Abstract
Cirrhosis commonly complicates portal hypertension worldwide but in Zambia hepatosplenic schistosomiasis (HSS) dominates as the cause of portal hypertension. We need easier and non-invasive ways to assess HSS. Transient elastography (TE), a measure of liver stiffness can diagnose liver cirrhosis. TE remains unexplored in HSS patients, who generally have normal liver parenchyma. We aimed to explore liver stiffness in HSS. This nested case control study was conducted at the University Teaching Hospital, Lusaka, Zambia between January 2015 and January 2016. We enrolled 48 adults with HSS and 22 healthy controls. We assessed liver stiffness using TE while plasma hyaluronan was used to assess liver fibrosis. Plasma tumor necrosis factor receptor 1 (TNFR1) and soluble cluster of differentiation 14 (sCD14) were used to assess inflammation. The median (interquartile range) liver stiffness was higher in patients, 9.5 kPa (7.8, 12.8) than in controls, 4.7 kPa (4.0, 5.4), P < 0.0001. We noted linear correlations of hyaluronan and TNFR1 with the liver stiffness, P = 0.0307 and P = 0.0003 respectively.
HSS patients seem to have higher liver stiffness than healthy controls. TE may be useful in identifying fibrosis in HSS. The positive correlations of inflammatory markers with TE suggest that HSS has both periportal and parenchymal pathophysiology.
Keywords: Metabolism, Liver stiffness, Hepatosplenic schistosomiasis, Fibrosis
Metabolism; Liver stiffness; Hepatosplenic schistosomiasis; Fibrosis.
1. Introduction
Hepatosplenic schistosomiasis (HSS) in the tropics is an important cause of portal hypertension and contributes significantly to mortality and morbidity (Berhe et al., 2007; Kibiki et al., 2004; Sinkala et al., 2016). Schistosomiasis is one of the neglected tropical diseases (Watts, 2017). Although cirrhosis is the leading cause of portal hypertension worldwide, in Zambia a large proportion of cases are non-cirrhotic and are due to schistosomiasis (Sinkala et al., 2016). Schistosomiasis is endemic in some districts of Zambia and sero-prevalence is as high as 88% (Chipeta et al., 2009; Payne et al., 2013; Mutengo et al., 2014).
To diagnose liver fibrosis, liver biopsy is required as the gold standard, but this is invasive and is associated with some risk of bleeding and sampling error (Ahmed et al., 2009; Seeff et al., 2010). Many authorities are now advocating for non-invasive means of diagnosing liver fibrosis (Marinho et al., 2010; Lombardi et al., 2015). Transient elastography (TE, FibroScan®) is promising to be a non-invasive tool for assessing liver fibrosis. Its clinical use in patients with liver disease is increasing and has proved to be reliable (Pang et al., 2014). It can be performed on an outpatient basis and this imaging technique takes about 5–10 min only. It does not require special preparation other than the patient starving for 2–3 h prior to the procedure (Wilder and Patel, 2014; Armstrong et al., 2013). Studies involving the TE in patients with chronic hepatitis B, C viral infections and non-alcoholic fatty liver disease have shown that TE could be superior in assessing liver fibrosis than the aspartate aminotransferase-to-platelet ratio index (APRI) score although it is not yet validated to replace liver biopsy (Myers et al., 2010; Liu et al., 2011). TE has been useful as a non-invasive tool in assessing liver fibrosis and cirrhosis in HBV and HCV infected patients. It is also useful in predicting variceal bleeding in patients with portal hypertension (Myers et al., 2010; Jung and Kim, 2012).
The use of TE in schistosomal liver disease remains unexplored especially in an African setting where it is quite common. It is not clear whether HSS is associated with increased liver stiffness considering that HSS is generally associated with periportal fibrosis and the liver parenchyma is spared (Sinkala et al., 2016; Rebouças, 1975). Furthermore, it is unknown if TE can be used to discriminate between cirrhosis and HSS and if it can be used to monitor disease progression in HSS. A study in Egypt by Esmat et al. showed that TE overestimated the degree of liver fibrosis in schistosomal liver disease patients who were co-infected with HCV (Esmat et al., 2013). However, this study did not assess the performance of TE in HSS patients who were mono-infected with schistosomiasis. We therefore aimed to evaluate liver stiffness in HSS patients seen at the University Teaching Hospital, Lusaka, Zambia.
2. Materials and methods
2.1. Ethics statement
Informed consent was obtained from all patients and controls. Our study was approved by the University of Zambia Biomedical Research Ethics Committee (ref: 006-07-12).
2.2. Study settings and patient recruitment
A nested case control study was carried out at the University Teaching Hospital in the Department of Internal Medicine between January 2015 and January 2016. During the rifaximin clinical trial of bacterial translocation in patients with HSS, FibroScan® became available in Lusaka, Zambia. After amending the trial protocol, 48 sequential patients with HSS among the patients in the rifaximin clinical trial were recruited. The rifaximin clinical trial was a randomized clinical trial in Zambia undertaken to test the hypothesis that rifaximin could reduce bacterial translocation in patients with HSS (Sinkala et al., 2018). Eighty-five (85) patients were eligible and randomized to either rifaximin with standard care or standard care only. Forty-four (44) patients received rifaximin and standard care while 41 received standard care only for 42 days (Sinkala et al., 2018). The standard care included propranolol which is a beta blocker and was taken orally. All the patients received praziquantel 40 mg per kg body weight orally in divided doses over a day.
In this nested case control study 22 controls alongside the trial were recruited. The controls were adults who were apparently healthy-looking individuals and sought treatment for non-specific abdominal pains. They were sero-negative for HIV and hepatitis B or C viruses. They had normal gastroscopy.
The inclusion criteria for cases were: haematemesis, varices on endoscopy, positive serology for schistosomiasis, ultrasound suggestive of periportal fibrosis and those aged ≥18 years. Exclusion criteria were: sero-positive for HIV and hepatitis B or C viruses, cirrhosis, pregnancy and inability to give consent. The case definition for HSS in this study was evidence of periportal fibrosis on ultrasound, positive serology for schistosomiasis supported by presence of varices on gastroscopy.
2.3. Study procedures
A questionnaire was administered to capture demographic data, medical history and social history. Patients and controls also underwent a thorough physical examination. Blood from cases and controls was drawn for full blood count (Sysmex 800i analyser, Koke, Japan). In HSS patients, the inflammatory markers measured were tumor necrosis factor receptor 1 (TNFR1) and soluble cluster of differentiation 14 (sCD14). TNFR1 was measured in plasma using ELISA (R&D Systems), Abingdon, UK, at 10-fold dilution while soluble cluster of differentiation 14 (sCD14) was measured by ELISA (R&D Systems) Abingdon, UK and was diluted 400-fold. Hyaluronan was measured as a marker of fibrosis using ELISA (R&D Systems) Abingdon, UK with a dilution factor of 80. The serology for schistosomiasis was performed using the microwell ELISA (SCI-MEDX Corporation, Denville, NJ, USA). This gives a qualitative determination of the immunoglobulins (IgG) to schistosoma species but does not differentiate between species (Sinkala et al., 2016).
TE (FibroScan® 402, Echosens, Paris, France) was carried out on 48 patients with schistosomiasis related portal hypertension and 22 controls. The transducer tip was placed on the lateral aspect of the liver on the right lobe. Ten measurements were taken on each patient and each control. Liver stiffness was taken to be valid if the success rate of valid measurement of liver stiffness was at least 60% and the median was taken as the representative measurement expressed in kiloPascals (kPa) (Mueller and Sandrin, 2010; Kircheis et al., 2012). One of the two operators of the FibroScan at the University Teaching Hospital with >1000 prior scan experience performed the scans.
2.4. Data analysis
Data analysis was carried out using STATA version 13.1 (Stata Corp, College Station, TX, USA) and GRAPHPAD PRISM 6.01 (GraphPad Software, San Diego, CA, USA). For data description, median with interquartile range was used. Mann-Whitney test was used to compare data between cases and controls. Spearman's rank test was used to check for association between liver stiffness and blood markers (inflammatory and fibrotic markers). A P value of less than 0.05 was considered significant.
3. Results
Of the 85 HSS patients in the rifaximin clinical trial (Sinkala et al., 2018), all the 48 patients who were evaluated underwent TE. Patients with HSS gave history of repeated exposure to natural water bodies through swimming, drawing water for domestic use, farming and swimming. Most of them reported exposure to water bodies during childhood. Liver ultrasound confirmed periportal fibrosis in all the patients while the controls had no evidence of periportal fibrosis. The controls had normal gastroscopy and gave no history of hematemesis or rectal bleeding. Liver ultrasound did not show any evidence of cirrhosis in cases and controls. Serum alanine aminotransferase levels in cases and controls were not significantly different but albumin levels were lower in the cases (Table 1). The renal function assessed by blood creatinine was normal and comparable in cases and controls (Table 1).
Table 1.
Basic demographic and laboratory data for cases and controls.
| Cases (n = 48) | Controls (n = 22) | P value | |||
|---|---|---|---|---|---|
| Age (years) | 40 (31,36) | 32 (27, 35) | 0.01 | ||
| Gender | Females | 25 | Females | 12 | 1.00 |
| Males | 22 | Males | 10 | ||
| BMI (kg/m2) | 22 (21, 25) | 23 (21, 26) | 0.39 | ||
| Spleen size (cm) | 17 (15, 18) | 10 (8, 11) | 0.0001 | ||
| Main portal vein (mm) | 12 (10, 14) | 8 (6, 8) | 0.0001 | ||
| WCC (x109/l) | 2.4 (1.6, 3.4) | 4.6 (3.8, 5.9) | 0.0001 | ||
| RBC (x1012/l) | 3.4 (2.8, 4.4) | 4.7 (4.4, 5.4) | 0.0001 | ||
| Haemoglobin (g/dl) | 8 (6, 11) | 14 (12, 15) | 0.0001 | ||
| Platelet (x109/l) | 49 (27, 77) | 188 (172, 295) | 0.0001 | ||
| ALT (U/L) | 33 (17, 36) | 16 (7, 30) | 0.08 | ||
| Albumin (g/dl) | 37 (35,41) | 43 (42,45) | 0.001 | ||
| Creatinine μmol/l | 72 (64, 84) | 75 (75, 88) | 0.3 | ||
All parameters are represented as median and interquartile range in the parenthesis.
Key: BMI – body mass index, ALT- Alanine aminotransferase, WCC- white cell count, RBC- red blood cell count.
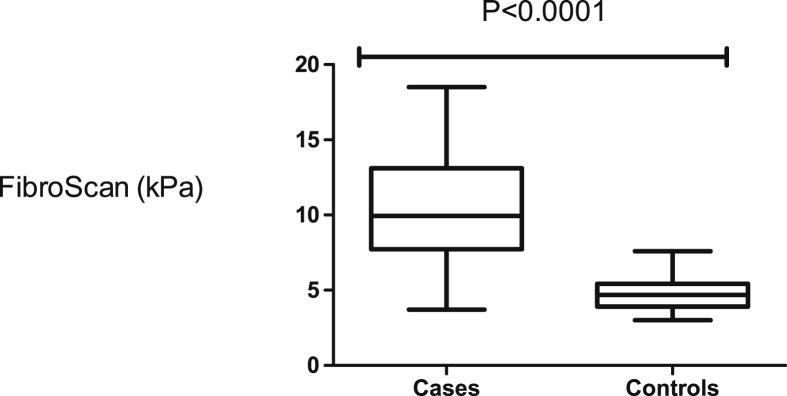
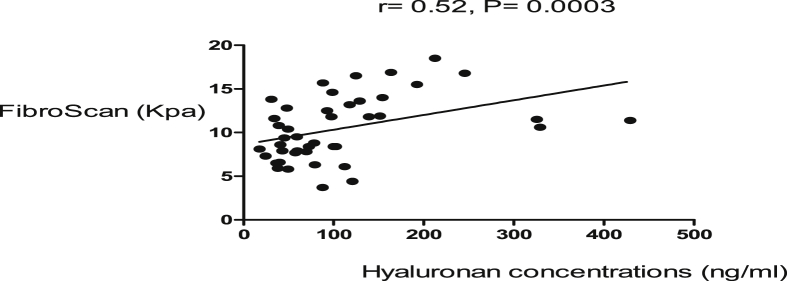
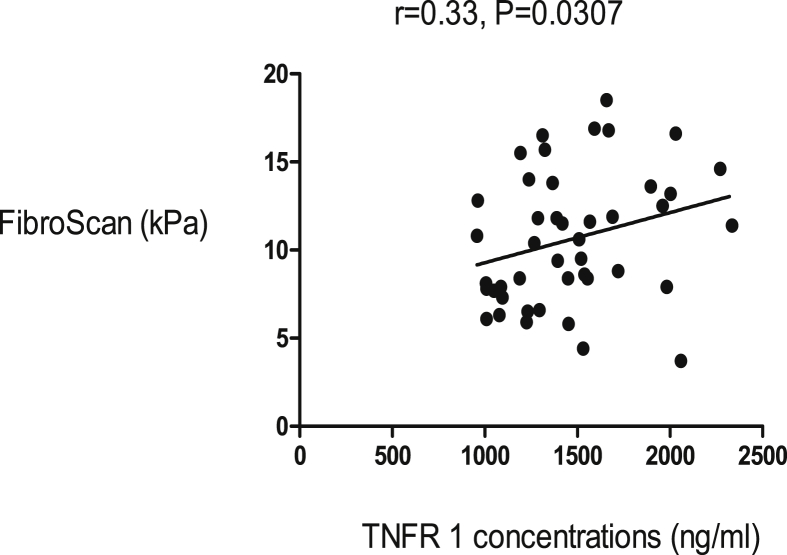
The body mass index (BMI) was similar in cases and controls. None of the cases and the controls were obese. The median age for controls was lower than in the cases (Table 1). The female to male ratio was similar in cases and controls (Table 1). Splenic size and main portal vein diameter were higher in cases than controls. Nine (9) cases with HSS had ascites. The full blood count showed that white cell count, red blood cell count and platelet count were reduced in cases compared to controls. This may be attributed to hypersplenism (Table 1). The stiffness of the liver was more pronounced in cases than controls (Figure 1). We noted a significant positive linear correlation of hyaluronan with TE. TNFR1 and TE showed positive linear correlation as well in HSS patients (Figures 2 and 3). However, there were no significant correlations between TE scores and other parameters.
Figure 1.
Transient elastography (FibroScan) was significantly pronounced in cases compared to controls.
Figure 2.
There was a positive correlation of FibroScan score and serum hyaluronan, a fibrotic marker in HSS patients.
Figure 3.
There was a positive correlation of FibroScan score and serum TNFR 1, an inflammatory marker in HSS patients.
4. Discussion
In this study, we measured liver stiffness in a well-characterized group of Zambians with advanced HSS and noted elevated liver stiffness compared with controls. This shows that TE could be an important non-invasive method of assessing liver stiffness in HSS patients. The role of TE in diagnosing liver disease has evolved over time such that it is now often used in the diagnosis of cirrhosis. There is great interest in using it instead of performing liver biopsy. Liver biopsy is an invasive procedure which is associated with the risk of bleeding, injury to surrounding structures and introduction of infection although mortality risk is as low as 0.03% (Seeff et al., 2010). Many studies of TE have been published in cirrhosis worldwide but to our knowledge this is the first in HSS related portal hypertension in an African setting where schistosomiasis is very common. A recent study was published in Brazil, which also showed that TE is elevated in HSS but this study did not evaluate the blood markers of inflammation and fibrosis in HSS patients (Veiga et al., 2017). When compared with the range of reported TE scores in cirrhosis worldwide (Göbel et al., 2015; Kircheis et al., 2012), the median TE scores seen in our HSS patients are lower. These data suggest that TE may be a useful tool to discriminate cirrhosis from HSS especially in HSS endemic areas.
HSS is generally characterised by normal liver cell function and the main pathology in these patients is periportal fibrosis (Rebouças, 1975; Da Silva et al., 2005; Sinkala et al., 2016). However, we have documented increased direct markers of fibrosis and inflammation. These data showed positive correlation of these markers with liver stiffness suggesting that hepatic parenchyma may be affected to some degree and inflammation could be driving fibrosis in HSS. Another study in animal models found that TNFR1 is a pro-fibrotic inflammatory marker in liver disease (Tarrats et al., 2011). We recently reported that hyaluronan and laminin may be important markers associated with fibrosis in HSS and that fibrosis may be driven by systemic inflammation in HSS patients (Sinkala et al., 2016). These findings therefore suggest that a combination of non-invasive blood markers and imaging tools such as TE may be useful in assessing and diagnosing HSS.
Although TE seems to be good in assessing hepatic fibrosis, it has its shortcomings. The scores tend to be influenced by obesity, acute hepatitis, cholestasis and performer experience (Chang et al., 2016). In this nested case control study, no patient had obesity and there was no evidence of acute hepatitis. TE was performed by an experienced person who had performed over a thousand scans. Therefore, the TE scores noted in our patients with HSS may reflect the actual stiffness of the liver. In our experience, substantial ascites results in failure to measure liver stiffness with FibroScan. The 9 HSS patients that we included in the analysis had trace or limited ascites detected on ultrasound and this did not result in failure of liver stiffness measurements using FibroScan. We suspect this minimal ascites might have increased the TE scores. We think the presence of ascites reflected the advanced disease in HSS.
This study had some limitations. Liver biopsies were not done and there were no cirrhotic patients as positive controls. We did not use the Niamey protocol score to determine the image pattern for HSS patients and therefore we acknowledge this as a limitation of the study. Another limitation was that we were not able to compare inflammatory markers in chronic patients with schistosomiasis without hepatosplenic disease and those with hepatosplenic disease. The small sample size did not allow us to perform adjusted analyses.
In conclusion, the elevated TE scores suggest that HSS patients despite the liver parenchyma being normal have increased liver stiffness. The positive and significant correlations of the liver stiffness with markers of fibrosis and inflammation support the view that inflammation may be a driver of fibrosis leading to liver stiffness in HSS patients. The positive correlations also suggest that HSS may have both periportal and parenchymal pathophysiology. A combination of non-invasive serum markers of fibrosis and inflammation together with imaging such as TE could be of value in clinical evaluation of HSS patients.
Declarations
Author contribution statement
Edford Sinkala, Michael Vinikoor: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Alice Miyanda Siyunda, Kanekwa Zyambo, Ellen Besa: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bright Nsokolo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gilles Wandeler: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Graham R Foster, Paul Kelly: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Wellcome Trust through the Southern Africa Consortium for Research (WT087537MA). We also received support from the U.S. National Institute of Health (NIH)-funded International Epidemiological Databases to Evaluate AIDS in Southern Africa (U01 AI069924). M.J.V. received support from the NIH Forgarty International Center (K01 TW009998) and G.W. was supported by an Ambizione- PROSPER fellowship from the Swiss National Science Foundation (PZ00P3 154730).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to our patients and controls for accepting to participate in this study. We want to thank the endoscopy nurses; Temba Banda, Rose Soko and Joyce Sibwani for assistance in recruitment. We are grateful to Mattias Egger and Gilles Wandeler for the loan of the FibroScan®.
References
- Ahmed L., Salama H., Ahmed R., Mahgoub A.M., Hamdy S., ABD Al Shafi S., Al akel W., Hareedy A., Fathy W. Evaluation of fibrosis sero-markers versus liver biopsy in Egyptian patients with hepatitis C and/or NASH and/or schistosomiasis. Parasitol United J. 2009;2:67–76. [Google Scholar]
- Armstrong M., Corbett C., Hodson J., Marwah N., Parker R., Houlihan D., Rowe I., Hazlehurst J., Brown R., Hübscher S. Operator training requirements and diagnostic accuracy of Fibroscan in routine clinical practice. Postgrad. Med. 2013 doi: 10.1136/postgradmedj-2012-131640. postgradmedj-2012-131640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe N., Myrvang B., Gundersen S.G. Intensity of Schistosoma mansoni, hepatitis B, age, and sex predict levels of hepatic periportal thickening/fibrosis (PPT/F): a large-scale community-based study in Ethiopia. Am. J. Trop. Med. Hyg. 2007;77:1079–1086. [PubMed] [Google Scholar]
- Chang P.E., Goh G.B.-B., Ngu J.H., Tan H.K., Tan C.K. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World J. Gastrointest. Pharmacol. Therapeut. 2016;7:91. doi: 10.4292/wjgpt.v7.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipeta J., Mwansa J., Kachimba J. Schistosomiasis disease burden in Zambian children: time for affirmative action is now. Med. J. Zambia. 2009;36:1–5. [Google Scholar]
- Da Silva L., Chieffi P.P., Carrilho F.J. Schistosomiasis mansoni–clinical features. Gastroenterol. Hepatol. 2005;28:30–39. doi: 10.1157/13070382. [DOI] [PubMed] [Google Scholar]
- Esmat G., Elsharkawy A., El Akel W., Fouad A., Helal K., Mohamed M.K., Attia D., Khattab H., Doss W., Labib S. Fibroscan of chronic HCV patients coinfected with schistosomiasis. Arab J.Gastroenterol. 2013;14:109–112. doi: 10.1016/j.ajg.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Göbel T., Schadewaldt-Tümmers J., Greiner L., Poremba C., Häussinger D., Erhardt A. Transient elastography improves detection of liver cirrhosis compared to routine screening tests. World J. Gastroenterol.: WJG. 2015;21:953. doi: 10.3748/wjg.v21.i3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K.S., Kim S.U. Clinical applications of transient elastography. Clin. Mol. Hepatol. 2012;18:163. doi: 10.3350/cmh.2012.18.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibiki G.S., Drenth J.P., Nagengast F.M. Hepatosplenic schistosomiasis: a review. East Afr. Med. J. 2004;81:480–485. [PubMed] [Google Scholar]
- Kircheis G., Sagir A., Vogt C., Vom DahL S., Kubitz R., Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J. Gastroenterol.: WJG. 2012;18:1077. doi: 10.3748/wjg.v18.i10.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-H., Liang C.-C., Huang K.-W., Liu C.-J., Chen S.-I., Lin J.-W., Hung P.-H., Tsai H.-B., Lai M.-Y., Chen P.-J. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin. J. Am. Soc. Nephrol. 2011 doi: 10.2215/CJN.04320510. 04320510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi R., Buzzetti E., Roccarina D., Tsochatzis E.A. Non-invasive assessment of liver fibrosis in patients with alcoholic liver disease. World J. Gastroenterol.: WJG. 2015;21:11044. doi: 10.3748/wjg.v21.i39.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho C.C., Bretas T., Voieta I., Queiroz L.C.D., Ruiz-guevara R., Teixeira A.L., Antunes C.M., Prata A., Lambertucci J.R. Serum hyaluronan and collagen IV as non-invasive markers of liver fibrosis in patients from an endemic area for schistosomiasis mansoni: a field-based study in Brazil. Memórias do Inst. Oswaldo Cruz. 2010;105:471–478. doi: 10.1590/s0074-02762010000400020. [DOI] [PubMed] [Google Scholar]
- Mueller S., Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepatic Med. 2010;2:49. doi: 10.2147/hmer.s7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutengo M.M., Mwansa J.C., Mduluza T., Sianongo S., Chipeta J. High Schistosoma mansoni disease burden in a rural district of western Zambia. Am. J. Trop. Med. Hyg. 2014;91:965–972. doi: 10.4269/ajtmh.13-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R.P., Elkashab M., Ma M., Crotty P., Pomier-Layrargues G. Transient elastography for the noninvasive assessment of liver fibrosis: a multicentre Canadian study. Canadian J. Gastroenterol. Hepatol. 2010;24:661–670. doi: 10.1155/2010/153986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J.X., Zimmer S., Niu S., Crotty P., Tracey J., Pradhan F., Shaheen A.A.M., Coffin C.S., Heitman S.J., Kaplan G.G. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PloS One. 2014;9 doi: 10.1371/journal.pone.0095776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L., Turner-Moss E., Mutengo M., Asombang A.W., Kelly P. Prevalence of schistosome antibodies with hepatosplenic signs and symptoms among patients from Kaoma, Western Province, Zambia. BMC Res. Notes. 2013;6:344. doi: 10.1186/1756-0500-6-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouças G. Clinical aspects of hepatosplenic schistosomiasis: a contrast with cirrhosis. Yale J. Biol. Med. 1975;48:369. [PMC free article] [PubMed] [Google Scholar]
- Seeff L.B., Everson G.T., Morgan T.R., Curto T.M., Lee W.M., Ghany M.G., Shiffman M.L., Fontana R.J., Di Bisceglie A.M., Bonkovsky H.L. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin. Gastroenterol. Hepatol. 2010;8:877–883. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkala E., Kapulu M.C., Besa E., Zyambo K., Chisoso N.A.J., Foster G.R., Kelly P. Hepatosplenic schistosomiasis is characterised by high blood markers of translocation, inflammation and fibrosis. Liver Int. 2016;36:145–150. doi: 10.1111/liv.12891. [DOI] [PubMed] [Google Scholar]
- Sinkala E., Zyambo K., Besa E., Kaonga P., Nsokolo B., Kayamba V., Vinikoor M., Zulu R., Bwalya M., Foster G.R., Kelly P. Rifaximin reduces markers of bacterial 16SrRNA in Zambian adults with hepatosplenic schistosomiasis: a radomized control trial. Am. J. Trop. Med. Hyg. 2018;98(4):1152–1158. doi: 10.4269/ajtmh.17-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrats N., Moles A., Morales A., García-Ruiz C., Fernández-Checa J.C., Marí M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–327. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga Z.S.T., Villela-Nogueira C.A., Fernandes F.F., Cavalcanti M.G., Figueiredo F.A., Pereira J.L., Pereira G.H., Moraes Coelho H.S., Peralta J.M., Marques C.E., Perez R.M., Fogaça H.S. Transient elastography evaluation of hepatic and spleen stiffness in patients with hepatosplenic schistosomiasis. Eur. J. Gastroenterol. Hepatol. 2017;29:730–735. doi: 10.1097/MEG.0000000000000853. [DOI] [PubMed] [Google Scholar]
- Watts C. Neglected tropical diseases: a DFID perspective. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder J., Patel K. The clinical utility of FibroScan® as a noninvasive diagnostic test for liver disease. Med. Dev. 2014;7:107. doi: 10.2147/MDER.S46943. (Auckland, NZ) [DOI] [PMC free article] [PubMed] [Google Scholar]