Highlights
-
•
Symptomatic aneurysms typically present with pain localised to the abdomen, back, or flank.
-
•
An extremely uncommon presentation of a symptomatic AAA is due to the sequelae of compression of the Inferior Vena Cava.
-
•
IVC compression secondary to AAA has previously been treated via open surgery.
-
•
Endovascular management of symptomatic AAA’s resulting in caval thrombosis may offer a viable alternative to open surgery.
Keywords: Abdominal aortic aneuryms, Pulmonary embolus, Caval thrombosis, Vascular
Abstract
Introduction
The abdominal aorta is the most common site of true arterial aneurysm, predominantly affecting the segment below the renal arteries [1]. Typically, they are now diagnosed as asymptomatic incidental findings on abdominal imaging for unrelated pathology/symptoms. Symptomatic aneurysms typically present with pain localised to the abdomen, back, or flank [2]. An extremely uncommon scenario is presentation due to the sequelae of compression of the Inferior Vena Cava (IVC). Previously, open surgical repair has been the treatment modality of choice in such cases [3].
Presentation of case
We describe the case of a symptomatic infra-renal AAA presenting with lower limb oedema and shortness of breath due to compression of the IVC which resulted in caval thrombosis and associated embolic disease in the form of a pulmonary embolus (PE). Novel endovascular techniques allowed for management via minimally invasive surgery in the form of endovascular aneurysm repair (EVAR) which avoided the high morbidity and mortality of the previous standard of care, open surgery.
Discussion
This resulted in a short duration of admission with resolution of clinical symptoms by follow-up at six-weeks post intervention.
Conclusion
This case highlights that the endovascular management of symptomatic AAA’s resulting in caval thrombosis may offer a viable alternative to open repair with decompression.
1. Introduction
The most strongly associated risk factors for development of an abdominal aortic aneurysm (AAA) are male gender, advanced age, and a history of tobacco use [4]. We may consider three clinical entities in regards to abdominal aortic aneurysms including: i) Non-inflammatory, non-infective which is the most common ii) Mycotic whereby primary bacterial infection of the aortic wall can lead to rapid expansion and iii) Inflammatory which is characterised by thickening of the adventitia with radiological guidelines highlighting >1 cm thick inflammatory rind surrounding the aorta on computed tomography as being definitive for diagnosis [5]. If symptomatic, they tend to present with acute onset abdominal/back/flank pain while rupture leads to progressive discomfort with associated haemodynamic instability.
In regards to the rare entity of IVC compression secondary to AAA, previously this would have been treated via open surgery to allow both exclusion of the aneurysm as well as resection so as to reduce caval compression. However, an endovascular approach is now being utilised in such a scenario. In this case, we present a symptomatic saccular AAA causing IVC occlusion and the utilisation of an endovascular approach in its acute management. This work has been reported in line with the SCARE criteria [6].
2. Presentation of case
A 77-year-old male presented to our tertiary hospital complaining of acute onset right lower limb swelling as well as a four-day history of shortness of breath on minimal exertion. This was on a background history of known peripheral vascular disease including a previous admission for acute limb ischemia in 2015 which was medically managed. He denied any recent travel, surgery, or period of prolonged immobility. Additionally, he had not had any recent symptoms of abdominal or back pain. Prior to this, he had been generally well with nil history of claudication, night pain, or rest pain.
Upon presentation, he was reviewed by emergency department staff who queried a deep venous thrombosis and the possibility of a pulmonary embolus. On examination, his abdomen was soft, non-tender while he had palpable pulses of the bilateral lower limbs. In regards to his right lower limb, this was notably swollen in comparison to the left while motor and sensory function was preserved. Following assessment, it was arranged for him to undergo a venous duplex of the right lower limb as well as a Computed Tomography Pulmonary Angiogram (CTPA).
Venous Doppler of the right lower limb highlighted slow flow throughout the right lower limb venous system with non-visualisation of the distal IVC. Additionally, it identified a saccular aneurysm of the distal abdominal aorta. CTPA highlighted right upper and lower lobe pulmonary emboli.
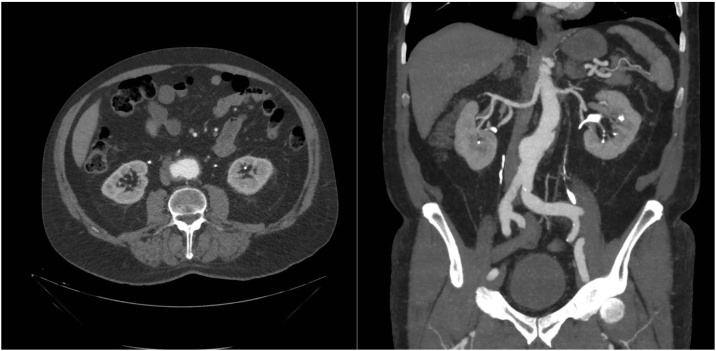
A tri-phasic CT Abdomen was subsequently performed which, in comparison to images from 2015 (Fig. 1), highlighted interval progression of an infra-renal saccular abdominal aortic aneurysm. It was found to be 5.5 cm in anterior-posterior (AP) diameter with a maximum transverse diameter of 7.5 cm (Fig. 2). CT identified thickening of the right lateral margin of the sac in keeping with intramural thrombus. The overall impression was that progressive enlargement of the aneurysmal sac had resulted in near complete occlusion of the adjacent IVC due to local mass effect/compression (Fig. 3).
Fig. 1.
Saccular abdominal aortic aneurysm (AAA) identified on computed tomography (2015).
Fig. 2.
Caval thrombosis secondary to abdominal aortic aneurysm compression identified on computed tomography.
Fig. 3.
Digital Subtraction Angiography of infra-renal abdominal aortic aneurysm (AAA).
The patient was urgently referred to the on-call vascular surgery team who began immediate pre-operative planning. He was commenced on an intravenous heparin infusion with a target activated partial thromboplastin time (APTT) of 70–95 seconds with close observation overnight. Following review and discussion, it was felt that acute intervention in regards to the saccular AAA was indicated. He was subsequently brought to the hybrid interventional surgical suite and underwent endovascular repair of abdominal aortic aneurysm (EVAR, Fig. 4) by the on-call vascular consultant. He had an uncomplicated post-operative course.
Fig. 4.
Completion Digital Subtraction Angiography post endovascular aneurysm repair (EVAR).
He subsequently attended his vascular out-patient clinic for follow-up at six weeks post-operatively. He highlighted nil concerns since discharge with right lower limb swelling now resolved, nil further issues as regards shortness of breath, and ongoing formal anticoagulation via a novel oral anticoagulant. Follow up imaging demonstrated reduction in size of noted aneurysm with a small type II endoleak secondary to a lumbar vessel identified. Additionally, follow up venous doppler highlighted resolution of right lower limb DVT at three months with a medical recommendation of six-months formal anticoagulation agreed between the haematology and vascular teams.
3. Discussion
Caval thrombosis secondary to extrinsic compression of the inferior vena cava (IVC) via an abdominal aortic aneurysm (AAA) is an extremely rare entity [3]. A review of the literature highlights minimal occurence with Coombe et al. describing 10 patients out of a series of 122 who presented with symptoms of iliocaval venous compression [3]. Additionally, the likelihood of caval compression has been shown to be related to AP aneurysm diameter with the majority of cases occurring with aneurysms greater than 6 cm [3]. This case stands alone in comparison to previously documented case reports as anterior-posterior (AP) diameter was just 5.5 cm.
Previously, the acute management in such scenarios would have been open repair to ensure exclusion of the aneurysm as well as relief of extrinsic caval compression [3,7,8]. However, the utilisation of EVAR (endovascular aortic aneurysm repair) is now being proposed with Li et al. (2017) identifiying the successful utilisation of EVAR in the treatment of a symptomatic AAA causing caval compression [9].
Endovascular management in this setting was believed to minimise peri-operative morbidity and mortality. The potential for large volume haemorrhage with an extensive aortic and caval exposure in a formally anticoagulated patient could be avoided while operative duration would be significantly reduced. It was also thought that minimal caval manipulation in the form of endovascular repair would also reduce our risk of further embolic burden to the pulmonary vasculature. Aligned to this, the potential contraindication of arterial compromise secondary to lower limb venous congestion was absent resulting in EVAR being an extremely viable option. The patient subsequently underwent successful endovascular repair and was discharged without complications.
Post-operative change in aneurysm diameter has varied in the literature but in those without shrinkage, resolution of symptoms is thought to be secondary to aneurysmal sac depressurisation via endovascular stent graft placement [10]. As highlighted by Lin et al. [9], the reduction in compression of the IVC due to noted depressurisation of the aneurysmal sac probably induced the restoration or recanalisation of the thrombosed IVC resulting in an increase in venous flow with subsequent symptom resolution. We also prescribed ongoing formal anticoagulation with bilateral thigh high compression stockings to alleviate the symptoms of post-thrombotic syndrome (PTS).
This case highlights the successful utilisation of EVAR in a presentation which would previously have been managed via an open aortic approach. Increasing surgical expertise as well as ongoing endovascular graft design advances will undoubtedly lead to increasing utilisation in such scenarios.
4. Conclusion
This case highlights that abdominal aortic aneurysms can result in IVC occlusion secondary to aneurysmal compression although it is a very rare occurrence. Endovascular treatment offers a surgical modality with reduced perioperative morbidity and mortality in comparison to open repair.
Conflicts of interest
Nil conflicts of interest to declare.
Funding
Nil funding received for this research.
Ethical approval
This study is exempt from the requirement for ethical approval at my institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Ian Barry was responsible for the study concept, methodology, data collection, data curation, and writing the paper – Original Draft.
Patrik Tosenovsky was responsible for reviewing/editing the original draft, project supervision, and project administration.
Guarantor
The guarantor is the corresponding author, Dr Ian Patrick Barry. I accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
This case report was drafted as part of my ongoing position as the Vascular Research Registrar at Fiona Stanley Hospital, Perth, Western Australia. I am grateful to Mr. Patrik Tosenovsky for the provision of facilities and support in the preparation and analysis of this manuscript.
References
- 1.Johnston K.W., Rutherford R.B., Tilson M.D. Suggested standards for reporting on arterial aneurysms. Subcommittee on reporting standards for arterial aneurysms, ad hoc committee on reporting standards, society for vascular surgery and North American chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1991;13(3) doi: 10.1067/mva.1991.26737. 452. [DOI] [PubMed] [Google Scholar]
- 2.ASSAR A.N., Zarins C.K. Ruptured Abdominal Aortic Aneurysm: a surgical emergency with many clinical presentations. Postgrad. Med. J. 2009;85(1003):268–273. doi: 10.1136/pgmj.2008.074666. [DOI] [PubMed] [Google Scholar]
- 3.Combe J., Besancenot J., Milleret P. Iliocaval venous compression due to aneurysm of the abdominal aorta: report of ten cases. Ann. Vasc. Surg. 1990;4:827954. doi: 10.1007/BF02042683. [DOI] [PubMed] [Google Scholar]
- 4.Chaikof E.L., Dalman R.L., Eskandari M.K. The society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018;67:2. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Pennell R.C., Hollier L.H., Lie J.T., Bernatz P.E., Joyce J.W., Pairolero P.C. Inflammatory abdominal aortic aneurysms (a thirty-year review) J. Vasc. Surg. 1985;2:859–869. [PubMed] [Google Scholar]
- 6.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus surgical case report (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Brandao D., Simoes J.C., Canedo A. Occlusion of inferior vena cava: a singular presentation of abdominal aortic aneurysm. Case Rep. Med. 2009;2009 doi: 10.1155/2009/827954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demircioglu F.F., Boke E., Demircin M. Abdominal aortic aneurysm with inferior vena cava obstruction: case report. Angiology. 1989;40 doi: 10.1177/000331978904000311. [DOI] [PubMed] [Google Scholar]
- 9.Li Nan, Xue Ming, Hong Deng-Ke, Chen Xing-Sheng. Endovascular repair of a large abdominal aortic aneurysm in a patient presenting with lower extremity edema as a result of inferior vena cava thrombosis. Ann. Vasc. Surg. 2017;42(July):300.e11–300.e14. doi: 10.1016/j.avsg.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Ahn K.T., Tanabe H., Kotani M. Endovascular treatment of aortoduodenal syndrome. Ann. Vasc. Surg. 2016;31:206.e1e3. doi: 10.1016/j.avsg.2015.08.007. [DOI] [PubMed] [Google Scholar]