Abstract
Biomedical research is dominated by relatively few nonhuman animals to investigate healthy and disease conditions. Research has overrelied on these models due to their well-described genomes, the capability to control specific genes, and the high rate of reproduction. However, recent advances in large-scale molecular sequencing experiments have revealed, in some cases, the limited similarities in experimental outcomes observed in common rodents (i.e., mice) compared with humans. The value of more varied comparative animal models includes examples such as long-term body weight regulation in seasonally breeding hamsters as a means to help understand the obesity epidemic, vocal learning in songbirds to illuminate language acquisition and maintenance, and reproduction in cichlid fish to discover novel genes conserved in humans. Studying brain genes in prairie voles and cichlids advanced knowledge about social behavior. Taken together, experiments on diverse animal species highlight nontraditional systems for advancing our understanding of human health and well-being.
Keywords: animal, model, neuroendocrinology, physiology, behavior
Introduction
During Charles Darwin’s travels on the Beagle, he conversed with a Spanish lawyer and a German naturalist, Renous. Renous asked the Spanish lawyer “what he thought of the King of England sending out a collector to their country, to pick up lizards and beetles, and to break stones?” To which the Spanish lawyer replied, “No man is so rich as to send out people to pick up such rubbish” (Darwin, 1839, p. 130). Investment in basic research has a long and distinguished tradition, and we are fortunate that governments continue to provide the essential financial support. Every year, nations throughout the world invest substantial amounts into basic and biomedical research with an aim of generating long-term translational outcomes that will benefit humankind. From 2000 to 2014, research spending adjusted for purchasing power has increased approximately 64% in the United States (~US$290-$450 billion), 57% in the European Union (~US$200-$350 billion), and 66% in the United Kingdom (~US$20-$30 billion, Research Council United Kingdom; Van Noorden, 2016). One of the key indicators of successful returns on research investment is the number of scientific publications. As evidenced by the MEDLINE database, the United States, European Union, and the United Kingdom contribute to approximately 32%, 26%, and 8% of the world’s scientific advancement through publications, respectively (Van Noorden, 2016). These patterns highlight a healthy balance between government expenditure and major scientific advances that in turn facilitate innovative technologies and knowledge that benefit human health and well-being.
The majority of biomedical research seeks to enhance knowledge at basic mechanistic and applied translational levels to better inform healthy and pathological conditions in humans. Most animals in biomedical research are mice and rats. The underuse of other “non-traditional” animals impedes our understanding of health and disease and the capability to develop medicines. Comparing the proportion of human publications in 2016 for a range of biomedical animals shows that publications using mice accounted for approximately 25% compared with human research (see Figure 1). The summed percentage of the nontraditional animals accounted for less than 2% illustrating a massively disproportionate amount of research funding on mice models. Yet, “non-traditional” animal models—hamsters, songbirds, voles, and cichlid fish—have aided discoveries. These nontraditional biomedical species offer specialized, adapted genomic, physiological, immunological, and behavioral traits. Comparing these traits across animals helps identify the common underlying causes of many human conditions (see also Remage-Healey, Krentzel, Macedo-Lima, & Vahaba, 2017). Here, we provide novel evidence to show that comparative animal studies yield major scientific advancements.
Figure 1.
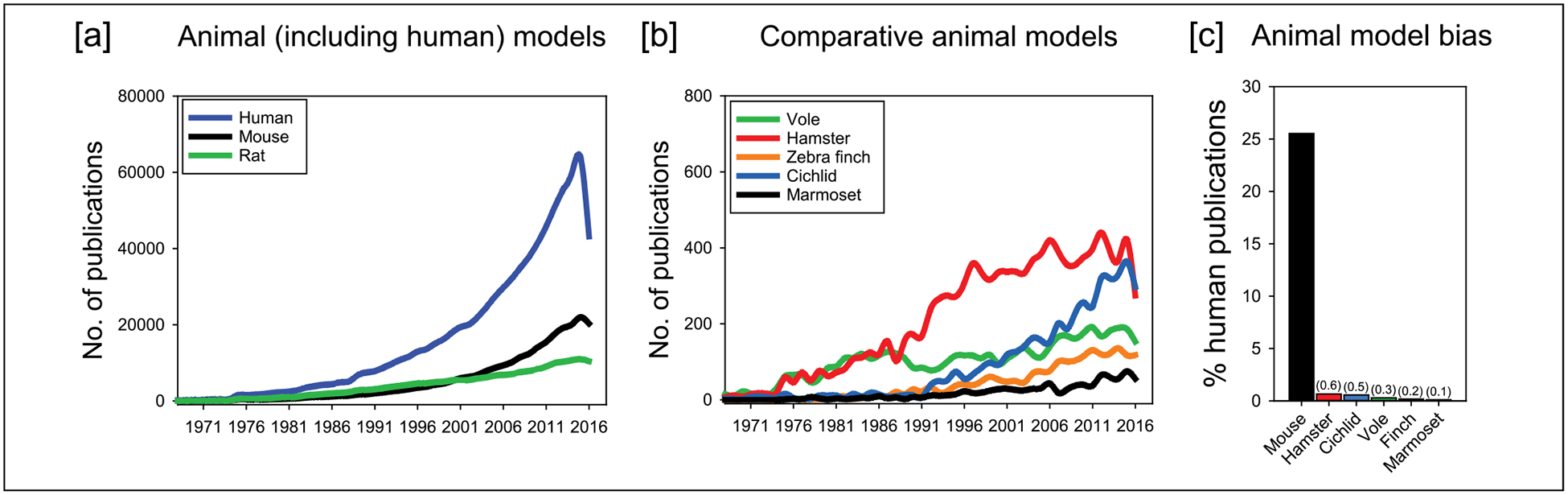
The lack of investment in comparative models for biomedical research
Note. (a, b) Annual number of publications in PubMed revealed that human and biomedical rodent models account for the vast majority compared with nontraditional models (approximately more than 100×). (c) For every four publications that used humans, there was one mouse publication (~25%). The summed percentage of nontraditional animals accounted for <2%. In many cases, nontraditional models are more suitable for biomedical research problems.
Biomedical Models for Scientific Research
Granted the exponential increase in publications using “human models” from 1968 to 2016 in PubMed, the two most common biomedical research animal models are mice and rats. The ability to generate genetically modified mice in the 1990s ushered in a new era of research that identified the functional significance of specific genes. Considering the traditionally female hormone estrogen, generating mice with a deleted estrogen-receptor gene illustrated how estrogen controls reproductive health in both females and males (Rissman, Wersinger, Taylor, & Lubahn, 1997). Subsequently, the capability to delete specific genes selectively in mice has increased the rate of using this model species.
However, mice are a limited model species to understand human function, so some findings do not translate well to human research, particularly in immunology (Bolker, 2012; de Souza, 2013; Drake, 2013). The comparative approach—going beyond mice, rats, and human—can identify commonalities and differences across organisms. Unfortunately, scientific funding is substantially lower for nontraditional animal species.
Krogh’s Principle and the Comparative Approach for Biomedical Animal Models
August Krogh was a Danish physiologist awarded the Nobel Prize in physiology or medicine in 1920 for his discovery of the circulatory systems’ ability to carry oxygen to muscles. In addition, Krogh (1929) proposed, “For a large number of [scientific] problems there will be some animal of choice or a few such animals on which it can be most conveniently studied … we must apply to the zoologists to find them” (p. 5). As noted, mice have contributed to the major scientific advancements in the last 20 years. However, as Krogh advised, many human conditions—physiological, immunological, neural, and behavioral—are best studied in an alternative model. Despite the comparative approach’s advantages to understand fundamental biomedical science or many pathological or disease conditions, research continues to overrely on mice (Beach, 1950). Substantial genomic developments such as genome sequencing (Koboldt, Steinberg, Larson, Wilson, & Mardis, 2013) and genome editing (Lee, Sundberg, Sigafoos, & Clark, 2016) now allow, respectively, sequencing whole genomes in a matter of days and conducting precise genomic manipulations. These two tools permit genomic analyses in a range of mammals, birds, and fish.
Below, four representative animal species illustrate physiology or behaviors different from other common biomedical models (i.e., mice) but facilitate our understanding of the human condition using Krogh’s principle. The comparative perspective below generates fundamental biological knowledge that can complement other biomedical models to provide a comprehensive understanding for human health and disease conditions.
Brain and Hormonal Control of Long-Term Body Weight Regulation in Hamsters
The World Obesity Federation estimates the prevalence of obesity is approximately 35% to 40% in the U.S. population. Healthy body weight regulation involves a complex interplay between behavioral (i.e., diet), physiological, and neural pathways (Yeo & Heisler, 2012). Most research has investigated short-term regulation, balancing food intake, and energy expenditure. Specific neuropeptides (e.g., agouti-related peptide, neuropeptide Y) signal energetic states: either low (undernourished) or high (overfed) body weight conditions. These systems determine short-term timing of meal intervals and compensatory responses to acute energy insufficiency, so genetic manipulation of such pathways often produces a clear phenotype. However, problems of healthy body weight maintenance extend beyond this well-defined neural system. Long-term hypothalamic mechanisms help regulate body weight (Ebling, 2015).
Seasonal animals provide a valuable opportunity to examine naturally occurring genomic, physiological, and behavioral changes with major translational implications for humans (Ebling, 2014; Morgan, Ross, Mercer, & Barrett, 2006; Stevenson et al., 2015). For example, the Siberian hamster (Phodopus sungorus) has been a valuable model to study long-term changes in physiological systems, such as losing body weight (Stevenson & Prendergast, 2013) and immune function (Stevenson, Onishi, Bradley, & Prendergast, 2014). Hamsters will show a reliable, robust, and repeatable cycle in body weight. In the laboratory, long days similar to the summer maintain “obese” hamsters. A simple change in the amount of light especially those that mimic short-winter days induces roughly 30% weight loss (Stevenson & Prendergast, 2015). The decrease in body weight represents a long-term change in homeostatic control of energy balance. Unfortunately, the common mouse models have lost the photoperiodic change in body weight and are, therefore, not suitable for examining long-term body weight regulation. In the hamster brain, a group of cells referred to as tanycytes controls the regulation of long-term changes in body weight (Lewis & Ebling, 2017). In mice, tanycytes monitor the levels of blood glucose, a strong measure of energetic state (Orellana et al., 2012). The current hypothesis is that the energetic state of an animal is detected by tanycytes (Bolborea & Dale, 2013), and then, tanycytes communicate this information to brain centers that control appetite (Yeo & Heisler, 2012). Experiments conducted in mice only focus on the acute, short-term impacts of diet and appetite on body weight regulation. Alternatively, hamsters have short-as well as long-term (e.g., months) cycles in body weight. The current evidence indicates that the action of thyroid hormones in hamster tanycytes controls long-term changes in body weight (Murphy et al., 2012). How the environment and diet affect the ability of tanycytes to detect energetic state and integrate thyroid hormone signals, and the subsequent impact on the short-term brain circuits are poorly understood. A greater understanding of the role of thyroid hormone action in the hamster tanycytes will better inform how obesity is maintained over longer time scales in humans.
Songbirds Enhance Our Understanding of Innately Programmed Learned Behavior: Language
The National Institute on Deafness and Other Communication Disorders (NIDCD; 2016) reports six to eight million people in the United States have language impairments. These include stutters, spasmodic dysphonia, and autism. The majority of disorders develop during childhood or adolescence and show remarkable sex-biases (Bale et al., 2010). Research into the underlying genetic, molecular, and neural control of language is hindered by the lack of animals that learn the species vocalizations. Songbirds, and in particular the zebra finch, represent the model system that has led to insights about the neurobiological control of learned vocalizations. A series of elegant experiments during the 1980s to 1990s demonstrated that discrete brain regions are necessary for songbirds to learn their songs (Fee & Scharff, 2010; reviewed in Alvarez-Buylla, Ling, & Nottebohm, 1992). The two main brain regions are essential for producing songbird vocalization such as HVC (acronym used as proper name) and caudal medial pallium (Bolhuis & Moorman, 2015). These two regions have functional analogy to areas in the human brain implicated in language (Broca’s and Wernicke’s areas), especially acquisition of language in infants and maintenance in adulthood (Bolhuis & Moorman, 2015).
Many songbirds, including the zebra finch, show substantial neurogenesis in the adult brain, that is, new neurons born and recruited to HVC (Alvarez-Buylla et al., 1992; Alward et al., 2015). Locally produced hormones in the pallium (e.g., HVC) improve the perception of the species’ own song (Remage-Healey, Jeon, & Joshi, 2013) and improve the birds’ ability to produce a high quality song (Alward, Balthazart, & Ball, 2013, Alward, Madison, Parker, Balthazart, & Ball, 2016; Rouse, Stevenson, Fortune, & Ball, 2015). The technological development of high-throughput analyses (such as microarray assays) has permitted the identification of large changes in gene expression in the songbird brain that are likely involved in vocal quality, a feature similar to how well humans produce speech (Replogle et al., 2008). These brain-derived hormones are likely regulating gene expression in brain regions involved in songbird vocalizations (Stevenson, Replogle, Drnevich, Clayton, & Ball, 2012). How the brain generates these new neurons, how the new neurons are recruited into HVC, and what triggers the functional outcome of these new neurons for songbird vocalizations remain unsolved. A deeper knowledge about these basic questions would aid treatment of language disorders in humans.
Elucidating the Neural Mechanisms of Social Behavior Using the Prairie Vole
In the past few decades, prairie voles have become a valuable animal for understanding the complex brain networks that control social behavior (Insel & Shapiro, 1992; McGraw & Young, 2010). Prairie voles have received substantial attention, largely based on (a) a rich literature on their natural history and behavioral ecology; (b) leveraging tools developed in classic rodent models, due to their close genetic relationship; and (c) rare but defining behaviors shared with humans but absent in common biomedical models (e.g., mice), such as communal living, social monogamy, and biparental care (Carter, 1998; Lukas, Clutton-Brock, 2012; McGraw & Young, 2010). In this respect, prairie voles illustrate the Krogh’s principle where general knowledge bridging behavioral ecology and neurobiology can be greatly expanded. Moreover, this species is well suited to serve as a model for several aspects of human social behavior and dysfunction (Carter, 2007; Young, 2001).
By far, the most commonly studied form of social behavior in prairie voles is pair bonding. Comparative studies in the 1990s made the remarkable discovery that the neuropeptides, vasopressin, and oxytocin are critical for monogamous relationships (Carter, DeVries, & Getz, 1995). These peptides were identified in numerous brain regions now known as critical for social behavior and characterized as a “pair bonding neural circuit” (Carter et al., 1997; Insel & Young, 2001; Young & Wang, 2004). Recent technological advances in molecular biology (e.g., epigenetic and optogenetic techniques) have expanded our understanding of the neural circuitry of reproductive decisions in prairie voles in a manner unavailable in common biomedical models (Amadei et al., 2017).
The value of prairie voles extends well beyond the study of pair bonding, and this species offers opportunities to address other pressing issues in behavioral neuroscience and general biology. For example, voles have been valuable to study biparental care (Bales et al., 2007; Hammock, 2015; Kelly, Hiura, Saunders, & Ophir, 2017; Prounis, Foley, Rehman, & Ophir, 2015; Wang & Novak, 1994), aggression (Gobrogge & Wang, 2011), social recognition (Blocker & Ophir, 2015; Zheng, Larsson, Phelps, & Ophir, 2013), neurogenomics of sociosexual behavior (McGraw et al., 2012), nonsexual relationships (Beery & Zucker, 2010), emotional regulation of the cardiovascular system and mind–heart interactions (Grippo et al., 2012), functional magnetic resonance imaging (fMRI) analysis of brain activity in awake animals (Yee et al., 2016), and reward and addiction (Aragona, Detwiler, & Wang, 2007; Ryabinin & Hostetler, 2016). Recently, prairie voles have also emerged as a promising system for studies that integrate the role of cognition (i.e., learning and memory) in mating systems to provide a more comprehensive understanding of the suite of factors that drive reproductive strategies and social decision making (Okhovat, Berrio, Wallace, Ophir, & Phelps, 2015; Ophir, 2017; Ophir, Wolff, & Phelps, 2008; Phelps & Ophir, 2009; Rice, Hobbs, Wallace, & Ophir, 2017). Thus, prairie voles represent a species with numerous uses in basic and translational research. With a fully sequenced genome (McGraw & Young, 2010) and an established network of researchers interested in understanding its behavioral ecology, development, neurobiology, and molecular genetics, prairie voles hold extraordinary potential to address questions focused on the neural and genetic basis of social behavior.
Cichlid Fish Species Facilitate Neurobiological Discoveries of Plasticity in Social Behavior
Behavior shaped by natural selection overwhelmingly results from selective pressures on social interactions. For example, group-living animals, including humans, form dominance hierarchies with obligatory social interactions. Nevertheless, most animal experiments are done on single individuals, typically in asocial, nonnatural conditions. This is partly because keeping rat and mouse model colonies and observing them during their normal nocturnal activities is hard, expensive, and impractical.
In contrast, fish offer unusual opportunities for understanding social behavior, its mechanistic underpinnings, and its role in reproduction. Cichlid fish that have evolved in the African rift lakes comprise more than 2,000 species, evolving a broad range of social systems including female, male, or biparental care; monogamous pairs with helpers; and polygamous harems with helpers (Awata, Munehara, & Kohda, 2005). Because cichlids offer experimental access at several levels of biological organization, their analysis has uncovered many widely conserved neural, physiological, and molecular mechanisms (Fernald, 2006; Ma, Juntti, Hu, Huguenard, & Fernald, 2015; Maruska & Fernald, 2014; White, Eisen, Kasten, & Fernald, 1998).
Here we describe research on a well-studied African cichlid fish, Astatotilapia burtoni, from Lake Tanganyika, East Africa. A. burtoni males in nature congregate around food sources and are either dominant or nondominant. Dominant males actively defend territories, court, and reproduce with females, whereas nondominant males look like the females that they mimic, do not reproduce, and school together (Fernald & Hirata, 1977). Crucially, these naturally occurring and reversible behavioral phenotypes can easily be observed in the laboratory, allowing experiments designed to answer questions at multiple biological levels.
The gonadotropin-releasing hormone (GnRH) is essential for regulating reproduction (Fernald & White, 1999; Stevenson, Hahn, MacDougall-Shackleton, & Ball, 2012). Release of GnRH from the brain’s hypothalamus controls the pituitary’s production of gonadotropins responsible for gonadal (sex organ) development in all vertebrates; this process has been highly conserved during 500 million years of vertebrate evolution. Using A. burtoni allowed the first cloning of the gene controlling this peptide in nonmammalian vertebrates (White, Kasten, Bond, Adelman, & Fernald, 1995), revealing not one but three genes. This discovery in fish then facilitated the identification of a second GnRH gene in humans (White et al., 1998) and other mammals (Kasten et al., 1996). What is particularly striking in this fish species are the observations that (a) the GnRH containing neurons increases 8× in volume when an animal becomes dominant (Francis, Soma, & Fernald, 1993), (b) the GnRH neurons are interconnected and fire synchronously in response to social ascent (Ma et al., 2015), (c) the GnRH production is regulated by social status (Soma, Francis, Wingfield, & Fernald, 1996), and (d) males reared with adults show delayed maturation relative to those reared without adults (Davis & Fernald, 1990). The availability of new genetic techniques has enhanced the roles of nontraditional fish model systems for discoveries with direct relevance to human health and wellness (Juntti, Hu, & Fernald, 2013).
An individual’s social status is important because the amount of GnRH in key brain regions is associated with the dominance rank, which is typically established via physical fights. Dominance is maintained through social signals including postures that demonstrate size or show teeth (Huntingford & Turner, 1987). In A. burtoni, nondominant males attend closely to dominant males, demonstrating that they anticipate movements of dominant males (Desjardins, Hofmann, & Fernald, 2012). But what do these animals know about their environment? Is it possible for cichlids to recognize the relative strength of other fish? In the field, colonies of A. burtoni range in size from a few dozen to hundreds (Fernald & Hirata, 1977) meaning that they could use a strategy of fighting with every male in the colony to identify one they could beat. However, by watching other male–male interactions, cichlids can infer their chances of winning a fight by viewing (i.e., “by-stander”) pairwise fights of other fish (Grosenick, Clement, & Fernald, 2007). This skill, known as transitive inference, is a form of deductive reasoning that allows inference of a relationship among items that have not been explicitly compared. Piaget (1928) described this as a key milestone in the development of human infants older than 3 years of age, and it has also been described for nonhuman primates (Rapp, Kansky, & Eichenbaum, 1996), such as rats (Roberts & Phelps, 1994) and birds (Bond, Kamil, & Balda, 2003).
Social information causes profound genomic, neural, physiological, and behavioral responses in A. burtoni (reviewed in Fernald, 2012; Maruska, 2015). When nondominant males have an opportunity to ascend in social rank, expression of the immediate early growth response gene 1 (egr-1) is upregulated within the preoptic area (POA), a critical hub within the social behavior network that mediates adaptive behavioral responses. Moreover, expression of gonadotropins and their receptors is higher in dominant males and increased in ascending males (Maruska, Levavi-Sivan, Biran, & Fernald, 2011). Within 30 min of social opportunity, expression levels of sex steroid hormones and their cognate receptors in the brain and gonads also increase (Maruska, Zhang, Neboori, & Fernald, 2013), as ascending males begin showing high levels of aggressive and reproductive behavior during social opportunity.
Many causal molecular mechanisms of A. burtoni behavior are unknown, but advances in genetic techniques have the potential to reveal new discoveries. For instance, recent work with gene editing technology (CRISPR-Cas9) identified a critical role of a receptor for one hormone (prostaglandin F2α) in regulating reproductive behavior in female A. burtoni (Juntti et al., 2013). New techniques have started a new era of animal research, allowing social behavior scientists to use diverse social systems present among many different taxa to understand the evolutionary trajectories of social behavior.
Conclusions and Future Directions
This article highlights the comparative approach in animal experimentation to better understand human health and diseases. Common biomedical models, particularly mice and rats, are well-established systems that have made significant gains in basic and translational research for the benefit of human health and wellness. However, in some cases highlighted here, other specialized physiological responses and social behaviors produced in nontraditional animal models (i.e., hamsters, songbirds, voles, and cichlids) are more suited for scientific investigation. Given the remarkable technological advances, such as whole genome sequencing and genome editing, the tools that were once the sole realm of mouse models can be readily applied to a range of animals. To successfully apply Krogh’s principle, large-scale funders need to incorporate strategic priorities that focus on the scientific gains afforded by the comparative approach.
Key Points.
Biomedical research mainly relies upon a few select animal models such as mice and rats.
Sixty percentage of nonhuman research published in 2016 used mice as the animal model.
For a large number of scientific problems, other non-traditional animals are more suitable.
Annual publications using nontraditional animals accounted for less than 2%.
Increased use of alternative animals in research would expedite advances in health and disease.
The creation of strategic funding priorities using non-traditional animals is critical.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Tyler J. Stevenson has received funding from the Leverhulme Trust. Francis J. P. Ebling is in receipt of funding from the Biotechnology and Biological Sciences Research Council (BBSRC; BB/M001555/1). The National Institutes of Health has funded Russell D. Fernald (NS 034950, NS093277, NIMH 087930), Alexander G. Ophir (HD079573, IOS-1354760), and Aubrey M. Kelly (HD081959). Beau A. Alward is an Arnold O. Beckman postdoctoral fellow.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alvarez-Buylla A, Ling CY, & Nottebohm F (1992). High vocal center growth and its relation to neurogenesis, neuronal replacement and song acquisition in juvenile canaries. Journal of Neurobiology, 23, 396–406. [DOI] [PubMed] [Google Scholar]
- Alward BA, Balthazart J, & Ball GF (2013). Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proceedings of the National Academy of Sciences United States of America, 110, 19573–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Madison FN, Parker SE, Balthazart J, & Ball GF (2016). Pleiotropic control by testosterone of a learned vocal behavior and its underlying neuroplasticity (1,2,3). eNeuro, 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Mayes WD, Peng K, Stevenson TJ, Balthazart J, & Ball GF (2015). Dissociable effects of social context on song and doublecortin immunoreactivity in male canaries. European Journal of Neuroscience, 40, 2941–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Liu RC (2017). Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature, 546, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Detwiler JM, & Wang Z (2007). Amphetamine reward in the monogamous prairie vole. Neuroscience Letters, 418, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awata S, Munehara H, & Kohda M (2005). Social system and reproduction of helpers in a cooperatively breeding cichlid fish (Julidochromis ornatus) in Lake Tanganyika: Field observations and parentage analyses. Behavioral Ecology and Sociobiology, 58, 506–516. [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarty MM, Nestler EJ (2010). Early life programming and neurodevelopmental disorders. Biological Psychiatry, 68, 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, & Carter CS (2007). Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience, 144, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA (1950). The snark was a boojum. American Psychologist, 5, 115–124. [Google Scholar]
- Beery AK, & Zucker I (2010). Oxytocin and same-sex social behavior in female meadow voles. Neuroscience, 169, 665–673. [DOI] [PubMed] [Google Scholar]
- Blocker TD, & Ophir AG (2015). Social recognition in paired but not single male prairie voles. Animal Behavior, 108, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolborea M, & Dale N (2013). Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends Neuroscience, 36, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, & Moorman S (2015). Birdsong memory and the brain: In search of the template. Neuroscience & Behavioral Review, 50, 41–55. [DOI] [PubMed] [Google Scholar]
- Bolker J (2012). Model organisms: There’s more to life than rats and flies. Nature, 491, 31–33. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, & Balda RP (2003). Social complexity and transitive inference in corvids. Animal Behavior, 65, 479–487. [Google Scholar]
- Carter CS (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology, 23, 779–818. [DOI] [PubMed] [Google Scholar]
- Carter CS (2007). Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioral Brain Research, 176, 170–186. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, & Getz LL (1995). Physiological substrates of mammalian monogamy: The prairie vole model. Neuroscience & Biobehavioral Review, 19, 303–314. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Taymans SE, Roberts RL, Williams JR, & Getz LL (1997). Peptides, steroids, and pair bonding. Annals of New York Academy Sciences, 807, 260–272. [DOI] [PubMed] [Google Scholar]
- Darwin C (1839). The voyage of the Beagle. London: Penguin Random Ltd. [Google Scholar]
- Davis MR, & Fernald RD (1990). Social control of neuronal soma size. Journal Neurobiology, 21, 1180–1188. [DOI] [PubMed] [Google Scholar]
- Desjardins JK, Hofmann HA, & Fernald RD (2012). Social context influences aggressive and courtship behavior in a cichlid fish. PLoS ONE, 7, e32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza N (2013). Model organisms: Mouse models challenged. Nature Methods, 10, 288. [DOI] [PubMed] [Google Scholar]
- Drake AC (2013). Of mice and men: What rodent models don’t tell us. Cellular Molecular Immunology, 10, 284–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ (2014). On the value of seasonal mammals for identifying mechanisms underlying the control of food intake and body weight. Hormones and Behavior, 66, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebling FJ (2015). Hypothalamic control of seasonal changes in food intake and body weight. Frontiers in Neuroendocrinology, 37, 97–107. [DOI] [PubMed] [Google Scholar]
- Fee MS, & Scharff C (2010). The songbird as a model for the generation and learning of complex sequential behaviors. Institute for Laboratory Animal Research Journal, 51, 362–377. [DOI] [PubMed] [Google Scholar]
- Fernald RD (2006). Casting a genetic light on the evolution of eyes. Science, 313, 1914–1918. [DOI] [PubMed] [Google Scholar]
- Fernald RD (2012). Social control of the brain. Annual Review Neuroscience, 35, 135–151. [DOI] [PubMed] [Google Scholar]
- Fernald RD, & Hirata NR (1977). Field study of Haplochromis burtoni: Habitats and co-habitants. Environmental Biology Fishes, 2, 299–308. [Google Scholar]
- Fernald RD, & White RB (1999). Gonadotropin-releasing hormone genes: Phylogeny, structure and functions. Frontiers in Neuroendocrinology, 20, 224–240. [DOI] [PubMed] [Google Scholar]
- Francis RC, Soma K, & Fernald RD (1993). Social regulation of the brainpituitary-gonadal axis. Proceedings of the National Academy Sciences United States of America, 90, 7794–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL, & Wang ZW (2011). Genetics of aggression in voles. Advanced Genetics, 75, 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Sgoifo A, Jepson AJ, Bates SL, Chandler DL, Preihs K (2012). The integration of depressive behaviors and cardiac dysfunction during an operational measure of depression: Investigating the role of negative social experiences in an animal model. Psychosomatic Medicine, 74, 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Clement TS, & Fernald RD (2007). Fish can infer social rank by observation alone. Nature, 445, 429–432. [DOI] [PubMed] [Google Scholar]
- Hammock EA (2015). Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology, 40, 24–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntingford FA, & Turner AK (1987). Animal conflict. London, England: Chapman & Hall. [Google Scholar]
- Insel TR, & Shapiro LE (1992). Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences United States of America, 89, 5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, & Young LJ (2001). The neurobiology of attachment. Nature Reviews Neuroscience, 2, 129–136. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Hu CK, & Fernald RD (2013). Tol2-mediated generation of a transgenic haplochromine cichlid, Astatotilapia burtoni. PLoS ONE, 8(10), e77647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten TL, White SA, Norton TT, Bond CT, Adelman JP, & Fernald RD (1996). Characterization of two new preproGnRH mRNAs in the tree shrew: First direct evidence for mesencephalic GnRH gene expression in a placental mammal. General & Comparative Endocrinology, 104, 7–19. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Saunders AG, & Ophir AG (2017). Oxytocin neurons exhibit extensive functional plasticity due to offspring age in mothers and fathers. Integrative & Comparative Biology, 57, 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt DC, Steinberg KM, Larson DE, Wilson RK, & Mardis ER (2013). The next-generation sequencing revolution and its impact on genomics. Cell, 155, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A (1929). The progress of physiology. American Journal of Physiology, 90, 243–251. [Google Scholar]
- Lee HB, Sundberg BN, Sigafoos AN, & Clark KJ (2016). Genome engineering with TALEN and CRISPR systems in neuroscience. Frontiers in Genetics, 7, Article 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JE, & Ebling FJP (2017). Tanycytes as regulators of seasonal cycles in neuroendocrine function. Frontiers in Neurology, 8, Article 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, & Clutton-Brock T (2012). Cooperative breeding and monogamy in mammalian societies. Proceedings of the Royal Society, Series B: Biological Sciences, 279, 2151–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Juntti SA, Hu CK, Huguenard JR, & Fernald RD (2015). Electrical synapses connect a network of gonadotropin releasing hormone neurons in a cichlid fish. Proceedings of the National Academy of Sciences United States of America, 112, 3805–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP (2015). Social transitions cause rapid behavioral and neuroendocrine changes. Integrative and Comparative Biology, 55, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, & Fernald RD (2014). Social regulation of gene expression in the African cichlid fish: Astatotilapia burtoni In Canli T (Ed.), The Oxford handbook of molecular psychology (pp. 52–78). Oxford: Oxford University Press. [Google Scholar]
- Maruska KP, Levavi-Sivan B, Biran J, & Fernald RD (2011). Plasticity of the reproductive axis caused by social status change in an African cichlid fish: I. Pituitary gonadotropins. Endocrinology, 152, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Zhang A, Neboori A, & Fernald RD (2013). Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. Journal of Neuroendocrinology, 25, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Davis JK, Thomas PJ, Program NCS, Young LJ, & Thomas JW (2012). BAC-based sequencing of behaviorally-relevant genes in the prairie vole. PLoS ONE, 7, e29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, & Young LJ (2010). The prairie vole: An emerging model organism for understanding the social brain. Trends in Neuroscience, 33, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Ross AW, Mercer JG, & Barrett P (2006). What can we learn from seasonal animals about the regulation of energy balance? Progress in Brain Research, 153, 325–337. [DOI] [PubMed] [Google Scholar]
- Murphy M, Jethwa PH, Warner A, Barrett P, Nilaweera KN, Brameld JM, & Ebling FJ (2012). Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinology, 153, 101–112. [DOI] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders. (2016). Statistics on voice, speech, and language. Retrieved from https://www.nidcd.nih.gov/health/statistics/statistics-voice-speech-and-language
- Okhovat M, Berrio A, Wallace G, Ophir AG, & Phelps SM (2015). Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science, 350, 1371–1374. [DOI] [PubMed] [Google Scholar]
- Ophir AG (2017). Navigating monogamy: Nonapeptide sensitivity in a memory neural circuit may shape social behavior and mating decisions. Frontiers in Neuroscience, 11, Article 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Wolff JO, & Phelps SM (2008). Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proceedings of the National Academy of Sciences United States of America, 105, 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Garcia MA (2012). Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia, 60, 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, & Ophir AG (2009). Monogamous brains and alternative tactics: Neuronal V1aR, space use and sexual infidelity among male prairie voles In Dukas R & Ratcliffe JM (Eds.), Cognitive ecology II (pp. 156–176). Chicago, IL: The University of Chicago Press. [Google Scholar]
- Piaget J (1928). Judgement and reasoning in the child. London, England: Kegan Paul, Trench, Trubner & Company. [Google Scholar]
- Prounis GS, Foley L, Rehman A, & Ophir AG (2015). Perinatal and juvenile social environments interact to shape cognitive behaviour and neural phenotype in prairie voles. Proceedings of the Royal Society, Series B: Biological Sciences, 282(1819). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, & Eichenbaum H (1996). Learning and memory for hierarchical relationships in the monkey: Effects of aging. Behavioral Neuroscience, 110, 887–897. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, & Joshi NR (2013). Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. Journal of Neuroendocrinology, 25, 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Krentzel AA, Macedo-Lima M, & Vahaba D (2017). Species diversity matters in biological research. Policy Insights in the Behavioral & Brain Sciences, 4, 210–218. [Google Scholar]
- Replogle K, Arnold AP, Ball GF, Band M, Bensch S, Brenowitz EA, Clayton DF (2008). The songbird neurogenomics (SoNG) initiative: Community-based tools and strategies for study of brain gene function and evolution. BMC Genomic, 9, Article 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MA, Hobbs LE, Wallace KJ, & Ophir AG (2017). Cryptic sexual dimorphism in spatial memory and hippocampal oxytocin receptors in prairie voles (Microtus Ochrogaster). Hormone and Behavior, 95, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, & Lubahn DB (1997). Estrogen receptor function as revealed by knockout studies: Neuroendocrine and behavioral aspects. Hormone and Behavior, 31, 232–243. [DOI] [PubMed] [Google Scholar]
- Roberts WA, & Phelps MT (1994). Transitive inference in rats: A test of the spatial coding hypothesis. Psychological Science, 5, 368–374. [Google Scholar]
- Rouse ML, Stevenson TJ, Fortune ES, & Ball GF (2015). Reproductive state modulates testosterone-induced singing in adult female European starlings (Sturnus vulgaris). Hormone and Behavior, 72, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, & Hostetler CM (2016). Prairie voles as a model to screen medications for the treatment of alcoholism and addictions. International Reviews Neurobiology, 126, 403–421. [DOI] [PubMed] [Google Scholar]
- Soma KK, Francis RC, Wingfield JC, & Fernald RD (1996). Androgen regulation of hypothalamic neurons containing gonadotropin-releasing hormone in a cichlid fish: Integration with social cues. Hormone and Behavior, 30, 216–226. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Hahn TP, MacDougall-Shackleton SA, & Ball GF (2012). Gonadotropin-releasing hormone plasticity: A comparative perspective. Frontiers in Neuroendocrinology, 33, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Onishi KG, Bradley SP, & Prendergast BJ (2014). Cell-autonomous iodothyronine deiodinase expression mediates seasonal plasticity in immune function. Brain, Behavior, and Immunology, 36, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, & Prendergast BJ (2013). Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proceedings of the National Academy of Sciences United States of America, 110, 16651–16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, & Prendergast BJ (2015). Photoperiodic time measurement and seasonal immunological plasticity. Frontiers in Neuroendocrinology, 37, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Replogle K, Drnevich J, Clayton DF, & Ball GF (2012). High throughput analysis reveals dissociable gene expression profiles in two independent neural systems involved in the regulation of social behavior. BMC Neuroscience, 13, Article 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Visser ME, Arnold W, Barrett P, Biello S, Dawson A, Helm B (2015). Disrupted seasonal biology impacts health food security and ecosystems. Proceedings of the Royal Society, Series B: Biological Sciences, 282, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noorden R (2016). China by the numbers. Nature, 534, 452–453. [DOI] [PubMed] [Google Scholar]
- Wang Z, & Novak M (1994). Alloparental care and the influence of father presence on juvenile prairie voles, Microtus Ochrogaster. Animal Behavior, 47, 281–288. [Google Scholar]
- White RB, Eisen JA, Kasten TL, & Fernald RD (1998). Second gene for gonadotropin-releasing hormone in humans. Proceedings of the National Academy of Sciences United States of America, 95, 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Kasten TL, Bond CT, Adelman JP, & Fernald RD (1995). Three gonadotropin-releasing hormone genes in one organism suggest novel roles for an ancient peptide. Proceedings of the National Academy of Sciences United States of America, 92, 8363–8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JR, Kenkel WM, Kulkarni P, Moore K, Perkeybile AM, Toddes S, Ferris CF (2016). BOLD fMRI in awake prairie voles: A platform for translational social and affective neuroscience. Neuroimage, 138, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS, & Heisler LK (2012). Unraveling the brain regulation of appetite: Lessons from genetics. Nature Neuroscience, 15, 1343–1349. [DOI] [PubMed] [Google Scholar]
- Young LJ (2001). Oxytocin and vasopressin as candidate genes for psychiatric disorders: Lessons from animal models. American Journal of Medical Genetics, 105, 53–54. [PubMed] [Google Scholar]
- Young LJ, & Wang Z (2004). The neurobiology of pair bonding. Nature Neuroscience, 7, 1048–1054. [DOI] [PubMed] [Google Scholar]
- Zheng DJ, Larsson B, Phelps SM, & Ophir AG (2013). Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behavioral Brain Research, 246, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]


