Highlights
-
•
Brain volumes at birth interacted with maternal sensitivity to predict child cognitive performance.
-
•
Larger total brain volume was associated with greater child susceptibility to maternal sensitivity.
-
•
Larger hippocampus and anterior cingulate gyrus contributed to greater child susceptibility.
-
•
Neonatal neurophenotypes may underlie susceptibility to parenting.
Keywords: Brain development, Newborn, Parenting, Differential susceptibility, Cognitive development, Executive function
Abstract
Parenting quality is associated with child cognitive and executive functions (EF), which are important predictors of social and academic development. However, children vary in their susceptibility to parenting behaviors, and the neurobiological underpinnings of this susceptibility are poorly understood. In a prospective longitudinal study, we examined whether neonatal total brain volume (TBV) and subregions of interest (i.e., hippocampus (HC) and anterior cingulate gyrus (ACG)) moderate the association between maternal sensitivity and cognitive/EF development across early childhood. Neonates underwent a brain magnetic resonance imaging scan. Their cognitive performance and EF was characterized at 2.0 ± 0.1 years (N = 53) and at 4.9 ± 0.8 years (N = 36) of age. Maternal sensitivity was coded based on observation of a standardized play situation at 6-mo postpartum. Neonatal TBV moderated the association between maternal sensitivity and 2-year working memory as well as all 5-year cognitive outcomes, suggesting that the positive association between maternal sensitivity and child cognition was observed only among children with large or average but not small TBV as neonates. Similar patterns were observed for TBV-corrected HC and ACG volumes. The findings suggest that larger neonatal TBV, HC and ACG may underlie susceptibility to the environment and affect the degree to which parenting quality shapes long-term cognitive development.
1. Introduction
Parental caregiving is one of the most important environmental factors laying the foundation for emotional, cognitive and behavioral development (Belsky and De Haan, 2011; Tottenham, 2017). A wide range of animal and human studies postulate that deprivation of parental care early in life has life-long and drastic consequences for development as well as psychiatric outcomes (Bos et al., 2009; Dozier and Peloso, 2006). However, not only deprivation and harsh parenting but also more subtle variation in the quality of care may alter child developmental outcomes (Curley and Champagne, 2016). For instance, parental warmth, sensitivity and intrusiveness have been shown to affect the development of temperament, affect regulation and cognitive function (e.g. Fay-Stammbach et al., 2014; Karreman et al., 2006).
While attachment formation and the parental buffering of early emotional reactivity has been a primary focus of parenting influences, there is increasing evidence for the effects of parenting on executive function (EF), which refers to higher-order cognitive functions used in goal-directed activities. EF skills undergo significant changes during early childhood (Diamond, 2013; Hendry et al., 2016), but the foundation for them is laid already in infancy, a developmental period characterized by rapid brain growth (Gilmore et al., 2018) and maturation of brain networks relevant for EF (Gao et al., 2013, 2009). Parenting behavior has been shown to be associated with the development of brain anatomy (e.g. amygdala, hippocampus and cingulate cortex) and connectivity (e.g. default mode, salience and central executive networks) (Dégeilh et al., 2018; Fareri and Tottenham, 2016; Hanson et al., 2019; Holmes et al., 2018; Kopala-Sibley et al., 2018; Rao et al., 2010; Rifkin-Graboi et al., 2015; Thijssen et al., 2017; Tomoda et al., 2009) relevant for cognitive and self-regulatory abilities, particularly EF (Cole et al., 2012; Heatherton and Wagner, 2011; Holmes et al., 2018; Tomoda et al., 2009). However, research on parenting and early childhood cognitive development and EF is still accumulating, and the findings have been partially inconsistent. Environmental (e.g. ethnicity, cigarette exposure) and child characteristics (e.g. temperament, physiological reactivity) have been suggested as crucial moderators explaining these inconsistencies (Fay-Stammbach et al., 2014). Because deficits in EF are cardinal symptoms of several neuropsychiatric disorders (Margari et al., 2016; Willcutt et al., 2005) and EF already in early childhood is a significant predictor of later social and cognitive functioning (Moffitt et al., 2011; Schoemaker et al., 2013), a better understanding of the early environmental determinants of EF is of high clinical relevance.
Interestingly, individuals differ in their degree to which they are amenable to environmental variation such as sensitive parenting (Belsky, 2016). Differences in genetic make-up and neural or behavioral phenotypes (Belsky and van IJzendoorn, 2017; Moore and Depue, 2016) have been shown to underlie interindividual differences in susceptibility. The models of environmental sensitivity (Pluess, 2015) include the differential susceptibility (Belsky et al., 2007), the biological susceptibility to context (Boyce, 2016), and the sensory processing sensitivity (Aron and Aron, 1997) hypotheses which all suggest that more sensitive individuals are not only more vulnerable to adverse environmental conditions (i.e. diathesis-stress, see Monroe and Simons, 1991), or sensitive to positive characteristics of the environment (i.e. vantage sensitivity, see Pluess and Belsky, 2013) but may be more susceptible to both conditions (“for better and for worse”). There is increasing evidence to support these frameworks (Belsky, 2016; Greven et al., 2019) which may have implications for identifying individuals who are most affected by environmental adversity (Meaney, 2018) and in turn, who benefit most from interventions (de Villiers et al., 2018).
However, although one of the key assumptions in the environmental sensitivity frameworks is the variability in the underlying neurobiology, the research on the neural correlates of interindividual differences in environmental susceptibility is sparse. Only one recent study has focused on early childhood neurophenotypes underlying susceptibility (Rifkin-Graboi et al., 2019) whereas most existing studies on the brain functional and structural characteristics reflecting differential susceptibility have been conducted in adult or adolescent populations (for instance, Doehrmann et al., 2013; Gard et al., 2018; Schriber et al., 2017; Whittle et al., 2011; Yap et al., 2008). In these studies, the environmental variable of interest functioning as the moderator (e.g., variation in parenting behavior) may also have contributed to the susceptibility marker (i.e., neurophenotype). Even though such development is inevitably a part of the process resulting in interindividual variation in susceptibility over time, we suggest that the use of brain phenotypes characterized at or shortly after birth (at which time they have been only minimally shaped by postnatal environmental influence) as markers of differential susceptibility may shed more light on the neural basis of interindividual variation in susceptibility to the environment.
Regarding cognition and EF as outcomes of interest, studies to date have not focused on neural phenotypes of interindividual variation in susceptibility to the environment. According to a recent meta-analysis, larger brain volume is linked to better cognitive performance across different ages (Pietschnig et al., 2015). Interestingly, animal studies suggest that a larger brain increases the complexity of behaviors and executive processing specifically (see a summary by Marino, 2005) which may provide adaptation advantage in novel environments (Gonzalez-Lagos et al., 2010; Sayol et al., 2016; Sol et al., 2008, 2005). This pattern may be driven by larger number of (larger) neurons, glial cells, and more gyrification which contribute to better cognitive capacity exposing the individual to tasks that in turn increase the brain size (Gabi et al., 2010; Gregory et al., 2016; Lefebvre and Sol, 2008). Furthermore, availability of neurotrophic factors like brain-derived neurotrophic factor (BDNF) and insulin-like growth factors (IGFs) influence brain size at all stages of brain development (Hansen-Pupp et al., 2013; Joseph D’Ercole and Ye, 2008; Nieto-Estévez et al., 2016) and at the same time have implications for neuroplasticity in the postnatal period (Dyer et al., 2016), proposing that brain growth broadly may also contribute to experience-dependent plasticity.
However, previous studies that reported an association between total brain volume and cognitive performance have typically reported modest effect sizes and some inconsistency in the direction of findings, which may be due to the fact that the impact of brain size on cognitive outcomes depends on the environmental context as suggested by the environmental sensitivity frameworks. Thus, overall brain size could be one plausible candidate neurophenotype of susceptibility to the parenting influences in terms of cognitive outcomes in childhood.
Prior research has furthermore emphasized the role of structures implicated in default mode and salience networks in differential susceptibility to the environment (Greven et al., 2019; Moore and Depue, 2016). Studies across age groups have shown that the hippocampus (HC), an early-developing limbic region that supports emotional and memory processes and is part of the salience network (Zheng et al., 2017) is pivotal for susceptibility (Rifkin-Graboi et al., 2019; Schriber et al., 2017; Whittle et al., 2011). HC size is associated with cognitive performance but study results are heterogeneous regarding the direction of the findings (Erickson et al., 2011; Van Petten, 2004), making HC a possible candidate for susceptibility to parenting in terms of cognition. Furthermore, the anterior cingulate cortex/gyrus (ACC/ACG) is a structure implicated in both interindividual differences in susceptibility (Greven et al., 2019; Mutschler et al., 2016; Rudolph et al., 2020; Yap et al., 2008) and cognitive and executive functioning, specifically in error detection and conflict resolution (Banich, 2009; Milham and Banich, 2005; Posner et al., 2007). ACC is an important hub for the salience network and is responsible for identification of important environmental and interpersonal stimuli (Menon and Uddin, 2010; Pujol et al., 2002), proposing that it may play a role in the susceptibility to parenting effects.
Thus, based on previous studies suggesting larger TBV as a structure that may benefit cognitive development and cross-species adaptation to context, the main focus of this study was to examine whether TBV moderates the association between early parenting and cognitive/EF development. We furthermore conducted exploratory analyses with subregions of interest examining whether the HC and ACG act as moderators of the association between parenting and cognitive/EF development. Maternal sensitivity, which refers to maternal warmth and ability to attune to child emotional states and allow adequate autonomy for the child (Skinner, 1986), was used to characterize maternal behavior. We measured cognitive development with a focus on EF in early childhood (at 2 years and at 5 years of age). Magnetic resonance imaging (MRI)-based TBV, HC and ACG volumes were tested as moderators of the association between maternal sensitivity and offspring EF. Based on previous observations (Rifkin-Graboi et al., 2019; Schriber et al., 2017; Whittle et al., 2011), we hypothesized that children with larger neonatal total brain volume (controlled for ICV) and larger HC/ACG (controlled for TBV) would exhibit heightened susceptibility to maternal sensitivity. Furthermore, in line with the differential susceptibility hypothesis, we anticipated that children with larger TBV and HC/ACG volumes would be more susceptible to both high as well as low maternal sensitivity.
2. Materials and methods
2.1. Participants and procedure
The participants were part of a prospective longitudinal study of mother-child dyads conducted at the University of California, Irvine, Development, Health and Disease Research Program (e.g. Rudolph et al., 2018), for which mothers were recruited during early pregnancy. For the analyses reported here, those mother-infant dyads were included who provided data for the following assessments: a newborn brain MRI scan at 27.6 ± 13 (M ± SD) days of age, maternal sensitivity assessment at 6.0 ± 0.3 (M ± SD) months postpartum, and child cognitive assessments at 2.0 ± 0.1 (M ± SD, range: 1.9–2.3) years (Y2) and/or at 4.9 ± 0.8 (M ± SD, range: 3.3–7.1) years of age (Y5). The sample comprised of N = 53 children who participated in the assessment of cognitive performance at Y2 and N = 36 children who participated in the assessment of cognitive performance at Y5. The participating mother-infant dyads did not differ from the total cohort of newborns with available MRI scans (N = 86, see e.g. Moog et al., 2018) in terms of brain structure (total brain, intracranial, grey matter or white matter volume) or in terms of any key sociodemographic and birth outcome variables (e.g., education and birth weight).
Exclusion criteria for the study were maternal use of systemic corticosteroids or psychotropic medications during pregnancy, infant birth before 34 weeks’ gestation, and infant congenital, genetic, or neurological disorder. All the children included had APGAR scores >8 five minutes after birth and weighed >1800 g. Demographic information is displayed in Table 1. The study was approved by the Institutional Review Board at the University of California, Irvine, and all mothers provided written informed consent.
Table 1.
Demographic information for the subsets of children having Y2 and Y5 cognitive outcomes.
| Y2 Outcomes N = 53 | Y5 Outcomes N = 36 | |
|---|---|---|
| Mean ± SD or N (%), observed range | ||
| Maternal variables | ||
| Maternal age in 1st trimester, years | 28.1 ± 4.9 | 27.5 ± 5.9 |
| SES index (education and income) | 3.1 ± 1.0 | 3.0 ± 1.0 |
| Maternal race/ethnicity | ||
| Non-hispanic White | 22 (42 %) | 14 (39 %) |
| Hispanic White | 15 (28 %) | 12 (33 %) |
| Other | 12 (22 %) | 9 (25 %) |
| Missing | 4 (8 %) | 1 (3 %) |
| Educational level | ||
| Less than high school | 2 (4 %) | 2 (5 %) |
| High school | 8 (15 %) | 8 (22 %) |
| Partial college or specialized training | 25 (47 %) | 16 (44 %) |
| Associate/Bachelor’s degree | 10 (19 %) | 6 (17 %) |
| Advanced | 6 (11 %) | 4 (11 %) |
| Yearly income per household | ||
| Below $15,000 | 6 (11 %) | 3 (8 %) |
| $15,000–$29,999 | 10 (19 %) | 9 (25 %) |
| $30,000–$49,999 | 14 (26 %) | 10 (28 %) |
| $50,000–$100,000 | 17 (32 %) | 9 (24 %) |
| Over $100,000 | 5 (9 %) | 4 (11 %) |
| Missing | 1 (2 %) | 1 (3%) |
| Birth outcomes and child variables | ||
| Gestational age at birth, weeks | 39.0 ± 1.6, 35–42 | 38.9 ± 1.3, 35–42 |
| Birth weight (grams) | 3290 ± 532, 1786–4906 | 3300 ± 573, 1786–4906 |
| Age at MRI scan, days | 27.6 ± 12.6, 8–64 | 27.3 ± 13, 8–64 |
| Child sex (male) | 33 (62 %) | 23 (64 %) |
| Total brain volume (cm3) | 427.86 ± 48.16 | 425.81 ± 50.66 |
| Intracranial volume (cm3) | 486.68 ± 58.77 | 486.16 ± 63.05 |
| Grey matter volume (cm3) | 262.33 ± 33.22 | 260.77 ± 34.77 |
| White matter volume (cm3) | 165.11 ± 18.95 | 165.04 ± 18.98 |
| Hippocampal volume, total (cm3) | 2.36 ± 0.27 | 2.34 ± 0.27 |
| Anterior cingulate gyri volume, total (cm3) | 3.36 ± 0.51 | 3.34 ± 0.56 |
| M6 Maternal sensitivity | 10.5 ± 2.6, 5–15 | 10.6 ± 2.6, 5–15 |
| Y2 Cognitive performance | ||
| Working memory (Spin the Pots) | 8.6 ± 4.2, 3–16 | |
| Inhibitory control (Snack Delay) | 5.3 ± 2.7, 1–9 | |
| Set-shifting (MEFS) (N = 33) | 11.3 ± 4.0, 5–19 | |
| Y5 Cognitive performance | ||
| General Abilities Index, z score (N = 27) | 92 ± 17, ± 57–124 | |
| Inhibition (EF Touch Arrows) | 0.75 ± 0.18, 0.33–1.00 | |
| Set-shifting (MEFS), z score | 98 ± 9, 76–120 | |
All the differences between the samples p > 0.05.
2.2. MRI acquisition and processing
2.2.1. MRI data acquisition
Infants were scanned during natural unsedated sleep using Siemens 3.0 T Scanner (TIM Trio, Siemens Medical Systems Inc., Germany) as previously described (Moog et al., 2018). T1-weighted images were obtained using a 3D magnetization prepared rapid gradient echo (MP-RAGE TR = 2400 ms, inversion time = 1200 ms, echo time = 3.16 ms, flip angle = 8°, resolution = 1 × 1 × 1 mm, 6.18 min), whereas T2-weighted images were acquired with a turbo spin echo sequence (TR 3200 ms; TE1 13 ms; TE2 135 ms; Flip Angle 180°; 4:18 min). The spatial resolution was a 1 × 1 × 1 mm voxel for T1-weighted images and 1 × 1 × 1 mm voxel with 0.5 mm interslice gap for T2-weighted images.
2.2.2. MRI data preprocessing
Tissue segmentation was performed using a neonate multi-atlas-based (https://www.nitrc.org/projects/unc_brain_atlas/) iterative expectation maximization segmentation algorithm as previously described (Cherel et al., 2015; Moog et al., 2018). The UNC neonatal multi-atlas was constructed from original and left-right mirrored images of 8 selected neonate datasets with manual expert tissue segmentations. Brain tissue was classified as gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). The first two tissue types were used to calculate total brain volume (TBV), and the three tissue types were used to calculate intracranial volume (ICV). The ACG was defined by multi-modality (employing both T1 and T2 weighted images) non-linear warping of a newborn average brain atlas to native space via the Advanced Neuroimaging Tools (ANTs) toolkit (Avants et al., 2011; Prastawa et al., 2005). The deformation field was then applied to the parcellation template as in Gousias et al. (2008) corresponding to the atlas, and combined with the GM tissue segmentation, resulting in parcellated volumes in native space.
Hippocampus segmentation was performed using a multi-modality, multi-template based automatic method combining T1- and T2-weighted high-resolution images (Wang et al., 2014) followed by manual correction in ITK-Snap (Yushkevich et al., 2006) as previously described (Moog et al., 2018). Manual corrections were performed with data re-aligned, the anterior-posterior direction being positioned along hippocampal long axis. Images were segmented in both original and left-right mirrored presentation to account for asymmetric presentation biases (Maltbie et al., 2012) and averaged for the final segmentation. Scan/rescan stability tests conducted in a separate sample set indicated reliable and stable results at coefficients of variance < 0.4 % for all structures. Reliability for manual correction was established for raters on this dataset via a standard reliability study, in which 5 datasets were triplicated and randomized. These 15 datasets were then segmented automatically and manually corrected by two raters. Correlation coefficients between the two raters and correlation coefficients within the same rater across the same dataset were both high (r > 0.98).
Finally, the residuals of TBV, intracranial volume (ICV), grey matter volume (GMV), white matter volume (WMV), hippocampus (HC) and anterior cingulate gyri (ACG) were generated to efficiently correct for length of gestation and newborn postnatal age at scan because of their strong association with brain volume (rpartial for TBV = 0.52–0.63, p < 0.001, for ICV = 0.46–0.62, p < 0.001, for GMV = 0.62–0.66, p < 0.001, for WMV = 0.24–0.46, p = 0.003–0.083, for HC (rpartial = 0.44–0.49, p < 0.001 and for ACG rpartial = 0.27–0.56, p < 0.001–0.057). Furthermore, because child sex was associated with all brain volumes in the Y2 subsample, it was included in all subsequent analyses.
2.3. Child cognitive outcomes and EF
2.3.1. Cognitive outcomes at 2 years of age (Y2)
The Spin the Pots task was used to measure visuospatial working memory (Hughes and Ensor, 2005). The Snack Delay task was used to measure inhibitory control (Kochanska et al., 2000). Categorization and set-shifting ability were assessed using the Executive Function Scale for Early Childhood (see reference in Section 2.3.2), where the child has to shift the sorting strategy of cards according to the varying instructions on a different level (from 1 to 7). For this task, only children passing the practice trials on Level 1 were included in the analyses (N = 33).
2.3.2. Cognitive outcomes at 5 years of age (Y5)
Inhibitory control at this age was measured using a computerized Spatial Conflict Arrows task from EF Touch (https://eftouch.fpg.unc.edu/) (Willoughby et al., 2012). Set-shifting was measured using age-relevant levels of the Minnesota Executive Functioning Scale, a computerized version of the Executive Function Scale for Early Childhood described in the previous paragraph (Carlson, 2017; Carlson and Zelazo, 2014). Overall cognitive performance was assessed in a smaller subpopulation of children (N = 28) using the General Abilities Index (GAI) from the Wechsler Preschool and Primary Scales of Intelligence IV (Wechsler, 2012), which is an index generated from the subscales Information, Similarities, Block Design and Matrix Reasoning.
In all tasks, higher scores reflected better performance.
2.4. Maternal sensitivity
At 6 months of age, infants and their mothers participated in a 15-minute standardized free play situation that was video recorded and coded afterwards by two trained and reliable observers. For coding of maternal behavior, the manual by NICHD Early Child Care Research Network (NICHD Early Child Care Research Network, 1999) was used. The maternal sensitivity coded using this paradigm has shown stability across early childhood (Mills-Koonce et al., 2008; NICHD Early Child Care Research Network, 1999). Overall maternal sensitivity was calculated by summing the scales for sensitivity to non-distress, positive regard and intrusiveness (reversed), which were measured on a 5-point Likert scale with higher points reflecting higher amounts of respective maternal behavior. All the scales showed high inter-rater reliability (Cohen’s K ranging from 0.83 to 1.00, being 0.86 for maternal overall sensitivity).
2.5. Covariates
Maternal educational level was reported during pregnancy at the first maternal visit (gestational week 10–12) and was assessed in categories from less than high school to advanced degree [master/doctorate] and then recoded into values from 1 to 5. Age at assessment was determined as years from birth date.
2.6. Analytic approach
All cognitive measures were used as separate outcomes across analyses. Based on the preliminary analyses (see the Appendix), the multivariable general linear models testing the interaction of neonatal TBV and maternal sensitivity in predicting each cognitive outcome were controlled for child sex, maternal education and age at the respective assessment (unless cognitive measures were already standardized for age). Next, a sensitivity analysis controlling for age-corrected ICV and then separately for birth weight as additional covariates was conducted to rule out the possibility of newborn head (including cerebrospinal fluid) or overall size, rather than specifically brain volume, underlying the observed associations. The interactions in focus in each set of analyses were corrected using Benjamini-Hochberg method (Benjamini and Hochberg, 1995) and False Discovery Rate p < .05.
These analyses were followed by a set of post-hoc analyses. Following the prior guidelines on examining differential susceptibility (Roisman et al., 2012), the significant interactions were probed conducting a set of simple slope analyses in Process Macro 3.3 (Hayes, 2017) controlling for the same covariates as in the main models and age-corrected ICV. More specifically, it was tested whether maternal sensitivity and child cognitive performance were related at high and low ends of TBV, specifically, at ±1 SD from the mean (Aiken and West, 1991). The figures were generated using the package ggplot2 (Wickham, 2009) in the R Program and the median split of TBV. The median split was only used in illustrating the data. Moreover, the regions of significance analysis for age-corrected TBV (Roisman et al., 2012) was performed as a supplementary analysis to examine the range of maternal sensitivity for which the brain volume and the cognitive outcome are associated (see the Table A3 Appendix).
Additionally, post-hoc analyses were conducted to test whether age-corrected GMV and WMV specifically moderate sensitivity to the early postnatal environment, and whether some specific aspects of maternal sensitivity (intrusiveness, sensitivity to non-distress and positive regard) interacted with neonatal TBV in predicting later cognitive outcomes. Finally, the same procedure described above was repeated for the subregions of interest, HC and ACG controlling for TBV in the analyses.
3. Results
3.1. Interaction of maternal sensitivity and TBV in predicting child cognitive performance
The interaction models are presented in Table 2. The interaction of age-corrected TBV and maternal sensitivity significantly predicted Y2 working memory (p = 0.010) and but not set-shifting (p = 0.089) or inhibitory control (p = 0.489). Furthermore, the interaction of age-corrected TBV and maternal sensitivity significantly predicted Y5 general abilities (p < 0.01), inhibitory control (p < 0.05) and set-shifting (p < 0.01). The results remained significant after FDR correction (see Table 2).
Table 2.
Multivariable General Linear Models for the Interaction of Newborn Total Brain Volume and Maternal Sensitivity in Predicting Cognitive Outcomes across Early Childhood.
| Unstandardized Beta Coefficients (Standard Error) |
||||||
|---|---|---|---|---|---|---|
| Y2 WM | Y2 IC | Y2 SS | Y5 GAI | Y5 ICa | Y5 SS | |
| Intercept | 4.89 (20.31) | 1.19 (13.40) | 1.71 (24.71) | 67.00 (14.94) | 0.34 (0.06) | 93.30 (6.67) |
| Child sex (ref: female) | –0.88 (1.26) | 0.14 (0.81) | –0.84 (1.67) | 0.19 (5.99) | 0.02 (0.02) | –4.61 (2.80) |
| Maternal education (ref: advanced) | ||||||
| Less than high school | 5.26 (3.29) | –2.55 (2.17) | 2.58 (4.17) | –7.56 (14.89) | 0.04 (0.04) | –8.13 (6.90) |
| High school | 0.77 (2.36) | 3.29 (1.47) | −1.18 (2.65) | –11.79 (12.48) | 0.01 (0.03) | –7.19 (5.06) |
| Partial college | 1.51 (1.97) | 0.83 (1.22) | 0.34 (2.09) | –8.75 (11.30) | 0.01 (0.03) | –9.88 (4.48) |
| Associates or Bachelor‘s degree | 3.71 (2.20) | 0.88 (1.41) | 2.72 (2.36) | –6.58 (12.80) | –0.01 (0.03) | –10.27 (5.43) |
| Age at assessmentb | 0.03 (0.81) | 0.06 (0.53) | 0.21 (0.99) | – | –0.05 (0.01) | – |
| TBVcorr | –5.69 (2.30) | –1.21 (1.47) | –3.30 (2.82) | –33.74 (10.87) | –0.10 (0.03) | –23.07 (5.95) |
| M6 Maternal Sensitivity | 0.16 (0.24) | 0.14 (0.15) | 0.38 (0.26) | 2.90 (1.06) | –0.001 (0.003) | 1.42 (0.56) |
| TBVcorr. × M6 Maternal Sensitivity | 0.59 (0.22) | 0.10 (0.45) | 0.45 (0.25) | 3.29 (1.03) | 0.008 (0.003) | 2.14 (0.57) |
| P value for the interaction term | 0.010 | 0.489 | 0.089 | 0.005 | 0.017 | 0.001 |
| FDR-corrected p-value | 0.020 | 0.489 | 0.107 | 0.015 | 0.023 | 0.006 |
| ƞ²p for the interaction | .16 | .01 | .12 | .35 | .20 | .34 |
| Degrees of freedom in the model | 49, 6 | 52, 6 | 33, 6 | 28, 5 | 36, 6 | 36, 5 |
The significant interactions are bolded.
WM = Working memory, IC = Inhibitory control, SS = Set-shifting, GAI = General Abilities Index, TBV = Total brain volume, standardized, corr = corrected for gestational age at birth and postnatal age at scan, corr = corrected for gestational age at birth and postnatal age at scan.
Logarithm-transformed scale.
Age at assessment was not included as a covariate in the models where the age-standardized test outcome was used.
3.1.1. Sensitivity analyses
In all models, the significant interactions remained significant after controlling for age-corrected ICV (p = 0.014 for Y2 working memory and p = 0.002–0.012 for Y5 outcomes) or birth weight (p = 0.019 for Y2 working memory and p = 0.002–0.019 for Y5 outcomes) even after FDR correction (all FDR-corrected p values < 0.019).
3.2. Post-hoc analyses
3.2.1. Probing the interactions of TBV
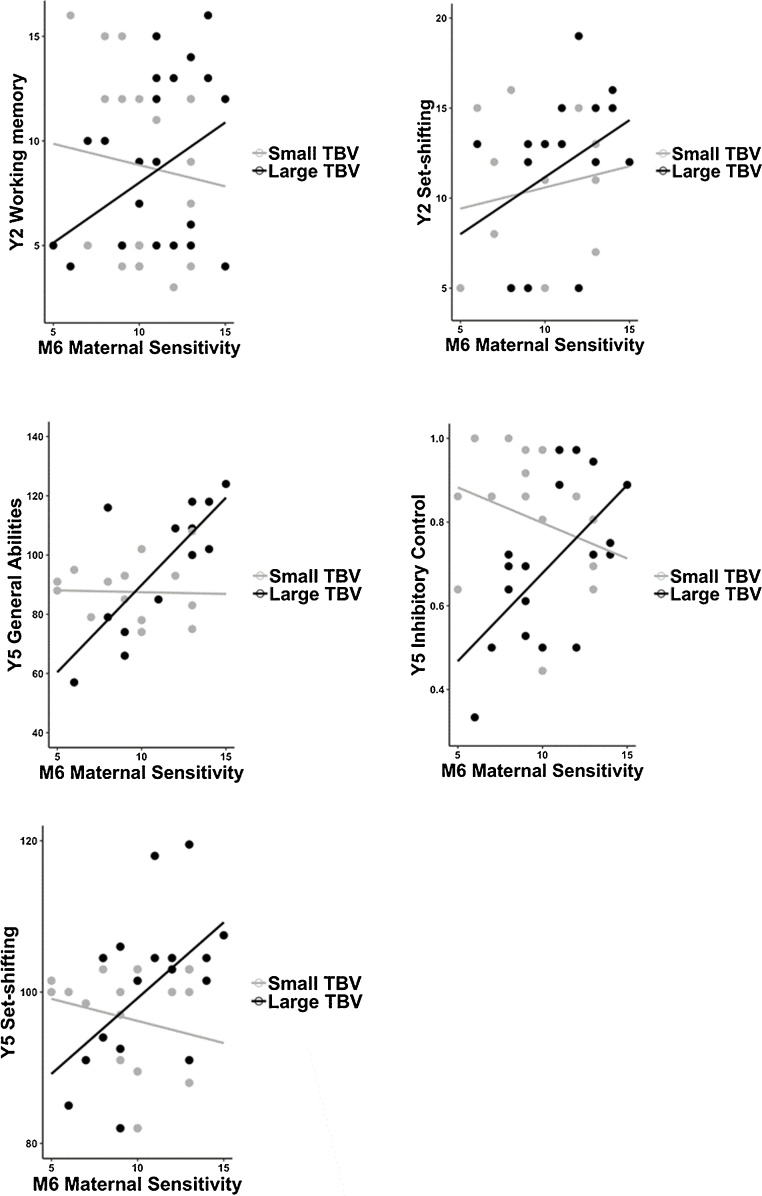
For Y2 outcomes, maternal sensitivity predicted Y2 working memory (B49,7 = 0.85, 95 % CI [0.19, 1.50] p = 0.013) when neonatal TBV was large (>1 SD from the mean) but not when TBV was average or small (p > 0.05). Regarding Y2 set-shifting, a similar pattern for larger TBV as a moderator was observed (B33,7 = 1.00, 95 % CI [0.19, 1.82] p = 0.018), even though the main interaction was not statistically significant (p = 0.089).
For Y5 outcomes, maternal sensitivity predicted Y5 inhibitory control (B36,7 = –0.011, 95 % CI [-0.020, -0.002], p = 0.021) only when neonatal TBV was large (>1 SD from the mean), and general abilities and set-shifting when TBV was large (for general abilities B28,6 = 6.46, 95 % CI [3.27, 9.67], p < 0.001 and for set-shifting B36,6 = 2.91, 95 % CI [1.30, 4.52], p < 0.001) or average-sized (for general abilities B28,6 = 3.30, 95 % CI [1.30, 5.30], p = 0.003 and for set-shifting B36,6 = 1.32, 95 % CI [0.21, 2.43], p = 0.021) but not when TBV was small (p > 0.05).
Thus, maternal sensitivity was positively associated with cognitive performance across different tasks and ages only in children who had a large or average but not a small TBV as newborns (see Fig. 1).
Fig. 1.
Maternal Sensitivity and Child Cognitive Performance by Small and Large Newborn Total Brain Volume (Based on Median Split).
3.2.2. Regions of significance for TBV
Regions of significance testing using TBV as predictor revealed that most interactions (Y2 WM, Y5 SS, Y5 GAI) supported differential susceptibility hypothesis (see Table A3 in the Appendix). For the other outcomes either vantage sensitivity (Y2 SS) or diathesis-stress models (Y5 IC) were suggested. However, due to the small sample size, the results of these analyses should be interpreted with caution.
3.2.3. Grey and white matter and the aspects of maternal sensitivity
Post-hoc analyses (see Table A4 in the Appendix) exploring brain tissue-specific effects showed that for Y2 set-shifting, only WMV interacted with maternal sensitivity in predicting child performance, while both WMV and GMV interacted with maternal sensitivity in predicting performance in other tasks. Thus, there was limited evidence for specificity of GMV or WMV underlying interindividual differences in susceptibility to parenting. Further, post-hoc analyses revealed that all three components of maternal sensitivity (intrusiveness, sensitivity to non-distress and positive regard) equally contributed to the observed interactions (see the Table A5 in the Appendix).
3.3. Exploratory analyses on the subregions: interaction of maternal sensitivity and HC and ACG in predicting child cognitive performance
The detailed results are reported in Table A6 in the Appendix. Regarding Y2 outcomes, the interaction of age-corrected left (p = 0.012) and total HC (p = 0.031) and left (p < 0.001) and total ACG (p = 0.028) and maternal sensitivity significantly predicted Y2 working memory after controlling for TBV. Further, total (p = 0.006), left (p = 0.002) and right (p = 0.030) HC moderated the association between maternal sensitivity and Y2 set-shifting.
Regarding Y5 outcomes, left (p < 0.001), right (p = 0.037) and total ACG (p = 0.003) moderated the association between maternal sensitivity and Y5 general abilities. Similarly, left (p = 0.014), right (p = 0.016) and total ACG (p = 0.004) moderated the association between maternal sensitivity and Y5 set-shifting. Furthermore, interaction of left (but not right or total) ACG and sensitivity predicted Y5 inhibitory control after controlling for TBV (p = 0.019). The interaction of the HC and maternal sensitivity did not predict any of the Y5 outcomes.
3.3.1. Probing the interactions of HC and ACG
In line with the TBV findings, the interaction probing suggested that the associations between maternal sensitivity and child cognitive outcomes were detected when the volume in respect was large or average-sized, but not small (see the Appendix section 6).
4. Discussion
EF and cognitive abilities in general are strongly related to socioemotional adaptation, mental and physical health, emphasizing the importance of understanding the determinants of cognitive and EF development in early childhood. In the current study, we tested whether a broad neonatal brain structural phenotype – larger total brain volume (TBV) – contributes to child sensitivity to the early postnatal environment, specifically to variation in maternal sensitivity, in terms of early childhood cognitive and EF. We found that larger neonatal TBV was associated with greater susceptibility to maternal parenting behavior in terms of the performance in almost all cognitive domains at 2 and 5 years of age. Further, the post-hoc analyses primarily suggested that the children with larger neonatal TBV showed better cognitive outcomes when exposed to higher maternal sensitivity in infancy and poorer cognitive outcomes when exposed to lower maternal sensitivity, a finding in concordance with the differential susceptibility hypothesis (“for better and for worse”). Additionally, a set of exploratory analyses revealed that also over and above TBV, larger hippocampus (HC) and larger anterior cingulate gyrus (ACG), regions indicated in the prior literature on differential susceptibility and cognitive functions, contributed to child susceptibility. Specifically, larger HC was related to susceptibility to parenting in terms of better Y2 cognitive performance, and larger ACG contributed to susceptibility in terms of both Y2 and Y5 outcomes. The findings emphasize the importance of quality of parenting for cognitive development, but also the significance of interindividual variation in neonatal neurophenotypes in affecting how amenable children are to parenting influences.
Environmental sensitivity hypotheses (Pluess, 2015) – including differential susceptibility (Belsky and Pluess, 2009), biological susceptibility to context (Boyce, 2016) and sensory processing sensitivity (Aron and Aron, 1997) models posit that individuals differ in their susceptibility to not only adverse but also to the positive aspects of the environment. This model is typically presented as an alternative to diathesis-stress (Monroe and Simons, 1991) and vantage sensitivity (Pluess and Belsky, 2013) models, describing the amenability of the individual to only adverse or beneficial characteristics in their environment. This variation in malleability for both better and for worse might make sense from an evolutionary point of view because different degrees of susceptibility provide advantages in different environmental conditions (see a review by Ellis et al., 2011): Individuals with lower responsivity to environmental conditions have advantages under adverse conditions, whereas individuals with greater susceptibility are adept to thrive in optimal environments.
Our findings along with other recent contributions (Rifkin-Graboi et al., 2019) suggest that neonatal neurophenotypes may underlie such interindividual differences in plasticity that allows different degrees of responding to the environment. The findings of this study are also partially in concordance with the wealth of literature showing that a larger brain is related to better cognitive performance (Pietschnig et al., 2015). Interestingly, however, no consistent main effects of either maternal sensitivity or total brain volume on child cognition were observed in the present study. This further indicates the importance of studying interactions of the environmental quality and neurophenotypes in young children, who are in general more amenable to early environmental exposures (Markant and Thomas, 2013; Weiss and Wagner, 1998) and in whom the main effects of parenting and brain size on performance may occur later in development along with increasing rank-order stability in cognitive and executive functioning (e.g. Bridgett et al., 2015). Further, our findings may provide one possible explanation for the generally modest effect sizes in previous studies focusing on total brain volume and cognitive performance, extending this literature to suggest that a larger brain volume might affect cognitive outcomes through heightened plasticity. However, because the sample size in the present study was small, the interactions detected as well as the type of susceptibility (differential susceptibility, diathesis-stress or vantage sensitivity) needs to be confirmed in the future studies.
Especially convincing is that the interaction of TBV and maternal sensitivity predicted almost all 2-year and 5-year outcomes except two – inhibitory control and set-shifting at 2 years. The 2-year set-shifting analyses were conducted in a smaller sample of toddlers due to the task characteristics, suggesting that the lack of statistically significant association may be related to low statistical power. Further, for both outcomes, the coefficients were in the same direction as for the pattern of findings in line with the hypothesis. The lack of significant interaction in predicting toddlerhood inhibitory control may also reflect the more emotional, reactivity-driven nature of the Snack Delay task (Hendry et al., 2016) in comparison to the more cognitive tasks used for assessing inhibitory control in early childhood, and other structures than TBV may prove relevant for this type of cognitive functioning. Also, the snack delay was the only task to measure inhibition related to food, with eating inhibition possibly having different underlying neurobiology than other EFs (see e.g. our previous study by Graham et al., 2017).
The underlying mechanisms of TBV as a contributor to susceptibility are not known. Larger brain is linked with larger number of larger-sized neurons and glia, and brain cell proliferation, neurogenesis, myelination, maturation and differentiation all contribute crucially to the number of neurons and the amount of glia and astrocytes, and eventually, brain size alterations in any of these processes may affect inter-individual differences in environmental sensitivity. Brain-derived neurotrophic factor (BDNF) and insulin-like growth factors (IGF-1 and -2) could play a role in determining susceptibility because they promote all stages of brain development described above (Joseph D’Ercole and Ye, 2008; Kowiański et al., 2018; Toro et al., 2009) as well as infant neurodevelopmental outcomes (Ghassabian et al., 2017; Hansen-Pupp et al., 2013; Hellström et al., 2016). Interestingly, IGFs also affect neuroplasticity postnatally (Dyer et al., 2016), and the BDNF Val66Met polymorphism has been linked to global brain and hippocampal volumes and cognitive performance (Jasińska et al., 2017) and susceptibility to parenting in previous studies (Dalton et al., 2014), suggesting a possible pathway linking brain growth, neuroplasticity and cognitive development. Finally, BDNF is reportedly also an important mediator of HC growth, one of our regions of interest, as well as spatial memory performance (e.g. Erickson et al., 2011; Figurov et al., 1996). Correspondingly, in the present study, larger HC was found to moderate the parenting influence specifically on spatial working memory and set-shifting tasks at 2 years.
The findings suggesting that certain individuals may benefit from positive environments more than others in terms of consequences for neurodevelopmental trajectories are well in line with the studies linking larger brain to better cognitive performance across ages. However, the question of why larger TBV (or other larger index structures) would be related to poorer development in a less favorable environment remains open. It is possible that the more malleable children respond to the poorer parenting quality by changing their behavior to match their environment (e.g. showing heightened emotional reactivity or shorter attention span), which in turn results in poorer cognitive outcomes and higher risk for psychopathology (Ellis et al., 2011) later in development. Another interesting perspective into how larger TBV can contribute to abnormal development may be provided by observations in children at high genetic risk for autism spectrum disorders who have been characterized with expansion of cortical surface area and larger TBV in the first years of life. Their larger TBV is assumed to be driven by increased proliferation of neural progenitor cells and associated with altered network connectivity regulating attentional and sensorimotor processes, resulting in abnormal experience-dependent development and later occurring symptoms (Piven et al., 2017). Although the sample in the present study comprised only typically-developing children, similar neural mechanisms may be involved in shaping how the child is affected by experiences given that the neural networks related to attentional and sensory processing also underlie the variation in susceptibility (i.e., salience and default mode networks). However, what underlies larger brain in the current samples of infants cannot be concluded from the data of the present study.
Finally, our findings are in concordance with the prior literature supporting the role of HC and ACG/ACC, structures implicated in the default mode and salience network, for susceptibility to environment (Greven et al., 2019; Moore and Depue, 2016). The preliminary findings of the present study also support the prior studies in proposing that over and above TBV, larger HC and ACG (Rifkin-Graboi et al., 2019; Schriber et al., 2017; Whittle et al., 2011) may be markers of heightened susceptibility, whereas smaller index regions may underlie lower responsivity to variations in parenting quality. Overall, our study is one of the first to suggest that larger HC and ACG may be relevant for postnatal plasticity to environment also in terms of cognitive development. However, the small sample size did not allow a brain-wide analysis on the specific structures and thus these findings remain preliminary so far.
The findings presented here and previous observations on interindividual differences in differential susceptibility may have important practical implications. Although high quality parenting is generally important for healthy child development, our findings support the prior views suggested by differential susceptibility frameworks that some children may on the one hand be harmed more by poor parenting than others and may on the other hand benefit more strongly from an intervention targeted to enhance parenting and family functioning. In turn, some children may need more intense, lengthy or alternative interventions to acquire and improve cognitive skills that more susceptible individuals acquire easily in a high-quality environment (de Villiers et al., 2018). However, much more research is needed on the generalizability of susceptibility to different environmental features in terms of cognitive performance vs. other outcomes and whether the susceptibility to positive environmental features can be also be acquired over time.
Further, to gain a better understanding which neurobiological mechanisms underlie increased environmental susceptibility in individuals born with larger brain volumes in terms of normal and pathological development, controlled experimental studies in animal models aiming at manipulating neurodevelopmental processes that will affect neonatal brain size are warranted. Interestingly, it has been proposed that the prenatal environment may program postnatal susceptibility to environmental conditions including parenting (Beijers et al., 2020; Hartman and Belsky, 2018), with stress-sensitive neurotrophic factors during fetal brain development potentially playing a role (Hellström et al., 2016). We have previously reported that maternal childhood maltreatment was associated with smaller neonatal brain size (Moog et al., 2018), a phenotype that we here observed to underlie lower sensitivity to parenting quality. This may be considered protective considering the risk of parenting difficulties in women exposed to childhood maltreatment (Bailey et al., 2012; Bouvette-Turcot et al., 2019; Pereira et al., 2012). Therefore, prenatal and genetic determinants of neonatal neurophenotypes affecting postnatal susceptibility are an important avenue for future research. Furthermore, as suggested by recent literature of environmental sensitivity studies characterizing specific structural and functional neonatal brain phenotypes (e.g., size of subregions, maturity of brain circuits), especially those involved in salience and default mode networks (Greven et al., 2019) and their interactive effects with postnatal experiences will shed light on the mechanisms of environmental sensitivity.
The strength of the current study is the longitudinal follow-up of newborns until childhood using robust indicators of cognitive and executive function. A limitation of the study is the lack of longitudinal brain MRI data given the fact that the trajectories of brain development are central for cognitive development and also psychopathology. Another limitation is the relatively small sample size with an overrepresentation of males; however, the sample had sufficient variability in the measures of neonatal brain volumes, maternal sensitivity, and cognition and the analyses showed a significant interaction between neonatal TBV and maternal sensitivity across the whole range of brain volumes and after controlling for the effects of sex.
5. Conclusions
We here provide evidence for neonatal TBV, HC and ACG volumes as neurophenotypes that underlie individual differences in susceptibility to maternal sensitivity and predicts cognitive and executive functioning across early childhood. These core functions are typically impaired in psychopathological development, emphasizing the clinical importance of these observations. Our findings further highlight the role of parenting as a predictor of major developmental outcomes, but also the interindividual differences in susceptibility to parenting influences that were related to neonatal brain volume. Further research is needed to identify the specific neural mechanisms as well as the origins of such interindividual differences in plasticity.
Funding
The work of SN was supported by Alexander von Humboldt Foundation, Signe & Ane Gyllenberg Foundation and Emil Aaltonen Foundation. Further, the research was supported by U.S. Public Health Service (National Institute of Health; R01 HD-060628 and R01 MH-105538 to PW, R01 MH-091351 to PW and CB and ECHO grant 5UG3OD023349 to PW and CB) and ERC Starting Grant (ERC-639766 to CB).
Declaration of Competing Interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2020.100826.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aiken L.S., West S.G. Sage Publications; 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Aron E.N., Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J. Pers. Soc. Psychol. 1997;73:345–368. doi: 10.1037/0022-3514.73.2.345. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey H.N., DeOliveira C.A., Wolfe V.V., Evans E.M., Hartwick C. The impact of childhood maltreatment history on parenting: a comparison of maltreatment types and assessment methods. Child Abus. Negl. 2012;36:236–246. doi: 10.1016/j.chiabu.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Banich M.T. Executive function. Curr. Dir. Psychol. Sci. 2009;18:89–94. doi: 10.1111/j.1467-8721.2009.01615.x. [DOI] [Google Scholar]
- Beijers R., Hartman S., Shalev I., Hastings W., Mattern B.C., de Weerth C., Belsky J. Testing three hypotheses about effects of sensitive-insensitive parenting on telomeres. Dev. Psychol. 2020;56:237–250. doi: 10.1037/dev0000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. The differential susceptibility hypothesis. JAMA Pediatr. 2016;170:321. doi: 10.1001/jamapediatrics.2015.4263. [DOI] [PubMed] [Google Scholar]
- Belsky J., De Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J. Child Psychol. Psychiatry Allied Discip. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Belsky J., Pluess M. The nature (and nurture?) of plasticity in early human development. Perspect. Psychol. Sci. 2009;4:345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Belsky J., van IJzendoorn M.H. Genetic differential susceptibility to the effects of parenting. Curr. Opin. Psychol. 2017;15:125–130. doi: 10.1016/j.copsyc.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Belsky J., Bakermans-Kranenburg M.J., Van Ijzendoorn M.H. For better and for worse: differential susceptibility to environmental influences. Curr. Dir. Psychol. Sci. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995:289–300. [Google Scholar]
- Bos K.J., Fox N., Zeanah C.H., Nelson C.A. Effects of early psychosocial deprivation on the development of memory and executive function. Front. Behav. Neurosci. 2009;3:16. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvette-Turcot A.A., Fleming A.S., Unternaehrer E., Gonzalez A., Atkinson L., Gaudreau H., Steiner M., Meaney M.J. Maternal symptoms of depression and sensitivity mediate the relation between maternal history of early adversity and her child temperament: the inheritance of circumstance. Dev. Psychopathol. 2019:1–9. doi: 10.1017/S0954579419000488. [DOI] [PubMed] [Google Scholar]
- Boyce W.T. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology. 2016;41:142–162. doi: 10.1038/npp.2015.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett David J., Burt Nicole M., Edwards Erin S., Deater-Deckard K. Intergenerational transmission of self-regulation: a multidisciplinary review and integrative conceptual framework. Psychol. Bull. 2015;141:602–654. doi: 10.1037/a0038662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.M. Reflection Sciences, Inc.; St. Paul, MN: 2017. Minnesota Executive Function Scale: Technical Report. [Google Scholar]
- Carlson S.M., Zelazo P.D. Reflection Sciences, Inc.; St. Paul, MN: 2014. Minnesota Executive Function Scale: Test Manual. [Google Scholar]
- Cherel M., Budin F., Prastawa M., Gerig G., Lee K., Buss C., Lyall A., Zaldarriaga Consing K., Styner M. Automatic tissue segmentation of neonate brain MR images with subject-specific atlases. In: Ourselin S., Styner M.A., editors. Proceedings of SPIE--The International Society for Optical Engineering. 2015. p. 941311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Yarkoni T., Repovs G., Anticevic A., Braver T.S. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J. Neurosci. 2012;32:8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley J.P., Champagne F.A. Influence of maternal care on the developing brain: mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016;40:52–66. doi: 10.1016/J.YFRNE.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton E.D., Hammen C.L., Najman J.M., Brennan P.A. Genetic susceptibility to family environment: BDNF Val66met and 5-HTTLPR influence depressive symptoms. J. Fam. Psychol. 2014;28:947–956. doi: 10.1037/fam0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers B., Lionetti F., Pluess M. Vantage sensitivity: a framework for individual differences in response to psychological intervention. Soc. Psychiatry Psychiatr. Epidemiol. 2018 doi: 10.1007/s00127-017-1471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégeilh F., Bernier A., Leblanc É., Daneault V., Beauchamp M.H. Quality of maternal behaviour during infancy predicts functional connectivity between default mode network and salience network 9 years later. Dev. Cogn. Neurosci. 2018;34:53–62. doi: 10.1016/j.dcn.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O., Ghosh S.S., Polli F.E., Reynolds G.O., Horn F., Keshavan A., Triantafyllou C., Saygin Z.M., Whitfield-Gabrieli S., Hofmann S.G., Pollack M., Gabrieli J.D. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70:87. doi: 10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M., Peloso E. The role of early stressors in child health and mental health outcomes. Arch. Pediatr. Adolesc. Med. 2006;160:1300–1301. doi: 10.1001/archpedi.160.12.1300. [DOI] [PubMed] [Google Scholar]
- Dyer A.H., Vahdatpour C., Sanfeliu A., Tropea D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience. 2016;325:89–99. doi: 10.1016/j.neuroscience.2016.03.056. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Boyce W.T., Belsky J., Bakermans-Kranenburg M.J., van Ijzendoorn M.H. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Dev. Psychopathol. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wojcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., Mcauley E., Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Tottenham N. Effects of early life stress on amygdala and striatal development. Dev. Cogn. Neurosci. 2016;19:233–247. doi: 10.1016/j.dcn.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay-Stammbach T., Hawes D.J., Meredith P. Parenting influences on executive function in early childhood: a review. Child Dev. Perspect. 2014;8:258–264. doi: 10.1111/cdep.12095. [DOI] [Google Scholar]
- Figurov A., Pozzo-Miller L.D., Olafsson P., Wang T., Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Gabi M., Collins C.E., Wong P., Torres L.B., Kaas J.H., Herculano-Houzel S. Cellular scaling rules for the brains of an extended number of primate species. Brain Behav. Evol. 2010;76:32–44. doi: 10.1159/000319872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Shen D., Smith J.K., Zhu H., Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb. Cortex. 2013;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A.M., Shaw D.S., Forbes E.E., Hyde L.W. Amygdala reactivity as a marker of differential susceptibility to socioeconomic resources during early adulthood. Dev. Psychol. 2018;54:2341–2355. doi: 10.1037/dev0000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A., Sundaram R., Chahal N., McLain A.C., Bell E., Lawrence D.A., Yeung E.H. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev. Psychopathol. 2017;29:1499–1511. doi: 10.1017/S0954579417000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lagos C., Sol D., Reader S.M. Large-brained mammals live longer. J. Evol. Biol. 2010;23:1064–1074. doi: 10.1111/j.1420-9101.2010.01976.x. [DOI] [PubMed] [Google Scholar]
- Gousias I.S., Rueckert D., Heckemann R.A., Dyet L.E., Boardman J.P., Edwards A.D., Hammers A. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage. 2008;40:672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Rudolph M.D., Heim C.M., Gilmore J.H., Styner M., Potkin S.G., Entringer S., Wadhwa P.D., Fair D.A., Buss C. Maternal systemic Interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2-years-of-age. Biol. Psychiatry. 2017:1–11. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M.D., Kippenhan J.S., Dickinson D., Carrasco J., Mattay V.S., Weinberger D.R., Berman K.F. Regional variations in brain gyrification are associated with general cognitive ability in humans. Curr. Biol. 2016;26:1301–1305. doi: 10.1016/j.cub.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven C.U., Lionetti F., Booth C., Aron E.N., Fox E., Schendan H.E., Pluess M., Bruining H., Acevedo B., Bijttebier P., Homberg J. Sensory Processing Sensitivity in the context of Environmental Sensitivity: a critical review and development of research agenda. Neurosci. Biobehav. Rev. 2019 doi: 10.1016/j.neubiorev.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Hansen-Pupp I., Hövel H., Löfqvist C., Hellström-Westas L., Fellman V., Hüppi P.S., Hellström A., Ley D. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr. Res. 2013;74:564–569. doi: 10.1038/pr.2013.135. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Gillmore A.D., Yu T., Holmes C.J., Hallowell E.S., Barton A.W., Beach S.R.H., Galván A., MacKillop J., Windle M., Chen E., Miller G.E., Sweet L.H., Brody G.H. A family focused intervention influences hippocampal-prefrontal connectivity through gains in self-regulation. Child Dev. 2019;90:1389–1401. doi: 10.1111/cdev.13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman S., Belsky J. Prenatal programming of postnatal plasticity revisited—and extended. Dev. Psychopathol. 2018;30:825–842. doi: 10.1017/S0954579418000548. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. 2nd ed. Guilford Press; New York, NY, US: 2017. Introduction to Mediation, Moderation, and Conditional Process Analysis: a Regression-based Approach in Series Methodology in the Social Sciences. [Google Scholar]
- Heatherton T.F., Wagner D.D. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström A., Ley D., Hansen-Pupp I., Hallberg B., Löfqvist C., van Marter L., van Weissenbruch M., Ramenghi L.A., Beardsall K., Dunger D., Hård A.-L., Smith L.E.H. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016;105:576–586. doi: 10.1111/apa.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A., Jones E.J.H., Charman T. Executive function in the first three years of life: precursors, predictors and patterns. Dev. Rev. 2016;42:1–33. doi: 10.1016/j.dr.2016.06.005. [DOI] [Google Scholar]
- Holmes C.J., Barton A.W., MacKillop J., Galván A., Owens M.M., McCormick M.J., Yu T., Beach S.R.H., Brody G.H., Sweet L.H. Parenting and salience network connectivity among african americans: a protective pathway for health-risk behaviors. Biol. Psychiatry. 2018;84:365–371. doi: 10.1016/j.biopsych.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Ensor R. Executive function and theory of mind in 2 year olds: A family affair? Dev. Neuropsychol. 2005;28 doi: 10.1207/s15326942dn2802. [DOI] [PubMed] [Google Scholar]
- Jasińska K.K., Molfese P.J., Kornilov S.A., Mencl W.E., Frost S.J., Lee M., Pugh K.R., Grigorenko E.L., Landi N. The BDNF Val66Met polymorphism is associated with structural neuroanatomical differences in young children. Behav. Brain Res. 2017;328:48–56. doi: 10.1016/j.bbr.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D’Ercole A., Ye P. Expanding the mind: insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreman A., Van Tuijl C., Van Aken M.A.G., Deković M. Parenting and self-regulation in preschoolers: a meta-analysis. Infant Child Dev. 2006;15:561–579. doi: 10.1002/icd.478. [DOI] [Google Scholar]
- Kochanska G., Murray K.T., Harlan E.T. Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Dev. Psychol. 2000;36:220–232. doi: 10.1037/0012-1649.36.2.220. [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley D.C., Cyr M., Finsaas M.C., Orawe J., Huang A., Tottenham N., Klein D.N. Early childhood parenting predicts late childhood brain functional connectivity during emotion perception and reward processing. Child Dev. 2018 doi: 10.1111/cdev.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowiański P., Lietzau G., Czuba E., Waśkow M., Steliga A., Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018;38:579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L., Sol D. Brains, lifestyles and cognition: are there general trends? Brain Behav. Evol. 2008;72:135–144. doi: 10.1159/000151473. [DOI] [PubMed] [Google Scholar]
- Maltbie E., Bhatt K., Paniagua B., Smith R.G., Graves M.M., Mosconi M.W., Peterson S., White S., Blocher J., El-Sayed M., Hazlett H.C., Styner M.A. Asymmetric bias in user guided segmentations of brain structures. Neuroimage. 2012;59:1315–1323. doi: 10.1016/j.neuroimage.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margari L., Craig F., Margari F., Legrottaglie A., Palumbi R., De Giambattista C. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2016;1191 doi: 10.2147/NDT.S104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino L. Big brains do matter in new environments. Proc. Natl. Acad. Sci. 2005;102:5306–5307. doi: 10.1073/PNAS.0501695102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J.M., Thomas K.M. Postnatal brain development. In: Zelazo P.D., editor. Oxford Handbook of Developmental Psychology, Vol. 1: Body and Mind. Oxford University Press; New York: 2013. pp. 129–163. [Google Scholar]
- Meaney M.J. Perinatal maternal depressive symptoms as an issue for population health. Am. J. Psychiatry. 2018;175:1084–1093. doi: 10.1176/appi.ajp.2018.17091031. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010 doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham M.P., Banich M.T. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum. Brain Mapp. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce W.R., Gariepy J.-L., Sutton K., Cox M.J. Changes in maternal sensitivity across the first three years: are mothers from different attachment dyads differentially influenced by depressive symptomatology? Attach. Hum. Dev. 2008;10:299–317. doi: 10.1080/14616730802113612. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Belsky D., Dickson N., Hancox R.J., Harrington H., Houts R., Poulton R., Roberts B.W., Ross S., Sears M.R., Thomson W.M., Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S.M., Simons A.D. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Moog N.K., Entringer S., Rasmussen J.M., Styner M., Gilmore J.H., Kathmann N., Heim C.M., Wadhwa P.D., Buss C. Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol. Psychiatry. 2018;83:120–127. doi: 10.1016/j.biopsych.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.R., Depue R.A. Neurobehavioral foundation of environmental reactivity. Psychol. Bull. 2016;142:107–164. doi: 10.1037/bul0000028. [DOI] [PubMed] [Google Scholar]
- Mutschler I., Ball T., Kirmse U., Wieckhorst B., Pluess M., Klarhöfer M., Meyer A.H., Wilhelm F.H., Seifritz E. The role of the subgenual anterior cingulate cortex and amygdala in environmental sensitivity to infant crying. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network Child care and mother-child interaction in the first 3 years of life. Dev. Psychol. 1999;35:1399–1413. doi: 10.1037/0012-1649.35.6.1399. [DOI] [PubMed] [Google Scholar]
- Nieto-Estévez V., Defterali Ç., Vicario-Abejón C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 2016;10:52. doi: 10.3389/fnins.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J., Vickers K., Atkinson L., Gonzalez A., Wekerle C., Levitan R. Parenting stress mediates between maternal maltreatment history and maternal sensitivity in a community sample. Child Abus. Negl. 2012;36:433–437. doi: 10.1016/j.chiabu.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Pietschnig J., Penke L., Wicherts J.M., Zeiler M., Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci. Biobehav. Rev. 2015;57:411–432. doi: 10.1016/j.neubiorev.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Piven J., Elison J.T., Zylka M.J. Toward a conceptual framework for early brain and behavior development in Autism. Mol. Psychiatry. 2017 doi: 10.1038/mp.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M. Individual differences in environmental sensitivity. Child Dev. Perspect. 2015;9:138–143. doi: 10.1111/cdep.12120. [DOI] [Google Scholar]
- Pluess M., Belsky J. Vantage sensitivity: individual differences in response to positive experiences. Psychol. Bull. 2013;139:901–916. doi: 10.1037/a0030196. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 2007 doi: 10.3758/CABN.7.4.391. [DOI] [PubMed] [Google Scholar]
- Prastawa M., Gilmore J.H., Lin W., Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med. Image Anal. 2005;9:457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pujol J., López A., Deus J., Cardoner N., Vallejo J., Capdevila A., Paus T. 2002. Anatomical Variability of the Anterior Cingulate Gyrus and Basic Dimensions of Human Personality. [DOI] [PubMed] [Google Scholar]
- Rao H., Betancourt L., Giannetta J.M., Brodsky N.L., Korczykowski M., Avants B.B., Gee J.C., Wang J., Hurt H., Detre J.A., Farah M.J. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L.W., Sanmugam S., Broekman B.F.P., Chen H., Wong E., Kwek K., Saw S.-M., Chong Y.-S., Gluckman P.D., Fortier M.V., Pederson D., Meaney M.J., Qiu A. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5:e668. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Tan H.M., Shaun G.K.Y., Sim L.W., Sanmugam S., Chong Y.S., Tan K.H., Shek L., Gluckman P.D., Chen H., Fortier M., Meaney M.J., Qiu A. An initial investigation of neonatal neuroanatomy, caregiving, and levels of disorganized behavior. Proc. Natl. Acad. Sci. U. S. A. 2019;116:16787–16792. doi: 10.1073/pnas.1900362116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman G.I., Newman D.A., Fraley R.C., Haltigan J.D., Groh A.M., Haydon K.C. Distinguishing differential susceptibility from diathesis-stress: recommendations for evaluating interaction effects. Dev. Psychopathol. 2012;24:389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Rudolph M.D., Graham A., Feczko E., Miranda-Dominguez O., Rasmussen J., Nardos R., Entringer S., Wadhwa P.D., Buss C., Fair D.A. Maternal IL-6 during pregnancy can be estimated from the newborn brain connectome and predicts future working memory performance in offspring. Nat. Neurosci. 2018:1–36. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K.D., Davis M.M., Modi H.H., Fowler C., Kim Y., Telzer E.H. Differential susceptibility to parenting in adolescent girls: moderation by neural sensitivity to social cues. J. Res. Adolesc. 2020;30:177–191. doi: 10.1111/jora.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayol F., Maspons J., Lapiedra O., Iwaniuk A.N., Székely T., Sol D. Environmental variation and the evolution of large brains in birds. Nat. Commun. 2016;7:13971. doi: 10.1038/ncomms13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker K., Mulder H., Deković M., Matthys W. Executive functions in preschool children with externalizing behavior problems: a meta-analysis. J. Abnorm. Child Psychol. 2013;41:457–471. doi: 10.1007/s10802-012-9684-x. [DOI] [PubMed] [Google Scholar]
- Schriber R.A., Anbari Z., Robins R.W., Conger R.D., Hastings P.D., Guyer A.E. Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clin. Psychol. Sci. 2017;5:632–649. doi: 10.1177/2167702617699277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner E.A. The origins of young children’s perceived control: mother contingent and sensitive behavior. Int. J. Behav. Dev. 1986;9:359–382. doi: 10.1177/016502548600900307. [DOI] [Google Scholar]
- Sol D., Duncan R.P., Blackburn T.M., Cassey P., Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Bacher S., Reader S.M., Lefebvre L. Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 2008;172:S63–S71. doi: 10.1086/588304. [DOI] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C., White T., Van Ijzendoorn M.H. Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29:505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Tomoda A., Suzuki H., Rabi K., Sheu Y.S., Polcari A., Teicher M.H. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47 doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R., Chupin M., Garnero L., Leonard G., Perron M., Pike B., Pitiot A., Richer L., Veillette S., Pausova Z., Paus T. Brain volumes and Val66Met polymorphism of the BDNF gene: local or global effects? Brain Struct. Funct. 2009;213:501–509. doi: 10.1007/s00429-009-0203-y. [DOI] [PubMed] [Google Scholar]
- Tottenham N. The fundamental role of early environments to developing an emotionally healthy brain. Policy Insights from Behav. Brain Sci. 2017 doi: 10.1177/2372732217745098. 237273221774509. [DOI] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004 doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wang J., Vachet C., Rumple A., Gouttard S., Ouziel C., Perrot E., Du G., Huang X., Gerig G., Styner M. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Front. Neuroinform. 2014;8:7. doi: 10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (WPPSI-IV): Administration and Scoring Manual. 4th ed. The Psychological Corporation; San Antonio, TX: 2012. Wechsler preschool and primary scale of intelligence. [Google Scholar]
- Weiss M.J.S., Wagner S.H. What explains the negative consequences of adverse childhood experiences on adult health? Insights from cognitive and neuroscience research. Am. J. Prev. Med. 1998;14:356–360. doi: 10.1016/s0749-3797(98)00011-7. [DOI] [PubMed] [Google Scholar]
- Whittle S., Yap M.B.H., Sheeber L., Dudgeon P., Yücel M., Pantelis C., Simmons J.G., Allen N.B. Hippocampal volume and sensitivity to maternal aggressive behavior: a prospective study of adolescent depressive symptoms. Dev. Psychopathol. 2011;23:115–129. doi: 10.1017/S0954579410000684. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer; 2009. Ggplot2: Elegant Graphics for Data Analysis. [DOI] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of Attention-Deficit/Hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willoughby M., Blair C.B., Wirth R.J., Greenberg M. The measurement of executive function at age 5: psychometric properties and relationship to academic achievement. Psychol. Assess. 2012;24:226–239. doi: 10.1037/a0025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.B.H., Whittle S., Yücel M., Sheeber L., Pantelis C., Simmons J.G., Allen N.B. Interaction of parenting experiences and brain structure in the prediction of depressive symptoms in adolescents. Arch. Gen. Psychiatry. 2008;65:1377. doi: 10.1001/archpsyc.65.12.1377. [DOI] [PubMed] [Google Scholar]
- Yushkevich P.A., Piven J., Hazlett H.C., Smith R.G., Ho S., Gee J.C., Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zheng J., Anderson K.L., Leal S.L., Shestyuk A., Gulsen G., Mnatsakanyan L., Vadera S., Hsu F.P.K., Yassa M.A., Knight R.T., Lin J.J. Amygdala-hippocampal dynamics during salient information processing. Nat. Commun. 2017;8:1–11. doi: 10.1038/ncomms14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.