Abstract
Background
The dual-mobility implant system has been shown to increase impingement-free range of motion and decrease dislocation risk by increasing the effective head size. In addition, the anatomic dual-mobility (ADM) cup offers relief between the acetabular shell rim and the iliopsoas tendon. This study was designed to review a series of hips implanted with the ADM acetabular cup to examine clinical outcomes after 5 years of implantation at multiple orthopaedic centers.
Methods
We retrospectively queried our prospectively collected total joint arthroplasty registry for patients who underwent total hip arthroplasty with an ADM cup from January 2008 to December 2012 at 4 different orthopaedic institutions and who had minimum 5-year follow-up. Harris Hip Scores and visual analog scale scores were evaluated. Postoperative complications, dislocations, and revisions for any reason were recorded.
Results
A total of 142 patients had a mean follow-up of 5.7 years (range: 5.0 to 8.0 years). Radiographic analysis showed no radiolucent lines, osteolysis, or acetabular loosening. There were no dislocations in this patient series. Two (1.2%) hips required a revision because of adverse local tissue reactions related to corrosion from a recalled modular neck stem, but this was unrelated to the ADM cup. The mean Harris Hip pain scores increased from 17 points preoperatively to 39 points at the most recent follow-up (P < .001). The mean Harris Hip function score increased from an average of 29 points preoperatively to 38 points at the most recent follow-up (P < .001). The mean visual analog scale score showed patient improvement from 6.5 preoperatively to 1.2 postoperatively (P < .001).
Conclusions
ADM prostheses were designed to reduce the risk of dislocation by increasing the size of the effective femoral head. In this multicenter study of ADM cups used in primary total hip arthroplasty, we demonstrated good clinical and radiographic outcomes, no dislocations, and no revisions at midterm 5-year minimum follow-up. Patient-reported outcome measures were also improved, supporting the use of this implant.
Keywords: Total hip arthroplasty, Instability, Dislocation, Dual mobility
Introduction
Total hip arthroplasty (THA) is an extremely successful surgery for alleviation of pain and restoration of function. Although rare, complications can occur, and the most common complication is instability accounting for more than 22.5% of revisions performed in the United States between 2005 and 2006 [1]. Postoperative hip dislocations, which occur in as high as 0.4% to 2% in the first year after hip arthroplasties, are devastating for patients both physically and psychologically and can be difficult to treat for the operative surgeon [2,3].
Numerous factors have been identified to influence the risk of dislocation after THA. These include patient factors such as noncompliance with restrictions postoperatively, spinopelvic disorders, neuromuscular disorders, osteonecrosis of the hip, and hip abductor dysfunction [2,[4], [5], [6]]. In addition, surgical factors such as cup position and orientation, cup and stem version, combined offset, head size, impingement, inadequate soft-tissue repair, and surgical approach also may influence the risk of instability [2,[4], [5], [6]].
Although dual-mobility (DM) cups have had a long and successful track record in Europe, they have only been recently introduced in the United States [7]. The construct was designed by Gilles Bousquet in France by combining the concepts of Charnley’s low-friction hip arthroplasty with the Mckee-Farrar theory of large-diameter femoral heads providing more stability [[7], [8], [9]]. The DM design allows for a larger effective head size by using a polyethylene ball with inner and outer bearing surfaces. This effectively reduces the risk of impingement by providing a larger head-to-neck ratio and improving the range of motion. The anatomic dual-mobility (ADM) cup has an anatomic shape that matches the rim of the native acetabulum and has a “left” and “right” configuration. It incorporates the fundamental DM bearing concept and also has a cutout placed along the iliopsoas groove anteriorly to reduce the risk of impingement and groin pain and also an extension of the cup surface posteriorly and inferiorly to provide greater stability in deep flexion. It also achieves implant rigidity via press-fitting, which allows for improved primary stability through rigid contact between the implant and the cortical bone. Of note is that the ADM cup is a monoblock cup which does not allow for screw fixation; therefore, adequate press-fit fixation must be achieved.
Currently, there are few studies reporting the midterm outcomes of the ADM construct in primary THA. We hypothesized that the ADM construct would provide a very low dislocation rate without an increased risk of complications related to the cup design. The purposes of our study were to determine the rate of postoperative dislocation and revision and to report on the patient-reported outcomes at a minimum 5-year follow-up.
Material and methods
After obtaining institutional review board approval from the 4 institutions, we retrospectively identified a cohort of all patients who underwent primary THA using the ADM (Stryker Corp., Mahwah, NJ) (see Fig. 1) acetabular component between January 1, 2008, and December 31, 2012. We used prospectively collected databases at all 4 high-volume arthroplasty centers. All primary THA procedures were performed by one of the 4 authors who were all fellowship-trained arthroplasty surgeons. The use of an ADM cup at each respective institution was surgeon preference in high-risk patients. The indications for an ADM cup included the following: (1) noninflammatory degenerative joint disease, (2) rheumatoid arthritis, and (3) femoral neck fractures of proximal femurs. While this clearly varied at each institution, most surgeons used the ADM cup in higher risk patients, including those with a body mass index of 30 kg/m2 or greater and with neurodegenerative diseases and aged 70 years or older. Demographics, operative details, and postoperative clinical and patient-reported outcomes were recorded.
Figure 1.

ADM cup (Stryker, Mahwah, NJ).
A total of 221 DM hips were implanted during the study period between the 4 institutions. Two hundred fifty fixed-bearing hips were also implanted among the institutions. An ADM cup was used in 184 of the 221 total hips. The remaining hips used a modular DM cup during the study period. Modular DM cups are used instead of ADM cups for patients with larger sized hips. While we only included patients with minimum 5-year follow-up, there were no major complications in patients at their last follow-up visits. Of the whole ADM cohort, 22 patients were deceased before 5-year follow-up for reasons unrelated to the surgery, leaving 162 hips. Of the remaining cohort, 142 patients had a minimum 5-year follow-up (88%). The mean age was 67 ± 10 years.
Surgical technique
Posterior approaches were solely used. Neuraxial anesthesia with either a spinal or epidural block was used in all cases. The ADM system is comprised of a monoblock cobalt chrome alloy cup with plasma-sprayed titanium surface overlaid with hydroxyapatite. A 28-mm delta-ceramic or cobalt-chromium head is captured within a highly cross-linked polyethylene ball, which articulates with the polished inner surface of the cup. The outer diameter of the polyethylene liner is always 6 mm smaller than the outer diameter of the cup. The polyethylene thickness varies from 5.9 mm with a 46-mm cup/40-mm polyethylene outer diameter ball to 14.9-mm thickness with a 64-mm cup/58-mm polyethylene outer diameter ball. The head-polyethylene ball is assembled with an intraoperative press before impaction onto the femoral trunnion.
The acetabulum is prepared by the removal of the labrum and medial soft tissue to expose the medial wall. The acetabular reamer is then used in the same orientation as the desired acetabular component with desired positioning of 45 degrees of abduction and 20 degrees of anteversion (range: 25-50 degrees). After every hip is appropirately templated, reaming of the acetabulum begins 6 mm smaller than the desired component and then is increased in 2 mm increments until circumferential bone contact is achieved. An ADM cup of the same diameter as the last reamer is then is then selected if a window trial provides a good fit. After reaming, the window trial is placed in the acetabulum to evaluate size, congruity, and depth. A marking pen is used circumferentially around the rim of the window trial to mark the desired location of the rim of the final component. The desired acetabular component is then opened and placed on the insertion handle with the laser marking at the 12 o’clock position. This is important as the cup has an anatomic shape (left and right) with a cutout anteriorly for the psoas tendon and a buildup posteriorly and inferiorly for enhanced stability in deep flexion. The cup is then impacted into the acetabulum and known to be well seated when it does not advance any further, and the rim of the cup is aligned with the blue mark that was previously made around the rim of the window trial. The position of the cup may be modified by using a rim impactor as needed. Osteophytes that protrude around the rim of the acetabular component are removed with an osteotome. After the proper-size femur broach is inserted, a trial reduction is performed with the head and DM liner trial. After reduction, the hip is brought through a full range of motion and checked for laxity and impingement. Acceptable stability was determined by greater than 90 degrees flexion, slight adduction, internal rotation to 60 degrees, and maximal external rotation with the hip extended. After the final stem is inserted, the real head and DM liner are assembled with an intraoperative press and then impacted onto the trunnion after it is cleaned and dried thoroughly. There were no cases in which the surgeon changed to a modular cup to use adjuvant screw fixation. The hip was then reduced and once again brought through a full range of motion and checked for stability.
Intraoperative adjustment of limb length and femoral offset were performed using a leg length and lateral offset measurement device. As per protocol, leg length discrepancy was uniformly maintained <5 mm and femoral offset ranged from 0 to 4 mm postoperatively. In addition, anteroposterior and lateral radiographs were taken preoperatively. Patient-anatomical landmark including the pubic tubercle and anterior superior iliac spines were used instead of target zones for anteversion and inclination in accordance with the posterior surgical approach.
A posterior capsule repair was performed in all cases and was consistent throughout surgeons. Postoperatively, patients were maintained on precautions 6 weeks postoperatively and mobilized with physical therapy beginning on the day of surgery or the following morning. While precautions may have varied between institutions, all the surgeons followed posterior hip precautions that limited flexion to 90 degrees and avoided internal rotation for 6 weeks. All patients were permitted full weight-bearing as tolerated.
Outcome measures
Primary outcome assessments were reoperation and dislocation after the index procedure. Visual analog scale (VAS) and Harris Hip Scores were secondary outcome measures. Scores were collected and analyzed at the preoperative and last postoperative visits.
Statistical methods
Statistical analyses were performed using SPSS, version 22 (IBM, Armonk, NY). Paired t-tests were used to compare preoperative and postoperative outcome measures. Statistical significance was set at P < .05.
Results
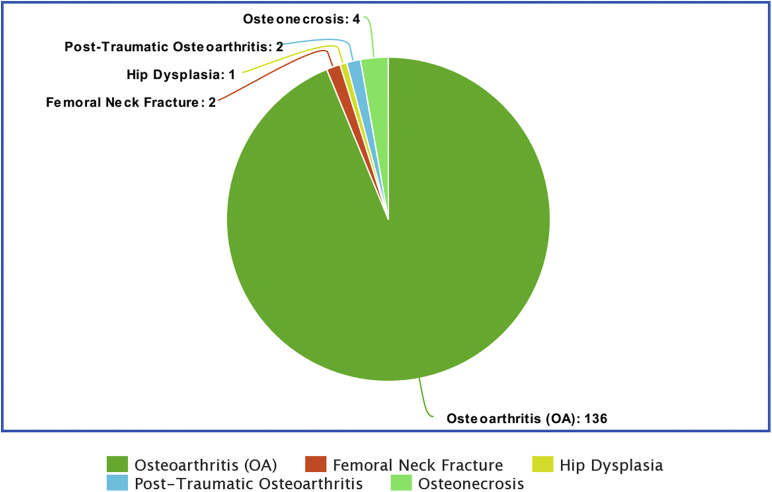
Indications for the index surgery included primary osteoarthritis, osteonecrosis, post-traumatic osteoarthritis, femoral neck fracture, and hip dysplasia (see Fig. 2). The mean acetabular component size used was 52 mm ± 3.5 mm. Metal heads were used in 90 of the 142 cases. Mean follow-up was 6 years (range: 5.0 to 8.0 years). There were no dislocations at the latest follow-up. There were 2 (1.2%) hips that required revision because of adverse local tissue reactions related to corrosion from a recalled modular neck femoral stem, but this was unrelated to the ADM cup. There were no ADM cups revised in this multicenter study, and there were no intraoperative fractures.
Figure 2.
Indications for the index surgery.
The mean Harris Hip pain scores increased from a mean of 17 points preoperatively to 39 points at the most recent follow-up (P < .001). The mean Harris Hip function scores increased from a mean of 29 points preoperatively to 38 points at the most recent follow-up (P < .001). The mean VAS scores improved from 6.5 preoperatively to 1.2 points postoperatively (P < .001) (Table 1).
Table 1.
Patient-reported outcome measurements at preoperative and 5-y follow-up visits.
| Patient reported outcome measures | Preoperative | 5-y follow-up | P-value |
|---|---|---|---|
| Harris Hip pain (mean) | 17 | 39 | P < .0001 |
| Harris Hip function (mean) | 29 | 38 | P < .0001 |
| VAS pain (mean) | 6.5 | 1.2 | P < .0001 |
Discussion
Instability after primary THA is an uncommon but devastating complication. DM cups have been shown to markedly reduce or eliminate the incidence of this problem [10,11]. However, there are limited reports on the ADM design since its introduction in the late 2000s. The goals of our study were to analyze the midterm outcomes of this implant, focusing on dislocation and revision as end points as well as functional improvement.
We acknowledge the limitations in the study. We did not have a control group of single-bearing primary THA for comparison, but dislocation rates with fixed-bearing hips are well studied in the literature with rates from 0.4% to 2.0% [2,3], which is consistent with the findings at our institutions. Second, a large number of patients were deceased or unable to be reached for follow-up at the 5-year postoperative time point. We determined 20 of 162 (13%) patients lost to follow-up, which is justifiable, given the nature of having a minimum of 5-year follow-up. Although we did not collect data on postoperative groin pain, a theoretical advantage of this design is a reduction in groin pain due to the recessed region for the psoas tendon in the ADM design. Although surgical techniques did vary, a multicenter approach still improves the generalizability of the results.
Nevertheless, we feel that our data clearly demonstrate the effectiveness and durability of the ADM cup at midterm follow-up, which is consistent with non-ADM primary THA. This is represented by comparable dislocation rates (average-0%), complication rates at the contributing centers (average - 1.7%), and lower accrued costs of DM THA than standard-bearing THA across the institutions. This supports the findings that DM THAs have lower accrued costs ($39,008 vs $40,031) and higher accrued utility (13.18 vs 13.13 QALYs) than standard-bearing THA [12]. Epinette et al. [13] found that risk difference translates into 3283 fewer dislocations per 100,000 patients for DM THA, which has the potential to save 39.6 million Euros.
No dislocations were observed in our study cohort of ADM cups for primary THA with minimum 5-year follow-up. There was a low all-cause revision rate of 1.2% for reasons unrelated to the design of the ADM cup. There were no revisions for failure of fixation of the ADM cup or subsequent aseptic loosening. Furthermore, there was excellent improvement in patient-reported outcome measures, with marked improvement in both Harris Hip Scores and VAS scores. These findings are consistent with those published in the existing literature.
Epinette et al. [14] reported on early outcomes of the ADM cup in 437 primary THAs with a 100% survivorship at 4-year mean follow-up in patients younger than 70 years and 99.7% in patients older than 70 years. Vigdorchik et al. [10] reported no dislocations at a minimum 2-year follow-up after primary THA in a cohort of 458 hips. Harwin et al. [15] published on 143 consecutive primary THAs using the ADM cup, showing no dislocations and a 98.6% all-cause survivorship at a mean of 6 years and 11-month follow-up. Darrith et al. [16] performed a meta-analysis of studies published between 2007 and 2016 reporting outcomes of DM cups in THA and found a 0.46% dislocation rate at a mean follow-up on 8.5 years in 10,783 primary THAs.
There have been some concerns in the literature specific to DM components including intraprosthetic dislocation (IPD) and potentially elevated wear rates. Neri et al. [17] reported that early designs of DM constructs resulted in IPD because of the use of 22-mm heads and wear of the first-generation polyethylene and subsequent failure of the rim’s retaining ring. With modern-day highly cross-linked polyethylene designs, this has become a rare occurrence. De Martino et al. [18] showed that majority of IPDs occur after attempting closed reduction of a dislocated DM construct. In our study, we did not have any IPDs, corroborating findings in the existing literature. IPDs can be overcome by requiring improved sedation for closed reduction or open surgery.
Furthermore, concerns have been proposed regarding potentially elevated wear rates in DM constructs compared with single-bearing constructs due to the presence of 2 bearing surfaces. However, recent studies have provided evidence refuting this hypothesis. Gaudin et al. [19] performed gravimetric wear analyses on DM vs single-bearing constructs put through a simulator to mimic 10-year follow-up. They determined that there are equivalent in vitro wear rates using conventional highly cross-linked polyethylene when comparing DM and single-bearing designs. Furthermore, D’Apuzzo et al. [20] demonstrated that retrieved DM constructs had very little wear on the outer bearing (with visualization of the original machine markings), with more of the wear occurring at the inner bearing. This study confirmed that most of the motion in DM hips indeed comes from the inner bearing-femoral ball articulation. Boyer et al. [21] also performed a retrieval analysis of 35 modern DM liners and found a median of 38 mm3/year of wear, equivalent or lower wear rates than the wear rates of single-bearing articulations.
Conclusions
Our data demonstrated that the ADM construct is promising for the prevention of postoperative instability after primary THA. At midterm follow-up, the ADM cup had excellent survivorship with no dislocations and no failures related to the cup or DM design. Although these results are encouraging, further long-term study will be needed to verify the continued durability and survivorship of the ADM construct.
Conflict of interest
G. Westrich receives royalties from Exactech, is a paid consultant for Stryker, Exactech, and DJO, receives research support from Stryker, Exactech, and DJO, and is a board member for the Eastern Orthopaedic Association; A. Malkani is a member of the Speakers bureau for Stryker, is a paid consultant for Stryker, receives research support from Stryker, receives royalties or financial or material support from Stryker, and is a member of the editorial board of the Journal of Arthroplasty; M. Mont receives royalties from Stryker and MicroPort, is a paid consultant for Stryker, CyMedica, DJ Orthopedics, Johnson & Johnson, OrthoSensor, Skye Biologics, TissueGene, Performance Dynamics, Pacira, PeerWell, and Pfizer, holds stock ownership in PeerWell and USMI, receives research support from Stryker, DJ Orthopedics, National Institutes of Health (NIAMS and NICHD), and TissueGene, receives royalties or financial or material support from Medicus Works LLC, Wolters Kluwer Health, and UpToDate, and is a board member for the AAOS, Journal of Arthroplasty, AAHKS, Journal of Knee Surgery, Knee Society, and Surgical Techniques International; K. Sharpe is a paid consultant for ConforMIS, holds stock ownership in Pacira, and receives research support from Stryker; all other authors declare no potential conflicts of interest.
Appendix A. Supplementary data
References
- 1.Bozic K.J., Kurtz S.M., Lau E., Ong K., Vail T.P., Berry D.J. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 2.Jolles B.M., Zangger P., Leyvraz P.-F. Factors predisposing to dislocation after primary total hip arthroplasty: a multivariate analysis. J Arthroplasty. 2002;17(3):282. doi: 10.1054/arth.2002.30286. [DOI] [PubMed] [Google Scholar]
- 3.Phillips C.B., Barrett J.A., Losina E. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85-A(1):20. doi: 10.2106/00004623-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Woo R.Y., Morrey B.F. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295. [PubMed] [Google Scholar]
- 5.Conroy J.L., Whitehouse S.L., Graves S.E., Pratt N.L., Ryan P., Crawford R.W. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867. doi: 10.1016/j.arth.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Nevelos J., Johnson A., Heffernan C., Macintyre J., Markel D.C., Mont M.A. What factors affect posterior dislocation distance in THA? Clin Orthop Relat Res. 2013;471(2):519. doi: 10.1007/s11999-012-2559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farizon F., de Lavison R., Azoulai J.J., Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22(4):219. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prudhon J.L., Verdier R., Caton J.H. Low friction arthroplasty and dual mobility cup: a new gold standard. Int Orthop. 2017;41(3):563. doi: 10.1007/s00264-016-3375-0. [DOI] [PubMed] [Google Scholar]
- 9.Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. J Bone Joint Surg Br. 1972;54(1):61. [PubMed] [Google Scholar]
- 10.Vigdorchik J.M., D’Apuzzo M.R., Markel D.C. Lack of early dislocation following total hip arthroplasty with a new dual mobility acetabular design. Hip Int. 2015;25(1):34. doi: 10.5301/hipint.5000186. [DOI] [PubMed] [Google Scholar]
- 11.Epinette J.-A., Harwin S.F., Rowan F.E. Early experience with dual mobility acetabular systems featuring highly cross-linked polyethylene liners for primary hip arthroplasty in patients under fifty five years of age: an international multi-centre preliminary study. Int Orthop. 2017;41(3):543. doi: 10.1007/s00264-016-3367-0. [DOI] [PubMed] [Google Scholar]
- 12.Barlow B.T., McLawhorn A.S., Westrich G.H. The cost-effectiveness of dual mobility implants for primary total hip arthroplasty: a computer-based cost-utility model. J Bone Joint Surg Am. 2017;99:768. doi: 10.2106/JBJS.16.00109. [DOI] [PubMed] [Google Scholar]
- 13.Epinette J.-A., Lafuma A., Robert J. Cost-effectiveness model comparing dual-mobility to fixed-bearing designs for total hip replacement in France. Orthop Traumatol Surg Res. 2016;102:143. doi: 10.1016/j.otsr.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Epinette J.-A., Béracassat R., Tracol P., Pagazani G., Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323. doi: 10.1016/j.arth.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Harwin S.F., Sultan A.A., Khlopas A. Mid-term outcomes of dual mobility acetabular cups for revision total hip arthroplasty. J Arthroplasty. 2018;33(5):1494. doi: 10.1016/j.arth.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Darrith B., Courtney P.M., Della Valle C.J. Outcomes of dual mobility components in total hip arthroplasty. Bone Joint J. 2018;100-B(1):11. doi: 10.1302/0301-620X.100B1.BJJ-2017-0462.R1. [DOI] [PubMed] [Google Scholar]
- 17.Neri T., Boyer B., Geringer J. Intraprosthetic dislocation of dual mobility total hip arthroplasty: still occurring? Int Orthop. 2018;43:1097. doi: 10.1007/s00264-018-4054-0. [DOI] [PubMed] [Google Scholar]
- 18.De Martino I., D’Apolito R., Soranoglou V.G., Poultsides L.A., Sculco P.K., Sculco T.P. Dislocation following total hip arthroplasty using dual mobility acetabular components. Bone Joint J. 2017;99-B(1_Supple_A):18. doi: 10.1302/0301-620X.99B1.BJJ-2016-0398.R1. [DOI] [PubMed] [Google Scholar]
- 19.Gaudin G., Ferreira A., Gaillard R., Prudhon J.L., Caton J.H., Lustig S. Equivalent wear performance of dual mobility bearing compared with standard bearing in total hip arthroplasty: in vitro study. Int Orthop. 2017;41(3):521. doi: 10.1007/s00264-016-3346-5. [DOI] [PubMed] [Google Scholar]
- 20.D’Apuzzo M.R., Koch C.N., Esposito C.I., Elpers M.E., Wright T.M., Westrich G.H. Assessment of damage on a dual mobility acetabular system. J Arthroplasty. 2016;31(8):1828. doi: 10.1016/j.arth.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer B., Neri T., Geringer J., Di Iorio A., Philippot R., Farizon F. Long-term wear of dual mobility total hip replacement cups: explant study. Int Orthop. 2018;42(1):41. doi: 10.1007/s00264-017-3525-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.