Abstract
Polyspike ictal-onset absence seizure has been reported in adult patients with genetic generalized epilepsy but is a novel pattern in the pediatric population. Absence seizures are usually associated with generalized spike-and-wave on EEG. However, we present the case of a 10-year-old girl with Down syndrome and developmental delays who presented with atypical absence seizure associated with an unusual electroencephalographic (EEG) pattern of polyspike ictal-onset. Recognition of this ictal pattern in the pediatric population, as previously reported in adult populations, is important as it can have therapeutic and prognostic implications.
Keywords: Absence seizure, Down syndrome, Polyspike ictal-onset, Ictal pattern
Highlights
-
•
Absence seizures are usually associated with generalized spike and wave, but here we report a polyspike ictal-onset pattern.
-
•
The pattern appeared as purely a polyspike ictal-onset, or polyspikes intermixed or preceding the generalized spike and wave.
-
•
Polyspike ictal onset is differentiated from generalized paroxysmal fast activity (GPFA), by morphology & other features.
1. Introduction
Absence seizures in children have been extensively described in the setting of unknown etiology, structural, or genetic generalized epilepsies [1]. Several types of absence seizures have been described like typical absence, atypical absence, absence with eyelid myoclonia, and myoclonic absence as outlined by the International League Against Epilepsy (ILAE)[2]. Ictal patterns in these children vary widely according to seizure type and epilepsy syndrome. The literature on the ictal EEG [3] recording for absence seizures describes 2.5 to 4 Hz generalized spike-and-wave (GSW) discharges in typical absence seizures, generalized polyspike waves at 3 to 6 Hz with eyelid myoclonia and slower frequencies of < 2.5 Hz in atypical absence seizures. Fakhoury & Abou-Khalil [4] & Tatum et al. [5] have described polyspike ictal-onset of absence seizure in adults. Here, we describe this novel EEG finding of our patient and review the literature on polyspike ictal patterns.
2. Case presentation
The patient is a 10-year-old girl with Down syndrome adopted four years ago from China. Due to her adoption, there is no information regarding her perinatal or family histories. The child lives with both adoptive parents and attends homeschooling. She presented to the neurology clinic due to new-onset of staring episodes, which started a month prior. Her mother described these as a complete behavioral arrest accompanied by eyes rolling upward. These events typically happen multiple times daily and last less than 30 s with no post-ictal changes. No eyelid myoclonia or automatisms or body jerks were associated with the seizures and no reported history of generalized tonic–clonic (GTC), or other seizure types. Her physical exam shows typical features of Down syndrome, and neurological examination was significant for mild developmental delay involving all areas of developmental milestones, especially in speech. Magnetic resonance imaging of the brain was unrevealing.
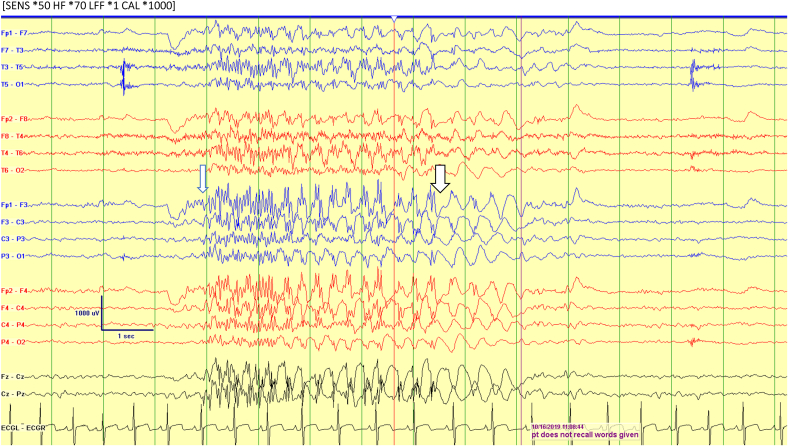
An EEG was done, which captured four stereotypical episodes lasting 5–8 s. Clinically, she was noted to have eye rolls associated with behavioral arrest and unresponsiveness. During the episode, she was unable to recall the word given to her. Ictal EEG recordings showed an initial high voltage (> 400 microvolts) generalized 14–18 Hz polyspikes, which occurred in isolation or followed by 2–3 Hz GSW discharges (Fig. 1). The faster ictal discharges were the dominant ictal activity and sometimes associated with preceding eye blinking and appeared to be embedded in some ictal discharges.
Fig. 1.
The ictal EEG (long arrow) with initial high amplitude (400–700 microvolts) frontally predominant generalized polyspikes (long arrow) followed by 2–3 Hz generalized spike and wave (short arrow). Also note the intermixed polyspikes with the spike and wave. The background dominant rhythm was slow for age.
The absence seizure was associated with staring, subtle behavioral arrest and up rolling of eyeballs. Patient was not able to recall the words given during this event.
Interictal EEG recordings showed few bursts of high voltage 4 to 6 Hz irregular GSW, 3 to 4 Hz polyspike and wave complex (PSW), as well as fragmentary bursts noted both during the awake and asleep state. Background rhythm revealed a poorly sustained 7–7.5 Hz posterior dominant rhythm for age. A typical event was induced by hyperventilation, but no abnormalities were elicited with the photic stimulation.
Based on the clinical profile and EEG findings, the patient was diagnosed with genetic generalized epilepsy and was started on levetiracetam given atypical EEG features. She has ongoing breakthrough seizures for which medication was optimized in the last clinical encounter.
3. Discussion
In the current ILAE classification [[2]], several types of absence seizures have been described like typical absence, atypical absence, absence with eyelid myoclonia, and myoclonic absence. Typical absence seizures usually present with complete impairment of awareness consisting of an abrupt start and end. Absence seizures are considered atypical if they are associated with a less clear abrupt onset, offset, and significant changes in tone. Awareness and responsiveness can be partially retained during some generalized seizures, like absence seizures with eyelid myoclonus, atypical absence, and myoclonic absence. However, our patient presents with an isolated seizure type consisting of absence seizures with predominant eye rolls.
The epileptiform discharges differ depending on the specific epilepsy syndrome and seizure types [3]. Typical absence seizures consist of 3-Hz frontally predominant generalized spike-and-wave (GSW) discharges in childhood absence epilepsy and relatively faster frequencies in juvenile absence seizures. In atypical absence seizures, the EEG will reveal irregular GSW discharges below 2.5 Hz. The ictal EEG pattern of eyelid myoclonia consists of generalized polyspikes waves at 3 to 6 Hz triggered by active eye-closure and photic stimulation [6]. Even though the eyelid myoclonia can be associated with generalized polyspikes, our patient did not have features of Jeavon syndrome-like clinical eyelid myoclonia, photosensitivity, and eye closure induced epileptic paroxysms. ILAE seizure classification [1,2] doesn't identify absence seizures with polyspike/fast rhythmic discharges EEG patterns such as the ones in our patient's EEG, as a distinct seizure type.
The paroxysmal event may be classified as atypical absence seizures due to unusual EEG findings that include polyspike ictal onset and an abnormally slow background. These findings were discussed with the family, and because of these atypical features, levetiracetam was chosen instead of ethosuximide. Atypical absence seizures are seen in cognitively impaired children with epilepsy. However, absence seizures are rare in patients with Down syndrome and therefore, are not typical for Down syndrome-associated epilepsy [7]. Patients with Down syndrome typically presents with infantile spasms and can have various other seizures types, including focal, myoclonic, GTC, and atonic seizures [7].
This ictal pattern of polyspikes can be confused with generalized paroxysmal fast activity (GPFA), which is differentiated mainly by morphology and other characteristics. GPFA is characterized by a diffuse paroxysmal rhythmic activity usually in the beta frequency (range of 8–26 Hz) with a low to medium voltage of 20–135 microvolts typically lasting for 2–50 s and posterior predominant in pediatric subjects [8]. Brenner & Atkinson [9] have previously defined GPFA as a generalized activity in the beta frequency lasting at least 1 s, usually preceded or followed by other generalized epileptiform abnormalities or slow waves. However, polyspikes are frontally predominant high voltage (> 200 microvolts) repetitive spikes (3 or more spikes) with a frequency of more than 10 Hz [10]. In our case, the EEG includes a high amplitude burst (> 400 microvolts) of several spikes occurring in a sequence resembling polyspikes. Aurlien et al. [11] defined ‘Polyspike’ as a multiple spike complex; several spikes occurring in a sequence without slow waves are usually no longer than 1 s and correlated with higher generalized epileptiform activity amplitude.
‘Polyspike slow-wave’ is defined as a sequence of multiple spikes and a slow wave [11]. Polyspike slow-wave discharges are typically found as an ictal pattern in epilepsies associated with myoclonus, photo-convulsive pattern, and observed in some patients with Childhood-Juvenile absence epilepsy. Still, polyspikes per se are not typically reported as an ictal pattern in case of absence seizures in children. Prominent polyspike discharges are noted interictally both in genetic generalized epilepsy, and in developmental and epileptic encephalopathy [12,13].
The pathophysiology for polyspike ictal-onset absence seizures in patients with generalized epilepsy has remained elusive [14]. Still, the presence of isolated polyspikes and intermixed with the generalized spike and wave associated with absence seizure suggests a common generator most likely arising from thalamocortical rhythms, which is the most accepted theory [14]. Seizures in patients with Down syndrome may be a secondary effect of both the functional and anatomical neurological decline [15].We hypothesized that the polyspike ictal pattern may be coincidental in this patient with Down syndrome and may represent the same spectrum as reported in adult literature due to similar clinical and EEG characteristics. The polyspikes as harmonic variations of the spike-and-wave activity are purely speculative. Whether this polyspike pattern evolves from 3 Hz GSW, faster 4–6 Hz GSW pattern or slower frequencies of < 2.5 Hz or is a modification of the typical electroclinical pattern is not proven. However, due to limited literature on polyspike ictal onset, it is unclear if the patient's Down syndrome plays a contributing part in her unique EEG pattern.
The phenomenon of fast rhythmic activity (polyspikes) seen in our pediatric case has been reported in the adult literature. Fakhoury & Abou-Khalil [4] reported the fast 10–15 Hz rhythmic discharges in 5 adult patients with absence seizures. Tatum et al. [5] reported absence seizures with polyspike ictal onset 3-Hz GSW were seen in seven adult patients. Michelucci et al. [16] described 3 of 12 patients with prolonged polyspike ictal-onset before 3-Hz GSW associated with absence seizures. Semiology in these studies [4,5,16] (total of 14 patients) consisted of mostly atypical absence seizures [4,16], like our patient, but few presented with typical absence seizures [5] are also described. The seizure onset was predominantly in the pediatric age group (range 4–32 years) with absence seizure type and developing other seizure types (generalized tonic–clonic, myoclonic) in their clinical course. In contrast, our patient seizure onset was at ten years old, with no reported history of other seizure types. Interictally, all of them [4,5,16] had at least 2.5–4 Hz generalized spike and wave as well as polyspikes and waves like our patient. The polyspike ictal-onset pattern was described with the following variations, as noted in our case. It represents rhythmic fast ictal discharge for > 50% of the ictus preceding the generalized spike and wave [4,5,16], an intermixed polyspike pattern with 2–3 Hz generalized spike and wave after onset [4,5,16], or purely a polyspike ictal-onset [4].
The patient's cognition was normal in most of them, with few having an intellectual disability, whereas our patient's impaired cognition was mainly related to Down syndrome. The prognosis was variable with a good response with valproic acid [4] or drug-resistant requiring lifelong anti-seizure medications [5,16]. Our patient has a shorter duration of epilepsy with ongoing breakthrough seizures for which levetiracetam is optimized. Hence, recognizing this ictal pattern could be of significance in children as it is reported with an unfavorable outcome with drug-resistant epilepsy in adult literature [5,16].
Some reports [17,18] have related fast rhythmic activity to atypical absence seizures, or the generalized tonic seizure in patients with Lennox-Gestaut syndrome (LGS). However, our patient does not meet the electroclinical criteria of LGS (no typical slow spike–wave, interictal paroxysmal fast rhythms (10–20 Hz) during sleep, and other seizures types like a tonic, atonic or generalized tonic–clonic seizures). GPFA in LGS is a sleep-dependent EEG phenomenon not seen in this patient. The cognitive delay and speech delay seen in our patient could be related to Down syndrome rather than being a feature of LGS.
There have been multiple reports [19,20] with no reference to polyspike ictal onset in children with the absence seizures. Electrographically, ictal patterns in children, can differ depending on multiple factors such as age, seizure and epilepsy type, and epileptic encephalopathies. Hence, an understanding of the full spectrum of ictal activity in children is necessary for neurophysiologists for diagnostic and therapeutic purposes.
4. Conclusion
In conclusion, this novel polyspike ictal-onset pattern for absence seizure though unusual in the pediatric population could be seen in subjects with genetic epilepsy, as in our case. This ictal pattern may represent the same spectrum as reported in adult literature due to similar clinical and EEG characteristics. This interesting pattern is speculated to be a harmonic variation of the spike-and-wave activity or modification of the typical electroclinical pattern, which could be secondary to the brain maturational process in Down syndrome. However, due to the lack of literature on polyspike-ictal onset in the pediatric patient population, it is unclear if the patient's Down syndrome plays a contributing part in her unique EEG pattern.
Ethical statement
I agree upon standards of expected ethical behavior as the author involved in the act of publishing. All authors have not received any honorarium, grant, or other forms of payment for the work. All authors have contributed equally to the writing of the manuscript and have seen and approved the submission of this version of the manuscript and take full responsibility for the manuscript. There are no prior publications or submissions with any overlapping information, including studies and patients. The paper will not be submitted to any other journal while under consideration at “Epilepsy & Behavior Reports.” We affirm that the work contains original information.
Financial disclosure
The authors received no financial support for the research and/or authorship of this article.
Declaration of competing interest
The author (Sherouk Abdelmoity) has written the first draft of the manuscript, and has not received any honorarium, grant, or other form of payment to produce this manuscript. The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017 Apr;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017 Apr;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 3.Spinosa M.J., Liberalesso P.B., Mehl L., Löhr Júnior A. Ictal patterns in children: an illustrated review. J Epilepsy Clin Neurophysiol. 2011;17(4):154–163. [Google Scholar]
- 4.Fakhoury T., Abou-Khalil B. Generalized absence seizures with 10–15 Hz fast discharges. Clin Neurophysiol. 1999 Jun 1;110(6):1029–1035. doi: 10.1016/s1388-2457(99)00028-0. [DOI] [PubMed] [Google Scholar]
- 5.Tatum W.O., Ho S., Benbadis S.R. Polyspike ictal onset absence seizures. J Clin Neurophysiol. 2010 Apr 1;27(2):93–99. doi: 10.1097/WNP.0b013e3181d64c7e. [DOI] [PubMed] [Google Scholar]
- 6.Striano S., Capovilla G., Sofia V., Romeo A., Rubboli G., Striano P. Eyelid myoclonia with absences (Jeavons syndrome): a well-defined idiopathic generalized epilepsy syndrome or a spectrum of photosensitive conditions? Epilepsia. 2009 May;50:15–19. doi: 10.1111/j.1528-1167.2009.02114.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg-Stern H., Strawsburg R.H., Patterson B., Hickey F., Bare M., Gadoth N. Seizure frequency and characteristics in children with Down syndrome. Brain Dev. 2001 Oct 1;23(6):375–378. doi: 10.1016/s0387-7604(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 8.Bansal L., Collado L.V., Pawar K., Nagesh D., Ilyas M., Hall A. Electroclinical features of generalized paroxysmal fast activity in typical absence seizures. J Clin Neurophysiol. 2019 Jan 1;36(1):36–44. doi: 10.1097/WNP.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 9.Brenner R.P., Atkinson R. Generalized paroxysmal fast activity: electroencephalographic and clinical features. Ann Neurol. 1982 Apr;11(4):386–390. doi: 10.1002/ana.410110412. [DOI] [PubMed] [Google Scholar]
- 10.Lüders H., Noachtar S. Saunders; 2000. Atlas and classification of electroencephalography. [Google Scholar]
- 11.Aurlien H., Gjerde I.O., Eide G.E., Brøgger J.C., Gilhus N.E. Characteristics of generalised epileptiform activity. Clin Neurophysiol. 2009 Jan 1;120(1):3–10. doi: 10.1016/j.clinph.2008.10.149. [DOI] [PubMed] [Google Scholar]
- 12.Smith S.J. EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry. 2005 Jun 1;76(Suppl. 2) doi: 10.1136/jnnp.2005.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini C., King M.A., Archer J.S., Newton M.R., Berkovic S.F. Idiopathic generalised epilepsy of adult onset: clinical syndromes and genetics. J Neurol Neurosurg Psychiatry. 2003 Feb 1;74(2):192–196. doi: 10.1136/jnnp.74.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gloor P., Fariello R.G. Generalized epilepsy: some of its cellular mechanisms differ from those of focal epilepsy. Trends Neurosci. 1988 Jan 1;11(2):63–68. doi: 10.1016/0166-2236(88)90166-x. [DOI] [PubMed] [Google Scholar]
- 15.Romano C., Tiné A., Fazio G., Rizzo R., Colognola R.M., Sorge G. Seizures in patients with trisomy 21. Am J Med Genet. 1990;37(S7):298–300. doi: 10.1002/ajmg.1320370758. [DOI] [PubMed] [Google Scholar]
- 16.Michelucci R., Rubboli G., Passarelli D., Riguzzi P., Volpi L., Parmeggiani L. Electroclinical features of idiopathic generalised epilepsy with persisting absences in adult life. J Neurol Neurosurg Psychiatry. 1996 Nov 1;61(5):471–477. doi: 10.1136/jnnp.61.5.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gastaut H. Clinical and electroencephalographical classification of epileptic seizures. Epilepsia. 1970 Mar;11(1):102–112. doi: 10.1111/j.1528-1157.1970.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 18.Berkovic S.F., Andermann F., Andermann E., Gloor P. Concepts of absence epilepsies: discrete syndromes or biological continuum? Neurology. 1987 Jun 1;37(6):993. doi: 10.1212/wnl.37.6.993. [DOI] [PubMed] [Google Scholar]
- 19.Penry J.K., Porter R.J., Dreifuss R.E. Simultaneous recording of absence seizures with video tape and electroencephalography. A study of 374 seizures in 48 patients. Brain. 1975 Sep;98(3):427–440. doi: 10.1093/brain/98.3.427. [DOI] [PubMed] [Google Scholar]
- 20.Panayiotopoulos C.P., Obeid T., Waheed G. Differentiation of typical absence seizures in epileptic syndromes: a video EEG study of 224 seizures in 20 patients. Brain. 1989 Aug 1;112(4):1039–1056. doi: 10.1093/brain/112.4.1039. [DOI] [PubMed] [Google Scholar]