Abstract
Whole blood donation rapidly removes approximately 10% of a donor's blood volume and stimulates substantial changes in iron metabolism and erythropoiesis. We sought to identify donors who benefit from iron supplementation, describe the nature of the benefit, and define the time course for recovery from donation. Blood samples were collected over 24 weeks following whole blood donation from 193 participants, with 96 participants randomized to 37.5 mg daily oral iron. Changes in total body, red blood cell (RBC), and storage iron, hepcidin, erythropoietin, and reticulocyte count were modeled using semiparametric curves in a mixed model. and the changes were compared among six groups defined by baseline ferritin (<12; 12–50; ≥50 ng/mL) and iron supplementation. The effect of oral iron on storage and RBC iron recovery was minimal in donors with baseline ferritin ≥50 ng/mL, but sizeable when ferritin was <50 ng/mL. Iron initially absorbed went to RBC and storage iron pools when ferritin was <12 ng/mL but went mostly to RBCs when ferritin was ≥12 ng/mL. Donors with ferritin ≥12 ng/mL had a “ripple” increase in reticulocytes ~100 days after donation indicating physiological responses occur months following donation. Thus, iron supplements markedly enhance recovery from whole blood donation in donors with ferritin <50 ng/mL. However, full recovery from donation requires over 100 days when taking iron. The findings also highlight the value of the study of blood donors for understanding human hemoglobin and iron metabolism and their usefulness for future studies as additional biomarkers are discovered.
1 |. INTRODUCTION
Whole blood donation results in the loss of 525 mL whole blood in 8 to 10 minutes and removes 238 to 265 mg iron, depending on the donor's hematocrit and sex.1 In the United States, whole blood donation is permitted every 56 days as long as fingerstick hemoglobin remains above 12.5 g/dL for females, or 13.0 g/dL for males. However, there is no requirement for maintenance of iron stores, which take 180 days or longer to recover.2–4 Consequently, many frequent donors of both sexes develop storage iron deficiency if not supplemented with oral iron to replace red blood cells (RBC) lost from donation.5–9 Industry consensus in the US recommends supplemental iron to “frequent” donors, generally agreed to represent two RBC units donated per year for premenopausal women and three for men and postmenopausal women, but there are no clear recommendations for other blood donors.
The acute blood loss associated with blood donation produces substantial immediate and longer term physiological responses that alter iron metabolism in order to produce new RBC.3,4,10,11 These responses are associated with changes in biomarkers of hemoglobin and iron status that can be assessed through laboratory testing of peripheral blood samples. These include ferritin, a measure of iron stores, which gradually decreases following blood donation;3,4 hepcidin, a hormone that decreases iron absorption from the gastrointestinal tract and iron release from macrophages, which rapidly decreases following blood donation4,12; and erythropoietin, a hormone that stimulates erythropoiesis, which rapidly increases following blood donation.4
The only pre-donation testing performed to assess a donor's hemoglobin and iron status is fingerstick hemoglobin measurement, which detects anemia, but does not accurately reflect the donor's iron stores, which can vary widely between donors. Approximately 17% of blood donors have absent iron stores, as defined by having plasma ferritin <12 ng/mL.13 Here, we utilized plasma samples from the National Heart Lung and Blood Institute (NHLBI) Recipient Epidemiology Donor Study III (REDS-III) Hemoglobin and Iron Recovery Study (HEIRS)3 to examine how oral iron supplementation supports post-whole blood donation recovery of peripheral blood biomarkers of hemoglobin and iron status in donors with differing severity of pre-donation iron deficiency. Changes in total body iron (TBI), RBC iron, storage iron, hepcidin, erythropoietin, and reticulocyte count were examined over the 24 weeks following donation of whole blood in 193 individuals, who were randomized to receive 37.5 mg daily oral iron or not. Distinct differences in RBC and iron parameters between donors with differing severity of iron deficiency at enrollment were identified in response to the acute blood loss and during the 24-week recovery period.
2 |. METHODS
2.1 |. Study enrollment
The REDS-III HEIRS study (clinicaltrials.gov identifier: NCT01555060) enrolled at US four blood centers as previously reported.3 The study was approved by institutional review boards at each site and the coordinating center. All subjects provided informed consent for participation in the study. Participants had not donated in the previous 4 months and were not taking iron supplements prior to enrollment. They donated whole blood and were randomized to take 37.5 mg elemental iron (ferrous gluconate) daily for 24 weeks, or no iron at their first follow-up visit 3 to 8 days after donation. There were 215 participants in the study. Here we report on data obtained from 193, who returned for all seven follow-up visits. Iron supplement compliance was 92.5%, and subjects verified they did not take non-study iron supplements.
2.2 |. Laboratory testing
Peripheral blood EDTA specimens were obtained at pre-donation and at seven post-donation visits: between days 3–8, and then at weeks 2, 4, 8, 12, 16, and 24. All plasma samples were batch tested at a central laboratory for ferritin, hepcidin, and erythropoietin. Ferritin was measured by immunoassay (ADVIA Centaur, Siemens, Deerfield, IL USA). Plasma hepcidin was measured by ELISA at Intrinsic LifeSciences (San Diego, CA USA). Plasma erythropoietin was measured by chemiluminescent immune assay (Access EPO, Beckman Coulter, Brea, CA USA). Plasma soluble transferrin receptor (sTfR) was measured on pre-donation and 24-week samples using an immuno-turbidometric assay (Tina-quant sTfR assay, Roche Diagnostics, Indianapolis, IN USA). Complete blood count (CBC) was performed on each sample. Reticulocyte count was performed at each time point, but only on samples from two of the four centers. CBC and reticulocyte analyses were performed locally within 24 hours of sample collection using Sysmex or Coulter hematology analyzers.
2.3 |. Calculation of TBI, RBC iron, and storage iron
The RBC iron was calculated using estimated blood volume and venous hemoglobin concentration. Storage iron was calculated based on sTfR and ferritin values as previously described.14 Multiple imputation with 10 imputed data sets was used to obtain storage iron values for time points between pre-donation and final sample without measured sTfR.3 The imputation regression model used gender and a quadratic curve for log-ferritin and was trained on the pre-donation and final visit data. The TBI was computed as the sum of RBC iron and storage iron in each dataset. Equations used for calculation of iron compartments have been reported.14,15
2.4 |. Statistical analyses
The main analytical approach was mixed effects modeling of measured values over time within each participant. This approach fits a separate trend curve for each subject in a way that the general shape of the curve is the same for all subjects, but the parameters have subject-specific values to capture between-subject heterogeneity. The trend model had a parametric backbone of an exponential curve with an asymptote to capture the overall expected pattern of a change from a baseline value to a potentially different stable value, and an additive smooth term describing local deviations from the main trend.16 This semi-parametric model can describe any trend over time that has a horizontal asymptote as time increases, while having an explicit parameter (d in the equation below) that can explicitly describe the difference between the baseline value and the asymptotic value. The model for the additive smooth term was selected to satisfy two requirements: (a) the smooth term needs to have an asymptotic value of zero, so that the exponential backbone can capture all the asymptotic behavior; and (b) the model needs to enable inference about quantities of interest such as values and locations of minima and maxima, rate of change, etc. We chose cubic splines, that is, smoothly connected piecewise cubic functions, to model the smooth terms, as they can be described with an equation that is easily differentiable for calculating rates of changes and extrema. The B-spline basis parametrization of cubic splines was chosen specifically, as the asymptotic restriction was easy to impose in this parametrization by using only components with a zero derivative at the upper boundary. The selecting trend model can be summarized using the following equation:
where A is the baseline value, d is the long-term shift in the baseline, and Bi(t) are the first k+1(out of k+3) cubic B-spline basis functions with k knots with weights ci. The boundary knots were at the extremes of the data (days 0 and 190), and the internal knots at days 9, 21, and 42. The parameters of the exponential curve (A, d) and the coefficients of the B-spline terms (ci) were allowed to vary by the fixed effects - randomization group, baseline ferritin group, and their interaction, as well as by donors via independently normally distributed random effects. Quantities of interest, such as maximum value, or highest rate of change were extracted from the fitted models using numerical optimization and/or root finding on the fitted function or its derivative. The fitting algorithm produced estimates and standard errors for the model coefficients, but not for the derived quantities of interest. Error propagation was used to estimate their standard errors, specifically they computed using the delta-method from the variance-covariance matrix of the model estimates and the Jacobian of the extraction function obtained via numerical approximation. For plotting, visit days were aligned between donors at the mean day of each visit. The actual days for visits were used during modeling. For storage and total body iron the analyses were performed on each imputed dataset, and the results combined using Rubin's formula.17 Analyses were performed using R 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
3 |. RESULTS
3.1 |. Study population and enrollment iron status
Of the 193 participants, 118 (61%) were female and 41 (21%) were ≥60 years old. Enrollment was targeted to recruit participants with baseline ferritin <26 or ≥26 ng/mL to delineate those with or without iron deficient erythropoiesis.3 However, for the present analysis participants were stratified into <12, 12–50 and ≥50 ng/mL enrollment ferritin groups. These cutoffs allowed examination of the response to, and recovery from, whole blood donation in groups in which all participants had severe iron deficiency (<12 ng/mL), all participants did not have iron deficiency (≥50 ng/mL), or some participants had iron deficiency (12–50 ng/mL).18 Thirty participants had ferritin <12 ng/mL - 13 took iron; 116 had ferritin 12–50 ng/mL - 61 took iron; and 47 had ferritin ≥50 ng/mL - 22 took iron. Demographic characteristics, enrollment TBI, RBC iron, storage iron, erythropoietin, hepcidin and reticulocyte count are presented in Table S1. Plasma biomarker recovery curves were analyzed as described in Methods. All analytic results are presented in Tables S2–7. These analyses were used to compare biomarker recovery between the six donor groups as presented below.
3.2 |. Iron supplements enhance recovery of TBI most strongly in donors with ferritin <50 ng/mL
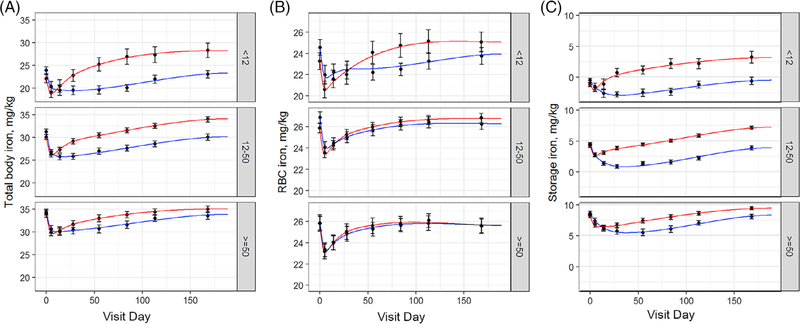
TBI represents combined RBC and storage iron. TBI decreased immediately following whole blood donation in all groups and then recovered towards a stable value at varying rates depending on baseline ferritin and iron use (Figure 1A). The maximal rate of TBI increase in those taking iron with ferritin <12 ng/mL peaked 2 weeks following donation at 17.2 mg/day, indicating the substantial ability of these donors to absorb supplemental iron (Figure S1). Donors with ferritin <50 ng/mL not taking iron had TBI below baseline about 16 to 20 weeks longer compared to those taking iron. In donors with ferritin ≥50 ng/mL the 24-week change in TBI was greater in those taking iron (1.1 mg/kg above baseline) than in those not taking iron (0.5 mg/kg below baseline). The corresponding improvement in TBI was 3- to 5-fold greater in those with baseline ferritin <50 ng/mL.
FIGURE 1.
Iron supplements enhance recovery of TBI and RBC iron following whole blood donation when baseline ferritin is < 50 ng/mL and degree and duration of storage iron deficiency following whole blood donation. A, Change in TBI following whole blood donation. Curves were analyzed for baseline, baseline shift at 24 weeks, maximum decrease from baseline, maximum rate of increase, and time of minimum. B, Change in RBC iron following whole blood donation. Curves were analyzed for baseline, baseline shift at 24 weeks, and maximal rate of increase. C, Change in storage iron following whole blood donation. Curves were analyzed for baseline, baseline shift at 24 weeks, maximal decrease from baseline, maximal rate of increase, and time of minimum. Red lines are donors who took 37.5 mg daily oral iron. Blue lines are donors who did not take iron
3.3 |. Iron supplements enhance recovery of RBC iron when baseline ferritin is <50 ng/mL
Baseline RBC iron in the ferritin <12 ng/mL group (24.0 mg/kg) was lower than in the 12–50 ng/mL (26.3 mg/kg; P = .075) or ≥50 ng/mL (25.8 mg/kg; P = .049) groups, but was not different between the 12–50 and ≥ 50 ng/mL groups (P = .46) (Table S1). Thus, on average, RBC iron does not decrease until ferritin is <12 ng/mL. RBC iron decreased 10% between baseline and the initial return visit in all groups (Figure 1B). Its recovery towards baseline depended on baseline ferritin and iron use. Iron supplements increased the maximal RBC iron recovery rate 1.9-fold in the 12–50 ng/mL group (P = .004). This increase was subtle, but sizeable, occurring in the first 25 days after donation. A similar increase was not present in the <12 ng/mL group. However, this was due to an initial burst of RBC iron between the second and third visits in the no iron group, which may represent stress erythropoiesis. Following this, iron supplements substantially increased the RBC iron recovery rate in the <12 ng/mL group (P < .0001 after day 21). Iron supplements had no effect on RBC iron recovery rate when ferritin was ≥50 ng/mL and returned to baseline at 24 weeks in these donors regardless of iron use.
3.4 |. Iron supplements substantially mitigate storage iron loss and decrease time to recovery when ferritin is <50 ng/mL
Storage iron decreased immediately following whole blood donation in all groups (Figure 1C). Iron supplements mitigated the maximum decrease of storage iron from baseline by two- to 3-fold in all baseline ferritin groups (P = .03; P <.001; P = .008 for ferritin <12; 12–50; ≥50 ng/mL, respectively) and decreased the time to minimum storage iron, from over 30 days in groups not taking iron to four, six and 13 days in <12 (P < .001), 12–50 (P <.001), and ≥50 (P = .04) ng/mL ferritin groups taking iron, respectively. Thus, iron supplements substantially decreased the degree and length of storage iron deficiency that occurs following whole blood donation when baseline ferritin was <50 ng/mL with more modest effects when ferritin was ≥50 ng/mL. Storage iron was above baseline at 24 weeks in all groups taking iron. The largest increase was in the ferritin <12 ng/mL group (4.26 mg/kg), and was progressively lower in the 12–50 (2.98 mg/kg), and ≥50 (1.26 mg/kg) ferritin groups (P < .001 for all). Among those not taking iron, the 12–50 ng/ mL ferritin group not did not recover baseline iron stores at 24 weeks (−0.56 mg.kg; P = .02), while those with ferritin <12 ng/mL or > 50 ng/ mL recovered to baseline. However, those with ferritin <12 ng/mL continued to have severe iron deficiency.
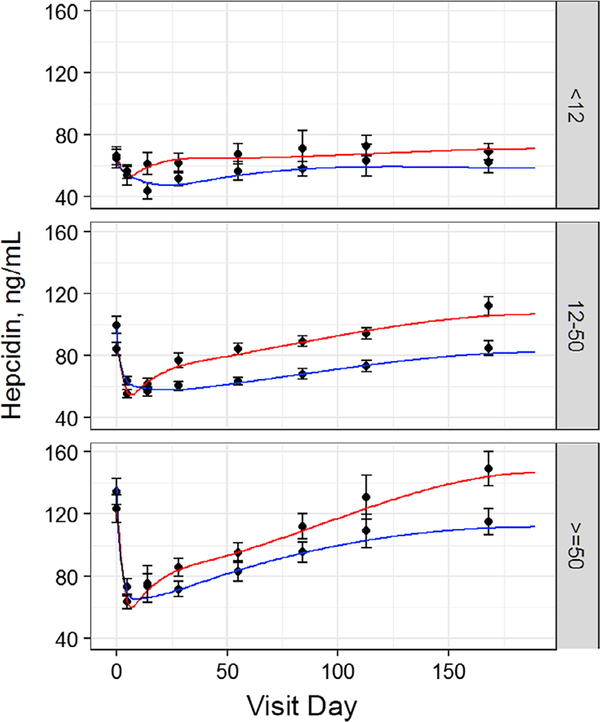
3.5 |. Iron supplements accelerate hepcidin recovery when baseline ferritin is ≥12, but not <12 ng/mL
Baseline hepcidin was progressively lower with lower baseline ferritin. Hepcidin dropped immediately following whole blood donation (Table S1; Figure 2). The magnitude of the drop progressively increased with higher baseline ferritin such that all groups had similar hepcidin (~50 ng/mL) at the first return visit. The rate and extent of hepcidin recovery varied depending on baseline ferritin and iron use. The estimated hepcidin nadir was Day 23 in the <12 and 12–50 ng/mL groups not taking iron vs approximately day 6 in those taking iron (P < .001 for both), mirroring changes observed in storage iron for these groups (Figure 1C). However, when ferritin was ≥50 ng/mL, the estimated hepcidin nadir was day 6 regardless of iron use, occurring earlier than the nadir of storage iron curve in the ≥50 ng/mL ferritin group not taking iron. Notably, the hepcidin nadir was the only difference observed when comparing the recovery curves of the <12 ng/mL ferritin groups with or without iron. These curves were remarkably flat regardless of iron supplements with no difference in 24-week hepcidin compared to baseline, reflecting continued iron deficiency in these donors.
FIGURE 2.
Iron supplements accelerate hepcidin recovery when ferritin is ≥12, but not < 12 ng/mL. Change in hepcidin following whole blood donation. Curves were analyzed for baseline, baseline shift at 24 weeks, maximum decrease from baseline, maximum rate of increase, and time of minimum. Red line is donors who took 37.5 mg daily oral iron. Blue line is donors who did not take iron
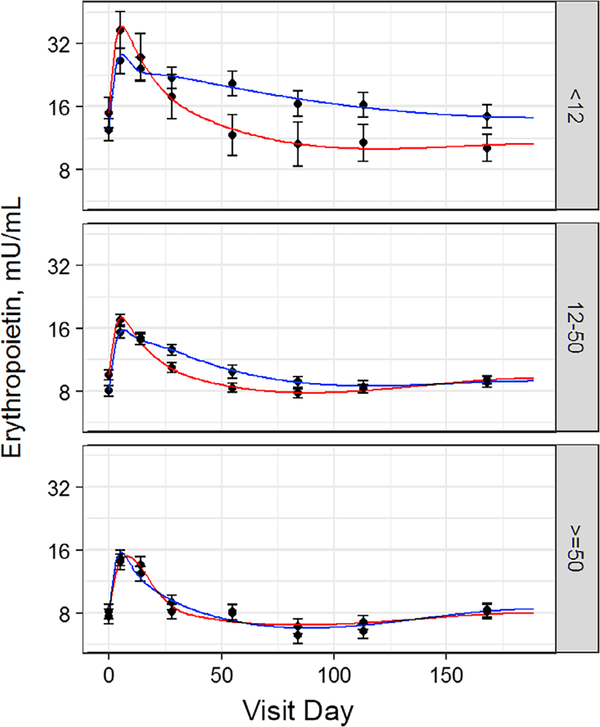
3.6 |. Iron supplements decrease the duration of elevated erythropoietin after donation when baseline ferritin is <50 ng/mL
Baseline erythropoietin was progressively reduced with higher ferritin (Table S1, Figure 3). Erythropoietin approximately doubled immediately following whole blood donation and then decreased towards baseline. A striking aspect of erythropoietin recovery was the more rapid decrease in those with ferritin <50 ng/mL taking iron when compared to those not taking iron. The <12 ng/mL ferritin group taking iron returned towards baseline at 4% per day, while those not taking iron returned at 1% per day (P = .002). The 12–50 ng/mL ferritin group taking iron returned at 3% per day, while those not taking iron returned at 1% per day (P < .001). In contrast, iron supplements had no effect in the ≥50 ng/mL ferritin group.
FIGURE 3.
Iron supplements decrease the duration of elevated erythropoietin following whole blood donation when baseline ferritin is < 50 ng/mL. Change in erythropoietin following whole blood donation. Curves were analyzed for baseline, baseline shift at 24 weeks, maximum increase, and maximum rate of decrease after day14. Red line is donors who took 37.5 mg daily oral iron. Blue line is donors who did not take iron
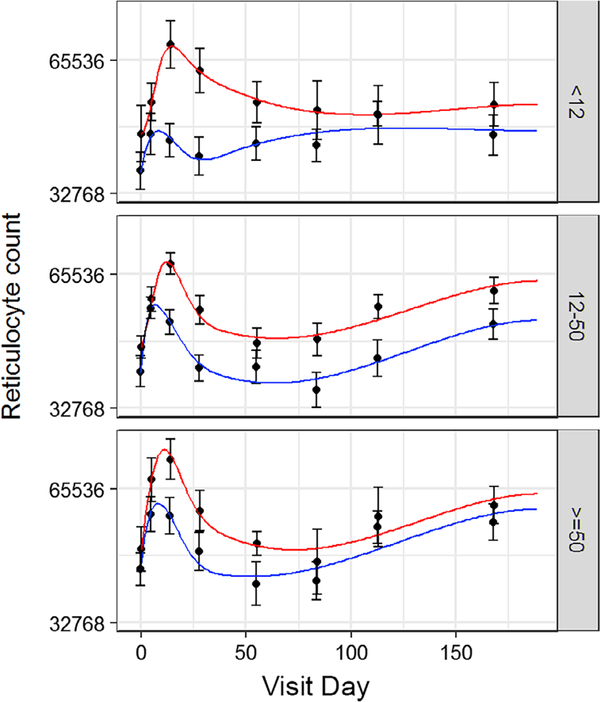
3.7 |. Iron supplements enhanced reticulocyte response
Reticulocytes increased immediately following whole blood donation in all groups (Figure 4). The initial reticulocyte peak was higher in donors taking iron, and iron supplements prolonged the period that reticulocyte count remained elevated. A secondary increase in the reticulocyte count occurred approximately 100 days after whole blood donation. This “ripple” effect was most prevalent in donors with ferritin ≥12 ng/mL and occurred regardless of iron use. However, it was not associated with observable changes in RBC iron.
FIGURE 4.
Iron supplements prolonged the post-whole blood donation elevation in reticulocyte count. Change in reticulocyte count following whole blood donation. Curves were analyzed for baseline, time of initial maximum, and maximum initial increase. Red line is donors who took 37.5 mg daily oral iron. Blue line is donors who did not take iron
4 |. DISCUSSION
In HEIRS, whole blood donors were randomized to take low-dose iron daily vs no iron for 24 weeks after donating.3 We previously found hemoglobin recovered at 4–5 weeks in those taking iron vs 11–23 weeks in those not taking iron. Ferritin recovered at 11 weeks in those taking iron vs >24 weeks in those not taking iron.3 There were no differences in recovery based on donor age (≥60 vs <60 years old) or sex.3 The HEIRS participants were recruited to have enrollment ferritin <26 or ≥ 26 ng/mL, which resulted in many having ferritin <12 or ≥ 50 ng/mL. This allowed for the analyses presented here examining how iron supplementation and the severity of iron deficiency altered post-whole blood donation recovery of peripheral blood biomarkers of iron metabolism and erythropoiesis. The findings highlighted a substantial effect of iron supplements on storage and RBC iron recovery when baseline ferritin was <50 ng/mL compared to more nominal effects when ferritin was ≥50 ng/mL. We estimate that over 50% of all female donors and over 25% of all male donors have ferritin <50 ng/mL.13 Several aspects of iron and erythropoiesis physiology occurring during recovery from whole blood donation were also identified. They included incorporation of approximately equal amounts of gastrointestinal absorbed iron into RBC, and storage iron pools during the period of maximal iron absorption in donors with baseline ferritin <12 ng/mL, while, iron incorporated primarily into the RBC pool in donors with ferritin ≥12 ng/mL; a blunted hepcidin response to iron when ferritin was <12 ng/mL, and effects on erythropoietin and reticulocytes that varied with baseline ferritin and iron supplement use.
Iron supplements increased the rate and extent of TBI recovery following whole blood donation. This was most evident when baseline ferritin was <50 ng/mL, where both RBC and storage iron recovered beyond baseline at 24 weeks, indicating that these donors had baseline iron stores and hemoglobin below their natural set point. Donors with ferritin <12 ng/mL had a substantial response to iron supplements approximately 2 weeks following whole blood donation when peak iron absorption was 17.2 mg/day, or 46% of the daily dose consumed by the donor. Increases in TBI in these donors were largest in the first 4 weeks suggesting that donating blood stimulates a rapid but transient increase in gastrointestinal iron absorption. Therefore, donors should be encouraged to take iron immediately following donation to take advantage of this physiologic response,1 rather than shortly before their next donation, which is a common donor behavior.19
The percentage of iron that incorporated into RBC vs storage iron varied with baseline ferritin and iron supplement use. In donors not taking iron, storage iron was used for new RBC synthesis, as indicated by the extended period of decreasing storage iron that occurred while RBC iron was recovering. This occurred even when baseline ferritin was ≥50, where iron stores decreased for 39 days in those not taking iron vs 12 days in those taking iron. Thus, iron supplements may benefit even iron replete donors by minimizing risk for transient iron deficiency in the post-donation period and associated possible adverse effects, including fatigue, pica or restless legs syndrome.20–24 In donors taking iron, the maximal rate of RBC iron increase was the same in all three ferritin groups, while the maximal rate of storage iron increase was 5-fold higher donors with ferritin <12 ng/mL than in the other donors. Thus, iron initially absorbed from the gastrointestinal tract both replaced iron stores and incorporated into new RBCs in severely iron deficient donors, while it primarily incorporated into new RBCs in other donors.
Iron deficiency, anemia, and increased erythropoietic activity are all associated with whole blood donation and with decreased plasma hepcidin.25,26 Accordingly, plasma hepcidin decreased following whole blood donation in all donors. Interestingly, hepcidin decreased considerably more in those with higher ferritin, such that at the first return visit hepcidin was approximately equal in all ferritin groups. This decrease to a common “floor” level presumably allowed for maximal iron absorption and mobilization of iron stores in response to the acute blood loss from donation. In contrast to donors with higher baseline ferritin, hepcidin remained low for the entire 24-week period in donors with ferritin <12 ng/mL, regardless of iron use, presumably to promote a prolonged period of maximal iron absorption in these severely iron deficient donors. However, TBI in the <12 ng/mL ferritin group taking iron began to level off after about 4 weeks, indicating that gastrointestinal iron absorption was decreasing despite the low hepcidin. Thus, there appear to be physiological mechanisms regulating post-donation iron absorption in donors with severe iron deficiency that are not associated with plasma hepcidin and distinct from donors with less severe iron deficiency. These findings are consistent with those of Roe and colleagues who found that plasma hepcidin levels account for only 36% of the variation in iron absorption by healthy men,27 and of Moretti and colleagues who found that 65% of fractional iron absorption variability can be explained by differences in TBI.28 Erythroferone,29 HIF2α,30,31 or another yet to be identified physiological regulator of iron absorption,27 also may play important roles in recovery from blood donation.
Baseline erythropoietin approximately doubled in a rapid response to whole blood donation. Following its initial peak, erythropoietin declined towards baseline. This decline was delayed in the <12 and 12–50 ng/mL ferritin groups not taking iron compared to other groups. Since erythropoietin senses hypoxemia, its slower decline in these donors may reflect their lower hemoglobin and prolonged relative cellular hypoxemia within the kidney. The initial increase in erythropoietin was associated with a corresponding increase in the reticulocyte count. Reticulocytes remained elevated longer in groups taking iron suggesting that the immediate reticulocyte response is dependent on iron availability as well as erythropoietin. Interestingly, the reticulocyte counts then began to rise again around day 100 in those with baseline ferritin ≥12 ng/mL. It is unclear why a similar response was not observed in those with ferritin <12 ng/mL but may be related to the continued low amount of storage iron in these donors. Since the lifetime of a human RBC is approximately 120 days, this “ripple” increase most likely represented new RBC synthesis to replace RBCs produced in response to the whole blood donation as they approached the end of their 120-day lifespan. This demonstrates that physiological responses to a whole blood donation occur well past 56 days, the minimum inter-donation interval for donors in the United States.
Whole blood donors are a unique population, because many have severe iron deficiency but are otherwise healthy, and undergo a procedure that rapidly removes approximately 10% of their RBC mass. HEIRS allowed for study of iron absorption and hemoglobin production following donation, and the hormonal regulation of these responses in donors with different degrees of iron deficiency. Although not powered to define age or sex associated differences in recovery, which may differ for some of the biomarkers examined, the findings provided new information of the biochemical and physiological mechanisms that modulate human iron absorption and erythropoiesis. This study also provided detailed information about the recovery iron and hemoglobin following blood donation and, therefore, information on donor management. Perhaps most importantly, the findings indicate that iron supplements markedly enhance recovery from blood donation in donors with ferritin <50 ng/mL and suggest that optimal donor management would include measurement of pre-donation ferritin, providing low-dose iron supplements to those with ferritin <50 ng/mL, and informing donors that full recovery from donation requires over 100 days, even if they take daily iron.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI contracts NHLBI HHSN2682011-00001I, −00002I, −00003I, −00004I, −00005I, −00006I, −00007I, −00008I, and −00009I. The authors would like to express their deep gratitude to the RBC-Omics research staff at the participating blood centers and testing lab for its exceptional performance and contribution to this project.
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), domestic component, is the responsibility of the following persons:
HUBS
A.E. Mast and J.L. Gottschall, Versiti, Milwaukee, Wisconsin.
D.J. Triulzi and J.E. Kiss, The Institute for Transfusion Medicine (now Vitalant Northeast Division), Pittsburgh, Pennsylvania.
E.L. Murphy and E.M. St. Lezin, University of California, San Francisco (UCSF), and Laboratory Medicine,
Department of Veterans Affairs Medical Center, San Francisco, California.
E.L. Snyder, Yale University School of Medicine, New Haven, Connecticut R.G. Cable, American Red Cross Blood Services, Farmington, Connecticut.
Footnotes
CONFLICT OF INTEREST
B.R.S. serves on the advisory board of HemaStrat. AEM receives research grant funding from Novo Nordisk and has received honoraria from Novo Nordisk for serving on Advisory Boards. The other authors have no competing interests.
DATA COORDINATING CENTER
D.J. Brambilla and M.T. Sullivan, Research Triangle International, Rockville, Maryland.
CENTRAL LABORATORY
M.P. Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, California.
PUBLICATION COMMITTEE CHAIRMAN
R.Y. Dodd, American Red Cross, Holland Laboratory, Rockville, Maryland.
STEERING COMMITTEE CHAIRMAN
S.H. Kleinman, University of British Columbia, Victoria, British Columbia, Canada.
National Heart, Lung, and Blood Institute, National Institutes of Health.
S.A. Glynn, K.B. Malkin Bethesda, Maryland.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Cable RG, Brambilla D, Glynn SA, et al. Effect of iron supplementation on iron stores and total body iron after whole blood donation. Transfusion. 2016;56:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mast AE, Lee TH, Schlumpf KS, et al. The impact of HFE mutations on haemoglobin and iron status in individuals experiencing repeated iron loss through blood donation*. Br J Haematol. 2012;156: 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schotten N, Pasker-de Jong PC, Moretti D, et al. The donation interval of 56 days requires extension to 180 days for whole blood donors to recover from changes in iron metabolism. Blood. 2016;128:2185–2188. [DOI] [PubMed] [Google Scholar]
- 5.Mast AE, Foster TM, Pinder HL, et al. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion. 2008;48:2197–2204. [DOI] [PubMed] [Google Scholar]
- 6.Finch CA, Cook JD, Labbe RF, Culala M. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood. 1977;50: 441–447. [PubMed] [Google Scholar]
- 7.Simon TL, Garry PJ, Hooper EM. Iron stores in blood donors. JAMA. 1981;245:2038–2043. [PubMed] [Google Scholar]
- 8.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milman N, Sondergaard M. Iron stores in male blood donors evaluated by serum ferritin. Transfusion. 1984;24:464–468. [DOI] [PubMed] [Google Scholar]
- 10.Di Angelantonio E, Thompson SG, Kaptoge S, et al. Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): a randomised trial of 45 000 donors. Lancet. 2017;390: 2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaptoge S, Di Angelantonio E, Moore C, et al. Longer-term efficiency and safety of increasing the frequency of whole blood donation (INTERVAL): extension study of a randomised trial of 20 757 blood donors. Lancet Haematol 2019;6:e510–e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. [DOI] [PubMed] [Google Scholar]
- 13.Spencer BR, Guo Y, Cable RG, et al. Iron status and risk factors for iron depletion in a racially/ethnically diverse blood donor population. Transfusion. 2019;59:3146–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. [DOI] [PubMed] [Google Scholar]
- 15.Bialkowski W, Kiss JE, Wright DJ, et al. Estimates of total body iron indicate 19 mg and 38 mg oral iron are equivalent for the mitigation of iron deficiency in individuals experiencing repeated phlebotomy. Am J Hematol. 2017;92:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wold S. Spline Functions in Data Analysis. Dent Tech. 1974;16(1): 1–11. [Google Scholar]
- 17.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 18.Ali MA, Luxton AW, Walker WH. Serum ferritin concentration and bone marrow iron stores: a prospective study. Can Med Assoc J. 1978; 118:945–946. [PMC free article] [PubMed] [Google Scholar]
- 19.Young S, Fink A, Geiger S, Marbella A, Mast AE, Schellhase KG. Community blood donors' knowledge of anemia and design of a literacy-appropriate educational intervention. Transfusion. 2010;50:75–79. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Larsh SE, Gurney CW. Iron therapy in chronically fatigued, nonanemic women: a double-blind study. Ann Intern Med. 1960;52: 378–394. [DOI] [PubMed] [Google Scholar]
- 21.Chansky MC, King MR, Bialkowski W, et al. Qualitative assessment of pica experienced by frequent blood donors. Transfusion. 2017;57: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant BJ, Yau YY, Arceo SM, Hopkins JA, Leitman SF. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer BR, Kleinman S, Wright DJ, et al. Restless legs syndrome, pica, and iron status in blood donors. Transfusion. 2013;53:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krayenbuehl PA, Battegay E, Breymann C, Furrer J, Schulthess G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118:3222–3227. [DOI] [PubMed] [Google Scholar]
- 25.Talbot NP, Smith TG, Lakhal-Littleton S, et al. Suppression of plasma hepcidin by venesection during steady-state hypoxia. Blood. 2016; 127:1206–1207. [DOI] [PubMed] [Google Scholar]
- 26.Nemeth E, Ganz T. Regulation of Iron Metabolism by Hepcidin. Annu Rev Nutr. 2006;26:323–342. [DOI] [PubMed] [Google Scholar]
- 27.Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ. Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr. 2009;89:1088–1091. [DOI] [PubMed] [Google Scholar]
- 28.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981–1989. [DOI] [PubMed] [Google Scholar]
- 29.Kautz L, Jung G, Valore EV, et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastrogiannaki M, Matak P, Keith B, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.