Abstract
The present manuscript has focused on the heavy metal; accumulation potential by common native plants i.e. Chenopodium album L., Ricinus communis, Ranunculus sceleratus, and Rumex dentatus growing on the disposed of pulp and paper mill effluent sludge. The sludge showed the abundance of benzene propanoic acid tert- butyldimethylsilyl ester, Octadecanoic acid, TMS, Hexadecanoic acid, TMS, cinnamic acid-α-phenyl-TMS ester, β-sitosterol TMS, 4-mercaptobenzoic acid as residual complex organic compounds along with heavy metals Fe (98.30 mg/L−1), Zn (51.00 mg/L−1), Cu (3.21 mg/L−1), Cd (9.11 mg/L−1), Mn (18.27 mg/L−1), Ni (5.21 mg/L−1), (Hg 0.014 mg/L−1) which were above the prescribed limit of environmental standard. The complexation of organic compounds with heavy metal restricts the bioavailability of metals to plants. But the metal analysis in various parts of the plant showed a significant amount of metal accumulation. Further, histological observations of root tissue through TEM showed apparent deposition of metal granules near the cell wall and vacuole as adoption features of plants. But the variable concentration of metal accumulation in different parts by various plants indicated the variable potential of tested plants with various metals. This also indicated their metal bio-availability and movement to plant tissue. Further, their bioconcentration factor (BCF) and translocation factor (TF) > 1.0 indicated the hyperaccumulation tendency of plants Mn was accumulated maximum in leaves C. album (69.38 mg/kg−1) followed by Cu (25.75 mg/kg −1), As (23.20 mg/kg −1), Fe (20.90 mg/kg −1) and Pb was maximum accumulated (22.41 mg/kg −1) in R. cummunis leaves. The result revealed that arsenic has been accumulated in higher amount root, shoot and leaves of all tested plants. The metal accumulator plants showed phytoremediation potential also by reducing various pollution parameters after growth on sludge. These potential plants may be used as biotechnological tools for the eco-restoration of polluted sites.
Keywords: Residual pollutants, Octadecanoic acid, Root histology, SEM-EDAX, Bioconcentration factor, Environmental analysis, Environmental hazard, Environmental pollution, Environmental toxicology, Environmental science, Toxicology
Residual pollutants; Octadecanoic acid; Root histology; SEM-EDAX; Bioconcentration factor, Environmental analysis; Environmental hazard; Environmental pollution; Environmental toxicology; Environmental science, Toxicology.
1. Introduction
Currently Industrial waste is one major source of heavy metals pollution in the environment worldwide. The agro-based industries i.e. distillery, Tannery and pulp and paper industries and thermal plants are contributing a significant amount of heavy metal contamination in soil and aquatic ecosystem along with their discharged waste (Chandra et al., 2011; Sivakumar et al., 2014; Satyawali and Balakrishnan, 2008; Sushil and Batra, 2006). The heavy metals concentration in the discharge of the above industry has been detected beyond the permissible level of environmental safety regulation (CPCB, 2007; EPA, 2003). The heavy metals pollution in the environment is not the only risk to microorganisms, zooplankton, phytoplankton, and wildlife, but also affects to human health due to its recycling through the food chain (Yang et al., 2005; Zhou et al., 2008). The paper industries manufacturing writing paper, Kraft paper, and hardboard discharge various metal mainly Fe, Mn, Zn, Cu, Cr, Cd, Ni, and Pb along with their waste (Singh and Chandra, 2019; Chandra and Singh, 2012). These heavy metals have a strong binding tendency with lignocellulosic waste due to their cations and result in the formation of the organometallic complex. Due to the complex nature of pollutants, these compounds behave as persistent organic pollutants. Therefore these heavy metals and persistent organic pollutants (POPs) are a threat to the environment for its persistence nature and harmful due to continuous bio-concentration to plant and animal tissue which adversely affects to food chain due to its tendency to accumulate in the food chain (Singh et al., 2015). In general, there is a discharge of 190–200m3 of wastewater per ton of paper production from paper industries containing a high amount of suspended particles and dissolved solids (Chandra and Singh, 2012). The total number of pulp and paper industries in India including small paper industries are more than 850 units as per the annual report of Central Pulp and Paper Research Institute 2016–17 (Annual report of CPPRI, 2016–17). This reflects the magnitude of environmental pollution caused by these industries in India for lignocellulosic waste along with heavy metals. In general, the wood digestion process, there is the discharge of lignocellulosic waste 55–60% containing lignocellulose, hemicellulose, and chlorolignin compounds; while remaining 40–45% pulp is obtained in the pulping process (Chandra and Singh, 2012). There are reported more than 200 organic and 700 inorganic compounds by various researchers (Lacorte et al., 2003). Such compounds contribute to the toxicity of the effluent and increase the chemical oxygen demand (COD) biological oxygen demand (BOD) and total dissolved solids (TDS) of the receiving aquatic resources which induce imbalance to aquatic life. The recent studies have also reported that the pollutants released from the pulp and paper industries not only contribute the toxicity and increase COD, but also a source of carcinogenic, mutagenic, and endocrine-disrupting chemicals which causing metabolic disorders by disturbing the food chain and adversely affect to human health also (Gustavo et al., 2015). The sludge generated from various industries is considered a major source of potentially toxic elements and its disposal is a threat to humans due to the presence of several heavy metals along with various unknown organic pollutants. Nowadays the production of sludge is increasing day by day as a result of wastewater treatment, it remains bulky with high moisture contents and their composition may range from a high content of organic to minerals and heavy metals depending on their origin. Sometimes, the industrial sludge after undergoing composting and vermicomposting is used in agricultural practices, but the application of sludge as a fertilizer in many countries are still warranted to assess their impact on soil, plant and microbial communities (Sun et al., 2013; Khan et al., 2010). Because, the trace elements bioavailability beyond the permissible limits has posed a crucial problem in agriculture and environmental studies (Bhargava et al., 2008). In the recent past several studies have been reported that phytoremediation and phytoextraction of heavy metals from the contaminated site by potential plants growing on polluted sites will be a prospective tool for eco-restoration as green technology (Mani and Kumar, 2014; Sun et al., 2014). Since, phytoremediation process essentially relates to the use of plants and associated soil microorganisms to decrease pollutant concentrations or toxic effects in the environment (Gouda et al., 2018; Segura et al., 2009). The process of phytoremediation is regulated by nature and concentration of organic pollutants, which determine the binding and bioavailability of metals, plant characteristics metal speciation and environmental condition are other factors which regulate the metal uptake in plants (Lasat, 2002; Kang et al., 2017). The phytoremediation potential of any plant is not only determined with plants it has a cumulative effect of metal speciation in soil. This may be exchangeable carbonate, Iron and manganese oxides, Organic and other residual pollutants. The movement of various metals with in ionic form is regulated by action pH and other factors present in the medium (Fu et al., 2019). The majority of studies of heavy metal removal by plants have been reported with pure metal in pot-study (Mahmood, 2010; Kumar et al., 2019). However, in industrial waste, the sludge remains a mixture of various metals along with various organic compounds (Yadav and Chandra, 2018). These pollutants present in several forms in the sludge remains extremely harmful. This poses a great threat to human health, food safety and the environment (Zhang et al., 2019). Moreover, the pulp paper mill sludge contains multi metal along with various organic compounds which makes complex of organometallic pollutants and aggravate the toxicity to any organism growing on sludge. Besides the presence of mutagenic compounds are also reported in the pulp and paper mill discharge. The complete knowledge of residual organic and inorganic pollutants in the pulp and paper industry waste is still lacking. Therefore, posing serious environmental issues, due to variation of process and pollutants from industry to industry. The strong binding tendency of heavy metals to soil particles creates an insoluble and heterogeneous environment (Meagher, 2000). Furthermore, indiscriminate use of the potential plant for metal accumulation and phytoremediation of any polluted site is also not recommended due to various applications of plants as medium & food in agriculture particles. The metal-binding with organic compounds in the environment a soil condition also restrict to plant for the metal accumulation from nature. The assessment of phytoremediation potential by native hyperaccumulator plants from complex organometallic sludge is an important burning issue for the eco-restoration and soil health for sustainable development. The selected site i.e. K. R. pulp and paper industry for this study is highly polluted by the discharge of a huge amount of pulp and paper industrial waste around the area which is damaging not only the soil properties but also continuously affecting water quality also. Then this study has been focused to investigate the potentiality of native hyperaccumulator plants growing on organometallic discharged waste from the pulp and paper industry that may open an opportunity for a technique that will be also used as a tool for eco-restoration of a polluted site.
2. Material and methods
2.1. Sample collection
The collection of effluent's sludge samples were done from M/s K.R. pulp and paper mill Ltd. located at Shahjahanpur (27º50′31.8″N 79º51′15.7″E), Uttar Pradesh, India. While, the potential plant species for heavy metals accumulation were done based on their abundant growth on disposed of pulp and paper sludge without showing any adverse symptoms. The four plant species i.e. Chenopodium album L. (Amaranthaceae), Ricinus communis (Euphorbiaceous), Ranunculus sceleratus (Ranunculaceae), and Rumex dentatus (Polygonaceae) were found abundantly growing on disposed sludge of pulp and paper industry. Therefore these plants were collected randomly from a different site from the same area. While the disposed of fresh pulp and paper sludge sample was collected as control from the pulp industry drainage area. Subsequently, after two months abundantly growing plants were uprooted, cleaned with sterilized distilled water and CaCl2 solution to remove the adherent sludge particle. Further root, shoot and leaves separated and it was kept in the pre-sterilized polythene bags and carried to the laboratory for analysis of accumulated metals in different parts (Chandra et al., 2017, Chandra et al., 2018).
2.2. Physico-chemical analysis
The Physico-chemical parameters were analyzed in the leachate of the sludge as per standard methods described in the American Public Health Association (APHA, 2012). The pH, TSS, TDS, TSS, electric conductivity (EC), chloride, sodium, and potassium of the pulp and paper wastewater sludge & leachate were also measured with the selective ion electrode of (Thermo Orion Model, 960). The lignin content present in the leachate was estimated according to the method of Pearl and Benson (1940). In this method, 1 ml CH3COOH (10%) and 1 ml NaNO2 (10%) were added to 50 ml of the sample. After 15 min, 2 ml of NH4OH was added then the mixture was left for 5 min and the absorbance was measured at 430 nm. For the blank, 1 ml CH3COOH (10%) was added to 50 ml distilled water and 2 ml NH4OH. After 15 min, 1 ml of NaNO2 (10%) was added. After 5 min, the absorbance was measured at 430 nm. The absorbance value was transformed into lignin content (ppm). Further, calibrate ion curve Quantification of PCP present in the leachate was analyzed by HPLC. Briefly, filtered leachate was acidified to pH 2.0 using 1 N HCl and subsequently extracted three times using ethyl acetate (99.5%) in a 1:1 ratio in a separating funnel by intermittent shaking. The extracted upper organic layer containing PCP was passed through sodium sulphate to absorb water. The ethyl acetate extract was evaporated up to dryness at room temperature, subsequently dissolved in 5.0 ml acetonitrile. The HPLC used was a Waters 515 model equipped with UV–vis (Waters2487, Milford, USA) detector operating at 320 nm. The separation was carried out with a reverse-phase Water's make 5 μm C-18 column (250 × 4.6 mm) and the isocratic mobile phase was acetonitrile and water (70:30, v/v) with a flow rate of 1 ml/min. PCP standard was analyzed under the same conditions and the reduction of PCP was estimated by measuring the peak area as describe earlier (Chandra et al., 2009). In addition, All the heavy metals were analyzed by the atomic absorption spectrophotometer (AAS, ZEEnit 700, and Analytic Jena, Germany) as previously described (Chandra et al., 2017).
2.3. Leachate preparation for analysis
Collected sludge samples were pooled mixed and it was air-dried, subsequently, it was ground with a pestle mortar to crush the entire available particle and it was sieved through a 63μm Pore size sieve to get a homogenous powder. Further, the solvent (Dichloromethane) extraction was carried out to obtain a 10% leachate of sludge as described earlier (Chandra et al., 2005). Briefly, 100g of sludge was added to 1000ml of dichloromethane (w/v) and the mixture was shaken continuously for 3–4 h at room temperature (25 ± 2 °C) and the suspension was filtered through 0.22μm syringe filter. The filtrate was used for further GC-MS analysis and UV-Vis scanning.
2.4. Scanning electron microscopic and UV-Vis analysis of effluent
The surface morphology of sludge pollutants was investigated by using scanning electron microscopy (SEM) as per the method described previously (Yadav and Chandra, 2018). To investigate the absorption behaviour of organic pollutants present in the leachate of post and pre-phytoextraction samples. The scanning of absorption spectrum analysis was done by a double beam UV-Vis spectrophotometer. The scanning absorption spectra of two leachate samples were done between 200-700 nm at room temperature by UV-Vis spectrophotometer (Thermo Fisher, Evolution, 2001; USA) as described previously (Chandra et al., 2018).
2.5. Analysis of organic pollutants from the sludge through GC-MS
The analyses of organic pollutants from the growing potential plants were done in the discharge sludge leachate of pulp and paper mill sludge. The extraction of an organic compound from sludge leachate of pulp and paper mill was done by using liquid-liquid extraction methods as described previously (Chandra et al., 2018) dichloromethane (DCM) was used as a solvent for extraction of organic pollutants from both the leachate i.e. pre and post phytoextracted sludge sample. Further, the characterization of organic pollutants was done by comparing with mass and charge number of ions (m/z) of chromatogram at different retention times (RT) from the NIST library available with instruments.
2.6. Estimation of total heavy metals
To estimate the accumulated concentration of heavy metals in the root, shoot, and leaves of the collected native potential plants. The different plant parts were separated into roots, shoot and leave and cut into small pieces and incubated at 65 °C for 7 days till the sample dry completely. Subsequently, samples were washed in a muffle furnace at 460 °C for 6h and digested with 10ml in 2% HNO3 (Horwitz, 2002). Which the leaves of the plant sample were crushed in fine powder after dry and, added 5.0 ml of HNO3 for digestion and continued until the generation of brown fumes were stopped (EPA, 2003). The concentrations of heavy metals like Mn, Pb, Cd, Zn, Cr, Fe, Cu, Ni and As in sludge as control and phytoextracted sample were measured by using an Inductive Coupled Plasma (ICP) (IRIS Intrepid II XDL: Thermo Electron, Waltham, Mass., USA) as per previously described method (Chandra et al., 2008, 2017).
2.7. Bioconcentration and translocation factor
The Bioconcentration factor (BCF) was determined by as previously described in-situ Phytoextraction potential in native hyperaccumulator plants (Yoon et al., 2006). While the translocation factor (TF) was evaluated by calculating the ratio of metal concentration in plant shoot and metal concentration in plant growth on sludge as per methods mention previously (Gupta and Sinha, 2008).
| BCF = Croot/Csludge |
| TF = Cshoot/Croot |
Where, Croot = concentration of metal in plant root (mg/kg−1), Csludge = concentration of metal in sludge of pulp and paper (mg/kg−1), and Cshoot = concentration of metal in mg/kg−1 as per the dry weight of plant shoot.
Both BCF and TF have to be considered for evaluating whether a plant is a metal hyperaccumulator. A hyperaccumulator plant should have BCF >1 or TF > 1, as well as total accumulation>1000 mg kg−1 of Cu, Co, Cr, Ni or Pb, or>10,000 mg/kg−1 of Mn or Zn (Baker and Brooks, 1989; Kabata-Pendias and Pendias, 2011).
2.8. Histological observation
The cellular observations of heavy metal accumulation inside the plant root tissue during the Phytoextraction by native potential metal accumulator plant and the plant root hyperaccumulator plants, plant root tips were cut (approx 2.0 mm) for section cutting and fixed in 2.5 % glutaraldehyde solution as previously described method (Chandra and Kumar, 2017) Sections were observed under the Transmission electron microscope TEM (FEI TecnaiTM G2 Spirit Twin, Hillsboro, USA) at an 80 kV voltage velocity.
2.9. Statistical analysis
All data were reported as means ± SD for triplicate samples to confirm the data variability and stability using Student's t-test (P < 0.001). The mean concentration of heavy metals in the root, shoot, and leaves were subjected for the statistical analyses by using the SPSS statistical software (version 17.0; SPSS Inc., Chicago, IL, USA).
3. Result and discussion
3.1. Physico-chemical characteristics of pre and post phytoremediation sludge sample
The Physico-chemical analysis of different pollutants in the leachate of sludge showed above the permissible limit in pre phytoremediation as shown in Table 1 where the leachate of discharged sludge from pulp and paper industry showed alkaline pH (8.1 ± 0.24), and high TDS (1856 ± 32.15 mg/L−1), TSS (82 ± 1.21 mg/L−1), with high BOD (18547 ± 182 mg/L−1), COD (37671 ± 174.00 mg/L−1) value. In addition, the high value of phenolic content (547 ± 22.21), Lignin (62102 ± 114.21 mg/L−1), Chlorophenols (388 ± 10.03 mg/L−1), chloride (4.55 ± 0.22 mg/L−1), and other salts were detected. Similarly, the value of different heavy metals i.e. Iron (Fe), Zinc (Zn), Copper (Cu), Cadmium (Cd), Manganese (Mn), Nickel (Ni) and Mercury (Hg+) was also found in high concentration (Table 1). While significant reduction was noted in various pollution parameters after plant growth on sludge. This showed the phytoextraction of heavy metal and phytoremediation of organic pollutants from the site. The value of Mercury (Hg+) in sludge was noted higher than the permissible level (i.e. 0.014 mg/L−1). The source of Hg+ in pulp paper mill effluent and sludge is mainly caustic soda which is heavily used in pulping and bleaching process. While the mercury (Hg+) is used in the production of caustic soda. The mercury concentration in caustic soda is reported as high as 22 gm per ton of caustic soda produced (Streets et al., 2019). Therefore even a low level of mercury in the effluent sludge contributes the toxicity to the aquatic organisms and other biotic components of the ecosystem. The high alkaline pH, TDS, TSS of the pulp and paper industry sludge might be due to the presence of residual content of sodium hydroxide and sodium sulphide utilized in the pulping process of the industry (Yadav and Chandra, 2018). Besides the presence of high TSS indicated mixing of Kraft paper industrial discharge due to utilization of sugarcane bagasse and recycled paper as raw material. Therefore, there is a discharge of suspended particles during the washing of their pulp in the manufacturing process. Moreover, the presence of cellulosic fine fibres given a piece of evidence for the discharged waste from the pulping and washing machine of the pulp and paper industries. The discharge of fine fibres in the effluent is well reported in the washing process of pulp previously (Chandra and Singh, 2012).
Table 1.
Physico-chemical characteristics and heavy metals content in pulp and paper mill effluents sludge leachate before and after phytoremediation.
| Parameters | Values (mean) before Phytoremediation | Values (mean) after Phytoremediation | Permissible limit (EPA, 2003) |
|---|---|---|---|
| pH | 8.1 ± 0.24 | 7.1 ± 0.21a | - |
| Color (Co–Pt) | 3341 ± 102 | 1940 ± 101 | Dark Brown |
| Total solid (mg/L−1) | 1524 ± 101 | 753 ± 1.10 | - |
| Total dissolved solid (mg/L−1) | 1856 ± 32.15 | 623 ± 0.1 | - |
| Total suspended solid (mg/L−1) | 82 ± 1.21 | 39 ± 3.11 | 35 |
| Chemical oxygen demand (mg/L−1) | 37671 ± 254.00 | 12670 ± 1.21a | 120 |
| Biological oxygen demand (mg/L−1) | 18547 ± 182 | 11254 ± 40a | 40 |
| Electrical conductivity (μmhoscm−1) | 1824 ± 81.00 | 526 ± 20a | 1000 |
| Total Phenols (mg/L−1) | 547 ± 22.21 | 159 ± 35c | 0.50 |
| Total nitrogen (mg/L−1) | 194 ± 4.11 | 71 ± 0.20c | 143 |
| Sulphate (mg/L−1) | 2569 ± 09.00 | 1027 ± 0.01a | 250 |
| Phosphorus (mg/L−1) | 204 ± 5.80 | 51 ± 0.04a | 200 |
| Cl− (mg/L−1) | 4.55 ± 0.22 | 2.21 ± 1.00b | 1500 |
| Na+ (mg/L−1) | 423 ± 11.20 | 124 ± 10.11b | 200 |
| K+ (mg/L−1) | 19.0 ± 0.70 | 10.01 ± 3.00a | - |
| Lignin (ppm) | 62102 ± 114.21 | 18542 ± 2.14a | - |
| Chlorophenols (mg/L−1) | 388 ± 10.03 | 83.70 ± 1.11a | 3.0 |
| Heavy metals | |||
| Iron (Fe) (mg/L−1) | 98.30 ± 1.80 | 24.21 ± 0.40 | 2.00 |
| Zinc (Zn) (mg/L−1) | 51.00 ± 0.00 | 18.01 ± 0.10b | 2.00 |
| Copper (Cu) (mg/L−1) | 3.21 ± 0.01 | 1.02 ± 0.94c | 0.50 |
| Cadmium (Cd) (mg/L−1) | 9.11 ± 0.01 | 4.31 ± 0.20 NS | 0.01 |
| Manganese (Mn) (mg/L−1) | 18.27 ± 0.20 | 10.22 ± 1.00b | 0.20 |
| Nickel (Ni) (mg/L−1) Arsenic (As) (mg/L−1) Mercury (Hg) (mg/L−1) |
5.21 ± 0.04 0.97 ± 0.01 0.014 ± 0.80 |
2.10 ± 1.00 NS 0.37 ± 0.00 0.001 ± 0.60 |
0.10 0.10 0.01 |
All the values are means of triplicate (n = 3) ±SD. Unit of all parameters are in (mg/L−1) except pH, color (Co–Pt. Unit) and EC (μmhoscm−1); aHighly significant at p < 0.001; bSignificant at p < 0.01; cLess significant at p < 0.05; NSNon-significant at p > 0.05.
Therefore, the value of five days BOD (17446 mg/L−1) and COD (35471 mg/L−1) of the sludge leachate before phytoextraction was calculated as the BOD/COD ratio showed 0.21. This indicated the less degradability nature of pollutants present in discharged wastewater. The degree of degradability of effluent was characterized based on the BOD: COD ratio of wastewater (Lee and Nikraz, 2014). Further, the high value of COD and organic content was found due to the release of various wood extracts along with chemicals used in the pulping process, which contribute complex chemicals in discharged effluent (Yadav and Chandra, 2018). A similar observation regarding the discharge of recalcitrant pollutants from pulp and paper mills of the biologically treated effluent has also been reported in previous work (Chandra et al., 2011). The presence of complex pollutants in discharged effluent might be due to imposing toxicity to the microbial community responsible for mineralization of organic pollutants in the effluent treatment plant working in the pulp and paper industry. Therefore, these pollutants have been noted in the discharge effluent above the permissible limit. The presence of various toxic pollutants in the pulp and paper effluent after secondary treatment corroborated to our previous observations (Yadav and Chandra, 2018; Bose and Bhattacharyya, 2008; Chandra et al., 2017). Similarly, the higher TDS might be due to the presence of dissolved lignocellulosic particles along with fine particles and fibers added in the effluent during pulp washing in the industry (Chandra and Singh, 2012). In addition, the higher concentration of Na+ and K+ in sludge might be added in the effluent during digestion of wood pulp which deposited in the sludge of industrial waste and contributed salinity and high pH which is also one important factor responsible for soil pollution and environmental toxicity (Wu and Guan, 2008). The toxicity of pulp and paper wastewater towards microplankton, which plays a very important role in the food chain of the aquatic ecosystem, has been reported by the previous worker (Gauthier and Archibald, 2001). The presence of various heavy metals beyond their prescribed limit and binding tendency towards sulfur and carboxylic acid (COOH) and a minor group of protein disturb various enzymatic activities of flora & fauna in the environment (Slavin et al., 2017).
3.2. Scanning of electron microscopy and UV-Vis scanning spectra pollutants
The SEM image of sludge showed as composed of various irregular particles. This provided a large surface area for the adsorption containing various pollutants along with heavy metals and lignin compounds as shown in (Figure 1a &b) and EDAX containing the elemental percentage composition i.e. O, Na, Mg, S, Cl, K shown in figure (1c & d). Further, the UV-Vis spectra wavelength range of 250–700 nm analysis showed the presence of various dissolved organic compounds in the sludge of the pulp and paper industry before and after phytoremediation with variable absorption peak (Figure 1e). This has given a shred of strong evidence for their absorption properties of soluble organic matter present in the UV-Vis range. The sludge sample prior to plant growth showed various mixed peaks which indicated a mixture of pollutants present in leachate. While the UV-Vis absorption spectrum pattern of phytoremediation sludge sample showed shifting of absorption and many peaks were even disappeared (Figure 1f). this indicated the transformation and degradation of various compounds after plant growth. Consequently, the reduction of pollutants might be occurred due to the combined effect of plant and rhizospheric microbial communities (Chandra et al., 2018). The underivatized lignin showed a granulated structure with grains or oval particles lightweight in different sizes. The crystal shape structure showed in the figure indicated the presence of lignin as a polymer along with the presence of different heavy metals and various particles (Yadav and Chandra, 2018). While the lignin was obtained by acid precipitation from industrial black liquor derived from the pulping process of bagasse soda (Slavin et al., 2017).
Figure 1.
Morphological view of sludge sample of pulp and paper industry after secondary treatment showing the several structures of the different organic polymer (A&B), elemental analysis from the sludge by EDAX pre and post phytoremediation (C&D) and UV-Vis spectra scanning (200–700 nm) analysis of sludge leachate from pulp and paper industry pre and post phytoremediation (E&F).
3.3. GC-MS analysis of organic pollutants from the paper mill sludge after plant growth
The GC-MS chromatogram and identified organic compounds in the Di-chloromethane (DCM) extract from the fresh paper mill discharged effluent's sludge and a native plant has grown sludge sample is shown in Figure 2a &b and Table 2. The compounds detected at RT 25.53, 28.10, 31.82, 33.81, and 43.77 are listed under endocrine disruptor screening as per program (USEPA, 2012). The presence of androgenic and estrogenic compounds in the industrial effluent has been also reported from various studies in the United States (Thomas et al., 2002; Harries et al., 1999).
Figure 2.
GC MS chromatogram of organic compounds extracted from the pulp and paper industry sludge by DCM. (a) Chromatogram of sludge before phytoremediation (b) Chromatogram of sludge after phytoremediation.
Table 2.
Identification of residual organic pollutants by GC-MS from TMS derivatized DCM extract from pulp and paper industry effluent sludge discharge after secondary treatment.
| Before phytoextraction | RT | Compounds | % similarity with NIST | Toxicity |
|---|---|---|---|---|
| 6.06 | 2-Pentadecanone | 75 | Hazardous to the aquatic environment, long-term hazard | |
| 10.27 | Nonacosane | 94 | Oral, dermal and inhalation toxicity | |
| 21.91 | Benzene Propanoic acid, tert-butyldimethylsilyl ester | 59 | Genotoxicity | |
| 25.53 | Octadecanoic acid, TMS | 92 | Endocrine disrupting chemicals (EDCs) | |
| 28.10 | Tetradecanoic methyl ester | 95 | EDCs, genotoxicity | |
| 31.82 | Heptacosane | 91 | EDCs | |
| 33.81 | Hexadecanoic acid, TMS | 97 | EDCs, mutagenic | |
| 35.60 | D-Lactic acid- DITMS | 89 | Genotoxicity | |
| 40.27 | Lactic acid, TMS ether, TMS | 93 | Genotoxicity | |
| 42.19 | Cinnamic acid-α-phenyl-TMS ester | 96 | Genotoxicity | |
| 43.77 | β- Sitosterol TMS | 98 | EDCs, genotoxicity, Mutagenic | |
| 47.01 | 4-Mercaptobenzoic acid | 86 | Carcinogenic | |
| After phytoextraction | RT | Compounds | % similarity with NIST | Toxicity |
| 6.31 | Pentatonic acid, TMS ester | 91 | Long-term hazard | |
| 19.39 | 1,4-Bis (hexadecyl)-2,5-divinylbenzene | 78 | Serious eye damage/eye irritation | |
| 21.76 | O-Trimethylsilyl Pyridine-2-Amidoxime | 81 | Unknown | |
| 27.68 | 6-Acetyl-1-acetoxy-10-hydroxy-neoline | 83 | Data not known | |
| 30.56 | 1-Methyl-3-(3,4-dimethoxyphenyl)-6,7-dimethoxyisochromene | 78 | Data not known | |
| 42.99 | Decane, 1 bromo-2-methy | 71 | Data not known | |
TMS: Trimethylsilyl; RT: Retention Time (Min).
The comparative result showed the disappearance of some peaks from chromatograms and some compounds were completely removed. Simultaneously, while some new compounds were also detected this indicated as a biotransformed product of compound due to plant growth. The bio-transformation and remediation of various organic pollutants from paper mill sludge and other organometallic complexes of industrial waste have also been reported in the previous study from a different region of disposed of pulp and paper mill effluent sludge (Chandra et al., 2018). The reduction of various pollutants might be the cumulative effect of plant microbe's interaction during phytoremediation of sludge due to the growth of potential plants. Soil microbial community alters the metal motilities for uptake to plants by producing cell exudes for (Lasat, 2002; Ojuederie and Babalola, 2017) the bacterial assisted phytoremediation activities also have been described in detail by various researchers (Ahmed, 2015). The discharged of various fatty acids and various lignin monomers during the secondary treatment of effluent has been well reported in the analysis (Yadav and Chandra, 2018) while phytoremediation of many compounds disappeared from the fresh sludge leachate sample this confirmed that the integration of bacteria and plant degraded the sludge compounds and some new products were generated. Therefore the change in chromatogram has been noted as shown in figure 2a and b.
3.4. Metal accumulation pattern in a different part of plants
Collected Potential heavy metal accumulator plants were from the pulp and paper industry disposal site was identified using the standard taxonomic method according to Duthie flora of Indo-Gangetic plain (Duthie et al., 1903). The identified plant showed the variable potential of metals accumulation pattern in their different part i.e. root, shoot, and leaves as shown in Table 3. The Chenopodium album L. showed maximum Mn (161.91 ± 0.9 mg/kg−1), accumulation potential followed by Cu (126.6 ± 1.0 mg/kg−1) the comparative analysis of metal accumulation potential of various plants was found different. However, the result showed a fast accumulation of mercury in the growing plant on the sludge. But the detected concentration was found within the permissible Level (Table 3) Hence, it needs more investigation from other industry to evaluate their environmental impact globally. The accumulation of Ag and Cu has been also reported by Eichharnia crassipes, Hydrilla verticillate, Lamna minor, Pistia stratiotes, Trapa natons drown in paper mill effluent of JK paper mill of Rayagada, Orissa (Mishra et al., 2013).
Table 3.
Heavy metal content (mg/kg−1) accumulated in the root, shoot, and leaves of various native plant species growing on the pulp and paper industry sludge.
| Plant name | Plant part | Mn | Pb | Cd | Zn | Cr | Fe | Cu | Ni | As | Hg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chenopodium album L | Root | 51.23 ± 0.3 | 1.36 ± 0.2 | 0.51 ± 0.3 | 35.70 ± 0.2 | 2.402 ± 0.3 | 35.0 ± 0.3 | 53.41 ± 0.4 | 4.104 ± 0.3 | 40.4 ± 0.3 | 0.010 ± 0.1 |
| Shoot | 41.30 ± 0.3 | 1.03 ± 0.3 | 1.23 ± 0.3 | 27.78 ± 0.3 | 2.421 ± 0.3 | 32.2 ± 0.3 | 47.44 ± 0.3 | 5.214 ± 0.3 | 51.01 ± 0.3 | 0.002 ± 0.2 | |
| Leaves | 69.38 ± 0.3 | 1.31 ± 0.3 | 2.20 ± 0.3 | 20.7 ± 0.3 | 2.841 ± 0.3 | 20.9 ± 0.3 | 25.75 ± 0.3 | 2.502 ± 0.3 | 23.20 ± 0.3 | 0.001 ± 0.4 | |
| Total | 161.91 ± 0.9 | 3.70 ± 0.8 | 3.94 ± 0.9 | 84.18 ± 0.8 | 7.664 ± 0.9 | 87.1 ± 0.9 | 126.6 ± 1.0 | 11.82 ± 0.9 | 114.61 ± 09 | 0.013 ± 0.7 | |
| Accumulation pattern | L>S>R | R>L>S | L>S>R | R>S>L | L>S>R | R>S>L | R>S>L | S>R>L | S>R>L | S>R>L | |
| Ricinus communis | Root | 25.03 ± 0.3 | 7.23 ± 0.1 | Nil ±0.2 | 16.13 ± 0.3 | 0.51 ± 0.4 | 41.7 ± 0.6 | 9.532 ± 0.1 | 21.36 ± 0.1 | 16.21 ± 0.3 | 0.002 ± 0.5 |
| Shoot | 22.36 ± 0.2 | BDL ±0.3 | BDL ±0.1 | 44.18 ± 0.5 | BDL ±0.3 | 16.6 ± 0.4 | 10.501 ± 0.2 | 14.21 ± 0.3 | 22.04 ± 0.2 | 0.001 ± 0.3 | |
| Leaves | 11.68 ± 0.1 | 22.41 ± 0.3 | BDL ±0.3 | 22.16 ± 0.4 | BDL ±0.3 | 27.5 ± 0.5 | 25.316 ± 0.3 | 11.21 ± 0.1 | 18.36 ± 0.3 | 0.001 ± 0.1 | |
| Total | 58.107 ± 06 | 29.64 ± 0.7 | 0.0 ± 0.6 | 72.47 ± 1.2 | 0.51 ± 1.0 | 85.8 ± 1.5 | 45.369 ± 0.6 | 46.78 ± 0.5 | 56.61 ± 0.8 | 0.004 ± 0.9 | |
| Accumulation pattern | R>S>L | L>R | BDL | S>L>R | BDL | R>L>S | L>S>R | R>S>L | S>L>R | R>L>S | |
| Ranunculus sceleratus | Root | 28.79 ± 0.6 | 11.20 ± 0.2 | 1.20 ± 0.5 | 17.25 ± 0.5 | 6.23 ± 0.5 | 42.24 ± 0.5 | 30.21 ± 0.5 | 21.60 ± 0.5 | 20.40 ± 0.5 | 0.010 ± 0.3 |
| Shoot | 31.50 ± 0.5 | 9.21 ± 0.4 | 1.05 ± 0.3 | 23.44 ± 0.5 | 14.43 ± 0.5 | 28.31 ± 1.0 | 25.20 ± 0.4 | 14.51 ± 0.5 | 19.20 ± 0.5 | 0.002 ± 0.1 | |
| Leaves | 35.10 ± 0.5 | 9.11 ± 0.3 | 4.70 ± 0.4 | 16.77 ± 0.5 | 8.55 ± 0.5 | 22.70 ± 0.5 | 37.69 ± 0.5 | 16.79 ± 0.5 | 18.01 ± 0.6 | 0.001 ± 0.1 | |
| Total | 94.139 ± 0.6 | 29.52 ± 0.9 | 6.95 ± 0.12 | 57.46 ± 1.5 | 29.21 ± 1.5 | 93.25 ± 2.0 | 93.1 ± 1.4 | 52.2 ± 1.5 | 57.61 ± 1.6 | 0.013 ± 0.5 | |
| Accumulation pattern | L>S>R | R>S>L | L>R>S | S>R>L | S>L>R | R>S>L | R > L>S | R>L>S | R>S>L | S>R>L | |
| Rumex dentatus | Root | 49.04 ± 0.5 | 7.26 ± 0.4 | 1.10 ± 0.5 | 11.32 ± 0.6 | 5.21 ± 0.5 | 40.30 ± 0.4 | 6.19 ± 0.4 | 21.54 ± 0.5 | 5.24 ± 0.5 | 0.002 ± 0.2 |
| Shoot | 44.37 ± 0.5 | 11.50 ± 0.1 | 1.49 ± 0.5 | 22.65 ± 0.5 | 10.60 ± 0.5 | 43.20 ± 0.4 | 19.12 ± 0.5 | 26.56 ± 0.5 | 7.71 ± 0 .5 | 0.003 ± 0.0 | |
| Leaves | 32.14 ± 0.5 | 7.10 ± 0.2 | 5.17 ± 0.5 | 8.13 ± 0.5 | 9.90 ± 0.5 | 23.90 ± 0.5 | 9.09 ± 0.5 | 18.76 ± 0.4 | 8.40 ± 0.3 | 0.001 ± 0.1 | |
| Total | 125.55 ± 1.5 | 25.86 ± 0.7 | 6.76 ± 1.5 | 42.00 ± 1.6 | 22.71 ± 1.5 | 107.4 ± 1.2 | 34.4 ± 1.4 | 66.86 ± 1.4 | 21.35 ± 1.3 | 0.005 ± 0.3 | |
| Accumulation pattern | R>L>S | S>R>L | L>S>R | S>R>L | S>L>R | S>R>L | S>L>R | S>R>L | L>R>S | S>R>L |
All the values are mean of three replicates (n = 3), (±) standard deviation (SD), BDL: Below detection limit, R: Root, S: Shoot, L: Leave.
This might be due to their variable accumulation potential and specific physiological property of the different plants based on their genetic properties. The variable metal accumulation pattern of various plant species has been also reported earlier from complex organometallic industrial waste (Kato et al., 2004) which supported the variable potential for metal accumulation of plant species from the complex environment. The variable potential of metal accumulation by the different plants has been also reported from another site in the previous study (Chandra et al., 2017). Moreover, the metal accumulation process in the plant at any polluted site is regulated by a mixed phenomenon of plant and microbes along with the type of soil, environmental factors, and category of heavy metals. The metals accumulation process from the lignocellulosic waste might be due to presence of various elements such as nitrogen, phosphorus, and potassium which are facilitated by existing lignocellulosic waste degrading bacterial communities Enterobacter sp., Pseudomonas sp., etc, and proving the source of carbon to growing plants (Chandra et al., 2011, 2017). Due to the influence of various factors on multi-metal speciation in the sludge, the existence of heavy metal in sludge becomes more complex heavy metals with the organic compound in the soil are also reported by the previous compound in (Zhang and Lin, 2014). The study has revealed for metal complexity in soil included concentration of metals, soil, Ph, soil organic content, soil texture, cation exchange capacity, redox potential, the interaction between element and micro-organism (Zhang and Lin, 2014).
3.5. Bioconcentration and translocation factor
The Bioconcentration factor of any plant is an important parameter for the health risk assessment of human. Thus, the plants growing on industrial sludge has accumulated the various metals in their root, and shoot indicated the nature of metal, mobility, and their binding potential with soil in sludge. The ratio of metal concentration in plant root to sludge which reflects the Bioconcentration factor (BCF) and the further ratio of root to shoot is also termed as Translocation factor (TF). Thus, Both BCF and TF are effective tools for estimation of plant's potential for metal accumulation and phytoremediation process from any polluted site with heavy metals as reported earlier (Chandra et al., 2017; Yoon et al., 2006). The BCF of all plant species was found more than one (>1) this supported that the plants are accumulating the metals from sludge. The BCF range among the tested plant was noted between 3.13- 64.80. The maximum BCF (64.80 mg/kg−1) was noted by R. dentatus for copper while minimum (3.13 mg/kg−1) was observed in R. sceleratus for arsenic as shown in Table 4.
Table 4.
Showing BCF and TF of different Heavy metal accumulation (mg kg−1) by various native plants.
| Native hyperaccumulators plants | Bioconcentration Factor (BCF) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Pb | Zn | Cr | Fe | Cu | Ni | As | Hg | |
| Chenopodium album L. | 54.1 | 51.00 | 9.270 | 10.21 | 29.10 | 48.11 | 14.19 | 5.33 | 3.99 |
| Ricinus communis | 48.70 | 31.60 | 4.210 | 21.88 | 27.06 | 31.80 | 6.28 | 4.26 | 5.48 |
| Ranunculus sceleratus | 18.33 | 17.00 | 7.661 | 18.54 | 30.40 | 22.00 | 9.13 | 3.13 | 2.23 |
|
Rumex dentatus |
25.40 |
18.05 |
11.61 |
20.31 |
33.11 |
64.80 |
10.13 |
5.04 |
4.08 |
|
Translocation Factor (TF) |
|||||||||
| Mn | Pb | Zn | Cr | Fe | Cu | Ni | As | Hg | |
| Chenopodium album | 6.02 | 7.01 | 8.25 | 4.01 | 17.37 | 21.50 | 2.55 | 1.04 | 0.04 |
| Ricinus communis | 8.07 | 6.46 | 5.24 | 5.04 | 14.04 | 16.80 | 3.37 | 1.03 | 0.03 |
| Ranunculus sceleratus | 6.24 | 5.60 | 6.76 | 8.00 | 15.03 | 13.40 | 3.29 | 1.20 | 0.02 |
| Rumex dentatus | 9.21 | 5.10 | 10.11 | 9.06 | 19.05 | 20.30 | 2.01 | 2.07 | 0.07 |
In the majority, the metal concentration in plant root was higher than sludge. This indicated the higher potential for metal accumulation (Chandra et al., 2017; Kumar et al., 2013). Further, the ability of all plants to transfer metals from root to shoot which is an important parameter of phytoremediation potential is also shown in Table 4. All these tested plants showed TF > 1. It was ranging from 1.03 to 21.50. This indicated high metal accumulation property of these plants for the translocation and metal movement to aerial parts of plants. This showed the phytoremediation potential also of plants growing on industrial waste contaminated soil. A similar, pattern of metal accumulation with more than one BCF and TF factor has been reported by another researcher for other plants as a potential metal accumulator (Stoltz and Greger, 2002). Thus, all collected plants were noted very potential metal accumulator and phytoremediator for heavy metal mixed lignocellulosic waste.
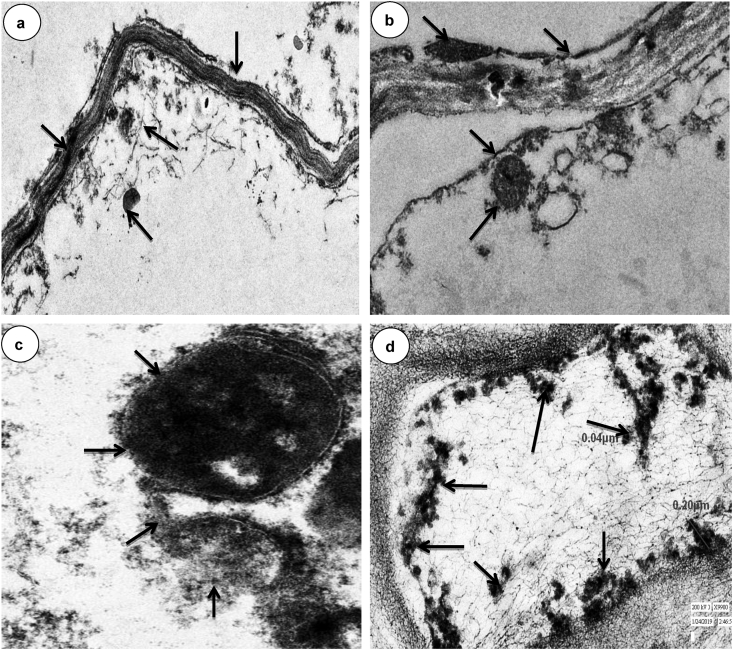
3.6. Histological observation of metal accumulation in root tissue of the plant
The transmission electron microscope (TEM) analysis of root tissue of collected native plants showed the presence of metals granules in their intracellular spaces i.e. cytoplasm, vacuole, and cell membrane as shown in Figure 3. The clear and scattered metal crystal is showed with an arrow in the cytoplasm of the C. album. The clump and glomerated structure with granule are also visible in some regions of the cytoplasm of the C. album (Figure 3a). The metal accumulation by C. album from other complex organo-metallic also has been shown in the form of granule in the vacuole as a depository layer and dense granular layer has been reported in the cell membrane during phytoremediation of the plant of industrial waste previously (Chandra et al., 2017, 2018; Tozser et al., 2019). Similarly, the deposited dense metal granules were noted near the cell membrane in the R. communis root TEM section. The clear cell membrane pores are also clearly visible in the cell of R. communis (Figure 3b). The nucleolus was also visible in section with scattered vacuole formation. This might the adaptive feature of plants for metal accumulation and phytoremediation of metal-containing lignocellulosic waste. A similar pattern of potential plant for a hyperaccumulation of heavy metal granules in their tissue is also observed previously from complex industrial sludge (Chandra et al., 2018, 2017). The R. sceleratus TEM section showed very densely deposited granules in the vacuole and other similar structures were noted at higher magnification (X9900). The anatomical observation of R. dentatus root tissue also showed a prominent depository granule of the continuous layer along with the cell membrane (Figure 3d).
Figure 3.
Showing the metal accumulation in root tissue of (a).Chenopodium album L. (b). Ricinus communis, (c). Ranunculus sceleratus (d). Rumex dentatus growing on disposed sludge of pulp and paper industry. Arrow (→) showed the accumulation of metals in different cell organelles in the cytoplasm.
All the plants are given clear cut evidence for metal deposition in their tissue as a potential metal accumulator for phytoremediation of lignocellulosic waste of paper industry.
4. Conclusions
The findings concluded that discharged waste content mixture of various complex organometallic pollutants above the permissible limit of environmental regulation. However, the presence of various toxic metals in waste along with mutagenic substances needs more study at large scale for environmental assessment. These native plants growing on pulp and paper mill sludge showed strong evidence of potential metal accumulation without showing any adverse effect this indicated hyperaccumulator tendency with >1.0 bioconcentration and translocation factor and they showed in-situ phytoremediation as a biotechnological tool. Thus, these plants can be recommended as tools for eco-restoration of the polluted site by industrial waste for sustainable development.
Declarations
Author contribution statement
Ram Chandra: Analyzed and interpreted the data; Wrote the paper.
Pooja Sharma: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sonam Tripathi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by DBT, vide letter no. BT/PR18896/BCE/8/1372/2016 Dated 28.3.2018 Govt. of India New Delhi.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Instrumentation facilities for Scanning Electron Microscopy (SEM) from USIC, BBAU Lucknow, India is gratefully acknowledged.
References
- Ahmed E.M. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 2015;6(2):105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annual report . U.P.; India: 2016-17. central Pulp Paper Research Institute (CPPRI) Saharanpur. [Google Scholar]
- APHA (American Public Health Association) twenty-second ed. 2012. Standard Method for Examination of Water and Wastewater. Washington, DC. [Google Scholar]
- Baker A.J., Brooks R. Terrestrial higher plants which hyper accumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1(2):81–126. [Google Scholar]
- Bhargava R.N., Chandra R., Rai V. Phytoextraction of trace elements and physiological changes in Indian mustard plants (Brassica nigra L.) grown in post methanated distillery effluent (PMDE) irrigated soil. Bioresour. Technol. 2008;99(17):8316–8324. doi: 10.1016/j.biortech.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Bose S., Bhattacharyya A.K. Heavy metal accumulation in a wheat plant grown in soil amended with industrial sludge. Chemosphere. 2008;70(7):1264–1272. doi: 10.1016/j.chemosphere.2007.07.062. [DOI] [PubMed] [Google Scholar]
- Chandra R., Abhishek A., Sankhwar M. Bacterial decolorization and detoxification of black liquor from rayon grade pulp manufacturing paper industry and detection of their metabolic products. Bioresour. Technol. 2011;102(11):6429–6436. doi: 10.1016/j.biortech.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Chandra R., Kumar V., Tripathi S., Sharma P. Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng. 2018;111:143–156. [Google Scholar]
- Chandra R., Kumar V. Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilized distillery sludge as a prospective tool for in situ phytoremediations of industrial waste. Environ. Sci. Pollut. Control Ser. 2017;24(3):2605–2619. doi: 10.1007/s11356-016-8022-1. [DOI] [PubMed] [Google Scholar]
- Chandra R., Singh R. Decolourisation and detoxification of rayon grade pulp paper mill effluent by mixed bacterial culture isolated from pulp paper mill effluent polluted site. Biochem. Eng. J. 2012;61:49–58. [Google Scholar]
- Chandra R., Raj A., Yadav S., Patel D.K. Reduction of pollutants in pulp paper mill effluent treated by PCP- degrading bacterial strains. Environ. Monit. Assess. 2009;155(1-4):1–11. doi: 10.1007/s10661-008-0413-4. [DOI] [PubMed] [Google Scholar]
- Chandra R., Yadav S., Yadav S. Phytoextraction potential of heavy metals by native wetland plants growing on chlorolignin containing sludge of pulp and paper industry. Ecol. Eng. 2017;98:134–145. [Google Scholar]
- Chandra R., Yadav S., Bhargava R.N., Murthy R.C. Bacterial pretreatment enhances the removal of heavy metals during treatment of post-methanated distillery effluent by Typha angustata L. J. Environ. Manag. 2008;88(4):1016–1024. doi: 10.1016/j.jenvman.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Chandra S., Chauhan L.K.S., Murthy R.C., Saxena P.N., Pande P.N., Gupta S.K. Comparative biomonitoring of leachates from hazardous solid waste of two industries using Allium test. Sci. Total Environ. 2005;347(1-3):46–52. doi: 10.1016/j.scitotenv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- CPCB . Central Pollution Control Board, Government of India; New Delhi: 2007. Transport Fuel Quality for the Year 2005. [Google Scholar]
- Duthie J.F., Parker R.N., Turrill W.B. Vol. 2. Office of the Superintendent of Government Print; 1903. Flora of the upper Gangetic plain, and of the adjacent Siwalik and sub-Himalayan tracts. [Google Scholar]
- PA, U. S. United States Environmental Protection Agency; Washington, DC: 2003. National Primary Drinking Water Standards. Office of Water, US Environmental Protection Agency. [Google Scholar]
- PA, U.S. Jointly developed by the Office of Chemical Safety & Pollution Prevention, the Office of Water and the Office of Research and Development; 2012. Environmental Protection Agency: Endocrine Disruptor Screening Program, Universe of Chemicals for Potential Endocrine Disruptor Screening and Testing; pp. 1–176. [Google Scholar]
- Fu J.T., Yu D.M., Chen X., Su Y., Li C.H., Wei Y.P. Recent research progress in geochemical properties and restoration of heavy metals in contaminated soil by phytoremediation. J. Mt. Sci. 2019;16(9):2079–2095. [Google Scholar]
- Gauthier F., Archibald F. The ecology of “fecal indicator” bacteria commonly found in pulp and paper mill water systems. Water Res. 2001;35(9):2207–2218. doi: 10.1016/s0043-1354(00)00506-6. [DOI] [PubMed] [Google Scholar]
- Gouda S., Kerry R.G., Das G., Paramithiotis S., Shin H.S., Patra J.K. Revitalization of plant growth-promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018;206:131–140. doi: 10.1016/j.micres.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Gupta A.K., Sinha S. Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour. Technol. 2008;98:1788–1794. doi: 10.1016/j.biortech.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Gustavo C., Ricardo B., Mauricio D., Meyling R., Paulina B., Kelly R. Munkittrick, Estrogencity, and intersex in juvenile rainbow trout (Oncorhynchusmy kiss) exposed to Pine/Eucalyptus pulp and paper production effluent in Chile. Aquat. Toxicol. 2015;164:126–134. doi: 10.1016/j.aquatox.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Harries J.E., Janbakhsh A., Jobling S., Matthiessen P., Sumpter J.P., Tyler C.R. Estrogenic potency of effluent from two sewage treatment works in the United Kingdom. Environ. Toxicol. Chem.: Int. J. 1999;18(5):932–937. [Google Scholar]
- Horwitz W. AOAC; 2002. AOAC: Requirements for Single Laboratory Validation of Chemical Methods. Draft 2002-11-07.http://www.aoac.org/AgMaterials/additives/aoac.slv.pdf [Google Scholar]
- Kabata-Pendias A., Pendias H. 2011. Trace elements in soils and plants. V. [Google Scholar]
- Kang X., Song J., Yuan H., Duan L., Li X., Li N.…Qu B. Speciation of heavy metals in different grain sizes of Jiaozhou Bay sediments: bioavailability, ecological risk assessment, and source analysis on a centennial timescale. Ecotoxicol. Environ. Saf. 2017;143:296–306. doi: 10.1016/j.ecoenv.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Kato S., Haruta S., Cui Z.J., Ishii M., Igarashi Y. Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol. Ecol. 2004;51(1):133–142. doi: 10.1016/j.femsec.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Khan M.U., Ahmed M., Nazim K., Shaukat S.S., Khan N. Effect of industrial waste on soil microbial community and seed germination of different plant species. Int. J. Biol. Biotechnol. 2010 [Google Scholar]
- Kumar N., Bauddh K., Kumar S., Dwivedi N., Singh D.P., Barman S.C. Accumulation of metals in weed species grown on the soil contaminated with industrial waste and their phytoremediation potential. Ecol. Eng. 2013;61:491–495. [Google Scholar]
- Kumar V., Singh J., Saini A., Kumar P. Phytoremediation of copper, iron, and mercury from aqueous solution by water lettuce (Pistia stratiotes L.) Environ. Sustain. 2019;2(1):55–65. [Google Scholar]
- Lacorte S., Latorre A., Barcelo D., Rigol A., Malmqvist A.T. Welander: organic compounds in paper-mill process waters and effluents. Trac. Trends Anal. Chem. 2003;22(10):725–737. [Google Scholar]
- Lasat M.M. Phytoextraction of toxic metals: a review of biological mechanisms. J. Environ. Qual. 2002;31(1):109–120. [PubMed] [Google Scholar]
- Lee A.H., Nikraz H. BOD: COD ratio as an indicator of pollutants leaching from landfills. J. Clean Energy Technol. 2014;2:263–266. [Google Scholar]
- Mahmood N.M.Q. Phytoremediation of arsenic (As) and mercury (Hg) contaminated soil. World Appl. Sci. J. 2010;8(1):113–118. [Google Scholar]
- Mani D., Kumar C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2014;11(3):843–872. [Google Scholar]
- Meagher R.B. Phytoremediation of toxic elemental and organic pollutants. Curr. Opin. Plant Biol. 2000;3(2):153–162. doi: 10.1016/s1369-5266(99)00054-0. [DOI] [PubMed] [Google Scholar]
- Mishra S., Mohanty M., Pradhan C., Patra H.K., Das R., Sahoo S. Physico-chemical assessment of paper mill effluent and its heavy metal remediation using aquatic macrophytes—a case study at JK Paper mill, Rayagada, India. Environ. Monit. Assess. 2013;185(5):4347–4359. doi: 10.1007/s10661-012-2873-9. [DOI] [PubMed] [Google Scholar]
- Ojuederie O.B., Babalola O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int. J. Environ. Res. Publ. Health. 2017;14(12):1504. doi: 10.3390/ijerph14121504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl I.A., Benson H.K. The determination of lignin in sulphide pulping liquor. Paper Trade J. 1940;111:35–36. [Google Scholar]
- Satyawali Y., Balakrishnan M. Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J. Environ. Manag. 2008;86(3):481–497. doi: 10.1016/j.jenvman.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Segura A., Rodríguez-Conde S., Ramos C., Ramos J.L. Bacterial responses and interactions with plants during rhizoremediation. Microbial Biotechnol. 2009;2(4):452–464. doi: 10.1111/j.1751-7915.2009.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Chandra R. Pollutants released from the pulp paper industry: aquatic toxicity and their health hazards. Aquat. Toxicol. 2019;88:214–219. doi: 10.1016/j.aquatox.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Singh R., Singh S., Parihar P., Singh V.P., Prasad S.M. Arsenic contamination, consequences, and remediation techniques: a review. Ecotoxicol. Environ. Saf. 2015;112:247–270. doi: 10.1016/j.ecoenv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Sivakumar D., Murugan N., Rajeshwaran R., Shobana T., Soundarya C., Vanitha V.S. Role of rice husk silica powder for removing Cr (VI) in a tannery industry wastewater. Int. J. ChemTech Res. 2014;6(9):4373–4378. [Google Scholar]
- Slavin Y.N., Asnis J., Hafeli U.O., Bach H. Metal nanoparticles: understanding the mechanisms behind the antibacterial activity. J. Nanobiotechnol. 2017;15:65–85. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz E., Greger M. Accumulation properties of As, Cd, Cu, Pb, and Zn by four wetland plant species growing on submerged mine tailings. Environ. Exp. Bot. 2002;47(3):271–280. May 1. [Google Scholar]
- Streets D.G., Horowitz H.M., Lu Z., Levin L., Thackray C.P., Sunderland E.M. Vol. 201. Atmospheric environment; 2019. pp. 417–427. (Global and Regional Trends in Mercury Emissions and Concentrations, 2010–2015). [Google Scholar]
- Sun L., Liao X., Yan X., Zhu G., Ma D. Evaluation of heavy metal and polycyclic aromatic hydrocarbons accumulation in plants from typical industrial sites: potential candidate in phytoremediation for co-contamination. Environ. Sci. Pollut. Control Ser. 2014;21(21):12494–12504. doi: 10.1007/s11356-014-3171-6. [DOI] [PubMed] [Google Scholar]
- Sun X., Sheng Z., Liu Y. Effects of silver nanoparticles on microbial community structure in activated sludge. Sci. Total Environ. 2013;443:828–835. doi: 10.1016/j.scitotenv.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Sushil S., Batra V.S. Analysis of fly ash heavy metal content and disposal in three thermal power plants in India. Fuel. 2006;85(17-18):2676–2679. [Google Scholar]
- Thomas K.V., Hurst M.R., Matthiessen P., McHugh M., Smith A., Waldock M.J. An assessment of in vitro androgenic activity and the identification of environmental androgens in United Kingdom estuaries. Environ. Toxicol. Chem.: Int. J. 2002;21(7):1456–1461. [PubMed] [Google Scholar]
- Tozser D., Tothmeresz B., Harangi S., Baranyai E., Lakatos G., Fülöp Z., Simon E. Remediation potential of early successional pioneer species Chenopodium album and Tripleurospermum inodorum. Nat. Conserv. 2019;36:47. [Google Scholar]
- Wu G., Guan Y.X. Zhan: effect of salinity on the activity, settling and microbial community of activated sludge in sequencing batch reactors treating synthetic saline wastewater. Water Sci. Technol. 2008;351:357. doi: 10.2166/wst.2008.675. [DOI] [PubMed] [Google Scholar]
- Yadav S., Chandra R. Detection and assessment of the Phytotoxicity of residual organic pollutants in sediment contaminated with pulp and paper mill effluent. Environ. Monit. Assess. 2018;190:581–591. doi: 10.1007/s10661-018-6947-1. [DOI] [PubMed] [Google Scholar]
- Yang X., Feng Y., He Z., Stoffel P.J. Molecular mechanisms of heavy metal hyperaccumulation, and phytoremediation. J. Trace Elem. Med. Biol. 2005;18:339–353. doi: 10.1016/j.jtemb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Yoon J., Cao X., Zhou Q., Ma L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006;368(2-3):456–464. doi: 10.1016/j.scitotenv.2006.01.016. Sep 15. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wen J., Hu Y., Fang Y., Zhang H., Xing L., Zeng G. Humic substances from green waste compost: an effective washing agent for heavy metal (Cd, Ni) removal from contaminated sediments. J. Hazard Mater. 2019;366:210–218. doi: 10.1016/j.jhazmat.2018.11.103. [DOI] [PubMed] [Google Scholar]
- Zhang T., Lin W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014;43(16):5982–5993. doi: 10.1039/c4cs00103f. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Zhang J., Fu J., Shi J., Jiang G. Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta. 2008;606(2):135–150. doi: 10.1016/j.aca.2007.11.018. [DOI] [PubMed] [Google Scholar]