Objective
Histological features of oral inflammation include infiltration of polymorphonuclear leukocytes [PMN], however studies have not examined the effects of interventions mitigating inflammation on oral PMN. Methods: This double-blind clinical study examined the effects of rinsing with mouthwashes formulated with chlorhexidine [CHX], an ingredient widely utilized in the dental clinic in comparison to a control on oral PMN representing a novel measure of inflammation. A concurrent evaluation of dental plaque and gingival inflammation using widely accepted clinical indices was included in the study. The study enrolled adult subjects providing informed consent, met study criteria and registered gingival index scores of 1.0 or more at the screening visit. Subjects [n = 90; age range 19–58 years] completed a washout phase prior to baseline evaluations for PMN and clinical assessments for dental plaque and gingivitis. Treatments [CHX or a control mouthwash] were randomly assigned to subjects for twice-daily use for the next two weeks. Post-treatment evaluations similar to baseline were conducted after one and two week use of assigned treatment. Results: At baseline, no statistically significant differences between treatment groups for PMN or clinical indices for dental plaque or gingivitis were noted. Rinsing with CHX demonstrated significant reductions for PMN and dental plaque, gingivitis in comparison to the control group. After one and two week use of CHX, PMN demonstrated a 35.9% and 54.9% reduction respectively in comparison to the control group representing significant differences [p < 0.05]. At the one and two week post-treatment evaluations, rinsing with CHX demonstrated 15% and 25% reductions in gingivitis respectively and were significantly different from the control [p < 0.05]. Rinsing with CHX also demonstrated significant reductions in dental plaque of 15% and 19% at the one and two-week post-treatment evaluations respectively in comparison to the control [p < 0.05]. The CHX group also demonstrated reductions in interproximal scores and registered the lowest frequency of gingival index or dental plaque scores on all oral surfaces. Conclusions: Results outline an objective approach to evaluate oral inflammation demonstrating a large and sustained reduction in oral PMN by CHX with these outcomes numerically higher than a clinical index evaluating gingivitis.
Keywords: Dental plaque, Gingivitis, Chlorhexidine, Inflammation, Mouthwash, Polymorphonuclear leukocytes
Highlights
-
•
Enumeration of oral polymorphonuclear leukocytes [PMN] estimates the oral inflammatory burden.
-
•
Rinsing with chlorhexidine demonstrated progressive PMN reductions corroborating gingivitis and dental plaque evaluations.
-
•
Rapid PMN assessment provides objective assessment of treatment effects applicable for dental chair-side setting.
1. Introduction
Gingivitis, an inflammation of the gums and tissues that supports the teeth is a commonly reported condition with a widespread prevalence [1]. The inception and progression of gingivitis is attributed to the natural formation of dental plaque comprising large densities of endogenous oral organisms leading to the accumulation of their metabolites and by-products with inflammatory potential [1,2]. Effective oral hygiene reduces accumulating dental plaque and other inflammatory components and may reverse the symptoms associated with gingivitis.
Chlorhexidine [CHX], a cationic bisbiguanide with antiseptic features is extensively used in medicine and surgery [3,4]. Based on the available evidence, CHX is widely regarded as a gold-standard. Notable features of CHX are its recognized safety and stability while delivering antibacterial effects for a sustained period due to its substantivity on oral surfaces [5] with substantial reductions in dental plaque and gingival index scores.
Tissue inflammation is characterized by histological features that include rapid infiltration of neutrophils [2,6] and are referred to as first responders. Estimates suggest about 1 billion neutrophils are produced per day per kilogram of body weight [6] representing the most abundant leukocyte population with effector functions on mucosal surfaces. During oral inflammation neutrophils [7] form a barrier between the plaque and the gingival tissue and wall off the growing microorganisms [2]. Following extravasation, neutrophils [PMN] accumulate in oral fluids with approximately 80% retaining viability for a short duration [8]. Noninvasive approaches to evaluate oral neutrophils in health and periodontal disease have been reported [[9], [10], [11], [12], [13]]. Surveys indicate that oral PMN increase from health to gingivitis with the highest numbers noted in periodontal disease (unpublished data) reflecting a relationship with the oral inflammatory burden. However, the effects of interventions that mitigate gingival inflammation on the numbers of oral PMN remain unknown. The aim of the present was to examine the effects of daily oral hygiene that included a CHX and a fluoride mouthwash on oral neutrophils as a novel and objective measure of inflammation to examine treatment related changes. Concurrent assessments of clinical parameters of dental plaque and gingivitis were included representing widely accepted measures to evaluate the effects of interventions.
2. Materials and methods
2.1. Study design
This single site, two-week parallel design study was double-blind with randomized treatment assignment that enrolled healthy volunteers. The study protocol was approved by the ethics board of the SDM Dental College and Hospital, Dharwad, India, prior to study enrollment and conducted at the dental clinic of the Dental College.
Volunteers of either gender from the local area who expressed an interest in study participation were invited to the dental clinic for a screening visit and allotted a unique identification number. Participants who voluntarily completed an informed consent form and consented to study participation were screened for study eligibility. The study dentist completed an oral examination that included the entire dentition, the tongue, palate and all soft tissue regions with these assessments conducted under constant lighting conditions. A medical history was taken and a whole mouth assessment of dental plaque and gingivitis conducted on evaluable teeth using the Turesky-Modification of the Quigley Hein Index [14] and Loe-Silness [15] Index respectively. Study eligibility was restricted to those of either gender between the age of 18–70 years and presented atleast 20 natural teeth. Subjects in good general health and registering dental plaque scores equal to or greater than 1.5 and gingival index equal to or greater than 1.0, were enrolled. Subjects with dentures, orthodontic bands were excluded. Exclusion criteria included pregnancy or breast feeding, systemic diseases, ongoing or scheduled medical or dental treatments during the study period. Those reporting medical or dental treatments or medications including antibiotics or anti-inflammatory treatments in the month preceding study screening or enrollment in a clinical study in that duration were excluded. Other exclusion criteria included those reporting allergies to oral hygiene formulations, a history of drug or alcohol abuse or with objects that pierce the lips of tongue. Subjects unable to refrain for upto 4 h from food and water were also excluded. Participants who satisfied the study inclusion and exclusion criteria were enrolled and provided a tube of commercially available fluoride toothpaste [Colgate Dental Cream, Great Regular Flavor, New York, NY] and a commercially available soft bristled toothbrush [Colgate Extra Clean, New York, NY] for use during the one week washout phase. Subjects were instructed to brush their entire dentition twice daily once in the morning and in the evening with the provided washout articles. Subjects were also instructed to not share any test related articles with anyone and only use the provided clinical supplies for the study duration.
After completing the washout phase, subjects refrained from oral hygiene for 12 h prior to their scheduled baseline visit at the dental clinic. Subjects were provided sterile saline to rinse their mouth for 30 s. These samples were expectorated into tubes labeled with unique subject identifiers and transported to the laboratory for microscopic analysis for PMN as described in section below. A dental examiner conducted an assessment of dental plaque and gingivitis.
Subjects were randomized and issued their treatments that comprised a commercially available fluoride mouthwash [Colgate Fluorigard, New York, NY; henceforth control] or a mouthwash formulated with 0.12% CHX [Colgate PerioGard, New York, NY; henceforth test]. All treatments were overwrapped and issued a unique code. To maintain blinding, randomization and product dispensation were conducted by study personnel who had no role in any other study related activities with these efforts scheduled in an area separate to that in which clinical assessments were conducted. All subjects were provided an identical tube of commercially available fluoride toothpaste [Colgate Dental Cream, Great Regular Flavor, New York, NY] and a commercially available soft bristled toothbrush and instructed to use allocated products twice daily for the 2 week duration of the study. Subjects instructed to brush their teeth twice daily with assigned toothpaste and then rinse for 30 s with 15 ml of assigned mouthwash.
Post-treatment evaluations were conducted after one and two week use of assigned treatments and were identical to those conducted during the baseline visit. Subjects refrained from oral hygiene for 12-h prior to their visits. Oral samples were evaluated for PMN in the laboratory and a dental examiner conducted clinical evaluations for dental plaque and gingivitis. A complete oral examination that included examination of the entire dentition, the tongue, palate and all soft tissue regions was conducted by a dentist at all visits. Product compliance was checked during visits and subjects interviewed for adverse events. At the final visit, subjects returned all study articles to the study site.
2.2. Clinical measurements
Subjects were assessed for dental plaque and gingivitis on all scorable teeth during all visits. Analysis of mouthwash efficacy was based on scores averaged over all sites of scorable teeth. A primary outcome measure was the gingival index [Loe-Silness Index [15]; assessment using a 3 point scale on 6 surfaces per tooth: (1)mesio-facial; (2) mid-facial; (3) disto-facial; (4) mesio-lingual; (5) mid-lingual; and (6) disto-lingual. The maximum score per tooth, therefore, is 18. Third molars and those teeth with cervical restorations or prosthetic crowns were excluded from the scoring procedure. A Loe-Silness Gingival Index score from 0 to 3 was assigned by the examining dental examiner to all scoreable surfaces of the maxillary and mandibular teeth using a dental light and dental mirror. A whole mouth mean score for each subject was determined by adding the values given by the dental examiner to each scorable surface and dividing that number by the total number of surfaces scored. The Loe-Silness criteria for the Gingival Index is as follows:
-
0
Absence of inflammation.
-
1
Mild inflammation-slight change in color and little change in texture
-
2
Moderate inflammation-moderate glazing, redness, edema and hypertrophy. Tendency to bleed upon probing
-
3
Severe inflammation-marked redness and hypertrophy. Tendency to spontaneous bleeding.
An additional primary outcome measure was a whole mouth plaque examination based on the Turesky Modified Quigley-Hein plaque index [14]. Dental plaque was disclosed using a red solution to dye the plaque and all scorable surfaces of the maxillary and mandibular teeth will be evaluated. A score from 0 to 5 was evaluated by a dental examiner for all scoreable surfaces of the maxillary and mandibular teeth using a dental light and dental mirror. Each tooth was scored on six surfaces: (1)mesio-facial; (2) mid-facial; (3) disto-facial; (4) mesio-lingual; (5) mid-lingual; and (6) disto-lingual. The scoring procedures excluded third molars and teeth with cervical restorations or prosthetic crowns. A whole mouth mean score for each subject was determined by adding the values given by the dental examiner to each scorable surface and dividing that number by the total number of surfaces scored. The Turesky Modification of the Quigley-Hein Plaque Index is as follows:
-
0
no plaque
-
1
separate flecks of plaque at the cervical margin of the tooth.
-
2
a thin continuous band of plaque (up to 1 mm) at the cervical margin of the tooth.
-
3
a band of plaque wider than 1 mm but covering less than one-third of the crown of the tooth.
-
4
plaque covering at least one-third but less than two thirds of the crown of the tooth.
-
5
plaque covering two-thirds or more of the crown of the tooth.
2.3. Oral PMN estimations
PMN estimations in oral samples were based on previously described procedures [9,13] with a few modifications. In brief, oral samples were briefly centrifuged and the pellet resuspended in an aliquot of fresh buffer. Samples were stained with acridine orange and the number of PMN estimated using a fluorescence microscopy with samples placed in a Neuber's chamber. These evaluations were conducted immediately after sampling in an adjacent laboratory with results that could be rapidly relayed chair-side.
2.4. Sample size calculations and statistical analysis
Sample size calculations for this study were based on historical data of gingival index scores as the response measure with a standard deviation of 0.3. Fifty (50) subjects enrolled per treatment group would allow detection of a 10% difference in gingival index scores between treatment groups at 80% power with a 15% attrition rate over the study duration.
At each time point of the study, descriptive statistics were separately computed for each treatment group that included subject age and gender with analysis conducted for subjects who completed the entire study and provided evaluable results. Frequency distributions for dental plaque and gingival index scores at each evaluation were computed. Summary statistics were calculated for the test and control groups along with mean scores for each outcome measure. Chi-square analysis and an analysis of variance (ANOVA) compared the two treatment groups with respect to demographic results and subject age respectively. Statistical analyses were conducted for PMN, gingival and plaque index scores at baseline and each post-treatment evaluation. Results of polymorphonuclear leukocytes (PMN) recorded as counts per milliliter were log transformed (log10) for analysis with percent differences between treatment groups determined as reported previously [16]. An analysis of variance (ANOVA) compared baseline scores for PMN, gingival and plaque index scores between treatment groups. Within-treatment comparisons from baseline to each post-treatment evaluation for PMN, gingival and plaque index scores were conducted by paired t-tests. Comparisons of treatment groups with respect to baseline-adjusted PMN, gingival and plaque index scores to all post-treatment examinations were conducted by analyses of covariance (ANCOVA's). All statistical tests of hypotheses were two-sided with statistically significant results reported at α = 0.05. Post-hoc statistical analyses were conducted for statistically significant results. Analyses were completed using commercially available statistical software (Minitab, State College, PA, USA).
3. Results
The population screened, evaluated for study enrolment and subject flow through the study is presented in the CONSORT diagram [Fig. 1]. One-hundred and twenty subjects who completed their voluntary informed consent were scheduled to visit the dental clinic for a screening examination conducted by a dentist to evaluate study eligibility. Nineteen subjects were excluded during the screening visit with 15 not meeting study criteria and 4 declining study participation. One hundred and one subjects met study criteria and were enrolled in the study and completed the washout phase with a commercially available fluoride toothpaste. Following the washout period, subjects arrived at the dental clinic for their baseline evaluations. 49 subjects and 52 subjects were randomized to the control and the test groups respectively and issued their treatments. Over the study period, 11 subjects discontinued study participation with these drop-outs unrelated to study procedures or products. Most of these subjects were lost during follow-up due to missed appointments. A total of 90 subjects completed the study with 45 subjects providing evaluable results from both the control and the test groups. No adverse events were reported by the subjects or dental examiners over the study period. Shown in Table 1 is a summary of demographic characteristics. Sixty eight women and twenty two men completed the study with an age range of 19–58 years and an average age of 34.37 years. Analysis indicate that the mean age in the control and test groups were 34.67 and 34.07 years respectively with no significant differences by ANOVA (p > 0.1). Similarly, there were no significant differences between the two treatment groups for gender by chi-square analysis (p > 0.1).
Fig. 1.
CONSORT 2010 flow diagram.
Table 1.
Demographics of subjects who completed the entire study.
| Total |
Control |
Test |
|
|---|---|---|---|
| n = 90 | n = 45 | n = 45 | |
| Age | |||
| Mean (SD)a | 34.37 (9.62) | 34.67 (9.97) | 34.07 (9.36) |
| Age Range | 19–58 | 20–58 | 19–56 |
| Genderb | |||
| Male | n = 22 | n = 11 | n = 11 |
| Mean (SD) | 34.82 (11.72) | 36.45 (12.68) | 33.18 (11.04) |
| Age Range | 19–56 | 22–55 | 19–56 |
| Female | n = 68 | n = 34 | n = 34 |
| Mean (SD) | 34.22 (8.93) | 34.09 (9.07) | 34.35 (8.92) |
| Age Range | 20–58 | 20–58 | 21–55 |
No significant differences between treatment groups for age by ANOVA (p > 0.1).
= No significant differences between treatment groups for gender by chi-square analysis (p > 0.1).
Results from the clinical evaluations conducted over the study period from subjects who completed the entire study is presented in Table 2, Table 3, Table 4. Table 2 provides a summary of results as mean and SD from all evaluations conducted twelve (12) hours after oral hygiene. Average baseline scores for oral PMN in the treatment groups were 5.4 with treatment groups recording average gingival index scores of 1.4 and dental plaque index scores of 2.6 at baseline. Baseline scores for gingival severity, plaque severity or interproximal measures indicate no significant differences between treatment groups (p > 0.1). Analyses indicate no significant differences between treatment groups for any recorded clinical outcome (p > 0.1) but progressive reductions in post-treatment scores over the study period.
Table 2.
Summary of subject Mean (SD) for Polymorphonuclear (PMN) Leukocytes and dental plaque, gingival index scores at all examinations for subjects who completed the entire study.
| Parameter | Treatment | Baseline Mean (S.D)a | 1 Week Mean (S.D) | 2 Week Mean (S.D) |
|---|---|---|---|---|
| PMN leukocytes (log10Count/ml) | Test | 5.474 (0.193) | 5.247 (0.192) | 5.082 (0.212) |
| Control | 5.450 (0.214) | 5.417 (0.214) | 5.404 (0.221) | |
| Gingival Index | Test | 1.47 (0.29) | 1.150 (0.21) | 0.96 (0.23) |
| Control | 1.46 (0.25) | 1.36 (0.25) | 1.28 (0.24) | |
| Plaque Index | Test | 2.64 (0.5) | 1.964 (0.59) | 1.75 (0.55) |
| Control | 2.65 (0.47) | 2.33 (0.44) | 2.17 (0.44) | |
| Gingival Severity | Test | 0.37 (0.18) | 0.20 (0.15) | 0.10 (0.13) |
| Control | 0.38 (0.17) | 0.32 (0.17) | 0.28 (0.18) | |
| Plaque Severity | Test | 0.42 (0.17) | 0.22 (0.17) | 0.19 (0.16) |
| Control | 0.42 (0.17) | 0.33 (0.16) | 0.30 (0.16) | |
| Gingival Interproximal | Test | 1.48 (0.28) | 1.20 (0.18) | 1.07 (0.17) |
| Control | 1.47 (0.25) | 1.36 (0.24) | 1.29 (0.23) | |
| Plaque Interproximal | Test | 2.68 (0.5) | 2.01 (0.59) | 1.80 (0.54) |
| Control | 2.68 (0.5) | 2.38 (0.43) | 2.23 (0.45) |
No statistically significant difference was indicated between the two treatment groups at baseline with respect to PMN leukocytes (log10Count/ml) samples (p = 0.576); gingival index (p = 0.952); plaque index (p = 0.773); gingival severity (p = 0.754); plaque severity (p = 0.916); gingival interproximal (p = 0.884); plaque interproximal (p = 0.939).
Table 3.
Summary of baseline-Adjusted Subject Mean (SE) for Polymorphonuclear (PMN), Gingival and Plaque Index, Gingival and Plaque Severity, Gingival and Plaque Interproximal scores at the one-week post-treatment evaluation for subjects who completed the entire study.
| Parameter | Treatment | Adj. 1 Week Mean (S.E.) | Within treatment comparisonsa | Between treatment comparisonsb |
|---|---|---|---|---|
| PMN leukocytes (log10Count/ml) | Test | 5.235 (0.008) | 40.7% | 35.9% |
| Control | 5.428 (0.008) | 7.5% | ||
| Gingival Index | Test | 1.15 (0.01) | 21.8% | 15.4% |
| Control | 1.36 (0.01) | 6.8% | ||
| Plaque Index | Test | 1.96 (0.05) | 25.8% | 15.9% |
| Control | 2.33 (0.05) | 12.1% | ||
| Gingival Severity | Test | 0.20 (0.01) | 45.9% | 37.5% |
| Control | 0.32 (0.01) | 15.8% | ||
| Plaque Severity | Test | 0.22 (0.02) | 47.6% | 31.3% |
| Control | 0.32 (0.02) | 21.4% | ||
| Gingival Interproximal | Test | 1.20 (0.01) | 18.9% | 11.8% |
| Control | 1.36 (0.01) | 7.5% | ||
| Plaque Interproximal | Test | 2.01 (0.05) | 25.0% | 15.5% |
| Control | 2.38 (0.05) | 11.2% |
Percent change exhibited by the one week mean relative to the baseline mean. A positive value indicates a reduction in the parameter by paired t-test. Statistically significant at p < 0.001.
Percent differences between the test and control treatments evaluated by ANCOVA utilizing baseline-adjusted means. Statistically significant at p < 0.001.
Table 4.
Summary of baseline-Adjusted Subject Mean (SE) for Polymorphonuclear (PMN), Gingival and Plaque Index, Gingival and Plaque Severity, Gingival and Plaque Interproximal scores at the two-week post-treatment evaluation for subjects who completed the entire study.
| Parameter | Treatment | Adj. 2 Week Mean (S.E.) | Within treatment comparisonsa | Between treatment comparisonsb |
|---|---|---|---|---|
| PMN leukocytes (log10Count/ml) | Test | 5.070 (0.012) | 59.4% | 54.9% |
| Control | 5.416 (0.012) | 10.1% | ||
| Gingival Index | Test | 0.96 (0.02) | 34.7% | 25.0% |
| Control | 1.28 (0.01) | 12.3% | ||
| Plaque Index | Test | 1.75 (0.05) | 33.7% | 19.4% |
| Control | 2.17 (0.05) | 18.1% | ||
| Gingival Severity | Test | 0.11 (0.01) | 73.0% | 60.7% |
| Control | 0.28 (0.01) | 26.3% | ||
| Plaque Severity | Test | 0.19 (0.02) | 54.8% | 36.7% |
| Control | 0.30 (0.02) | 28.6% | ||
| Gingival Interproximal | Test | 1.07 (0.02) | 27.7% | 17.7% |
| Control | 1.30 (0.02) | 12.2% | ||
| Plaque Interproximal | Test | 1.80 (0.05) | 32.8% | 19.3% |
| Control | 2.23 (0.05) | 16.8% |
Percent change exhibited by the one week mean relative to the baseline mean. A positive value indicates a reduction in the parameter by paired t-test. Statistically significant at p < 0.001.
Percent differences between the test and control treatments evaluated by ANCOVA utilizing baseline-adjusted means. Statistically significant at p < 0.001.
Analysis of the one-week post-treatment results is presented in Table 3. All treatments demonstrating significant reductions from their corresponding baselines for clinical outcomes evaluated (p < 0.001). Analysis by ANCOVA comparing the two treatment groups indicates significant greater reductions in the test group in comparison to the control (p < 0.001). Baseline-adjusted PMN scores for the test and control were 5.235 and 5.428 respectively representing a statistically significant difference of 35.9% between the treatment groups (p < 0.001). At the one-week post-treatment evaluation, the gingival index scores for test and control groups were 1.15 and 1.36 respectively for a 15.4% difference between treatments (p < 0.001). Plaque index scores for the test and control were 1.96 and 2.33 respectively after one-week use of assigned test treatments. A significant difference of 15.9% in dental plaque scores were observed with lower plaque levels observed in the test group in comparison to the control (p < 0.001). Gingival and plaque severity scores amongst the test were 37.5% and 31.3% respectively and significantly lower than the control (p < 0.001). Additionally, the test demonstrated a significantly lower interproximal gingival index and dental plaque scores of 11.8% and 15.5% respectively in comparison to the control (p < 0.001).
Shown in Table 4 is a summary of the analysis of results from the two-week post-treatment evaluations. Both treatment groups demonstrated reductions from their corresponding baseline by paired t-tests for each evaluated outcome measure (p < 0.001). The test group demonstrated significantly greater reductions than the control by ANCOVA for PMN and the clinical indices evaluating gingivitis and dental plaque (p < 0.001). Baseline-adjusted PMN scores for the test and control were 5.07 and 5.41 respectively representing a statistically significant difference of 54.9% between the treatment groups (p < 0.001). Gingival index scores for the test and control were 0.96 and 1.28 respectively representing a 25% difference between treatment groups (p < 0.001). At the two-week assessment, plaque index scores for the test and control were 1.75 and 2.17 respectively. A significant difference of 19.4% in dental plaque scores were observed between the treatment groups with lower scores registered with the test group in comparison to the control (p < 0.001). The test demonstrated significantly greater reductions of 60.7% and 36.7% for gingival and plaque severity scores respectively in comparison to the control (p < 0.001). A significantly lower interproximal gingival index and dental plaque score of 17.7% and 19.3% respectively for the test was noted in comparison to the control (p < 0.001).
Gingival index frequency scores from evaluations over the study period is shown in Fig. 2. At baseline, approximately 60% of surfaces in the treatment groups registered a score of 1. Scores of 2 were noted on approximately 28% and 25% surfaces of the control and test groups respectively at the baseline evaluation. At the one-week post-treatment evaluations, both treatment groups registered reductions in the number of sites with a score of 3. Scores of 0 and 1 increased in the test group to 6.2% and 74.1% respectively in contrast to corresponding scores of 1.2% and 66.9% respectively for the control group. Frequencies for score 2 and 3 decreased in both treatment groups at the two-week evaluation with a score of 2 noted on 26% of the control and 10% of the test groups. The test group demonstrated increases in the frequencies of score 0 and 1 than the control. Sites with the highest scores were 0.5% in the test and 1.8% in the control. Approximately 15% of surfaces in the test group registered a gingival index score of 0 in contrast to 2.2% in the control group.
Fig. 2.
Frequency distribution of gingival index scores in treatment groups over the study period.
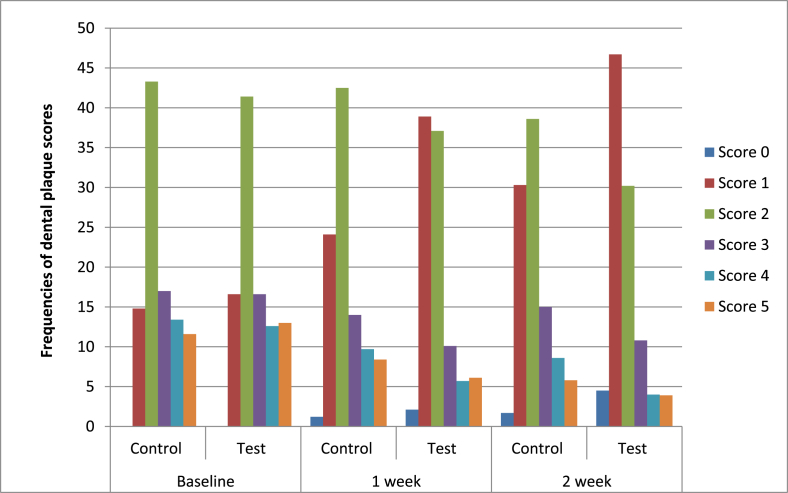
Shown in Fig. 3 are frequency scores from dental plaque index evaluations recorded over the study. Irrespective of treatment group, scores of 1,2 and 3 were noted on approximately 16%, 43% and 17.0% of surfaces at baseline. Both treatment groups demonstrated improvements at the one-week evaluation with between 24 and 38% of the surfaces registering a score of 1 and between 37 and 42% of the surfaces registering a score of 2. Less than 15% of surfaces registered scores of 4 or more with the test group registering lower frequencies of these scores at post-treatment evaluations. The two-week evaluation registered additional improvements in the test group with 4% of surfaces free of dental plaque and a score of 1 observed on 46% of surfaces and a score of 2 registered on 30% of surfaces. In contrast, for the control group, a score of 0 was observed on 1.7% of the surfaces and scores of 1 and 2 noted on 30% and 38% of surfaces respectively.
Fig. 3.
Frequency distribution of dental plaque index scores in treatment groups over the study period.
4. Disscussion
Neutrophils are critical effector cells, referred to as the first responders [17] that are produced in large numbers and reported from mucosal surfaces including the eye, nose, saliva and other locations to protect these barriers [6,13,18]. Earlier investigations report the ability of dental plaque, isolated oral bacteria or their components [19] on PMN functions with differences noted between virulent and avirulent strains [20]. Salivary PMN has been enumerated in samples collected from subjects with reports indicating differences between healthy and periodontal disease [[8], [9], [10], [11], [12], [13]].
This double-blind study investigated the preventative effect of a well-established oral therapeutic i.e. CHX [[3], [4], [5], [21]] with regards to mitigating gingival inflammation and included an estimation of oral neutrophils as an objective measure of inflammation. This study utilized commercially available formulations and enrolled adult subjects of either gender with gingivitis from the general population who were not seeking any medical or dental care. Test conditions were standardized with a washout phase included to reduce the influences of previously utilized formulations with treatments assigned randomly.
Notable features of this study utilizing CHX an established ingredient are relevant in this effort examining effects on the numbers of oral PMN. These include the relatively short duration of the study with subjects visiting the dental clinic weekly for regularly scheduled visits for evaluations. In addition to examining progressive clinical changes, the study design allowed assessments of adverse events and determine study compliance. Oral health status was determined using traditional clinical indices utilized extensively [5].
Over the study period both the gingival and plaque indices scores decreased from their corresponding baselines and corroborated published results. Additionally, results evaluated effects on different sites of the mouth, the frequencies of clinical scores over the study period and effects on interproximal and other hard to reach sites. The CHX group demonstrated reductions of 0.2 for gingival index and 0.4 for plaque index in this study with progressive effects observed over the study period. Furthermore, these effects were observed on all oral regions and surfaces providing a comprehensive treatment assessment. These results also support the compliance of study procedures by subjects.
Investigators have evaluated CHX mouthwashes in clinical trials of different designs for effects on cytokines and other inflammatory mediators with these studies also concurrently evaluating clinical indices for dental plaque and gingivitis [[21], [22], [23]][. Whereas, clinical indices offer subjective assessments of oral health they have an extended history and are accepted by regulatory agencies [24]. In this study, oral PMN were collected by non-invasive sample collection with assessments conducted immediately after sampling for chair-side reporting. PMN numbers are related to oral health status in surveys with their numbers increasing from health to those with gingivitis and the highest numbers noted in subjects with periodontal disease (unpublished data). Estimation of PMN likely provides an assessment of the inflammatory continuum representing changes in healing and resolution in an interventional clinical setting and is relevant to oral health. Further, PMN dynamics may allow an evaluation of incipient disease in contrast to classical clinical indices. Biomarkers related to PMN activity such as lactoferrin and PMN-elastase have been cleared by the FDA for clinical use in USA and Europe [25] with studies reporting correlations between other PMN derived components such as myeloperoxidase and clinical indices [26].
In the present study, PMN scores of the two treatment groups at baseline were similar with no statistically significant differences. Oral PMN scores in the control group decreased modestly from 5.45 to 5.40 over the study period representing between 7.5 and 10% differences, however, the effects in the CHX group were substantially higher and ranged between 40 and 59.4%. The CHX group consistently demonstrated statistically significant differences from the control with a 35% difference noted at the first week and 54% noted at the two-week evaluation. These results demonstrate progressive reductions in PMN counts over the study period representing cumulative treatment effects on the oral inflammatory burden. While PMN observations corroborated those noted with the conventional clinical indices the differences between the test and control groups for neutrophils were numerically higher than those for the gingival index but similar to gingival severity scores.
This study enrolled adults with gingivitis. Availability of the present results allows future studies that can examine the effects of other interventions amongst selected populations with common oral diseases. PMN and its activities are widely acknowledged as key determinants in host defense [2] and likely reflect changes in the oral microbiome including microbial and fungal constituents. These results faciliate evaluations of common oral therapeutics and may likely contribute to the development of strategies that could augment or enhance neutrophil functions. For instance, a recent report proposes vitamin E supplementation as a nutritional interventional strategy for oral therapeutics to enhance neutrophil functions and is applicable to persons of all ages [27]. Similarly, dietary supplements reportedly aid patients with systemic inflammation and neutrophil function suggesting the availability of potential approaches for investigation [28].
In summary, the results from this double-blind clinical study demonstrate progressive reductions from the one-week to the two-week evaluation of subjects’ twice-daily brushing with a commercially-available fluoride toothpaste and rinsing with a commercially available 0.12% chlorhexidine mouthwash. Samples from subjects taken 12 h after oral hygiene with chlorhexidine had significantly greater reductions in the numbers of oral PMN compared to those rinsing with a commercially available fluoride mouthwash and brushing with a commercially-available fluoride toothpaste. Correspondingly, the use of chlorhexidine demonstrated significantly greater reductions in dental plaque and gingivitis than the control group at all post-treatment evaluations. A simultaneous assessment of clinical outcomes in conjunction with immunological characteristics will likely enhance an understanding on the progression of common oral conditions to design and evaluate measures for their prevention and control.
Acknowledgements
The Study was funded by Colgate-Palmolive Company (USA).
References
- 1.Arweiler N.B., Netuschil L. The oral microbiota. Adv. Exp. Med. Biol. 2016;902:45–60. doi: 10.1007/978-3-319-31248-4_4. [DOI] [PubMed] [Google Scholar]
- 2.Uriarte S.M., Edmisson J.S., Jimenez-Flores E. Human neutrophils and oral microbiota: a constant tug-of-war between a harmonious and a discordant coexistence. Immunol. Rev. 2016 Sep;273(1):282–298. doi: 10.1111/imr.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solderer A., Kaufmann M., Hofer D., Wiedemeier D., Attin T., Schmidlin P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: a systematic review. Clin. Oral Investig. 2019;23(1):21–32. doi: 10.1007/s00784-018-2761-y. [DOI] [PubMed] [Google Scholar]
- 4.James P., Worthington H.V., Parnell C., Harding M., Lamont T., Cheung A., Whelton H., Riley P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017;3:CD008676. doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Weijden F.A., Van der Sluijs E., Ciancio S.G., Slot D.E. Can chemical mouthwash agents achieve plaque/gingivitis control? Dent. Clin. N. Am. 2015;59(4):799–829. doi: 10.1016/j.cden.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ley K., Hoffman H.M., Kubes P., Cassatella M.A., Zychlinsky A., Hedrick C.C., Catz S.D. Neutrophils: New insights and open questions. Sci Immunol. 2018 Dec 7;3(30) doi: 10.1126/sciimmunol.aat4579. [DOI] [PubMed] [Google Scholar]
- 7.Kornman K.S., Page R.C., Tonetti M.S. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol. 2000;14(1997):33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 8.Delima A.J., Van Dyke T.E. Origin and function of the cellular components in gingival crevice fluid. Periodontol. 2000;31(2003):55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 9.Akpek G., Knight R.D., Wright D.G. Use of oral mucosal neutrophil counts to detect the onset and resolution of profound neutropenia following high-dose myelosuppressive chemotherapy. Am. J. Hematol. 2003;72(1):13–19. doi: 10.1002/ajh.10250. [DOI] [PubMed] [Google Scholar]
- 10.Bender J.S., Thang H., Glogauer M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. J. Periodontal. Res. 2006;41(3):214–220. doi: 10.1111/j.1600-0765.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 11.Lukac J., Mravak-Stipetić M., Knezević M., Vrcek J., Sistig S., Ledinsky M., Kusić Z. Phagocytic functions of salivary neutrophils in oral mucous membrane diseases. J. Oral Pathol. Med. 2003;32(5):271–274. doi: 10.1034/j.1600-0714.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 12.Takubo T., Yamane T., Tsuda I., Tagawa S., Tatsumi N. Polymorphonuclear neutrophils in saliva and blood: a comparative study of morphology, function and phenotype. Br. J. Biomed. Sci. 1997;54(4):260–266. [PubMed] [Google Scholar]
- 13.Wright D.G., Meierovics A.I., Foxley J.M. Assessing the delivery of neutrophils to tissues in neutropenia. Blood. 1986;67(4):1023–1030. [PubMed] [Google Scholar]
- 14.Turesky S., Gilmore N.D., Glickman I. Reduced plaque formation by the chloromethyl analogue of vitamin C. J. Periodontol. 1970;41:41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 15.Löe H., Silness J. Periodontal disease in pregnancy. Acta Odontol. Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 16.Haraszthy V.I., Sreenivasan P.K. Microbiological and clinical effects of an oral hygiene regimen. Contemp Clin Trials Commun. 2017;8:85–89. doi: 10.1016/j.conctc.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenne C.N., Liao S., Singh B. Neutrophils: multitasking first responders of immunity and tissue homeostasis. Cell Tissue Res. 2018;371(3):395–397. doi: 10.1007/s00441-018-2802-5. [DOI] [PubMed] [Google Scholar]
- 18.Meyle J., Dommisch H., Groeger S., Giacaman R.A., Costalonga M., Herzberg M. The innate host response in caries and periodontitis. J. Clin. Periodontol. 2017;44(12):1215–1225. doi: 10.1111/jcpe.12781. [DOI] [PubMed] [Google Scholar]
- 19.Lareau D.E., Herzberg M.C., Nelson R.D. Human neutrophil migration under agarose to bacteria associated with the development of gingivitis. J. Periodontol. 1984;55(9):540–549. doi: 10.1902/jop.1984.55.9.540. [DOI] [PubMed] [Google Scholar]
- 20.Taichman N.S., Hammond B.F., Tsai C.C., Baehni P.C., McArthur W.P. Interaction of inflammatory cells and oral microorganisms. VII. In vitro polymorphonuclear responses to viable bacteria and to subcellular components of avirulent and virulent strains of Actinomyces viscosus. Infect. Immun. 1978;21(2):594–604. doi: 10.1128/iai.21.2.594-604.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Costa L.F.N.P., Amaral C.D.S.F., Barbirato D.D.S., Leão A.T.T., Fogacci M.F. Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: a meta-analysis. J. Am. Dent. Assoc. 2017;148(5):308–318. doi: 10.1016/j.adaj.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Türkoğlu O., Becerik S., Tervahartiala T., Sorsa T., Atilla G., Emingil G. The effect of adjunctive chlorhexidine mouthrinse on GCF MMP-8 and TIMP-1 levels in gingivitis: a randomized placebo-controlled study. BMC Oral Health. 2014 May 20;14:55. doi: 10.1186/1472-6831-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramberg P., Albertsson K.W., Derks J., Van Dijken J. A randomized controlled cross-over study of the effect of alcohol-free chlorhexidine and essential oils on interleukin-1 levels in crevicular fluid. Swed. Dent. J. 2016;40(2):143–152. [PubMed] [Google Scholar]
- 24.McCracken G.I., Preshaw P.M., Steen I.N., Swan M., deJager M., Heasman P.A. Measuring plaque in clinical trials: index or weight? J. Clin. Periodontol. 2006;33(3):172–176. doi: 10.1111/j.1600-051X.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 25.Langhorst J., Boone J., Lauche R., Rueffer A., Dobos G. Faecal lactoferrin, calprotectin, PMN-elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with Mild to moderate ulcerative colitis: post hoc analysis of a prospective clinical trial. J Crohns Colitis. 2016;10(7):786–794. doi: 10.1093/ecco-jcc/jjw044. [DOI] [PubMed] [Google Scholar]
- 26.Güncü G.N., Tözüm T.F., Güncü M.B., Yamalik N., Tümer C., Karabulut E., Kilinç K. Myeloperoxidase as a measure of polymorphonuclear leukocyte response in inflammatory status around immediately and delayed loaded dental implants: a randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2008 Mar;10(1):30–39. doi: 10.1111/j.1708-8208.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- 27.Bou Ghanem E.N., Lee J.N., Joma B.H., Meydani S.N., Leong J.M., Panda A. The alpha-tocopherol form of vitamin E boosts elastase activity of human PMNs and their ability to kill Streptococcus pneumoniae. Front Cell Infect Microbiol. 2017 May 3;7:161. doi: 10.3389/fcimb.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faintuch J., Bortolotto L.A., Marques P.C., Faintuch J.J., França J.I., Cecconello I. Systemic inflammation and carotid diameter in obese patients: pilot comparative study with flaxseed powder and cassava powder. Nutr. Hosp. 2011;26(1):208–213. [PubMed] [Google Scholar]