Abstract
Background
PRR (Pattern Recognition Receptor) agonists have been widely tested as potent vaccine adjuvants. TLR7 (Toll-Like Receptor 7) and NOD2 (nucleotide-binding oligomerization domain 2) are key innate receptors widely expressed at mucosal levels.
Methods
Here, we evaluated the immunostimulatory properties of a novel hybrid chemical compound designed to stimulate both TLR7 and NOD2 receptors.
Finding
The combined TLR7/NOD2 agonist showed increase efficacy than TLR7L or NOD2L agonists alone or combined in different in vitro models. Dual TLR7/NOD2 agonist efficiently stimulates TLR7 and NOD2, and promotes the maturation and reprogramming of human dendritic cells, as well as the secretion of pro-inflammatory or adaptive cytokines. This molecule also strongly induces autophagy in human cells which is a major intracellular degradation system that delivers cytoplasmic constituents to lysosomes in both MHC class I and II-restricted antigen presentation. In vivo, TLR7/NOD2L agonist is a potent adjuvant after intranasal administration with NP-p24 HIV vaccine, inducing high-quality humoral and adaptive responses both in systemic and mucosal compartments. Use of TLR7/NOD2L adjuvant improves very significantly the protection of mice against an intranasal challenge with a vaccinia virus expressing the p24.
Interpretation
Dual TLR7/NOD2L agonist is a very potent and versatile vaccine adjuvant and promote very efficiently both systemic and mucosal immunity.
Funding
This work was supported by Sidaction.
Keywords: Adjuvants, TLR7, NOD2, Vaccine, Chimeric
Research in context.
Evidence before this study
Immune adjuvants are components that stimulate, potentiate, or modulate the immune response to an antigen. They are crucial elements for both prophylactic and therapeutic vaccines. Within the framework of vaccine adjuvant design, targeting endosomal and intracellular receptors to enhance and guide the immune response is a highly promising approach. A major impediment to the development of vaccines is their inability to effectively induce mucosal immunity. TLR7 and NOD2 receptors are constitutively expressed in the intestinal and nasal mucosa. No study has so far demonstrated the vaccinal interest of being able to specifically target these two receptors in order to induce a mucosal response.
Added value of this study
Here we have chosen to develop a new class of chemical adjuvant capable of simultaneously stimulating both TLR7 and NOD2 receptors in order to evaluate their adjuvant properties at the mucosal levels. This new type of chimeric adjuvant could make it possible to strongly induce a protective mucosal response in many infectious models, but could also have properties of stimulating or regulating the immune response in different context.
Implications of all the available evidence
Our data demonstrates that the chimeric TLR7/NOD2 adjuvant is highly potent to stimulate DC maturation both in vitro and in vivo. The use of this molecule with a model HIV vaccine, allow the induction of a strong and specific adaptive immunity allowing to protect against a lethal challenge. The new knowledge presented here has immediate value for the design of new vaccine candidates for mucosal pathogen agents.
Alt-text: Unlabelled box
1. Introduction
Most modern vaccines are composed of recombinant proteins. Unlike entire pathogens, these molecules are often considered by the organism as negligible inconvenience, and no immune response is induced against the antigen [1]. To stimulate the immune system, pathogen-associated danger signals are required [2]. PAMPs (Pathogen Associated Molecular Patterns) are components of pathogens that stimulate immune responses through the activation of PRRs. They act as vaccine adjuvants, considered as “immunologist's dirty little secret” [3], to stimulate immunity against antigens. In 2017, only one PRR agonist (MPLA) was approved for use as vaccine adjuvant in humans. This molecule targets TLR4 and stimulates immunity against influenza antigens [4]. Other PRR agonists are currently studied as vaccine adjuvants in clinical trials, such as TLR3 [5], TLR7 [6] or TLR9 [7] ligands. In this study, we evaluated the effect of TLR7 and NOD2 agonists. TLR7 recognizes viral single-stranded RNA. Its stimulation induces the secretion of pro-inflammatory cytokines and type I IFN. The stimulation of this receptor is known to induce CD4+ and CD8+ T cell responses in vivo [8,9]. NOD2 is a cytosolic receptor recognizing muramyl dipeptide (MDP), a minimal bioactive peptidoglycan motif common to Gram-negative and Gram-positive bacteria [10]. NOD2 agonists are known to be efficient mucosal adjuvants [11,12]. Moreover, a recent sudy showed that NOD2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera-toxin [13]. Many evidences show the interest of the crosstalk between PRR agonists for vaccine adjuvantation [14]. Even if this interaction has been studied for numerous PRRs [15], [16], [17], the synergy between TLR7 and NOD2 is poorly described. The synergistic induction of the production of IL-1β and IL-23 in monocyte-derived DCs (moDCs) has been reported after stimulation with NOD2 and TLR7/8 agonists [18]. Recently, the cooperation between TLR7 and NOD2 signaling was demonstrated in response to Candida parapsilosis infection [19]. As part of the evaluation of the interest of the crosstalk between PRR agonists, the combination of multiple agonists in a single molecule showed promising effects in multiple studies [20], [21], [22]. Most pathogens enter the body through mucosa. Therefore, effective vaccines inducing a protection at these portal of entry of pathogens are needed [23]. Induction of mucosal immunity, including secretion of secretory IgA and IgG [24] and cytotoxic immune response, at the site of pathogen entry may be critical for protection against multiple pathogens. This kind of immune responses can be induced by nanoparticulate vectors combined with immune adjuvants [25].
In this study, we evaluated the immunostimulatory properties of a molecule combining a TLR7 and NOD2 agonist for mucosal vaccination. The ability of the molecule to stimulate both TLR7 and NOD2 as well as the induction of moDC maturation and production of multiple cytokines were assessed in vitro. The induction of autophagy by this molecule was evaluated in vitro in a reporter cell model. Systemic and mucosal immune responses after intranasal immunization were also assessed in vivo, using a mouse model of vaccination with nanoparticulate HIV-1 p24. The ability of the molecule to stimulate humoral and cellular immune responses after intranasal administration was studied, as well as the protection induced after a viral challenge with a gag encoding vaccinia virus.
2. Materials and methods
2.1. TLR ligands and HIV-1 p24
The TLR7/NOD2 agonist is a hybrid molecule incorporating the TLR7 agonist CL239 covalently linked to the NOD2 agonist MDP designed to stimulate both TLR7 and NOD2 (Fig. 1C; synthesis described in supplementary informations). The whole synthesis of CL325 is provided in the supplementary material. The ligands specific activities were determined using the HEK-Blue-hTLR7, HEKBlue-hNOD2 reporter cell lines (InvivoGen) to monitor the activation of the NF-kB and AP-1 pathways using a secreted embryonic alkaline phosphatase (SEAP). Stimulation with ligands activates NF-kB and AP-1, which induce the production of SEAP. The level of SEAP can be easily quantified with a detection medium that turns purple/blue in the presence of alkaline phosphatase (HEK-Blue Detection; InvivoGen). HIV-1 p24 antigen was produced and purified by PX'Therapeutics. The purity of p24 was higher than 97% with endotoxin content lower than 5 EU/mg of p24 protein. All other reagents were of analytical grade and were procured from commercial sources.
Fig. 1.
Dual TLR7/NOD2L agonist stimulates both TLR7 and NOD2 and induces strong autophagy activity. (A) In vitro assessment of ligand-specific activation via the TLR7 and NOD2 pathways in HEK-Blue hTLR7 and hNOD2 cells. (B) Evaluation of the induction of autophagy in reporter cells, derived from HeLa cells, expressing a fluorescent GFP-LC3 fusion protein, after 8 hrs stimulation with 10 µM of ligands or 50 µM of Tamoxifen. Data are representative of 3 independent experiments. (C) Chemical structures and data sizing of the TLR7/NOD2L agonist. CL325 was solubilized as before at 4.2 mg/ml in acetone/buffer pH 9 and then diluted to PBS at 10 µg/ml. Nanosizer size measurement was possible. The CL325 is organized into particles with an average particle size of 300 nm +/- 70 nm. Even after sonication, the particle size remains the same, indicating a stable size.
2.2. Analysis of the induction of autophagy
Autophagy was studied by immunofluorescence analysis of LC3 in HeLa-Difluo™ hLC3 reporter cells (InvivoGen). Cells were seeded in 24-wells plates (50,000 cells per well) and stimulated with 10 µM of TLR7, NOD2, TLR7/NOD2 ligands or 50 µM Tamoxifen as a positive control (InvivoGen). After stimulation (8 h), cells were observed using fluorescent microscopy, and quantification of cells containing LC3-positive autophagosomes was performed.
2.3. Preparation of poly (lactic acid) nanoparticles
Nanoparticles (NPs) were prepared by nanoprecipitation as described previously [25,26]. Briefly, 110 mg of polymer were dissolved in 5.5 mL of acetone and gradually added to 3.5 mL of aqueous solution (57% v/v of ethanol in water) under slow stirring. Organic solvents were then removed under reduced pressure at 30 °C. Particle size, polydispersity and surface charge were determined at 25 °C using a Zetasizer Nano ZSP (Malvern). The p24 protein was diluted in PBS at 1 mg/mL. PLA NPs were diluted at a concentration of 25 mg/mL in PBS and one volume of protein solution was added to one volume of NPs. The solution was incubated for 2 h at room temperature under moderate end-overhead stirring. Unbound p24 protein was collected in the supernatant by centrifugation at 10,000 x g for 10 min and quantified by Bradford protein assay (Bio-Rad). The absorbance of the samples was measured at 595 nm using a microplate reader. Nanoparticle size was determined using a Zetasizer Nano ZS (Malvern Instruments).
2.4. moDC maturation assay and transcriptomic profile
Monocytes were purified from peripheral human blood, obtained from EFS (Etablissement Français du Sang) by density gradient centrifugations using Ficoll-Paque™ plus and Percoll (GE Healthcare) as previously described [12]. Remaining erythrocytes, NK, B- and T- cells were then depleted by anti-glycophorin A, anti-CD56, anti-CD19 and anti-CD3 antibodies (Beckman Coulter) respectively, using Dynabeads® Pan Goat antimouse IgG (Invitrogen). The purified monocytes (0.5 × 106 cell/ml) were cultured in RPMI medium supplemented with 10% heat-inactivated fetal calf serum (FCS), gentamycin (50 U/ml), 100 U/ml penicillin and 100 μg/ml streptomycin in the presence of 62.5 ng/ml of human interleukin-4 (IL-4) (R&D systems) and 75 ng/ml of human granulocyte macrophage colony stimulating factor (GM-CSF) to differentiate into moDCs. This process led to the differentiation of more than 96% of the cells. After 6 days, 1 × 106 moDCs per condition were incubated for 24 h in the absence or presence of various ligands at individual concentrations of 10 μM. Cells were then stained with α-CD1a-FITC together with α-CD40-PE, α-CD80-PE, α-CD86-PE, or α-MHC-II-PE (BD Biosciences). Data were acquired using a FACS Canto I flow cytometer (BD Biosciences) and analyzed with FlowJo software version 9.3.7 (Tree Star Inc.). RNA was extracted from moDCs using an RNeasy Mini Kit (Qiagen), and the expression of 84 genes was quantified using an RT2 Profiler PCR Arrays Human Toll-Like Receptor Signaling Pathway Kit (Qiagen). The experiments were repeated with 4 donors.
2.5. Quantitation of cytokines by Luminex
Cytokines and chemokines present in moDC cultures supernatants were measured using a Luminex T200 instrument in combination with human Bio-Plex Immunoassay Kits (Bio-Rad). All concentrations were determined as the mean of two replicates.
2.6. In vitro priming of effector CD8+T cells
The in vitro priming assay was performed as described previously [27]. Briefly, PBMCs (Peripheral Blood Mononuclear Cells) from healthy HLA-A2+ individuals were supplemented with FLT3L (FMS-like tyrosine kinase 3 ligand; 50 ng/ml; R&D Systems) and cultured at 5 × 106 cells/well in a 24-well plate. After 24 h, maturation of DCs was induced with different ligands (at individual concentrations of 10 μM or 10 µM of each for the combination) or a standard cocktail of inflammatory cytokines comprising TNF (1000 U/mL), IL-1β (10 ng/mL), IL-7 (0.5 ng/mL) and prostaglandin E2 (PGE2; 1 μM) (R&D Systems) or the ssRNA40 TLR8L (0.5 μg/mL; Invivogen), added together with the ELA-20 peptide (YTAAEELAGIGILTVILGVL; Melan-A/MART-1 residues 21–40A27L) at a final concentration of 10 µM. ELA-specific CD8+ T cell frequency and phenotype were determined on day 10. Data were acquired using an LSR Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star). The experiments were repeated with 5 donors.
2.7. Mice and immunization protocol
Female CB6F1 mice (Charles River) were hosted at the PBES (ENS de Lyon, France). The protocol was approved by the Ethics Committee of the Comité Rhône-Alpes d'Ethique pour l'Experimentation Animale (France). The vaccine formulations were prepared by mixing NP with each molecular adjuvant. Seven-week-old mice were anesthetized with isoflurane and immunized intranasally with 20 µL (10 µL in each nostril) of the formulation. Groups of 5 mice were immunized with 10 µg HIV-1 Gag p24 (PX Therapeutics) adsorbed onto PLA NPs (NP-p24), 10 µg NP-p24 plus TLR7L (20 nmol, 1 µg) or NOD2L (20 nmol; 1 µg) or TLR7L (20 nmol; 1 µg) + NOD2L (20 nmol; 1 µg) or TLR7/NOD2L (20 nmol; 1 µg) (InvivoGen). A combination of 10 µg p24 + 2.5 µg heat-labile enterotoxin (HLT; Sigma-Aldrich) were used as a control. Three doses of each formulation were administered at weekly intervals, followed by an equivalent booster dose after a further 3 weeks.
2.8. p24-specific IFNγ and IL-17 ELISPOT assays
Detection of p24-specific CD4+and CD8+ T lymphocytes were measured by ELISPOT in the spleen and the lungs of vaccinated mice. Mice were killed by means of cervical dislocation after achievement of anesthesia at day 42. ELISpot assays were performed with cells isolated from the spleen and lungs. Cells (3 × 105 cells per well) were directly plated and cultured for 24 h with or without stimulation with recombinant p24 antigen (1 µg/ml) or p24-specific MHC1 peptide (10 µM) as previously decribed [12,21,26,28] . The plates were assayed for IFNg and IL-17 according to the procedure provided by the manufacturer (Mabtech, Cincinnati, Ohio). Spot-forming cells were counted on an ELISpot reader (BIOREADER 3000; BIO-SYS). The results are expressed as the number of spots per 1 × 106 cells after subtracting the number of spots in unstimulated wells.
2.9. Blood, feces and vaginal secretion recovery
Biological fluids were recovered before immunization (D0), at D28 and D49 to examine their antibody content. Serum samples were obtained from whole blood recovered by retro-orbital vein puncture. Vaginal secretions were collected by vaginal lavages with 50 µL of PBS (two times) placed in the vagina of the animal using an adapted pipette (Pipette M100, Gilson), and tips (CP100, Gilson). Ten microliters of 10x Halt™ Protease Inhibitor Cocktail (Thermo Scientific) were then added to the vaginal lavages to protect antibodies from degradation. Fresh feces were also collected from each animal and diluted at 100 mg/mL with 1x Halt™ Protease Inhibitor Cocktail. Feces were strongly mixed to dislocate and then centrifuged at 16,000 x g. Supernatants were collected and stored at −20 °C until further use. Sera were then collected by centrifugation at 16,000 x g and stored at −20 °C until use.
2.10. Viral challenge
Viral challenge was performed with the vDK1 recombinant vaccinia virus expressing HIV-1 p24 gag (this reagent was obtained through the National Institutes of Health AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: vDK1 was from Dr Daniel R. Kuritzkes) as previously described [28,29]. A dose of 4 × 107 plaque- forming units of the virus in 40 µL was administered through the intranasal route 2 weeks after the last immunization. Mice were then weighed daily for up to 7 days.
2.11. Statistical analysis
All statistical analyses were performed with the InStat version 5.02 software from GraphPad Software. A nonparametric t-test or one-way ANOVA followed by Bonferroni post hoc test was used where appropriate. The p values lower than 0.05 (marked by *), lower than 0.01 (**), or lower than 0.005 (***) were considered as significant. Statistically significant differences between groups are emphasized by bars connecting the relevant columns. All data generated or analyzed during this study are included in this published article (and its supplementary information files).
3. Results
3.1. The hybrid molecule (TLR7/NOD2L) activates both TLR7 and NOD2 (Fig. 1C)
The specific activity of TLR7L, NOD2L or the chimeric TLR7/NOD2L was evaluated in vitro using cell lines expressing one unique PRR (TLR7 or NOD2). We observe that the TLR7/NOD2 agonist stimulates significantly both TLR7 and NOD2 in a dose-dependent manner (Fig. 1A). The TLR7 part only activates HEK-Blue hTRL7 cells while the NOD part only stimulates HEK-Blue hNOD2 cells. Moreover, TLR7/NOD2L is a better activator of TLR7 than TLR7L alone. To evaluate if the structure of the chimeric molecule could be implicated in it better activity, we proceed to a size analyzing of the different ligands with a nanosizer. For MDP and CL239, no significant size measurements were possible. These results were expected from compounds that are highly soluble in water. The CL325 is organized into particles with an average particle size of 300 nm +/- 70 nm (Fig. 1C). Even after sonication, the particle size remains the same, indicating a stable size. Due to the chemical structure, a liposomal organization is possible. This confirms the particulate suspicion of this compound which would favor multi-affinity presentation and uptake by Antigen Presenting Cells (APCs).
3.2. TLR7/NOD2 chimeric molecule strongly induces autophagy in human transfected cells
The mechanism of autophagy plays an important role in innate immunity and the fight against pathogen. To analyze its induction, autophagy reporter cells, derived from HeLa cells, expressing a fluorescent GFP-LC3 fusion protein were used. These cells enable monitoring of autophagic flux by fluorescence microscopy. Tamoxifen was used as a positive control. Eight hours after stimulation with the different molecules, cells were rinsed and observed by fluorescence microscopy. The number of cells containing at least one GFP-LC3+ autophagosome was counted (Fig. 1B). When the cells are treated with the positive control tamoxifen, 89% of the cells contain at least one autophagosome, whereas only 3% of the cells contain autophagosomes in the untreated population of cells. While TLR7L and NOD2L did not stimulate autophagy, 58% of the cells contained autophagosomes after stimulation with the TLR7/NOD2L. This experiment shows the ability of the TLR7/NOD2 agonist to induce autophagy in human cells.
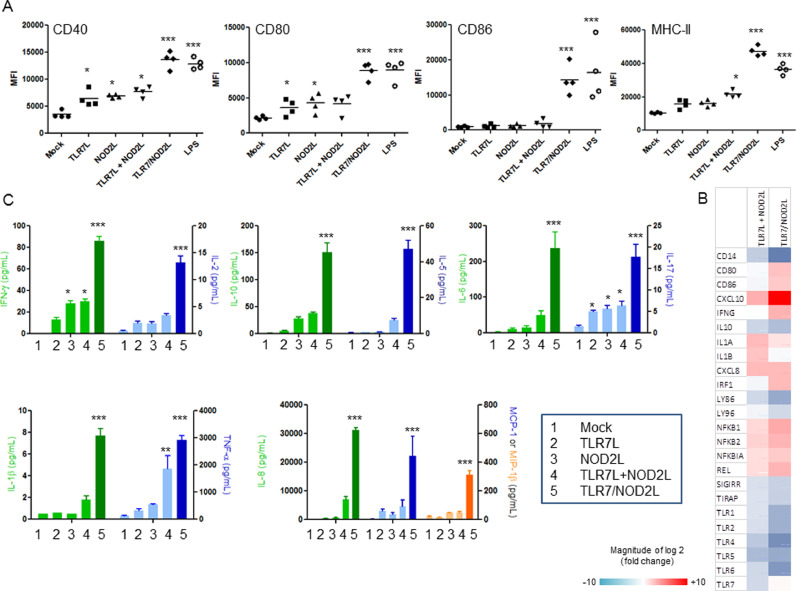
TLR7/NOD2L chimeric molecule stimulates human DCs in a synergistic or additive way and primes cytotoxic and central memory CD8+ T lymphocytes. Dendritic cells are central effectors of immunity, acting as messengers between the innate and adaptive immune system. They express a repertoire of PRR, allowing the recognition of a large range of pathogens. Their stimulation induces their maturation, the presentation of antigen fragments and the production of cytokines that direct the immune response. To assess the adjuvant potential of the TLR7/NOD2L, we measured its ability to induce DC maturation. Human monocyte-derived DCs were stimulated with equimolar concentrations of TLR7L, NOD2L, TLR7L+NOD2L or TLR7/NOD2L for 24 h and the expression of markers of maturation was analyzed by flow cytometry (Fig. 2A). The different ligands tested didn't show any sign of toxicity in vitro (data not shown). While 10 µM of TLR7L or NOD2L alone did not induce any significant overexpression of maturation markers, the combination of both ligands at high concentration (10 µM + 10 µM) and more significantly the use of the hybrid TLR7/NOD2 agonist at both 1 and 10 µM induced significant expression of CD40, CD80, CD86 and MHC-II (p < 0.01). To go further in the study of moDC maturation, we analyzed the expression of 84 genes associated with TLR signaling pathway after stimulation with the different agonist (Fig. 2B). These data showed that the co-administration of TLR7 and NOD2 ligands induces a strong modification in the expression pattern of multiple genes in moDCs. The TLR7/NOD2L impacts the same genes than the combination but with a more pronounced effect. It increases the expression of CD80 and CD86, confirming the observations made at protein level (Fig. 2A), but also IFN-γ, pro-inflammatory cytokines and the NF-kB genes. It also induces a large overexpression of CXCL10 mRNA (. Interestingly, those molecules seem to decrease the expression of TLR genes. The maturation of DCs is associated with the secretion of cytokines that stimulate inflammation and adaptive immunity (Fig. 2C). While the co-administration of TLR7 and NOD2 ligands induced the overexpression of multiple cytokines, the hybrid molecule enhanced synergistically this effect. In fact, TLR7/NOD2L induced significantly the secretion of Th1 (IFN-γ and IL-2, p<0.05), Th2 (IL-10 and IL-5, p<0.05), Th17 (IL-17, p<0.05) and pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α, p<0.05). It also induces chemokines (IL-8, MCP-1 and MIP-1β, p < 0.05).
Fig. 2.
Dual TLR7/NOD2L agonist stimulates human DC maturation, reprogram TLR expression and cytokine secretion in vitro. (A) Expression (MFI Median Fluorescence Intensity) of the co-stimulatory molecules CD40, CD80, CD86 and MHC-II on the surface of CD1a+ CD14– moDCs generated in vitro was determined by flow cytometry 24 hr after stimulation with the indicated ligands at a concentration of 1 (low) or 10 µM (high). (B) Transcriptomic profile of TLR pathways genes after 24 hrs stimulation with TLR7 plus NOD2 or TLR7/NOD2 agonist. Results are indicated on fold change within the unstimulated control (C) Expression profile of multiple cytokines after stimulation of moDCs with the different agonists at 10 µM during 24 h using luminex assay. The data represent two independent experiments done in duplicates (n = 4). Statistical significance between two groups was determined using an uncorrected Student's t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In an in vitro well described CD8+ T-cell priming assay, TLR7/NOD2L primed significantly a substantial population of ELA-specific CD8+ T-cells (Fig. 3). Around 80% of the primed cells expressed CCR7, while only 10% expressed CD45RA (Fig. 3), the lack of expression of CD45RA (Fig. 3) being a hallmark of central memory cells [30,31]. Moreover, the dual agonist was also able to prime significantly cytotoxic perforin+ and granzyme B+ CD8+ T cells (Fig. 3). The importance of central memory cells subsets but also the activation of CTLs for the effectiveness of vaccines has been shown in several studies, confirming the strong potential of TLR7/NOD2L as a vaccine adjuvant.
Fig. 3.
Dual TLR7/NOD2L agonist prime efficiently human CD8+T cells with cytolytic potential and central memory T cells phenotype in vitro. Primes PBMCs from healthy HLA-A2+ individuals were stimulated with FLT3L and primed with the Melan-A/MART-1 epitope ELA for 10 days in the presence or absence of the indicated ligands at a concentration of 2.5 µM. Tetramer-binding CD3+ CD8+ cells and intracellular expression of perforin, granzyme B CD45RA and CCR7 were quantified by flow cytometry. The data represent four independent experiments. Statistical significance between two groups was determined using an uncorrected Student's t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Chimeric TLR7/NOD2L induces in vivo protective mucosal immune responses against the HIV-1 p24 antigen. To assess the ability of the TLR7/NOD2 agonist to stimulate an immune response in vivo, CB6F1 mice (27) were intranasally immunized with HIV p24 coated on PLA NPs co-administrated with TLR7, NOD2, TLR7+NOD2L or TLR7/NOD2 agonists as adjuvants. HLT mucosal adjuvant was used as positive control. Any safety concerns using the single ligand, the combination of ligands or the chimeric molecule was observed (no local reaction, no weight drop, no diarrhea). We did not observe any sign of pulmonary toxicity after intranasal administration looking on the lung of vaccinated mice (data not shown).
To assess systemic and mucosal immunity, p24-specific IgG and IgA were measured in serum, feces and vaginal washes (Fig. 4). Use of TLR7/NOD2L induced higher IgG titers than the unadjuvanted NP-p24 (p < 0.01), and all other formulations (Fig. 4A). Endpoint specific IgG1 and IgG2A were also measured in the serum to evaluate in vivo the orientation of the immune response (Fig. 4A). TLR7L induced lower IgG1 and higher IgG2a concentrations than the NP-p24 alone, leading to a lower IgG1/IgG2a ratio (p< 0.05). The predominant IgG subclass in the group immunized with the TLR7/NOD2L was IgG2a (p<0.05), showing its potential to induce a strong Th1 response. Use of TLR7/NOD2L adjuvant induces significantly a systemic and mucosal IgA response (Fig. 4B). After intranasal immunization, NP-p24 alone was not able to induce detectable IgG or IgA responses (Fig. 4A-B). The administration of TLR7L, NOD2L or co-administration of both did not induce any increase in IgG or IgA at mucosal sites. However, the administration of the TLR7/NOD2L induced the production of p24-specific IgG/IgA response in feces or vaginal lavages. The effect of TLR7/NOD2L is significantly higher to promote p24-specific antibody responses than the well-known HLT mucosal adjuvant.
Fig. 4.
Use of dual TLR7/NOD2 adjuvant with NP-p24 HIV vaccine enhances systemic and mucosal immune humoral responses in CB6F1 mice after intranasal administration. Six groups of mice (n = 5 per group) were immunized intranasally with NP-p24 in the presence or absence of the indicated ligands on days 0, 7, 14 and 28. (A) Serum and mucosal (fecal and vaginal) p24-specific IgG titers were measured by ELISA on day 35 (see supplemental methods). Serum IgG1 and IgG2a isotypes were also measured on day 35. (B) Serum, fecal, and vaginal p24-specific IgA were measured by ELISA after intranasal immunization (see supplemental methods). Statistical significance between two groups was determined using an uncorrected Student's t-test: ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
TLR7/NOD2L ability to promote a p24-specific Th1 (Fig. 5A) and Th17 (Fig. 5B) systemic and mucosal responses was also confirmed by ELISPOT. Stimulation of splenocytes or lung CD4+ and CD8+ lymphocytes with p24 Ag and/or p24-specific MHC-I peptide induced a high number of IFN-γ or IL-17-producing cells (p < 0.01). Stimulation with p24 Ag or with a MHC-I peptide induced a 10-times higher number of IFN-γ-secreting cells (p < 0.0001) when the mice were immunized with NP-p24+TLR7/NOD2L (Fig. 5A). Similarly, a higher number of IL-17 secreting cells were observed (Fig. 5B, p < 0.01). This observation confirmed the ability of TLR7/NOD2L to stimulate both CD4+ and CD8+T cells at both systemic and mucosal levels.
Fig. 5.
Use of dual TLR7/NOD2 adjuvant with NP-p24 HIV vaccine enhances systemic and mucosal T cell response in CB6F1 mice after intranasal administration and protect mice for a lethal intranasal challenge. IFN-γ and (A) IL-17 (B) secreting CD4+ and CD8+T cells were analyzed by ELISPOT in the spleen or in the lung of vaccinated mice at day 42. IFN-γ and IL-17 secretion were measured after stimulation with p24 recombinant antigen (CD4 response) or p24-specific MHC-I peptide. Results are expressed as number of spots per 1.106 splenocytes. (see supplemental methods). (C) Disease course of the mice assessed by weight loss after challenge with vDK1 recombinant vaccinia virus expressing HIV-1 p24 gag (two independent experiments). Vaccinia titers were measured in the lungs at the time of sacrifice as previously described [28,29]. A nonparametric t-test or one-way ANOVA followed by Bonferroni post hoc test was used where appropriate. P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To measure the quality of the induced-immune response, protection against an intranasal challenge with recombinant vaccinia virus encoding the p24 model antigen was analyzed in all groups of mice (Fig. 5C). All mice, except those immunized intranasally with NP-p24+TLR7/NOD2L and to a less extent with p24+HLT, experienced significant weight loss after viral challenge (p<0.0001 for the non-immunized mice and for the mice immunized with NP-p24, p<0.05 for those immunized with HLT+p24). The co-administration of the TLR7/NOD2L induced a significant protection of immunized mice and a high significant reduction of viral titers in the lung of immunized mice (Fig. 5C). Protection for the viral vaccinia challenge has been described to be mainly T-dependent as confirmed here in our study.
4. Discussion
The most important outcome of this study was the identification of a versatile adjuvant able to induce balanced systemic and mucosal immune responses after mucosal administration. Here, we showed that a newly designed molecule targets both TLR7 and NOD2, and that the hybrid ligand is significantly more efficient than separated ligands to stimulate TLR7 (Fig. 1–5). Whereas NOD2 is a cytosolic receptor, TLR7 is an endosomal receptor. Its stimulation requires the engulfment within endosomes. It has been demonstrated that large complexes generated when TLR agonists are conjugated to NPs are better recognized and engulfed by macrophages and DCs [32,33]. As the hybrid molecule is twice bigger than that the TLR7 side, the recognition and uptake by immune cells might be facilitated, leading to a better stimulation of TLR7. The CL325 is organized into large particles and a liposomal/micelle organization could be suspected (Supplemental Fig. 6). We think that this confirms the particulate suspicion of this compound which would favor multi-affinity presentation and uptake by Antigen Presenting Cells (APCs).
We also showed that the TLR7/NOD2 agonist induces autophagy in human cells (Fig. 1B). This property is highly wanted for adjuvant development. This phenomenon is known to be important for innate cytokines production [34]. It was also demonstrated that autophagy is an essential process for the survival of memory B cells in mice in the case of influenza virus infection [35]. The ability of the ligand to stimulate autophagy might enhance the immune response of the host and in particular cross-presentation, and give an improved protection upon immunization.
A previous study showed the interest of cooperative stimulation of DCs with TLR7 and NOD2 ligands to induce a synergistic release of pro-inflammatory mediators which promote the activation of IL-17-producing T cells [18]. In this study, we showed the interest of stimulating these two receptors with a single molecule for the induction of an optimal DC maturation process (Fig. 2). In fact, the molecule induces the expression of maturation markers as well as the secretion of multiple cytokines, necessary for the induction of a proinflammatory and adaptive immune response (Fig. 2). Moreover, we also showed in an in vitro priming assay of specific T cells, that the TLR7/NOD2L dual agonist is a potent inducer of central memory T cells and is also able to prime CTLs (Fig. 3). It will be of a great interest to understand how mechanistically the chimeric molecule improves the priming of CTLs response. Ability of TLR7/NOD2L to induce autophagy is probably involved in the effect.A pharmacokinetic analysis will be interested to compare the stability of the chimeric adjuvant and the single molecule. It's also possible to imagine that the physical or chemical properties of adjuvanted particles could stimulate different signaling pathway.
Finally, we showed that the intranasal administration of the molecule with HIV p24 coated on PLA NPs induces the production of IgG and IgA, at systemic and mucosal level (Fig. 5). The TLR7 agonist support the activation of IgG2a compared to the NP-p24 alone. This feature was conserved with the TLR7/NOD2L, showing its potential to induce Th1 immune response. Mucosal IgG response observed could be due to the transudation and/or FcRn transport to mucosal surfaces [36]. Chimeric adjuvant also induces a strong IFN-γ-and IL-17 CD4+ and CD8+ p24-specific response [37], both at systemic and mucosal levels. The induced immune response induced by the combination between the NP-p24 vaccine and the TLR7/NOD2L enables the protection of mice against a viral challenge.
To conclude, we showed that the TLR7/NOD2 agonist seems to be a potent adjuvant both in vitro and in vivo. This activity seems to be mediated by an optimal DC maturation process, which could induce strong B and T cell stimulation. This molecule is a potent versatile tool for adjuvantation of prophylactic but also therapeutic vaccines.
Declaration of Competing Interest
The authors declare that there are no competing interests.
Acknowledgments
Acknoledgements
This work was supported by Sidaction. The following reagent was obtained through the National Institutes of Health (NIH) AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH: vDK1 from Dr Daniel R. Kuritzkes.
Author contribution
Alice Gutjahr designed and performed the research, analyzed data and wrote the paper. Laura Papagno designed and performed the research, analyzed data and wrote the paper. Fabienne Vernejoul designed and performed the research. Thierry Lioux designed and performed the research. Fabienne Jospin performed the research. Blandine Chanut performed the research. Eric Perouzel designed the research. Nicolas Rochereau designed the research. Victor Appay designed the research, analyzed data and wrote the paper. Bernard Verrier designed the research. Stéphane Paul designed the research, analyzed data and wrote the paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102922.
Appendix. Supplementary materials
References
- 1.Geeraedts F., Goutagny N., Hornung V. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 2008;4(8) doi: 10.1371/journal.ppat.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 3.Janeway C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 4.McKee A.S., Marrack P. Old and new adjuvants. Curr Opin Immunol. 2017;47:44–51. doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada H., Kalinski P., Ueda R. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung I.F., Zhang A.J., To K.K. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin Infect Dis. 2014;59(9):1246–1255. doi: 10.1093/cid/ciu582. [DOI] [PubMed] [Google Scholar]
- 7.Eng N.F., Bhardwaj N., Mulligan R., Diaz-Mitoma F. The potential of 1018 ISS adjuvant in hepatitis B vaccines: HEPLISAV review. Hum Vaccin Immunother. 2013;9(8):1661–1672. doi: 10.4161/hv.24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowling D.J., van Haren S.D., Scheid A. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight. 2017;2(6):e91020. doi: 10.1172/jci.insight.91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wille-Reece U., Flynn B.J., Lore K. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102(42):15190–15194. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardin S.E., Boneca I.G., Viala J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 11.Jackson E.M., Herbst-Kralovetz M.M. Intranasal vaccination with murabutide enhances humoral and mucosal immune responses to a virus-like particle vaccine. PLoS ONE. 2012;7(7):e41529. doi: 10.1371/journal.pone.0041529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavot V., Climent N., Rochereau N. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials. 2016;75:327–339. doi: 10.1016/j.biomaterials.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Kim D., Kim Y.G., Seo S.U. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med. 2016;22(5):524–530. doi: 10.1038/nm.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutjahr A., Tiraby G., Perouzel E., Verrier B., Paul S. Triggering Intracellular Receptors for Vaccine Adjuvantation. Trends Immunol. 2016;37(10):716. doi: 10.1016/j.it.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Kornbluth R.S., Stone G.W. Immunostimulatory combinations: designing the next generation of vaccine adjuvants. J Leukoc Biol. 2006;80(5):1084–1102. doi: 10.1189/jlb.0306147. [DOI] [PubMed] [Google Scholar]
- 16.Lovgren T., Sarhan D., Truxova I. Enhanced stimulation of human tumor-specific T cells by dendritic cells matured in the presence of interferon-gamma and multiple toll-like receptor agonists. Cancer Immunol Immunother. 2017;66(10):1333–1344. doi: 10.1007/s00262-017-2029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netea M.G., Ferwerda G., de Jong D.J. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174(10):6518–6523. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz H., Posselt G., Wurm P., Ulbing M., Duschl A., Horejs-Hoeck J. TLR8 and NOD signaling synergistically induce the production of IL-1beta and IL-23 in monocyte-derived DCs and enhance the expression of the feedback inhibitor SOCS2. Immunobiology. 2013;218(4):533–542. doi: 10.1016/j.imbio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Patin E.C., Jones A.V., Thompson A. IL-27 Induced by select Candida spp. via TLR7/NOD2 signaling and IFN-beta production inhibits fungal clearance. J Immunol. 2016;197(1):208–221. doi: 10.4049/jimmunol.1501204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutjahr A., Papagno L., Nicoli F. Cutting Edge: a Dual TLR2 and TLR7 ligand induces highly potent humoral and cell-mediated immune responses. J Immunol. 2017;198(11):4205–4209. doi: 10.4049/jimmunol.1602131. [DOI] [PubMed] [Google Scholar]
- 21.Pavot V., Rochereau N., Resseguier J. Cutting edge: new chimeric NOD2/TLR2 adjuvant drastically increases vaccine immunogenicity. J Immunol. 2014;193(12):5781–5785. doi: 10.4049/jimmunol.1402184. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L., Dewan V., Yin H. Discovery of small molecules as multi-toll-like receptor agonists with proinflammatory and anticancer activities. J Med Chem. 2017;60(12):5029–5044. doi: 10.1021/acs.jmedchem.7b00419. [DOI] [PubMed] [Google Scholar]
- 23.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 24.Boyaka P.N. Inducing mucosal iga: a challenge for vaccine adjuvants and delivery systems. J Immunol. 2017;199(1):9–16. doi: 10.4049/jimmunol.1601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutjahr A., Phelip C., Coolen A.L. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines (Basel) 2016;4(4) doi: 10.3390/vaccines4040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavot V., Rochereau N., Primard C. Encapsulation of Nod1 and Nod2 receptor ligands into poly(lactic acid) nanoparticles potentiates their immune properties. J Control Release. 2013;167(1):60–67. doi: 10.1016/j.jconrel.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Lissina A., Briceno O., Afonso G. Priming of qualitatively superior human effector CD8+ T Cells Using TLR8 ligand combined with FLT3 ligand. J Immunol. 2016;196(1):256–263. doi: 10.4049/jimmunol.1501140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rochereau N., Pavot V., Verrier B. Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells. Eur J Immunol. 2015;45(3):773–779. doi: 10.1002/eji.201444816. [DOI] [PubMed] [Google Scholar]
- 29.Rochereau N., Pavot V., Verrier B. Delivery of antigen to nasal-associated lymphoid tissue microfold cells through secretory IgA targeting local dendritic cells confers protective immunity. J Allergy Clin Immunol. 2016;137(1):214–222. doi: 10.1016/j.jaci.2015.07.042. e2. [DOI] [PubMed] [Google Scholar]
- 30.Takata H., Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177(7):4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 31.Willinger T., Freeman T., Hasegawa H., McMichael A.J., Callan M.F. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175(9):5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 32.Shima F., Uto T., Akagi T., Akashi M. Synergistic stimulation of antigen presenting cells via TLR by combining CpG ODN and poly(gamma-glutamic acid)-based nanoparticles as vaccine adjuvants. Bioconjug Chem. 2013;24(6):926–933. doi: 10.1021/bc300611b. [DOI] [PubMed] [Google Scholar]
- 33.Xiang S.D., Scholzen A., Minigo G. Pathogen recognition and development of particulate vaccines: does size matter? Methods. 2006;40(1):1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Morris S., Swanson M.S., Lieberman A. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J Immunol. 2011;187(8):3953–3961. doi: 10.4049/jimmunol.1100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M., Hong M.J., Sun H. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat Med. 2014;20(5):503–510. doi: 10.1038/nm.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo T.T., Baker K., Yoshida M. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30(6):777–789. doi: 10.1007/s10875-010-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malyguine A.M., Strobl S., Dunham K., Shurin M.R., Sayers T.J. ELISPOT assay for monitoring cytotoxic T Lymphocytes (CTL) activity in cancer vaccine clinical trials. Cells. 2012;1(2):111–126. doi: 10.3390/cells1020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.