Abstract
Chimeric antigen receptor (CAR) T cells use re-engineered cell surface receptors to specifically bind to and lyse oncogenic cells. Two clinically approved CAR-T–cell therapies have significant clinical efficacy in treating CD19-positive B cell cancers. With widespread interest to deploy this immunotherapy to other cancers, there has been great research activity to design new CAR structures to increase the range of targeted cancers and anti-tumor efficacy. However, several obstacles must be addressed before CAR-T–cell therapies can be more widely deployed. These include limiting the frequency of lethal cytokine storms, enhancing T-cell persistence and signaling, and improving target antigen specificity. We provide a comprehensive review of recent research on CAR design and systematically evaluate design aspects of the four major modules of CAR structure: the ligand-binding, spacer, transmembrane, and cytoplasmic domains, elucidating design strategies and principles to guide future immunotherapeutic discovery.
Keywords: Chimeric antigen receptor, CAR-T, Immunotherapy, Cancer, Cell engineering, Synthetic biology
1. Introduction
T cells expressing chimeric antigen receptors (CAR) have displayed remarkable efficacy at treating malignant cancers, particularly liquid tumors. The ability to custom design CARs for specific oncological applications has made them an attractive alternative to conventional cancer treatments such as radiation and chemotherapy. CARs consist of an extracellular ligand-binding domain, most commonly a single chain variable fragment (scFv), a spacer domain, a transmembrane domain, and one or more cytoplasmic domains [1]. First-generation CARs contain a single activatory domain, which in most cases is the CD3ζ cytoplasmic domain, while a few studies used the γ chain of Fc receptors. Second-generation CARs commonly contain an activatory domain (CD3ζ/γ chain of Fc receptors) connected to co-stimulatory domains obtained from native co-stimulatory molecules such as CD28 and 4–1BB [1]. More recent optimization has led to the development of third-generation constructs incorporating CD3ζ with two co-stimulatory cytoplasmic domains. The design of each module of the CAR structure can contribute to CAR-T–cell signaling mechanisms, effector functions, and its eventual efficacy and toxicity. It is evident that modules such as the scFv and intracellular cytoplasmic domains play a key role in ligand recognition and signaling. It has recently become clear that non-signaling domains such as the spacer and transmembrane domains can also influence CAR functions.
Highly encouraging preclinical and clinical results have spurred massive research efforts into CAR-T cell therapy, yet challenges still lie ahead of full clinical adoption due to their toxicity and challenges associated with non-hematological cancers. So far only two CAR-T–cell therapies, Kymriah® and Yescarta®, are clinically approved yet they have exhibited serious, sometimes lethal, side-effects. Cytokine release syndrome, the rapid release of cytokines into the bloodstream following administration of immunotherapies, as well as neurotoxicity have been frequently reported [2,3]. In addition to these side effects, responses to CAR-T therapy have been variable due to both heterogeneity in target-antigen expression in malignant cells as well as high rates of antigen escape and downregulation of target cancer markers [4,5]. CAR-T cells continue to be minimally effective against solid tumors, in part due to their inability to persist and maintain their effector functions in the neoplastic microenvironment [6]. Recent literature suggests that appropriate compositional and structural design of CARs can reduce off-target effects and enhance tumor eradication and persistence. While it is recognized that small variations in these CAR modules and characteristics can be critical functionality determinants, relationships among these factors are complicated, and currently no general design rules can predict in vivo functions. In this review, we will discuss recent advances in engineering the extracellular, spacer, transmembrane, and cytoplasmic domains of CARs and how they affect CAR-T function. We summarize a list of design parameters tested in literature for each module and describe their effects on the functionality of CAR-T cells (Fig. 1). This systematic analysis can help uncover design principles, which can be broadly applied toward future designer immunotherapies.
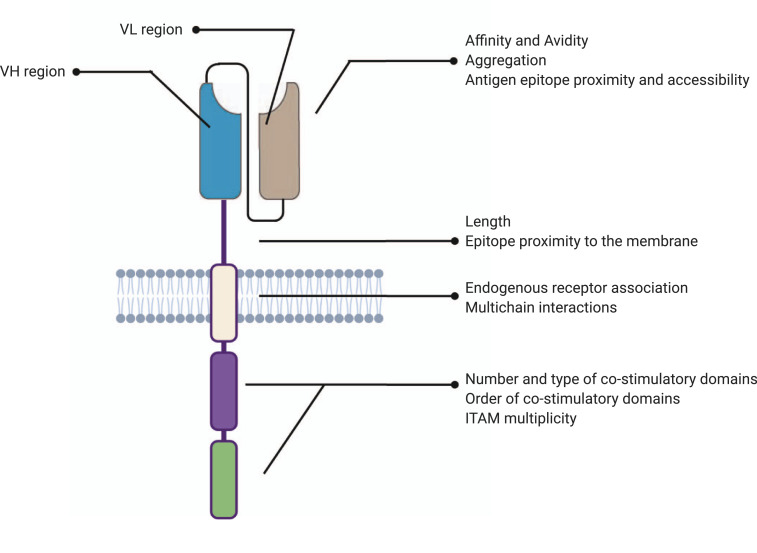
Fig. 1.
Design parameters of each module of the CAR tested in literature.
2. Ligand-binding domain
scFvs are the most commonly used ligand-binding domains in CAR structures, although other domains such as nanobodies, ligands to cognate receptors, native receptors against targets—including those such as NKG2D and T1E that target multiple ligands—and small peptides have been used [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Fig. 1 and Fig. 2 highlight critical design parameters of ligand-binding domain including affinity, avidity, antigen epitope location, and accessibility, as well as how they affect CAR-T–cell functionality. Interested readers can also refer to Supplementary Table 1 for a detailed list of representative publications that highlight the importance of these parameters.
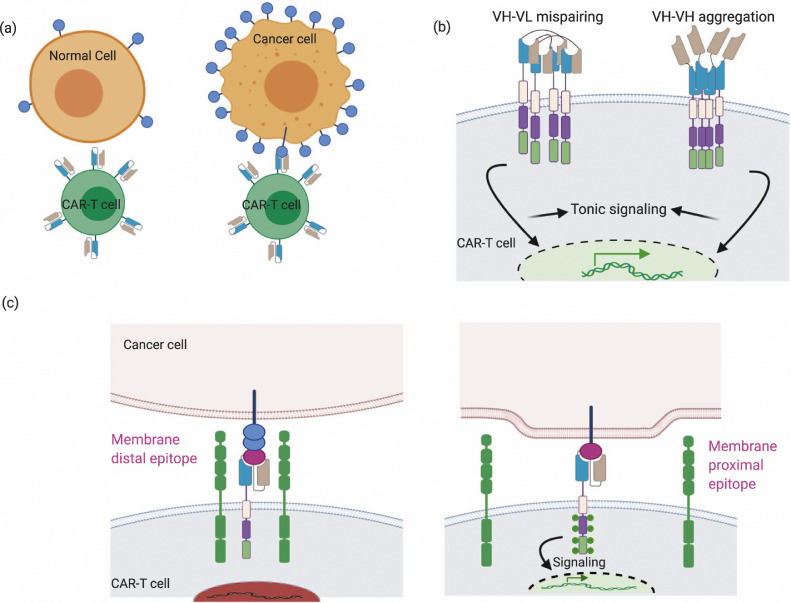
Fig. 2.
scFv properties such as affinity, avidity, aggregation propensity, and its antigen epitope location are critical parameters that can affect CAR function. (a) scFv affinity and avidity can be modulated to improve selective recognition of target cells bearing higher ligand density, thus reducing on-target off-tumor effects.
(b) CAR surface aggregation can cause VH-VL mispairing, which can occur at high expression levels or with sub-optimal linker design that limits stabilizing inter-domain interactions. (c) Location of epitope targeted by scFv dictates synaptic cleft distances, which are important for kinetic segregation of phosphatases like CD45.
2.1. Affinity and avidity of ligand-binding domain
scFv affinity is a key parameter that has been modulated to improve specificity of the CAR and reduce “on-target, off-tumor” side effects, which is of particular importance when the target antigen is ubiquitously expressed on healthy tissue. For instance, CARs constructed from an anti-ErbB2 scFv with a KD (dissociation constant) of 0•3 μM showed selective cytotoxicity towards cells highly expressing ErbB2 while CARs bearing high-affinity scFv sequences (KD <0•01 μM) ErbB2 did not [17]. Similarly, in another study anti-ErbB2 CARs were constructed from affinity-modulated scFv sequences derived from monoclonal antibody mAb 4D5. CAR-T cells using a lower-affinity 4D5 variant (KD ~ 1 μM) showed an increased therapeutic index in mice compared to CAR-T cells bearing a high-affinity 4D5 variant (KD ~ 0•6 nM) [18]. This was attributed to the ability of low-affinity scFv CARs to selectively discriminate between tumors which typically express ErbB2 at higher densities compared to normal tissues. Caruso et al. compared the specificity of anti-EGFR CARs constructed from Cetuximab and Nimotuzumab, which has a 10-fold lower affinity than Cetuximab [19]. Nimotuzumab-based CARs showed EGFR-density dependent activation in vitro and did not show potent recognition of low-density EGFR cells in vivo, unlike the Cetuximab-based CAR. In another study, an anti-CD38 CAR with a low-affinity scFv (KD in the micromolar range), obtained from an affinity-tuned antibody library, was specifically cytotoxic to cells highly expressing CD38 both in vitro and in vivo, while having minimal effect on healthy CD38+ hematopoietic cells [20]. Similarly, LFA-1 I domains modulated for micromolar affinity to ICAM-1 were more selective to cells expressing high levels of the target antigen (ICAM-1). CAR-T cells incorporating micromolar-affinity I domains specifically cleared thyroid carcinoma xenografts in mice without systemic toxicity [9].
While reducing affinity has been shown to improve CAR-T–cell specificity, it can reduce anti-tumor potency in certain cases. Anti-ROR1 CARs constructed from a higher-affinity scFv (R12) showed greater anti-tumor potency compared to CARs constructed from a 2A2 scFv which has a 50-fold lower affinity [21]. Similarly, higher-affinity anti-FRβ CARs (KD ~54•3 nM) showed specific and complete abrogation of tumors in mouse models of acute myeloid leukemia compared to lower-affinity anti-FRβ CARs (KD ~2•4 nM) which were ineffective against the disease [22]. In another study, anti-GD2 CARs constructed from a low-affinity 14G2a scFv were ineffective against rapidly proliferating tumors, while a high-affinity scFv obtained by rationally engineering the 14G2a scFv conferred significant anti-tumor potency. However, the increased sensitivity and potency also resulted in severe neurotoxicity due to non-specific off-tumor effects [23]. Ligand-binding affinities should therefore be optimized by finely balancing the desired strength of anti-tumor response with the potential risk of on-target, off-tumor toxicity. Lastly, affinity thresholds are likely not universal and depend on interconnected factors such as antigen densities on target cells, CAR expression levels, and binding epitope location [24,25].
Affinity modulation can impact CAR signaling and other effector functions such as cytokine secretion, proliferation, and persistence. Low-affinity anti-CD19 CAR (CAT-CAR) (KD = 14•3 nM) showed increased antigen-specific proliferation and increased persistence in vivo compared to the conventional FMC63-based CARs (KD = 0•32 nM), even though both were found to target similar epitopes on the CD19 antigen. IL-2 and IFNγ secretion levels were comparable for the two CARs, while TNFα showed a small increase in the case of the low-affinity CAT-CAR (both in vitro and in vivo) [26]. The authors also note that the faster dissociation constant (Koff) of the scFv used in the CAT-CAR (3 × 10−3 s − 1) compared to the FMC63 scFv (6•8 × 10−5 s − 1) contributes to its low affinity and could result in reduced duration of receptor-ligand interactions. Faster Koff values could also result in increased serial killing and hence improved therapeutic performance. On the contrary, another work using anti-CD123 CAR-T cells indicated that effector functions such as cytokine secretion levels and proliferation were more dependent on CAR expression levels than on affinity [25]. It is likely that once the affinity is sufficiently high, further enhancements to affinity do not translate to further enhancements in CAR performance [17,25]. Indeed, how ligand-binding domain affinity mechanistically affects CAR-T–cell effector functions is as of yet unclear. In physiological TCRs, the strength and duration of TCR-pMHC interactions influence early signaling events as well as later effector responses [27], [28], [29]. Similar relationships between signal strength and affinity parameters such as Kon and Koff may also impact how ligand-binding domain affinities impact CAR function.
The affinity of the ligand-binding domain has proved to be a crucial parameter in CAR design. Yet, it is still a measure of monovalent receptor-ligand interactions. In CAR-T cells as well as in native T cells, the overall strength of interactions is additionally determined by multiple receptor-ligand interactions occurring both due to multiple synapses at the T cell–target cell interface, as well as clustering of receptors at the immune synapse [30], [31], [32]. Avidity is a parameter that accounts for multiple receptor-ligand interactions and is influenced by CAR expression levels, ligand densities on target cells, and affinity of individual ligand-binding domains. In one study, CARs were constructed from scFv sequences targeting HLA-A2 displaying WT1 (Wilms tumor suppressor gene 1) peptide. High affinity as well as high avidity of CARs (concomitant with high CAR expression levels) were implicated in non-specific cross-reactivity with pMHCs displaying irrelevant peptides [32]. In another study, a high-affinity anti-CD123 CAR (KD = 2 nM) expressed at relatively low levels showed significantly lower proliferation and cytokine production despite similar cytotoxicity against target cells compared to a similarly high-affinity anti-CD123 CAR (KD = 1 nM) expressed at a much higher level, indicating avidity-related effects on effector functions [25]. Similarly, Weitjens et al. noted that cytokine secretion (TNFα, IL2) correlated with expression levels of anti-G250 CARs when the CAR-T cells were exposed to high antigen densities [33]. Considerations of avidity necessitate evaluation of ligand-binding domains in the context of CAR-T cells. For instance, in a recent report a library of ~120 affinity-modulated scFv sequences was constructed against CD38. Some of these candidates did not show detectable binding using standard affinity measurement techniques such as bilayer interferometry, but did display binding when tested with ligand-coated beads. Ligand-binding was further confirmed by CAR-T cell killing, indicating avidity-reinforced activation of CAR-T cells [20].
Lastly, multiple molecular engineering methods exist for controlling CAR expression. For example, self-inactivating lentiviral vectors with EF1α promoter have been shown to result in reduced CAR expression levels compared to LTR (long terminal repeat) promoter-based gammaretroviral vectors [34]. Furthermore, integrating CARs into the TRAC locus of T cells resulted in lower but dynamically regulated CAR surface expression compared to retrovirally integrated CARs, and T cells expressing CARs from the TRAC locus exhibited reduced tonic signaling and improved in vivo anti-tumor efficacy [35].
2.2. scFv aggregation
scFv aggregation also plays a role in regulating CAR-T–cell activity, where it has been implicated in tonic signaling. Excessive tonic signaling—signaling in an antigen-independent manner—can eventually cause early exhaustion of T cells [34,[36], [37], [38]]. In one study, framework regions of anti-GD2 14G2a scFv were proposed to be responsible for CAR surface aggregation resulting in tonic signaling and exhaustion [36]. In the same study, tonic signaling was observed in several other CARs such as an anti-ErbB2 CAR (4D5 scFv) and anti-CD22 (H22 and m971 scFv) CARs but not an anti-CD19 CAR (FMC63 scFv). In this study, the authors found that replacing the framework regions of anti-CD19 FMC63 CAR-scFv with the framework regions of anti-GD2 14G2a scFv resulted in increased exhaustion. However, anti-GD2 14G2a CAR modified with framework regions from FMC63 scFv did not express on the cell surface, making it difficult to ascertain whether removal of scFv aggregative sequences would prevent tonic signaling. Another study on tonic signaling also identified that antigen-independent proliferation without exogenous IL-2 was observed in CD28-CD3ζ second-generation anti–c-Met-and anti-Mesothelin CAR-T cells but not in CD28-CD3ζ FMC63-based anti-CD19 CAR-T cells [24]. In addition, the authors also noted a correlation between higher CAR expression and increased continuous antigen independent proliferation. Although the authors did not specifically implicate scFv aggregation, the combinatory effect of the scFv and CD28 costimulatory domain is likely the cause of the continuous proliferation phenotype observed. scFv aggregation or misfolding could be caused by low folding stabilities of the VH or VL domain or exposure of hydrophobic residues at the VH-VL interface; scFv linkers can sterically constrain VH-VL domain interaction and result in oligomerization [37,39]. Elevated CAR expression levels can facilitate dynamic swapping of VH-VL domains between different CAR units and enhance aggregation potential on the cell surface [37]. Particularly, in cases where antigen densities on target cells require higher CAR expression, one should carefully balance the trade-off between high expression and aggregation propensities.
2.3. Antigen epitope location and accessibility
The flexibility of the CAR's modular structure allows for targeting difficult epitopes including larger, bulky cell surface receptors, especially heterogeneously glycosylated tumor-associated molecules like MUC1 or mesothelin (MSLN). Suboptimal performance of an anti-MUC1 SM3-scFv–based CARs was attributed to glycosylation independent steric hindrance [40]. CARs based on an scFv targeting the membrane-proximal region (Region III) of the MSLN molecule showed increased functional response (both cytotoxicity and cytokine secretion) in vitro and in vivo compared to a membrane-distal epitope targeting CAR. The authors attributed this to the rigid structure of the membrane-proximal region that enabled better signal transduction. Additionally, the membrane-distal region of MSLN functionally interacts with proteins such as CA125 (MUC16), which might impede CAR binding [41]. This suggests that apart from steric availability, structural as well as functional aspects of the target epitope need to be included in design considerations for CARs. Novel CAR designs such as bispecific CARs utilizing tandemly connected scFv sequences targeting two antigens may require additional design efforts to identify appropriate CAR structures that allow accessibility to both targets [42]. Epitope location is also important to modulate immune synapse distance, which determines effective cytotoxic granule delivery and kinetic segregation of phosphatases [8,43,44]. A CAR targeting the membrane-distal epitope of CD22 was found to have weaker signaling, lower lytic efficiency, and defective degranulation compared to CARs binding to a membrane-proximal epitope [45]. While the immune synapse distance can be more easily tuned by the spacer domain, as we will articulate below, it is still important to consider epitope location along with functional and structural constraints imposed by the nature of the target.
3. Spacer domain
Spacer domains that connect the scFv to the transmembrane domain lend flexibility to the scFv and help improve efficacy. Functional effects of spacer design modulations are illustrated in Fig. 3. A comprehensive list of selected publications investigating various spacer designs is available in Supplementary Table 2. Appropriate spacer domain engineering can enable recognition of target epitopes that are otherwise sterically inaccessible. The use of a highly flexible IgD hinge instead of a CD28 hinge resulted in better recognition of the sterically hindered MUC1 epitope [40]. Spacer domain modulation can also be used to regulate synaptic cleft distances and hence signaling phenomena such as kinetic segregation. To maintain optimal synapse distance, membrane-distal epitopes usually require shorter spacers whereas membrane-proximal epitopes require longer spacers [21]. Apart from limiting exclusion of inhibitory phosphatases, increasing epitope-paratope distance can also result in impaired delivery of granzymes and perforins to the target cell, thus reducing lytic efficiency. In a physiological T-cell setting, the highly dense immune synapse hinders diffusion of lytic granules, which enhances pore formation by perforins and granzyme delivery [46]. Despite the non-classical nature of CAR-T immune synapses, kinetic segregation and lytic granule delivery are still considered key to CAR-T–cell signaling and killing mechanisms [43,47]. Thus, altering spacer lengths can have a profound effect on cytolytic activity and signaling of CAR-T cells. In an earlier report, first-generation anti-CEA CARs, which bound to a membrane-distal epitope of CEA, were tested with either an IgG1-Fc spacer or no spacer [48]. It was found that addition of the IgG1-Fc spacer reduced secretion of IFNγ without a concomitant loss in lytic efficiency. In an effort to evaluate whether this effect was due to epitope location, the authors tested the same CARs in cell lines that expressed a truncated form of the antigen in a membrane-proximal position. However, this did not affect the trend in IFNγ or lytic efficiency that was noted earlier, which the authors attributed to possible steric hindrances. This study underscores the fact that spacer-domain design considerations should take into account factors such as steric accessibility and ligand density. Spacer length has also been purported to affect mechano-transduction of ligand recognition. CARs with longer spacers (IgG4-Fc) that were generated against soluble homo-dimeric TGF-β showed decreased activation profiles compared to shorter (IgG4 hinge only) spacers [49]. Given that these novel CARs respond to soluble antigens and a synaptic cleft does not exist in this case, it clearly exemplifies the role of the spacer region in mechanically transducing ligand-recognition to T cells.
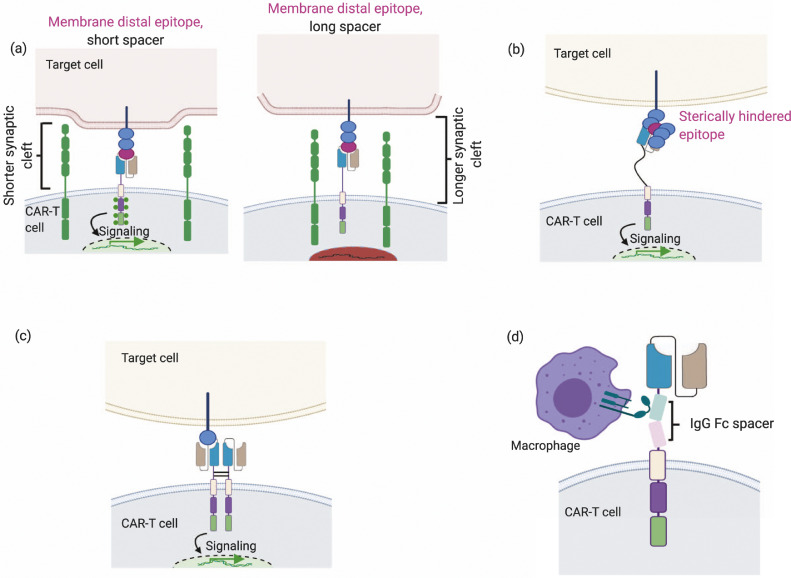
Fig. 3.
Spacer design can be used to modulate synaptic cleft distances, allow flexibility and dimerization and reduce non-specific innate immune responses. (a) Spacer length can be modulated to control synaptic cleft distances, which can possibly regulate signaling. When targeting membrane distal epitopes, short spacers (left) shorten the synaptic cleft, enabling exclusion of phosphatases such as CD45 and hence enhancing phosphorylation of cytoplasmic ITAMs while long spacers (right) lengthen the synaptic cleft and possibly do not exclude phosphatases. (b) Flexible spacers can enable access to sterically hindered epitopes. (c) Dimerization of spacer domains results in increased signal strength and activation stimulus. (d) FcγR interactions arising from IgG based spacers results in activation of innate immune system and decreased efficiency.
Interestingly, although use of a long IgG-Fc spacer can produce the strongest in vitro response for some CARs, overtly strong CAR signaling can also result in impaired in vivo function due to activation-induced cell death (AICD) [50]. In addition to fratricidal AICD, non-specific FcγR interactions can trigger AICD and also elicit an innate immune response, limiting CAR-T–cell persistence in vivo [51], [52], [53], [54]. For CARs where a long spacer is required to achieve optimal spacing between T cells and target cells, IgG1-Fc and IgG4-Fc–based spacers can be mutated to minimize FcγR interactions by substituting the CH2 domain with an IgG2 CH2 domain and/or introducing mutations in other regions that minimize interactions with FcγR. A list of these mutational modifications in IgG1 and IgG4 spacers tested in CAR-Ts is available in Supplementary Table 2. Despite its extremely low binding affinity to FcγR, IgG2-based spacers have only been sparsely used in CARs [55].
Non-IgG–based spacers such as CD8 and CD28 hinge regions have proved equally effective and have been used in clinically approved CAR-T–cell therapies. Alabanza et al. reported that the CD28α hinge region incorporated into an anti-CD19 CAR was shown to increase AICD compared to CD8α hinge CARs [56]. Cytokine production levels (IFNγ and TNFα) were also elevated in CD28 hinge-incorporating CAR-T cells, although there was no significant difference in cytotoxicity or in vivo tumor control. The authors attributed this to structural aspects of the CD28 hinge, which is more prone to dimerization than the CD8α hinge. The authors hypothesized that increased dimerization of CD28 hinge-CARs on the cell surface results in increased activation signals and consequently, greater AICD. Recent work from Majzner et al. demonstrated that CD28 hinge and transmembrane domains decrease the antigen-density threshold for T-cell activation in CD19 CARs compared to their CD8 counterparts [57]. We note in the two examples above the hinge and transmembrane domains are varied together as a single variable, so it is difficult to determine whether the observed differences in CAR function are due to the hinge or transmembrane domain alone or their combination. Additionally, size differences in the CD28 hinge region (45 amino acids) and the CD8 hinge region (39 amino acids) may also contribute to their differing functionalities [57]. Furthermore, another study showed that enhancing antigen-independent dimerization of CARs with S228P mutation in IgG4 hinge improved both in vitro cytotoxicity and in vivo tumor regression [58]. While this study did not delve into AICD, it is evident that spacer-mediated CAR dimerization caused an anti-tumor response different from that reported by Alabanza et al. [56]. Thus, while structural aspects of the hinge region can be exploited to modulate CAR avidity and valency, it is clear from these studies that the functional effects of such changes are not generalizable.
In addition, it is possible that spacer domains can alter the CAR's structural conformation. For instance, in one study the length of CD8α hinge was modulated by truncating or adding a few residues [59]. These modifications produced CARs with altered extracellular conformations, and resulted in dramatically different in vivo and in vitro responses. In this study, the authors were able to identify a novel modified CD8α hinge CAR that elicited no severe cytokine release or neurotoxicity in vivo. While the mechanistic effect of these modifications to CD8α is not yet understood, this study underscores that fact that spacer design critically contributes to CAR-T efficacy.
Apart from playing a key role in CAR signaling, spacers are also commonly used to quantify and purify CAR-positive subsets of T cells after engineering. Anti-Fc antibodies are commonly used for quantifying cell surface expression of IgG-Fc spacer-based CARs. A novel design incorporating Strep-TagII sequence in the CAR spacer region has also been used for detection and purification of CAR-positive cells [60].
4. Transmembrane domain
Transmembrane domains in CAR structures serve as a fulcrum for transducing ligand recognition signals to the intracellular cytoplasmic domain. In a physiological T cell, the transmembrane domains of the TCR-CD3 complex play an imperative role in the assembly of the complex. Of relevance to the CAR structure is the CD3ζ transmembrane domain incorporated in cis with the CD3ζ cytoplasmic domain in first-generation CARs. Dimerization of CD3ζ is mediated by a cysteine residue at position two in the transmembrane domain of CD3ζ. First-generation anti-CEA CARs constructed with a C2G mutation in the transmembrane domain that abrogates dimerization showed a significant impairment in CD69 upregulation when incubated with antigen compared to CARs using the native CD3ζ transmembrane domain [61]. Interestingly, exogenous expression of the CD3ζ domain in a first-generation CAR format led to increased expression of CD3ε as well. In the same study it was noted that first-generation CD3ζ CAR interacts with TCRα and TCRβ chains via ionic interactions between transmembrane domains. These interactions result in both cis and trans signaling mechanisms where cytoplasmic signaling units of endogenous TCR-CD3 complex are involved [61,62]. In another study, Guedan et al. noted that third-generation CARs incorporating ICOS, 4–1BB, and CD3ζ cytoplasmic domains had significantly improved anti-tumor potency when the ICOS cytoplasmic domain was connected to an ICOS transmembrane domain (ICOSTM-ICOS-41BB-CD3ζ) as opposed to a CD8α transmembrane (CD8TM-ICOS-41BB-CD3ζ) [63]. While Wan et al. reported that the transmembrane domain of ICOS constitutively associates with Lck and promotes proximal signaling, it remains to be conclusively tested if this is responsible for increased anti-tumor potency and persistence of the third-generation ICOSTM-ICOS-41BB-CD3ζ observed by Guedan et al. [63,64].
Transmembrane-mediated interactions can also be used to generate novel CAR designs such as split CAR systems that can deliver trans-cytoplasmic domain signaling. Anti-Mesothelin and anti-CD19 CAR systems were constructed by using an scFv-linked KIR domain and a DAP12 domain. This design exploited the natural transmembrane interactions between the KIR2DS2 domain and DAP12 [65]. T cells maintained more stable surface expression of KIR2DS2/DAP12 CARs as compared to CD3ζ CARs, and the authors attributed this to the stability of the KIR2DS2/DAP12 domain in the plasma membrane. Similarly, NKG2D CARs constructed with full-length NKG2D receptors containing the native NKG2D extracellular, transmembrane and cytoplasmic domains allow for transmembrane-mediated interactions with DAP10 and offer the potential for additional “in-trans” co-stimulation with DAP10 [13,14]. Another CAR transmembrane design was based on the FcεRI domain, which consists of three subdomains: α, β, and γ, which are associated through transmembrane interactions. A second-generation “trans” signaling CAR was constructed by replacing the native cytoplasmic domains of γ and β with CD3ζ and 4–1BB respectively. The extracellular domain of the α domain was replaced with an anti-CD19 scFv [66]. Such novel designs have the potential to offer different functionality and modularity to CARs. While the actual signaling mechanisms of “trans” configurations (as opposed to linear CARs) have not been fully elucidated, they may mimic multi-chain TCR architecture and can potentially result in a more effective physiological signaling profile. Fig. 4 and Supplementary Table 3 elucidate the novel engineering strategies afforded by the transmembrane domain.
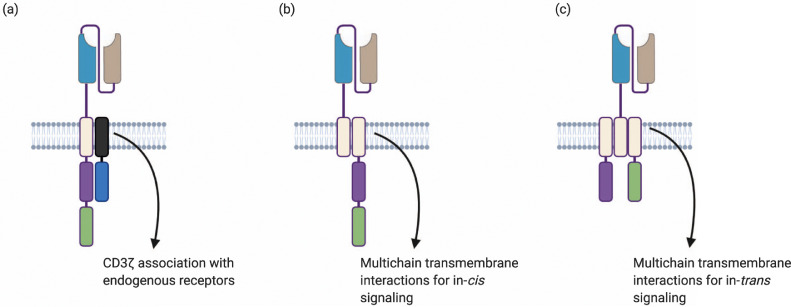
Fig. 4.
Transmembrane domain interactions can afford novel CAR designs. (a) Association of CAR transmembrane domain with endogenous receptors/endogenous transmembrane domains. (b) Multichain transmembrane association for split CAR systems. (c) Multichain transmembrane associations to create in trans co-stimulatory domain signaling.
5. Cytoplasmic domains
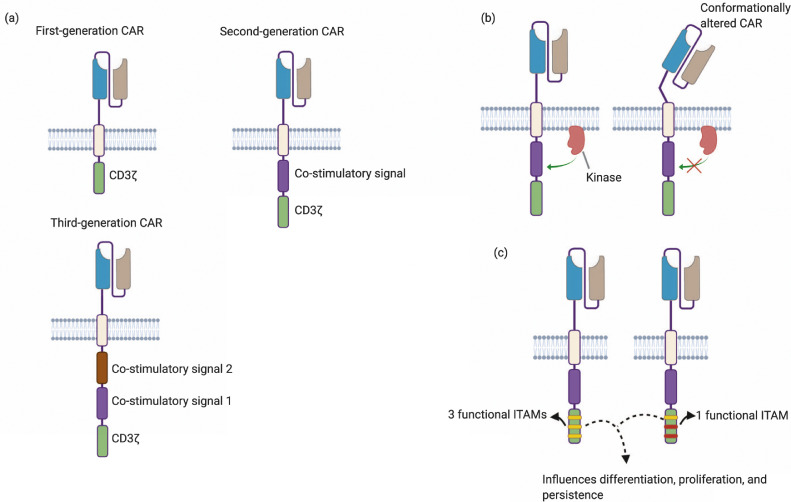
In T cells, the intracellular signaling domain of the TCR-CD3 complex transduces the necessary “signal 1″ to kick-start the signaling cascade. Co-stimulatory receptors, especially CD28, convey “signal 2″ which is important for sustained signaling, prevention of anergy, and proliferation. 4–1BB, ICOS, and OX40 are other co-stimulatory receptors that affect T-cell differentiation pathways, metabolic cycles, as well as apoptosis and activation-induced cell death [67]. In CAR-T cells, co-stimulatory signals are usually included in-cis with the CD3ζ cytoplasmic domain. The number and type of co-stimulatory signals, as well as their order and proximity to the membrane, are critical parameters that have been addressed in literature (Fig. 5). Immunoreceptor Tyrosine Activation Motifs (ITAMs) present on cytoplasmic domains of TCR-CD3 complexes are the phosphorylation sites, which recruit ZAP70, critical for signaling cascades. In T cells, ITAM diversity and number of functional CD3ζ ITAMs are important for optimal signaling [68,69]. In CAR-T cells, the number of functional ITAMs is gaining attention as an important design strategy to ensure efficacy. We have organized a concise list of relevant publications investigating these parameters in Supplementary Table 4. We acknowledge that the breadth of literature in this field is large and encourage readers to also refer to other key review papers [67,70].
Fig. 5.
Number, type and order of co-stimulatory domains as well as ITAM multiplicity can affect CAR-T functionality. (a) Based on the number of co-stimulatory domains used, CARs are classified as first-generation
(no co-stimulatory domain), second-generation (one co-stimulatory domain), or third-generation (two co-stimulatory domains). (b) The order of the co-stimulatory domains can possibly dictate structural compatibility with the transmembrane region and influence conformation of the CAR. It can also affect accessibility of membrane proximal kinases that are critical for signaling. (c) The number of ITAMs on CD3ζ can be modulated to alter effector functions. Mutations in signaling residues in co-stimulatory domains can also be used to regulate effector functions.
5.1. Number and type of co-stimulatory domains
First-generation CARs are typically engineered with primary signaling motifs that provide activating signal (signal 1) upon ligand engagement. Second- and third-generation CARs are engineered to provide one or more co-stimulatory signals (signal 2) along with activating signal (signal 1) upon ligand engagement. Most first-generation CAR-T cells used only the intracellular CD3ζ domain as the primary signaling motif, and showed lack of proliferation and persistence in vivo [71]. Early studies also used the γ-chain of Fc receptors as the primary activating domain, as reviewed by Sadelain et al. [72]. One particular study by Haynes et.al compared first-generation anti-CEA CARs containing FcεR1-γ chain versus CD3ζ cytoplasmic domain [73]. This study showed that CD3ζ-based CARs elicited greater cytotoxicity and IFNγ production compared to FcεR1-γ based CARs, potentially due to the presence of more ITAMs in the CD3ζ domain (3 ITAMs in monomeric CD3ζ vs 1 ITAM in FcεR1-γ). The effects of ITAM multiplicity are discussed in Section 5.3 below.
Second-generation CARs, especially CD28– and 4–1BB–based CARs, have become particularly attractive due to their ability to confer functionalities such as long-term persistence and increased efficacy and are currently utilized in clinically approved therapies, namely Kymriah® and Yescarta® [70,[74], [75], [76], [77], [78], [79]]. Both 4–1BB and CD28 are intensely investigated in literature and some of the key functional modulations they affect are listed in Table 1. It is worth noting that despite poorer in vitro performance compared to its CD28 counterpart, 4–1BB–based CARs tend to result in greater long-term persistence, and the two co-stimulatory domains’ relative clinical efficacy remains unclear due to a lack of head-to-head clinical comparisons [80], [81], [82], [83], [84]. Xiong et al. made the interesting observation that 4–1BB–based CARs form higher-quality immunological synapses than CD28-based CARs and argued that synapse quality can be used as a predictor of in vivo efficacy [85]. In another study, the signaling of CD28– and 4–1BB–based second-generation CARs were compared using a phosphoproteomic mass spectroscopic analysis [86]. Interestingly, it was shown that CARs with CD28 co-stimulatory domains showed faster and higher intensities of phosphorylation, indicating higher signal strengths compared to CARs with a 4–1BB domain. Interestingly, divergent phosphorylation pathways were not detected, suggesting that differences in signaling kinetics and intensity—rather than the types of signaling pathways activated—explain the diverse functional effects of the CD28 and 4–1BB second-generation CARs. In T cells, the signal strength received by the TCRs is dependent on pMHC affinity and density [28,87]. In CAR-T cells, the signal strength is dependent on all of its structural components, as each of them affects signal transduction. In the case of T cells, TCR signal strength is critical in determining positive and negative selection of T cells, differentiation phenotypes, as well as cytokine secretion [27]. Signal strengths can similarly influence CAR function and hence their ultimate efficacy.
Table 1.
Comparison of functional aspects of CD28 and 4–1BB co-stimulatory domains.
| CD28 | 4–1BB |
|---|---|
| - Lower persistence and differentiation towards effector memory phenotype compared to 41BB second-generation CARs[120] -More prone to tonic signaling and causes early exhaustion[24] -Imparts resistance to Tregs in-vitro, in-vivo models however suggested that CD28 co-stimulation causes increased infiltration of Tregs and were less effective against tumors in presence of Tregs[121,122] -Resistant to CTLA4 inhibition[123] -Faster and higher signaling intensity[86] -Does not alter scFv “affinity ceiling” –affinity beyond which IFNγ, IL2 secretion and cytotoxicity do not increase[124] |
-Greater persistence and differentiation towards central memory phenotype compared to CD28 second- generation CARs[120] - Can reduce tonic signaling at optimal expression levels and decrease exhaustion[24,34,36] -Slower and less intense signaling[86] |
Third-generation CARs utilizing both CD28 and 4–1BB domains have been tested against various targets such as CD19, PSMA, GD2 and mesothelin [76,[88], [89], [90], [91]]. In one particular study, differences in intracellular signaling were evaluated for anti-CD19 second-generation CARs with a CD28 co-stimulatory domain and compared with third-generation CARs containing 4–1BB and CD28 co-stimulatory domains. This study revealed that third-generation CAR-T cells showed an overall increase in the phosphorylation status of signaling proteins, indicating potentially higher signal strengths for the third-generation CARs compared to second-generation CARs [92]. Preclinical studies of third-generation anti-PSMA and anti-mesothelin CD28–4–1BB–CD3ζ CARs indicated superior tumor eradication and an increased persistence compared to their second-generation formats [89,91]. Similarly, third-generation ICOS–4–1BB–CD3ζ–based anti-mesothelin CARs had increased anti-tumor potency as well as increased persistence [63]. With regards to B-cell malignancies, a direct comparative clinical study of second-generation CD28-based CARs to third-generation 4–1BB–CD28–based CARs indicated higher persistence and expansion of the third-generation CARs compared to the second-generation formats, particularly in cases where disease burden was low [88]. However, the superiority of third-generation CARs over second-generation counterparts is still a subject of debate. For instance, a study by Abate-Daga et al. comparing the efficacy of second-generation, CD28-based anti-PSCA CARs with third-generation CD28–4–1BB–based CARs indicated that while in vivo persistence of third-generation CARs were generally improved in pre-clinical mouse xenograft models of pancreatic cancer, the second-generation CARs still outperformed the third-generation formats in terms of anti-tumor potency [93]. In another case, in vitro cytokine secretion (IL-2, TNFα) and proliferation were improved in third-generation anti-GD2 CARs containing CD28-OX40-CD3ζ domains as compared to second-generation (CD28-CD3ζ or OX40-CD3ζ) and first-generation (CD3ζ) formats [94]. However, a clinical study from the same group revealed that the clinical performance of the third-generation CAR was not significantly improved compared to previous studies on first-generation CARs reported by Pule et al. and Louis et al. [95], [96], [97]. Apart from the structural differences in co-stimulatory domains, patient heterogeneity and differing treatment regimens may also contribute to the lack of improvement from third-generation CARs. Another study by Hombach et al. reported that third-generation, anti-CEA CD28-CD3ζ-OX40 CARs performed suboptimally compared to second-generation CD28-CD3ζ CARs [98]. The fact that this study used cytokine-induced killer cells (CIKs) makes it difficult to generalize these results to conventional CAR-T cell engineering. Parallel clinical comparisons and detailed mechanistic studies on different co-stimulatory designs will be required to affirm which of these designs are clinically effective. Lastly, Zhao et al. analyzed seven chimeric antigen receptors structures and showed that a second-generation CD28-based CAR co-expressed with 4–1BB ligand (4–1BBL) performed better than a third-generation CAR with both 4–1BB and CD28. This study suggests that both the type and the spatial configuration of co-stimulatory modules matter in CAR function [76].
Other co-stimulatory domains such as OX40 and ICOS also have been utilized in various studies. ICOS signaling domains have been shown to promote Th17 polarization [99]. Anti-GD2 third-generation CARs constructed with CD28 and OX40 co-stimulatory domains showed improved proliferation and expansion compared to second-generation CARs using only the CD28 co-stimulatory domain [94]. Another interesting CAR construct is a third-generation CAR using CD28 and the cytoplasmic domain from TLR2 (Toll-like receptor 2), which not only increased in vivo tumor eradication but also upregulated genes related to migration, synaptic transmission, and cell adhesion [100]. Another novel design utilizes truncated IL2Rβ along with a STAT3-binding motif with a CD28 and CD3ζ domain to activate downstream JAK-STAT cytokine signaling pathways [101]. These studies indicate that guided modulation of the cytoplasmic domain can equip CAR-T cells with novel functionalities.
Multiple co-stimulatory domains and their combinations have been tested in vitro and in vivo, yet there is no consensus on the ideal design for CAR-T cells. The choice of co-stimulatory domain is likely dependent on clinical indications as well as on other CAR modules. Tonic signaling in anti-GD2 second-generation CARs caused by scFv-mediated aggregation was eliminated when a 4–1BB cytoplasmic domain was used instead of CD28 [36]. Anti-GD2 CARs based on the same scFv were tested in second-generation format with 4–1BB co-stimulation or third-generation format with both 4–1BB and CD28 [34,90]. Interestingly, these studies showed a uniform distribution of CAR molecules on the surface and minimal surface aggregation unlike the aggregated punctae of CARs observed in the CD28-based second-generation CAR in the study by Long et al. [36]. These studies indicate that co-stimulatory domains could influence CAR surface expression and distribution. Affinity thresholds, most commonly associated with the ligand-binding domain, can also be altered by the number and type of cytoplasmic domains used. The in vitro lytic efficiency of 4–1BB–based CARs was reduced with a lower-affinity scFv, while CD28-based CARs showed comparable lytic efficiencies with a wide range of scFv affinities [102]. Second-generation anti-PSCA CARs with a 4–1BB domain were less reactive to target cells presenting lower antigen densities compared to CD28-based CARs [103]. In light of these studies, it is evident that the optimal design for the cytoplasmic domain should be based on its synergistic effect with other components of the CAR.
5.2. Order of co-stimulatory domains
For second-generation and subsequent CAR designs, the order of the co-stimulatory domains has been found to influence their effector functions. An early study exploring functional differences between CD28-CD3ζ CARs and CD3ζ-CD28 CARs found that CD28-CD3ζ T cells secreted higher levels of IL-2 and importantly were able to undergo repeated cycles of stimulation and expansion, critical for sustained activity [74]. The authors posit that the superior performance of CD28- CD3ζ could be due to better structural integrity, which enhances signal transduction or the proximity of CD28 to membrane-proximal signaling kinases such as Lck.
Recent work has shown that CD28-CD3ζ, OX40-CD3ζ and CD28-OX40-CD3ζ produced comparable amounts of IL-10, an anti-inflammatory cytokine that is known to reduce anti-tumor efficacy [94]. Hombach et al. found that CD28-CD3ζ-OX40 T cells secreted significantly lower levels of IL-10 compared to a CD28-CD3ζ control [104]. It would be interesting to test whether these contradictory effects of OX40 are due to different positioning of the cytoplasmic domains or due to differences in the nature of the scFvs, target antigen, or other components of the CAR. Guedan and coworkers explored a variety of cytoplasmic domain combinations of 4–1BB, ICOS, and CD3ζ and found that T cells expressing a CAR with an ICOS-4–1BB-CD3ζ cytoplasmic domain and ICOS transmembrane domain resulted in enhanced expansion and persistence in vivo and 100% tumor regression within 35 days. Notably, a CAR with ICOS–4–1BB–CD3ζ but using a CD8 transmembrane domain displayed only modest tumor regression. Given that this CD8-modified CAR also expressed lower levels of IL-13 and IL-6, the authors posit that the membrane-proximal section of the cytoplasmic domain has a significant role in determining the CAR-T–cell's cytokine profile [63].
5.3. ITAM multiplicity
ITAMs are the sequence motifs (YXXL/I) in the cytoplasmic domains of receptors in hematopoietic cells [105]. In physiological T cells, ZAP70, a protein kinase, is recruited to doubly phosphorylated ITAMs resulting in activation of the signaling cascade. CAR-T cells, despite forming non-classical immune synapses, have also been shown to activate the ZAP70 kinase in an antigen-dependent manner [47,92]. Key differences exist between T cells and conventional CAR-T cells in terms of the number of ITAMs engaged in signaling (three for monomeric and six for homodimeric CARs vs. ten for T cells) [106]. Significant differences in activity of CARs and TCRs have been attributed to the difference in number of ITAMs [106,107]. Increasing the number of ITAMs from three to six by having two CD3ζ domains in cis resulted in an increased fraction of activated cells. Reducing the number of ITAMs from three to two enabled selective recognition of cells expressing high target densities [108]. Majzner et al. also demonstrated that doubling of CD3ζ ITAMs from three to six in 4–1BB–based second-generation CARs increased T-cell proliferation, IL-2 production, as well as in vivo anti-tumor response [57]. In another study each of the ITAMs of CD3ζ were mutated and it was found that both the number of functional ITAMs and their position influenced CAR-T–cell proliferation, in vivo tumor eradication, as well as differentiation phenotype [109]. In the same study, the authors showed that a single membrane-proximal functional ITAM in conjunction with a CD28 cytoplasmic domain was able to sustain proliferation, persistence, and cytotoxicity. In physiological T cells, ITAM multiplicity has been implicated in negative selection of T cells in the thymus, proliferation, and cytokine secretion [110]. In CAR-T cells, signaling mechanisms that are affected by ITAMs are not clear. Particularly, the availability of in-trans ITAMs potentiates distinct signaling mechanisms in T cells. The position of ITAMs themselves influences its affinity towards ZAP70 kinase in T cells [105]. These aspects of ITAMs have implications in CAR design and improved understanding of these aspects of signaling could immensely inform CAR-T design.
6. Conclusions and outstanding questions
Each module of the CAR structure influences CAR function both independently and synergistically. Reducing the affinity of scFv has been shown to improve selective recognition of tumor and thus reduce on-target, off-tumor toxicity. However, lowering the affinity can also result in reduced activation and anti-tumor cytotoxicity. Choosing the right cytoplasmic domains can augment these signals. For example, in one study, lytic efficiencies of CARs incorporating a CD28 cytoplasmic domain were not affected by the scFv affinities, while 4–1BB–incorporating CARs showed differential lytic efficiencies correlating with scFv affinities [102]. Multiple parameters such as CD28 co-stimulation, elevated levels of CAR expression, and aggregation-prone scFv sequences have been implicated in tonic signaling, a phenomenon that can result in early CAR-T–cell exhaustion and reduced tumor eradication. The length and nature of spacer domains impact immune synapse distances and are critical in determining cytolysis. In conjunction with scFv, the spacer domain is crucial in enabling target-epitope accessibility. Transmembrane domains are key structural components that have the potential to be exploited for novel CAR systems with in trans coupling of co-stimulatory domains and CD3ζ domains. In this review, we have focused on the design of the CAR molecule itself, but it is also important to acknowledge that parameters other than the CAR protein can significantly impact CAR-T–cell function. For example, choice of vector system for transduction and site of insertion of CARs have a deep impact on efficacy [34,35,111].
Lastly, a number of novel innovations in CAR designs has led to development of “universal” and switchable systems which can be used to conveniently tune CAR-modified T cells to target, in principle, any antigen of interest [93,112]. These novel designs usually consist of T cells engineered with an extracellular adapter domain that binds to soluble ligand-binding domains. Similar to conventional CARs, the adapter domain is connected to transmembrane and intracellular cytoplasmic signaling units. SUPRA CARs utilize a leucine-zipper extracellular domain connected to the transmembrane and cytoplasmic signaling domains (zipCAR); scFvs targeting the antigen of interest are fused to a cognate leucine-zipper domain that binds to the extracellular moiety of the zipCAR [113]. Other designs use specific affinity tags such as biotin or peptide neoepitopes (PNE) [58,114]. These novel “universal” designs offer a number of functional benefits, such as targeting multiple antigens simultaneously and allowing for regulation of effector functions by modulating the dose of the ligand-binding moiety. Despite these advantages, the design of novel CAR systems still suffers from similar challenges as conventional CARs, and their implementation requires additional soluble components, each with its own design and delivery considerations. Due to the functional similarity of conventional and novel CARs, design principles and strategies developed for conventional CARs can be translated to novel CAR design and development.
Many studies have employed rational protein design and/or library screening to optimize CAR sequences. CAR components can be modified in a combinatorial or sequential manner depending on their function in vitro and in vivo. For example, CARs targeting the orphan G protein-coupled receptor class C group 5 member D (GPRC5D) were constructed using select scFv sequences obtained from a phage-display library generated against the target epitope. scFv sequences were chosen based on binding capacity and incorporated into CARs in VH/VL or VL/VH formats. A combinatorial library was constructed with varied spacer domains and orientations of the select scFvs [55]. In another study, a bispecific CAR using two distinct scFv sequences targeting CD19 and CD20 sequentially identified spacer and scFv linker requirements to enable optimal targeting of both antigens [42]. While these studies and many others have provided immense information about CAR function and design strategies, they are largely empirical, tedious, and are limited in the number of designs one can exploit in an experiment. The optimal CAR design for a given application will likely depend on interrogation of many variables of each module in an interdependent and combinatorial fashion, a process that can benefit from single-cell–based, high-throughput functional assays in the future [115].
A second major hurdle in CAR-T development is to identify in vitro quantitative parameters that can be correlated to in vivo efficacy. It has been shown that parameters such as in vitro activation and cytokine release do not always correlate with desired in vivo outcomes [53,[116], [117], [118]]. Ultimately, in vitro functional assays for CAR-T design need to be developed and validated in correlation with in vivo outcomes not only from animal models but also from clinical data. Along this line, polyfunctionality, namely the ability of T cells to produce more than one type of cytokine, has gained attention as an in vitro measurement that correlates with in vivo efficacy. Pre-infusion anti-CD19 CAR-T products were tested for 32 different cytokines and chemokines using a novel single-cell–based assay, and it was reported that increased polyfunctionality correlated with improved patient response [119]. Functional assay platforms that can quantitatively interrogate parameters that are predictive of in vivo CAR-T–cell function would greatly facilitate the elucidation of relevant CAR design principles, enable better understanding of CAR-T–cell biology, and facilitate the discovery of CAR formats with novel functionalities [115]. Ultimately, such efforts can streamline the immunotherapeutic discovery process, resulting in effective CAR-T candidates at lower costs and reduced development time.
7. Search strategy and selection criteria
Relevant papers on CAR-T design were chosen from searches on Google scholar and PUBMED. Search terms “CAR-T design”, “CAR-T cytoplasmic domain”, “CAR-T spacer”, “CAR-T scFv”, “CAR-T biology” were used to identify literature relevant to this article. In addition, we also used “TCR-CD3 complex”, “signal strengths” “polyfunctionality”, “TCR biology” to identify relevant articles from T-cell biology to corroborate and compare functional mechanisms between T cells and CAR-T cells (in some instances). We explored relevant and selected literature ranging from 1997 - March 2020.
Funding sources
This work was supported by the NIH R21CA219225, DOD/CDMRP W81XWH-17–1–0522, AR3T P2CHD086843, and NSF SBIR #1,913,404 to W.Z. J.J was supported by Otto W. Shaler scholarship. M.M was supported by NSF GRFP (USA) (DGE-1,839,285). R.D was supported by UC Irvine School of Medicine Dean's Summer Research Scholarship Award (USA). A.J.H was supported by the Mark Foundation of Cancer Research (grant to Y.Y.C.).
Declaration of interests
Ms. Jayaraman reports funding Otto W. Shaler Scholarship. Mr. Mellody reports funding from the National Science Foundation. Ms. Desai reports funding from the Dean's Summer Research Scholarship. Mr. Hou, Ms. Fung, and Ms. Pham have nothing to disclose. Dr. Chen reports funding from the Mark Foundation for Cancer Research and patents US62/248,685, US62/091,854, and US2019/036,731 pending. Dr. Zhao reports grants from the NIH, the DOD/CDMRIP, NSF, and Amberstone Biosciences Inc.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102931.
Appendix. Supplementary materials
References
- 1.Sadelain M., Brentjens R., Riviere I. The basic principles of chimeric antigen receptor (CAR) design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng P.P., Kros J.M., Li J. Approved CAR T cell therapies: ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov Today. 2018;23:1175–1182. doi: 10.1016/j.drudis.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z., Chen Y., Francisco N.M., Zhang Y., Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8:539–551. doi: 10.1016/j.apsb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi B.D., Yu X., Castano A.P., Bouffard A.A., Schmidts A., Larson R.C. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 5.Majzner R.G., Mackall C.L. Tumor antigen escape from car t-cell therapy. Cancer Discov. 2018;8:1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 6.D'Aloia M.M., Zizzari I.G., Sacchetti B., Pierelli L., Alimandi M. CAR-T cells: the long and winding road to solid tumors review-article. Cell Death Dis. 2018;9:282–293. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y.J., Dougan M., Jailkhani N., Ingram J., Fang T., Kummer L. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc Natl Acad Sci USA. 2019;116:7624–7631. doi: 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S., Riddell S.R. Engineering CAR-T cells : design concepts. Trends Immunol. 2015;8:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S., Shevlin E., Vedvyas Y., Zaman M., Park S., Hsu Y.M.S. Micromolar affinity CAR T cells to ICAM-1 achieves rapid tumor elimination while avoiding systemic toxicity. Sci Rep. 2017;7:1–15. doi: 10.1038/s41598-017-14749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahlon K.S., Brown C., Cooper L.J.N., Raubitschek A., Forman S.J., Jensen M.C. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 11.Brown C.E., Aguilar B., Starr R., Yang X., Chang W.C., Weng L. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol Ther. 2018;26:31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Starr R., Chang W.C., Aguilar B., Alizadeh D., Wright S.L. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med. 2020;12:1–12. doi: 10.1126/scitranslmed.aaw2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber A., Rynda A., Sentman C.L. Chimeric NKG2D Expressing T Cells Eliminate Immunosuppression and Activate Immunity within the Ovarian Tumor Microenvironment. J Immunol. 2009;183:6939–6947. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sentman C.L., Meehan K.R. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–159. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dotti G., Gottschalk S., Savoldo B., Brenner M.K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies D.M., Foster J., van der Stegen S.J.C., Parente-Pereira A.C., Chiapero-Stanke L., Delinassios G.J. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med. 2012;18:565–576. doi: 10.2119/molmed.2011.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chmielewski M., Hombach A., Heuser C., Adams G.P., Abken H. T Cell Activation by Antibody-Like Immunoreceptors: increase in Affinity of the Single-Chain Fragment Domain above Threshold Does Not Increase T Cell Activation against Antigen-Positive Target Cells but Decreases Selectivity. J Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Jiang S., Fang C., Yang S., Olalere D., Pequignot E. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caruso H.G., Hurton L V. Najjar A, Rushworth D, Ang S, Olivares S, et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drent E., Themeli M., Poels R., de Jong-Korlaar R., Yuan H., de Bruijn J. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol Ther. 2017;25:1946–1958. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudecek M., Lupo-Stanghellini M.T., Kosasih P.L., Sommermeyer D., Jensen M.C., Rader C. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynn R.C., Feng Y., Schutsky K., Poussin M., Kalota A., Dimitrov D.S. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30:1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman S.A., Nunez-Cruz S., Moghimi B., Li L.Z., Gershenson Z.T., Mourelatos Z. High-Affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res. 2018;6:36–46. doi: 10.1158/2326-6066.CIR-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.M Frigault M., Lee J., Basil M.C., Carpenito C., Motohashi S., Scholler J. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3:356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcangeli S., Rotiroti M.C., Bardelli M., Simonelli L., Magnani C.F., Biondi A. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol Ther. 2017;25:1933–1945. doi: 10.1016/j.ymthe.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghorashian S., Kramer A.M., Onuoha S., Wright G., Bartram J., Richardson R. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25:1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 27.Gascoigne N.R.J., Rybakin V., Acuto O., Brzostek J. TCR Signal Strength and T Cell Development. Annu Rev Cell Dev Biol. 2016;32:327–348. doi: 10.1146/annurev-cellbio-111315-125324. [DOI] [PubMed] [Google Scholar]
- 28.Corse E., Gottschalk R.A., Allison J.P. Strength of TCR–Peptide/MHC Interactions and In Vivo T Cell Responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 29.Wu H., Witzl A., Ueno H. Assessment of TCR signal strength of antigen-specific memory CD81 T cells in human blood. Blood Adv. 2019;3:2153–2163. doi: 10.1182/bloodadvances.2019000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viganò S., Utzschneider D.T., Perreau M., Pantaleo G., Zehn D., Harari A. Functional avidity: a measure to predict the efficacy of effector T cells. Clin Dev Immunol. 2012;2012:1–14. doi: 10.1155/2012/153863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong S., Malecek K., Johnson L.A., Yu Z., De Miera E.V.S., Darvishian F. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci USA. 2013;110:6973–6978. doi: 10.1073/pnas.1221609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oren R., Hod-Marco M., Haus-Cohen M., Thomas S., Blat D., Duvshani N. Functional Comparison of Engineered T Cells Carrying a Native TCR versus TCR-like Antibody–Based Chimeric Antigen Receptors Indicates Affinity/Avidity Thresholds. J Immunol. 2014;193:5733–5743. doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]
- 33.Weijtens M.E.M., Hart E.H., Bolhuis R.L.H. Functional balance between T cell chimeric receptor density and tumor associated antigen density: CTL mediated cytolysis and lymphokine production. Gene Ther. 2000;7:35–42. doi: 10.1038/sj.gt.3301051. [DOI] [PubMed] [Google Scholar]
- 34.Gomes-Silva D., Mukherjee M., Srinivasan M., Krenciute G., Dakhova O., Zheng Y. Tonic 4-1BB Costimulation in Chimeric Antigen Receptors Impedes T Cell Survival and Is Vector-Dependent. Cell Rep. 2017;21:17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eyquem J., Mansilla-Soto J., Giavridis T., Van Der Stegen S.J.C., Hamieh M., Cunanan K.M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long A.H., Haso W., Shern J., Wanhainen K., Murgai M., Ingaramo M. 4-1BB Costimulation Ameliorates T Cell Exhaustion Induced by Tonic Signaling of Chimeric Antigen Receptors. Nat Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajina A., Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther. 2018;17:1795–1815. doi: 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan M.A., Schambach A. Engineering CAR-T Cells for Improved Function Against Solid Tumors. Front Immunol. 2018;9:1–17. doi: 10.3389/fimmu.2018.02493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gil D., Schrum A.G. Strategies to stabilize compact folding and minimize aggregation of antibody-based fragments. Adv Biosci Biotechnol. 2013;4:73–84. doi: 10.4236/abb.2013.44A011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkie S., Picco G., Foster J., Davies D.M., Julien S., Cooper L. Retargeting of Human T Cells to Tumor-Associated MUC1: the Evolution of a Chimeric Antigen Receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z., Jiang D., Yang H., He Z., Liu X., Qin W. Modified CAR T cells targeting membrane-proximal epitope of mesothelin enhances the antitumor function against large solid tumor. Cell Death Dis. 2019;10:1–12. doi: 10.1038/s41419-019-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zah E., Lin M.Y., Anne S.B., Jensen M.C., Chen Y.Y. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benmebarek M.R., Karches C.H., Cadilha B.L., Lesch S., Endres S., Kobold S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci. 2019;20:1–21. doi: 10.3390/ijms20061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang Z.N.L., CARs Chen YY. Synthetic Immunoreceptors for Cancer Therapy and Beyond. Trends Mol Med. 2017;23:430–450. doi: 10.1016/j.molmed.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.James S.E., Greenberg P.D., Jensen M.C., Lin Y., Till B.G., Raubitschek A.A. Antigen sensitivity of CD22-specific chimeric T cell receptors is modulated by target epitope distance from the cell membrane. J Immunol. 2008;180:7028–7038. doi: 10.4049/jimmunol.180.10.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodsworth D.J., Dunsing V., Coombs D. Design Parameters for Granzyme-Mediated Cytotoxic Lymphocyte Target-Cell Killing and Specificity. Biophys J. 2015;109:477–488. doi: 10.1016/j.bpj.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davenport A.J., Cross R.S., Watson K.A., Liao Y., Shi W., Prince H.M. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci USA. 2018;115:2068–2076. doi: 10.1073/pnas.1716266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guest R.D., Hawkins R.E., Kirillova N., Cheadle E.J., Arnold J., O'Neill A. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–211. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 49.Chang Z.L., Lorenzini M.H., Chen X., Tran U., Bangayan N.J., Chen Y.Y. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol. 2018;14:317–324. doi: 10.1038/nchembio.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuünkele A., Johnson A.J., Rolczynski L.S., Chang C.A., Hoglund V., Kelly-Spratt K.S. Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell fas-FasL-Dependent AICD. Cancer Immunol Res. 2015;3:368–379. doi: 10.1158/2326-6066.CIR-14-0200. [DOI] [PubMed] [Google Scholar]
- 51.Jonnalagadda M., Mardiros A., Urak R., Wang X., Hoffman L.J., Bernanke A. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23:757–768. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hombach A., Hombach A.A., Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc spacer domain in the extracellular moiety of chimeric antigen receptors avoids off-target activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 53.Almåsbak H., Walseng E., Kristian A., Myhre M.R., Suso E.M., Munthe L.A. Inclusion of an IgG1-Fc spacer abrogates efficacy of CD19 CAR T cells in a xenograft mouse model. Gene Ther. 2015;22:391–403. doi: 10.1038/gt.2015.4. [DOI] [PubMed] [Google Scholar]
- 54.Hudecek M., Sommermeyer D., Kosasih P.L., Silva-Benedict A., Liu L., Rader C. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith E.L., Harrington K., Staehr M., Masakayan R., Jones J., Long T.J. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11:1–14. doi: 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alabanza L., Pegues M., Geldres C., Shi V., Wiltzius J.J.W., Sievers S.A. Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol Ther. 2017;25:2452–2465. doi: 10.1016/j.ymthe.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majzner R.G., Rietberg S.P., Sotillo E., Dong R., Vachharajani V.T., Labanieh L. Tuning the Antigen Density Requirement for CAR T Cell Activity. Cancer Discov. 2020;10:702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodgers D.T., Mazagova M., Hampton E.N., Cao Y., Ramadoss N.S., Hardy I.R. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci USA. 2016;113:459–468. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ying Z., Huang X.F., Xiang X., Liu Y., Kang X., Song Y. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25:947–953. doi: 10.1038/s41591-019-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L., Sommermeyer D., Cabanov A., Kosasih P., Hill T., Riddell S.R. Inclusion of Strep-tagII in design of antigen receptors for T-cell immunotherapy. Nat Biotechnol. 2016;34:430–434. doi: 10.1038/nbt.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bridgeman J.S., Hawkins R.E., Bagley S., Blaylock M., Holland M., Gilham D.E. The Optimal Antigen Response of Chimeric Antigen Receptors Harboring the CD3ζ Transmembrane Domain Is Dependent upon Incorporation of the Receptor into the Endogenous TCR/CD3 Complex. J Immunol. 2010;184:6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 62.Bridgeman J.S., Ladell K., Sheard V.E., Miners K., Hawkins R.E., Price D.A. CD3ζ-based chimeric antigen receptors mediate T cell activation via cis- and trans-signalling mechanisms: implications for optimization of receptor structure for adoptive cell therapy. Clin Exp Immunol. 2014;175:258–267. doi: 10.1111/cei.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guedan S., Posey A.D., Shaw C., Wing A., Da T., Patel P.R. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3:1–17. doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan Z., Shao X., Ji X., Dong L., Wei J., Xiong Z. Transmembrane domain-mediated Lck association underlies bystander and costimulatory ICOS signaling. Cell Mol Immunol. 2020;17:143–152. doi: 10.1038/s41423-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang E., Wang L.C., Tsai C.Y., Bhoj V., Gershenson Z., Moon E. Generation of potent T-cell immunotherapy for Cancer using DAP12-based, multichain, chimeric immunoreceptors. Cancer Immunol Res. 2015;3:815–826. doi: 10.1158/2326-6066.CIR-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juillerat A., Marechal A., Filhol J.M., Valton J., Duclert A., Poirot L. Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep. 2016;6:1–7. doi: 10.1038/srep18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinkove R., George P., Dasyam N., McLellan A.D. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunol. 2019;8:1–14. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ardouin L., Boyer C., Gillet A., Trucy J., Bernard A.M., Nunes J. Crippling of CD3-ζ ITAMs does not impair T cell receptor signaling. Immunity. 1999;10:409–420. doi: 10.1016/s1074-7613(00)80041-2. [DOI] [PubMed] [Google Scholar]
- 69.Bettini M.L., Chou P.-.C., Guy C.S., Lee T., Vignali K.M., Vignali D.A.A. Cutting Edge: CD3 ITAM Diversity Is Required for Optimal TCR Signaling and Thymocyte Development. J Immunol. 2017;199:1555–1560. doi: 10.4049/jimmunol.1700069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Der Stegen S.J.C., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brocker T. Chimeric Fv-ζ or Fv-ε receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 72.Sadelain M., Brentjens R., Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haynes N.M., Snook M.B., Trapani J.A., Cerruti L., Jane S.M., Smyth M.J. Redirecting Mouse CTL Against Colon Carcinoma: superior Signaling Efficacy of Single-Chain Variable Domain Chimeras Containing TCR-ζ vs FcεRI-γ. J Immunol. 2001;166:182–187. doi: 10.4049/jimmunol.166.1.182. [DOI] [PubMed] [Google Scholar]
- 74.Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 75.Savoldo B., Brenner M., Dotti G. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Z., Condomines M., van der Stegen S.J.C., Perna F., Kloss C.C., Gunset G. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finney H.M., Akbar A.N., Lawson A.D.G. Activation of Resting Human Primary T Cells with Chimeric Receptors: costimulation from CD28, Inducible Costimulator, CD134, and CD137 in Series with Signals from the TCRζ Chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 78.Finney H.M., Lawson A.D., Bebbington C.R., Weir A.N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 79.Imai C., Mihara K., Andreansky M., Nicholson I.C., Pui C.H., Geiger T.L. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 80.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:1–6. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porter D.L., Hwang W.T., Frey N.V. Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:1–13. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P.T., Carpenter R.O., Maryalice S.S. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiong W., Chen Y., Kang X., Chen Z., Zheng P., Hsu Y.H. Immunological Synapse Predicts Effectiveness of Chimeric Antigen Receptor Cells. Mol Ther. 2018;26:963–975. doi: 10.1016/j.ymthe.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salter A.I., Ivey R.G., Kennedy J.J., Voillet V., Rajan A., Alderman E.J. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal. 2018;11:1–18. doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Panhuys N. TCR signal strength alters T-DC activation and interaction times and directs the outcome of differentiation. Front Immunol. 2016;7:1–14. doi: 10.3389/fimmu.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramos C.A., Rouce R., Robertson C.S., Reyna A., Narala N., Vyas G. In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin's Lymphomas. Mol Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong X.S., Matsushita M., Plotkin J., Riviere I., Sadelain M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI 3 kinase/AKT/Bcl-X L activation and CD8 T cell-mediated tumor eradication. Mol Ther. 2010;18:413–420. doi: 10.1038/mt.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quintarelli C., Orlando D., Boffa I., Guercio M., Polito V.A., Petretto A. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology. 2018;7:1–16. doi: 10.1080/2162402X.2018.1433518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carpenito C., Milone M.C., Hassan R., Simonet J.C., Lakhal M., Suhoski M.M. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karlsson H., Svensson E., Gigg C., Jarvius M., Olsson-Strömberg U., Savoldo B. Evaluation of intracellular signaling downstream chimeric antigen receptors. PLoS ONE. 2015;10:1–20. doi: 10.1371/journal.pone.0144787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abate-Daga D., Lagisetty K.H., Tran E., Zheng Z., Gattinoni L., Yu Z. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25(12):1003–1012. doi: 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pulè M.A., Straathof K.C., Dotti G., Heslop H.E., Rooney C.M., Brenner M.K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 95.Heczey A., Louis C.U., Savoldo B., Dakhova O., Durett A., Grilley B. CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma. Mol Ther. 2017;25:2214–2224. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V. Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hombach A.A., Rappl G., Abken H. Arming Cytokine-induced Killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation. Mol Ther. 2013;21:2268–2277. doi: 10.1038/mt.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guedan S., Chen X., Madar A., Carpenito C., McGettigan S.E., Frigault M.J. ICOS-based chimeric antigen receptors program bipolar TH17/ TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai Y., Weng J., Wei X., Qin L., Lai P., Zhao R. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia. 2018;32:801–808. doi: 10.1038/leu.2017.249. [DOI] [PubMed] [Google Scholar]
- 101.Kagoya Y., Tanaka S., Guo T., Anczurowski M., Wang C.H., Saso K. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drent E., Poels R., Ruiter R., Van De Donk N.W.C.J., Zweegman S., Yuan H. Combined CD28 and 4-1BB costimulation potentiates affinity-tuned chimeric antigen receptor-engineered t cells. Clin Cancer Res. 2019;25:4014–4025. doi: 10.1158/1078-0432.CCR-18-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Priceman S.J., Gerdts E.A., Tilakawardane D., Kennewick K.T., Murad J.P., Park A.K. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology. 2018;7:1–15. doi: 10.1080/2162402X.2017.1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hombach A.A., Heiders J., Foppe M., Chmielewski M., Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hayes S., Love E. ITAM-mediated Signaling by the T-Cell Antigen Receptor. Cold Spring Harb Perspect Biol. 2010;2:1–11. doi: 10.1101/cshperspect.a002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harris D.T., Kranz D.M. Adoptive T Cell Therapies: a Comparison of T Cell Receptors and Chimeric Antigen Receptors. Trends Pharmacol Sci. 2016;37:220–230. doi: 10.1016/j.tips.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harris D.T., Hager M.V, Smith S.N., Cai Q., Stone J.D., Kruger P. Comparison of T Cell Activities Mediated by Human TCRs and CARs That Use the Same Recognition Domains. J Immunol. 2018;200:1088–1100. doi: 10.4049/jimmunol.1700236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.James J.R. Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. Sci Signal. 2018;11:1–9. doi: 10.1126/scisignal.aan1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feucht J., Sun J., Eyquem J., Ho Y.J., Zhao Z., Leibold J. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pitcher L.A., Van Oers N.S.C. T-cell receptor signal transmission: who gives an ITAM. Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Zhang C., Liu J., Zhong J.F., Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:3–8. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao J., Lin Q., Song Y., Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11:25–29. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cho J.H., Collins J.J., Wong W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell. 2018;173:1426–1438. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lohmueller J.J., Ham J.D., Kvorjak M., Finn O.J. mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology. 2017;7:1–6. doi: 10.1080/2162402X.2017.1368604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Segaliny A.I., Li G., Kong L., Ren C., Chen X., Wang J.K. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip. 2018;18:3733–3749. doi: 10.1039/c8lc00818c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of t cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song D.G., Ye Q., Carpenito C., Poussin M., Wang L.P., Ji C. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB) Cancer Res. 2011;71:4617–4627. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gattinoni L., Klebanoff C.A., Palmer D.C., Wrzesinski C., Kerstann K., Yu Z. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rossi J., Paczkowski P., Shen Y.W., Morse K., Flynn B., Kaiser A. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018;132:804–814. doi: 10.1182/blood-2018-01-828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawalekar O.U., O’ Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey A.D. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 121.Loskog A., Giandomenico V., Rossig C., Pule M., Dotti G., Brenner M.K. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 122.Kofler D.M., Chmielewski M., Rappl G., Hombach A., Riet T., Schmidt A. CD28 costimulation impairs the efficacy of a redirected T-cell antitumor attack in the presence of regulatory T cells which can be overcome by preventing lck activation. Mol Ther. 2011;19:760–767. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Condomines M., Arnason J., Benjamin R., Gunset G., Plotkin J., Sadelain M. Tumor-targeted human T cells expressing CD28-based chimeric antigen receptors circumvent CTLA-4 inhibition. PLoS ONE. 2015;10:1–15. doi: 10.1371/journal.pone.0130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chmielewski M., Hombach A.A., Abken H. CD28 cosignalling does not affect the activation threshold in a chimeric antigen receptor-redirected T-cell attack. Gene Ther. 2011;18:62–72. doi: 10.1038/gt.2010.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.