associations were similarly strong between NEP activity and NEP expression (rs = 0.78; p < 0.001) as well as NEP concentration (rs = 0.59; p < 0.001) and modest for NEP expression and NEP concentration (rs = 0.30; p = 0.029). However, there was no correlation of any of the tissue measures with either plasma or liquor NEP concentration or activity when using all tissues together (p = NS for all comparisons) or when only using kidney samples (p = NS for all comparisons).
Figure 4.
Relationship Between Tissue NEP Measurements and Between Plasma And Tissue NEP Measurements for Different Organ Systems
(A) Linear regression models were calculated for tissue NEP expressions and concentrations, as well as for tissue NEP concentrations and NEP activities and (B) relationship between plasma and tissue NEP concentrations and activities. Linear regression curves were built by considering each Y value as individual point without constrains showing the 95% confidence band of the best fit line. Goodness of fit (R2) and the p value were indicated in the plot. Abbreviation as in Figure 2.
Discussion
This is the first study to investigate NEP regulation under the pathophysiological condition of HF as well as the relationship between tissue NEP and circulating NEP to gain further insights into the mechanism of NEP inhibition and the diagnostic value of plasma NEP as a biomarker. We demonstrated: 1) that NEP is a ubiquitous enzyme with the highest expression, concentrations, and activity in the kidneys, followed by the lungs and liver; 2) NEP mRNA and protein are detectable in the myocardium and show different concentrations and enzymatic activities in the ventricles and atria; 3) that overall tissue NEP expression as well as concentrations and activity are reduced in the condition of HF; and 4) that plasma NEP is not related to tissue NEP activity. In summary, the NEP system appears to be downregulated in HF and circulating NEP fails to sufficiently reflect tissue NEP status. Downregulation of NEP may represent a physiological counter-regulatory mechanism within the context of neurohumoral dysregulation.
Rationale for NEP in HFrEF
LCZ696, the first-in-class ARNI, proved to be superior to enalapril in the PARADIGM-HF (Prospective Comparison of ARNI with ACE-I to determine Impact on global Mortality and Morbidity in Heart Failure) study (1). With a 1B recommendation for symptomatic patients in current guidelines, chronic NEP inhibition was introduced for HFrEF therapy (2). The main suggested rationale for NEP inhibition in HF was the augmentation of the natriuretic peptide system, especially BNP, as a consequence of reduced inactivation by the enzyme (22). Elevated plasma levels of BNP were confirmed in patients who received ARNI, which was accompanied by reduced concentrations of NT-proBNP, which was not affected by NEP action (23). This probably indicated both reduced BNP de novo synthesis and general improvement of HF. However, NEP was responsible for the degradation of a variety of substrates equally implicated in cardiovascular homeostasis, including adrenomedullin, endothelin-1, substance P, or angiotensin II (6,24). These peptides exert partially opposing roles, so that the net effect of NEP inhibition would depend on the particular pattern of the prevailing substrates, which leaves the crucial therapeutic mode of action of ARNI a subject of debate (22). Similarly, NEP is a membrane-bound enzyme with broad tissue expression. Labeling of NEP by infusion of an intravenous substrate revealed high concentrations of the enzyme in the kidneys, lymph nodes, and lungs (25). Significant concentrations of NEP could similarly be found in other organs with functional barriers that were inaccessible for intravenous labeling, which indicated important NEP actions beyond inactivation of circulating substrates (25). Therefore, it was likely that substantial effects of NEP dysregulation and inhibition were due to tissues where NEP and its substrates colocalized. Alterations of NEP concentration and activity by HF or therapeutic intervention might not be necessarily reflected by circulating biomarkers.
NEP in various tissues
NEP expression was reported and compared for different tissues in individuals without HF(26). NEP mRNA abundance was highest in kidneys and duodenum and was approximately 100-fold higher compared with that in the myocardium and was approximately 10-fold higher than that in the lungs and liver. Our animal data confirmed these findings in healthy animals and animals with HF. Moreover, animals with HF showed a systemic down-regulation of NEP, with overall reduced expression that translated into reduced concentrations and activity across various tissues. A recent study investigated NEP release and expression in myocardial tissue samples in patients with HFrEF and found plasma NEP was higher in the coronary sinus compared with samples of the cubital vein. These results were found together with an increased but variable expression of NEP in LV myocardial tissue compared with that in healthy individuals (27). Our study confirmed detectable NEP expression in cardiac tissues with regional differences with similar expression levels in both ventricles, including the infarcted areas, but found much lower expression in the LA and particularly the RA. However, in contrast to the previously described findings, our results showed no upregulation but rather unchanged expression in ischemic HF. These differences might be explained HF etiology and severity, and confounding factors in the small human cohort. NEP expression was shown to be dynamically regulated by hypoxia and oxidative stress (28), androgens (29) and estrogens (30), somatostatin (31), vitamin D (32), and others (33), and to decrease with age (34). In contrast to multiple confounding factors in human samples, the data from our animal model offered the possibility to delineate effects from HF and other variable factors. Strong NEP down-regulation in HF was found in duodenum and frontal cortex. A lower NEP expression in the brain could be a factor for increased risk of dementia and Alzheimer’s disease in patients with HF, possibly stimulated by hypoxia.
Pathophysiological NEP regulation
NEP is not only implicated in cardiovascular disease but also plays a major role in the central nervous and inflammatory systems, as well as malignant diseases (35). In the brain, NEP degrades the ß-amyloid peptide involved in Alzheimerŕs disease. Consequently, NEP upregulation has been postulated as a therapeutic concept in translational studies (36). NEP has been located on the surface of neutrophils and in the lungs, which limits neurogenic inflammation in the respiratory system by degrading proinflammatory peptides (14). Airway irritants such as smoke or allergens downregulate NEP, thus potentiating the inflammatory response, whereas anti-inflammatory properties of inhalative corticosteroids have been attributed to an enhanced NEP expression (37). The deletion of NEP has exacerbated intestinal inflammation in an experimental model (38). It has also been suggested that NEP down-regulation may be associated with cancer development and progression. For cancer cell lines, a negative, yet also a positive regulation, of NEP as a response to hypoxia have been reported (39,40). Altogether, a negative regulation of NEP is commonly found in disease-associated conditions; however, data are ambiguous. Our study was the first to investigate NEP expression in HF, equally proposing a pathophysiological down-regulation of the NEP system across various tissues, which might well be affected by HF as a systemic disease. NEP down-regulation might represent an already naturally initiated counter-regulatory mechanism that might be further aggravated by ARNI therapy. The exact mechanisms still remain to be elucidated.
NEP as biomarker
Because NEP inhibition is related to markedly improved outcomes in HF, NEP has developed a growing interest as a biomarker. Circulating NEP concentrations and activity were investigated by several studies. Vodovar et al. (9) reported higher plasma concentrations and activity of NEP in patients with chronic HF compared with patients with acutely decompensated HF, yet no overall correlation between NEP concentration and activity could be detected. The study found no correlation between plasma NEP concentrations and BNP, but an inverse relationship between plasma NEP activity and BNP was demonstrated for a mixed cohort of patients with HFrEF (9). In the study of Bayes-Genis et al. (17), increased circulating NEP concentrations were significantly associated with cardiovascular death after multivariate adjustment in 1,069 ambulatory patients with chronic stable HFrEF. However, no correlation between NEP concentrations and NT-proBNP could be found, raising even more questions about the nature of circulating NEP (17,41). The same group reported a dynamic regulation of circulating NEP concentrations with increased levels at admission and a significant association of elevated admission values with worse short-term outcome in acute HF (18). It was recently reported that NEP plasma concentrations increased upon recovery of patients admitted with acute HF and dyspnea of noncardiac origin (42). However, another group reported that NEP plasma levels were not affected in patients who recovered from acute decompensated HF (43).
The investigations aimed at establishing plasma NEP as a biomarker are complicated by pre-analytical considerations and lack of associations between commercially available immunoassays (4). Apart from technical considerations, several aspects shed doubt on the use of plasma NEP as a HF biomarker. NEP is primarily a membrane-bound enzyme and the effects of several of its substrates act similarly on the tissue level. Therefore, the origin of circulating NEP is unclear, and it might represent shuddered ecto-domains from tissue itself or neutrophils that potentially contaminate samples during plasma preparation. If NEP originates from tissue, the strong NEP expression and concentration in kidneys and liver, given organ mass and perfusion, indicate that these 2 organs are more plausible sources than the heart. Ectodomain cleavage and exosomal release are possible modes of action for producing circulating NEP (44). The extensive number of substrates and its dynamic expressional regulation appear to hamper its biomarker usefulness for HF. The lack of association between NEP levels in tissue and circulation in our data are an indication that plasma NEP may not be an appropriate biomarker. These issues could explain the conflicting results, as well as the lack of association with other prognostic variables, especially NT-proBNP. The association of tissue NEP with disease severity remains to be proven.
Study limitations
One limitation of this study was the limited sample size, but we followed the 3R rules (reduce, refine, replace) of the European Union. In addition, the costs of the investigation of animals in a translational study with this complexity were high. We also refrained from a sham control group that underwent cardiac catheterization due to ethical reasons. On 1 hand, cardiac catheterization is usually part of the diagnostic tree in patients with HF, and on the other hand, diagnostic cardiac catheterization without complications before the final 6-month follow-up was neglectable in terms of the outcome for the investigated parameters.
Conclusions
In summary, we concluded that HF was characterized by an overall systemic NEP down-regulation, as well as reduced concentrations and activity in various organs. The success of ARNI in HF might lie in the strengthen of the already initiated pathophysiological down-regulation of the natural NEP action. The regular function of the ubiquitous transmembrane NEP is located at the tissues, and because plasma NEP concentrations and activities seem not to reflect tissue levels, they might not be an appropriate biomarker in HF.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE 1: HF seems to be characterized by an overall systemic NEP down-regulation. This is a primarily counterintuitive finding that suggests that the success of ARNI in HF might lie in the strengthening of the already initiated pathophysiological down-regulation of the natural NEP action.
COMPETENCY IN MEDICAL KNOWLEDGE 2: The regular function of the ubiquitous transmembrane NEP enzyme is located in the tissues, and because plasma NEP concentrations and activities does not correlate with tissue levels, circulating NEP may not be an appropriate biomarker of HF.
TRANSLATIONAL OUTLOOK 1: Investigations on the mechanism of actions of ARNI should include the kidneys because the contribution of the kidneys to systemic NEP actions seems to be the highest.
TRANSLATIONAL OUTLOOK 2: HF seems to be characterized by an overall systemic NEP down-regulation. This is a primarily counterintuitive finding that suggests that the success of ARNI in HF might lie in the strengthening of the already initiated pathophysiological down-regulation of the natural NEP action. This hypothesis needs to be tested in future studies.
TRANSLATIONAL OUTLOOK 3: The regular function of the ubiquitous transmembrane NEP enzyme is located in the tissues, and because plasma NEP concentrations and activities does not correlate with tissue levels, circulating NEP may not be an appropriate biomarker of HF.
Acknowledgments
The authors would like to thank the whole veterinarian team of the University of Kaposvar for their help and professionalism in terms of animal keeping and handling.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
For supplemental figures, please see the online version of this paper.
Appendix
References
- 1.McMurray J.J., Packer M., Desai A.S. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure. J Am Coll Cardiol. 2015;65:102–141. doi: 10.1016/j.jacc.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Bayes-Genis A., Barallat J., Richards A.M. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68:639–653. doi: 10.1016/j.jacc.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 5.Erdos E.G., Skidgel R.A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3:145–151. [PubMed] [Google Scholar]
- 6.Roques B.P., Noble F., Dauge V., Fournie-Zaluski M.C., Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- 7.Spillantini M.G., Sicuteri F., Salmon S., Malfroy B. Characterization of endopeptidase 3.4.24.11 (“enkephalinase”) activity in human plasma and cerebrospinal fluid. Biochem Pharmacol. 1990;39:1353–1356. doi: 10.1016/0006-2952(90)90012-a. [DOI] [PubMed] [Google Scholar]
- 8.Yandle T., Richards M., Smith M., Charles C., Livesey J., Espiner E. Assay of endopeptidase-24.11 activity in plasma applied to in vivo studies of endopeptidase inhibitors. Clin Chem. 1992;38:1785–1791. [PubMed] [Google Scholar]
- 9.Vodovar N., Seronde M.F., Laribi S. Elevated plasma B-type natriuretic peptide concentrations directly inhibit circulating neprilysin activity in heart failure. J Am Coll Cardiol HF. 2015;3:629–636. doi: 10.1016/j.jchf.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Emrich I.E., Vodovar N., Feuer L. Do plasma neprilysin activity and plasma neprilysin concentration predict cardiac events in chronic kidney disease patients? Nephrol Dial Transplant. 2019;34:100–108. doi: 10.1093/ndt/gfy066. [DOI] [PubMed] [Google Scholar]
- 11.Simonini G., Azzari C., Gelli A.M. Neprilysin levels in plasma and synovial fluid of juvenile idiopathic arthritis patients. Rheumatol Int. 2005;25:336–340. doi: 10.1007/s00296-004-0447-z. [DOI] [PubMed] [Google Scholar]
- 12.Standeven K.F., Hess K., Carter A.M. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond) 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson A.R., Coalson J.J., Ashton J., Larumbide M., Erdos E.G. Neutral endopeptidase in serum samples from patients with adult respiratory distress syndrome. Comparison with angiotensin-converting enzyme. Am Rev Respir Dis. 1985;132:1262–1267. doi: 10.1164/arrd.1985.132.6.1262. [DOI] [PubMed] [Google Scholar]
- 14.Borson D.B. Roles of neutral endopeptidase in airways. Am J Physiol. 1991;260:L212−25. doi: 10.1152/ajplung.1991.260.4.L212. [DOI] [PubMed] [Google Scholar]
- 15.Miners J.S., Barua N., Kehoe P.G., Gill S., Love S. Aβ-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol. 2011;70:944–959. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- 16.Willard J.R., Barrow B.M., Zraika S. Improved glycemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2017;60:701–708. doi: 10.1007/s00125-016-4172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayes-Genis A., Barallat J., Galan A. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J Am Coll Cardiol. 2015;65:657–665. doi: 10.1016/j.jacc.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 18.Bayes-Genis A., Barallat J., Pascual-Figal D. Prognostic value and kinetics of soluble neprilysin in acute heart failure: a pilot study. J Am Coll Cardiol HF. 2015;3:641–644. doi: 10.1016/j.jchf.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Goliasch G., Pavo N., Zotter-Tufaro C. Soluble neprilysin does not correlate with outcome in heart failure with preserved ejection fraction. Eur J Heart Fail. 2016;18:89–93. doi: 10.1002/ejhf.435. [DOI] [PubMed] [Google Scholar]
- 20.Bayes-Genis A., Prickett T.C., Richards A.M., Barallat J., Lupon J. Soluble neprilysin retains catalytic activity in heart failure. J Heart Lung Transplant. 2016;35:684–685. doi: 10.1016/j.healun.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLOS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh J.S.S., Burrell L.M., Cherif M., Squire I.B., Clark A.L., Lang C.C. Sacubitril/valsartan: beyond natriuretic peptides. Heart. 2017;103:1569–1577. doi: 10.1136/heartjnl-2017-311295. [DOI] [PubMed] [Google Scholar]
- 23.Zile M.R., Claggett B.L., Prescott M.F. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2016;68:2425–2436. doi: 10.1016/j.jacc.2016.09.931. [DOI] [PubMed] [Google Scholar]
- 24.Campbell D.J. Long-term neprilysin inhibition - implications for ARNIs. Nat Rev Cardiol. 2017;14:171–186. doi: 10.1038/nrcardio.2016.200. [DOI] [PubMed] [Google Scholar]
- 25.Sales N., Dutriez I., Maziere B., Ottaviani M., Roques B.P. Neutral endopeptidase 24.11 in rat peripheral tissues: comparative localization by 'ex vivo' and 'in vitro' autoradiography. Regul Pept. 1991;33:209–222. doi: 10.1016/0167-0115(91)90215-3. [DOI] [PubMed] [Google Scholar]
- 26.Fagerberg L., Hallstrom B.M., Oksvold P. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrigo M., Vodovar N., Nougue H. The heart regulates the endocrine response to heart failure: cardiac contribution to circulating neprilysin. Eur Heart J. 2018;39:1794–1798. doi: 10.1093/eurheartj/ehx679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisk L., Nalivaeva N.N., Boyle J.P., Peers C.S., Turner A.J. Effects of hypoxia and oxidative stress on expression of neprilysin in human neuroblastoma cells and rat cortical neurones and astrocytes. Neurochem Res. 2007;32:1741–1748. doi: 10.1007/s11064-007-9349-2. [DOI] [PubMed] [Google Scholar]
- 29.Yao M., Nguyen T.-V.V., Rosario E.R., Ramsden M., Pike C.J. Androgens regulate neprilysin expression: role in reducing β-amyloid levels. J Neurochem. 2008;105:2477–2488. doi: 10.1111/j.1471-4159.2008.05341.x. [DOI] [PubMed] [Google Scholar]
- 30.Liang K., Yang L., Yin C. Estrogen stimulates degradation of β-amyloid peptide by up-regulating neprilysin. J Biol Chem. 2010;285:935–942. doi: 10.1074/jbc.M109.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito T., Iwata N., Tsubuki S. Somatostatin regulates brain amyloid β peptide Aβ42 through modulation of proteolytic degradation. Nat Med. 2005;11:434. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- 32.Grimm M.O.W., Lehmann J., Mett J. Impact of vitamin D on amyloid precursor protein processing and amyloid-β peptide degradation in Alzheimer's disease. Neurodegen Dis. 2014;13:75–81. doi: 10.1159/000355462. [DOI] [PubMed] [Google Scholar]
- 33.Grimm M.O.W., Mett J., Stahlmann C.P., Haupenthal V.J., Zimmer V.C., Hartmann T. Neprilysin and Aβ clearance: impact of the APP intracellular domain in NEP regulation and implications in Alzheimer’s Disease. Front Aging Neurosci. 2013;5:98. doi: 10.3389/fnagi.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nalivaeva N.N., Belyaev N.D., Zhuravin I.A., Turner A.J. The Alzheimer's amyloid-degrading peptidase, neprilysin: can we control it? Int J Alzheimers Dis. 2012;2012:383796. doi: 10.1155/2012/383796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner A.J., Isaac R.E., Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Spencer B., Marr R.A., Rockenstein E. Long-term neprilysin gene transfer is associated with reduced levels of intracellular Abeta and behavioral improvement in APP transgenic mice. BMC Neurosci. 2008;9:109. doi: 10.1186/1471-2202-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sont J.K., van Krieken J.H., van Klink H.C. Enhanced expression of neutral endopeptidase (NEP) in airway epithelium in biopsies from steroid- versus nonsteroid-treated patients with atopic asthma. Am J Respir Cell Mol Biol. 1997;16:549–556. doi: 10.1165/ajrcmb.16.5.9160837. [DOI] [PubMed] [Google Scholar]
- 38.Kirkwood K.S., Bunnett N.W., Maa J. Deletion of neutral endopeptidase exacerbates intestinal inflammation induced by Clostridium difficile toxin A. Am J Physiol Gastrointest Liver Physiol. 2001;281:G544–G551. doi: 10.1152/ajpgi.2001.281.2.G544. [DOI] [PubMed] [Google Scholar]
- 39.Mitra R., Chao O.S., Nanus D.M., Goodman O.B., Jr. Negative regulation of NEP expression by hypoxia. Prostate. 2013;73:706–714. doi: 10.1002/pros.22613. [DOI] [PubMed] [Google Scholar]
- 40.Leithner K., Wohlkoenig C., Stacher E. Hypoxia increases membrane metallo-endopeptidase expression in a novel lung cancer ex vivo model - role of tumor stroma cells. BMC Cancer. 2014;14:40. doi: 10.1186/1471-2407-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards A.M. Plasma Neprilysin concentrations: a new prognostic marker in heart failure? Rev Esp Cardiol (Engl Ed) 2015;68:1053–1055. doi: 10.1016/j.rec.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Arrigo M., Nougué H., Launay J.-M., Mebazaa A., Vodovar N. Plasma neprilysin concentration during recovery from acute illness. Eur Heart J. 2018;39:3474–3475. doi: 10.1093/eurheartj/ehy456. [DOI] [PubMed] [Google Scholar]
- 43.Takahama H., Minamino N., Izumi C. Plasma soluble neprilysin levels are unchanged during recovery after decompensation of heart failure: a matter of the magnitude of the changes in systemic haemodynamics? Eur Heart J. 2018;39:3472–3473. doi: 10.1093/eurheartj/ehy454. [DOI] [PubMed] [Google Scholar]
- 44.Kuruppu S., Rajapakse N.W., Minond D., Smith A.I. Production of soluble neprilysin by endothelial cells. Biochem Biophys Res Commun. 2014;446:423–427. doi: 10.1016/j.bbrc.2014.01.158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.