Abstract
Exercise is a major challenge for cardiovascular apparatus since it recruits chronotropic, inotropic, pre-load, and afterload reserves. Regular physical training induces several physiological adaptations leading to an increase in both cardiac volume and mass. It appears that several gender-related physiological and morphological differences exist in the cardiovascular adjustments and adaptations to dynamic exercise in humans. In this respect, gender may be important in determining these adjustments and adaptations to dynamic exercise due to genetic, endocrine, and body composition differences between sexes. Females seem to have a reduced vasoconstriction and a lower vascular resistance in comparison to males, especially after exercise. Significant differences exist also in the cardiovascular adaptations to physical training, with trained women showing smaller cardiac volume and wall thickness compared with male athletes. In this review, we summarize these differences.
Keywords: Sex hormones, blood pressure, stroke volume, cardiac output, training, exercise
1. INTRODUCTION
There are several genetic, anatomical, and hormone differences between males and females. Moreover, the effects of sex hormones vary during menstrual cycle and after menopause. These sex-related differences can impact the cardiovascular functions, thus they should be considered when conducting research in this field and when selecting optimum diagnostic and therapeutics procedures [1-9].
In this review, we summarize the main gender-related differences in the hemodynamic adjustments and adaptations to dynamic exercise with large muscle mass, such as running, cycling, and rowing. We will first provide a brief general view of changes which acutely occur in the cardiovascular system during dynamic exercise involving large muscle mass (i.e. the cardiovascular adjustments to dynamic exercise) and to the chronic adaptations induced by regular physical training.
2. THE CARDIOVASCULAR ADJUSTMENTS AND ADAPTATIONS TO EXERCISE: A GENERAL BRIEF VIEW
It is well known that there is a close relationship between the energetic demand of exercising muscles and circulation. During dynamic efforts involving large muscle mass, such as cycling, rowing, swimming, and running, the cardiovascular system is finely adjusted so that cardiac output (CO) rises linearly as a function of O2 uptake (VO2). At least in healthy humans, a constant linear relationship between the increase in VO2 and CO exists. It has been found that about 6 liters of CO is required for every liter of VO2 increment above rest, regardless of age and fitness level of the subject [10-15].
According to the Fick principle, CO can be expressed as CO=VO2 / arterio-venous oxygen difference (a-vO2D). Inasmuch as CO can be expressed also as CO= stroke volume (SV) • heart rate (HR), it is possible to re-write the equation as: SV•HR=VO2/a-vO2D.
a-vO2D has been found to increase almost linearly and in a predictable way with respect to workload, with the slope of this relationship depending on arterial O2 content (CaO2) [11-14]. CaO2, in turn, depends on the oxygen carried by hemoglobin, according to the following formula: CaO2 = Hb • 1.34 • HbO2Sat [11],
where Hb is hemoglobin concentration in the blood (measured in g/dl) and HbO2Sat is the percentage of Hb being saturated with O2. For an Hb concentration of 15g and a HbO2Sat of 99%, the CaO2 is equal to 19.8 ml for every 100 ml of blood. A further little quantity of O2 (about 0.3 ml for every 100 ml of blood) is physically dissolved in plasma [16]. This little amount sums to the amount of O2 carried by hemoglobin. As a result, for an individual with an Hb concentration of 15g and a HbO2Sat of 99%, the total CaO2 is 20.1 ml/100 ml.
Hence, the capacity of the cardiovascular system to supply the working muscle with O2 depends on four factors: 1) possibility to increase CO, which in turn depends on HR and SV reserves; 2) quantity of Hb in the blood; 3) quantity of Hb saturated with O2; and finally 4) amount of O2 extracted from blood by the muscle, also termed O2 extraction; this quantity in turn depends on the driving force for the O2, i.e. the force that allows the oxygen to be carried from the blood to the muscle mitochondria [17, 18].
With aging, maximal CO during exercise progressively declines because of a reduction in maximum HR and SV, and this reduction explains a significant portion of the age-related decline in maximum oxygen uptake (VO2max). The age-related CO reduction does not seem to be influenced by gender [19, 20], although it has been suggested that men have a significantly greater reduction in maximum CO than women [21], but this finding has never been confirmed.
It should be also considered that the cardiovascular regulation during effort has many facets and that to deliver oxygen to working muscles is not the only task that cardiovascular controlling mechanisms have to deal with during dynamic exercise. Another challenge is to avoid blood pressure drops due to metabolic-induced arteriolar vasodilation in the working muscle, which markedly reduces systemic vascular resistance (SVR). This phenomenon would cause a decrease in blood pressure and would impair perfusion of organs, such as the brain, if control mechanisms did not contemporary augment CO. However, this is almost never the case, as healthy subjects usually exercise without any symptom of blood pressure fall. Indeed, in healthy subjects CO rises, due to an increase in HR and SV, and counteracts the reduction in SVR via a flow-increment mechanism. As a matter of fact, dynamic exercise is characterized by an increase in mean arterial pressure (MAP) notwithstanding the marked SVR reduction due to the metabolic-induced vasodilation [14, 22].
A few neural cardiovascular control mechanisms are responsible for the fine hemodynamic regulation that guarantees blood supply to exercising muscle and avoids excessive MAP changes, namely 1) central command, 2) exercise pressor reflex, and 3) arterial baroreflex [23-25]. The central command arises from regions of the brain responsible for motor unit recruitment. This mechanism sets a basic level of sympathetic activation and vagal withdrawal closely related to motor drive from the motor cortex. Exercise pressor reflex works on the basis of peripheral signals arising from mechano- and metabo- receptors within the muscle (type III and IV nerve endings). This mechanism adjusts blood pressure, HR, cardiac pre-load, myocardial performance, and SVR by reflexively modulating sympathetic and parasympathetic tone to heart and vessels on the basis of the mechanical and metabolic status of the working muscle [23, 26-28]. The result of the activation of central command and exercise pressor reflex is that sympathetic activity raises while the parasympathetic one decreases as a function of exercise intensity. The autonomic response arising from the central command and the exercise pressor reflex is in turn modulated by the arterial baroreflex, which opposes any mismatch between SVR and CO by controlling muscle vasodilation and cardiac chronotropism [24, 28-31].
In summary, a precise regulation of chronotropism, inotropism, pre-load, and after-load is necessary in order to reach the target cardiovascular activity during exercise. In short, the cardiovascular system must be finely adjusted to provide nutrients and O2 to the muscle, to guarantee metabolic washout, and to maintain the target blood pressure.
As far as cardiovascular adaptations are concerned, it is well ascertained that regular physical training enhances the heart’s capacity to pump blood in circulation. Training induces cardiac hypertrophy and increases cardiac chamber size. A 15-20% greater left ventricular wall thickness (LVWT) and 10% greater left ventricular cavity size (LVCS) are reported in athletes as compared to sedentary people. These adaptations lead to an increase in left ventricular mass (LVM). Moreover, diastolic ventricular filling is enhanced, with a disproportionate increase in pulmonary arterial pressures during exercise. This causes greater afterload on the right ventricle and may render endurance athletes more vulnerable to ventricular arrhythmias due to right ventricular dysfunction. Overall, the result is that SV is larger in athletes than sedentary people. In short, physical training improves the SV reserve.
Finally, a well recognised feature of the athlete’s heart is the reduction in HR at rest and at a given workload with respect to sedentary subjects. Thus, the HR reserve is also enhanced [32-35].
3. SEARCH STRATEGY
Potential studies were identified by two unbiased reviewers using PubMed and Scopus databases. Search terms used were “gender” and “sex” and “cardiovascular regulation”, “cardiovascular adjustments”, “cardiovascular adaptations”, “exercise pressor response”, “blood pressure”, “central command”, “metaboreflex”, “mechanoreflex”, “baroreflex”, and “exercise” respectively. The language was English. Only studies involving healthy humans were taken into account. Animal studies and investigations involving humans with cardiovascular, metabolic, and neurological diseases were excluded [36]. Reference lists of articles retrieved were manually checked for additional articles.
4. GENDER-RELATED DIFFERENCE IN THE MAIN HEMODYNAMIC PARAMETERS DURING AND AFTER DYNAMIC EXERCISE
Female sexual hormones exert a relaxing effect on peripheral resistance vessels [1, 37]. Furthermore, young women show lower resting muscle sympathetic activity in support to blood pressure than men [38, 39], whilst menopause is accompanied by an accelerated rise in sympathetic activity [9]. Yet, young women have a reduced sympathetic activity and arterial vasoconstriction during orthostatic stress [40-42]. This can explain the reason why blood pressure level at rest has often been found lower in women than in men at similar ages. Moreover, women tend to have higher CO and lower SVR at rest, thereby minimizing blood vessel injury. Thus, men are considered to be at greater risk for cardiovascular and renal disease than age-matched, pre-menopausal women.
However, it is to be highlighted that a direct scientific evidence regarding a link between endogenous sex hormones and blood pressure levels is still lacking, being sex-related differences in blood pressure probably multifaceted and multi-factorial. Indeed, in women, after menopause hormone replacement therapy in most cases does not significantly reduce blood pressure, thereby suggesting that the drop in estrogen levels may not be the only cause responsible for the higher blood pressure in post-menopausal women [43]. Furthermore, estrogen therapy does not seem to attenuate progressive stiffening in postmenopausal women [44].
As far as sex-related difference in blood pressure response during physical exercise is concerned, the scientific literature is scarce. It has been found that males have higher systolic blood pressure during exercise than females [45]. This is probably due to females’ blunted sympathetic response and higher vasodilatory state of women in comparison with men [46]. However, potentially confounding factors, such as phase of menstrual cycle, exercise model, length of exercise session, as well as environmental conditions, may be involved in this phenomenon [47]. It should be considered that, in a more recent study, gender-related differences in systolic blood pressure during exercise disappeared when adjusting for body mass index (BMI), exercise duration, and resting systolic blood pressure [48]. Moreover, some studies conducted during exercise reported no difference in terms of SVR level between genders [21, 41]. Thus, the concept that females have a reduced capacity to vasoconstrict the circulation during exercise is not unanimously accepted and deserves further investigation.
A further potential gender-related hemodynamic difference is the lower maximum level of SV reached during dynamic exercise by women in comparison with men. It has been proposed that this difference is mainly due to females’ smaller cardiac size; particularly the left ventricular volume and mass. This lower SV in turn explains the women’s lower maximum CO, since maximum HR is not different between genders [49-51] as maximum HR achieved during exercise depends mainly on the age than on the gender of a subject [21, 51, 52]. Hence, the smaller cardiac size due to smaller body may be responsible for the reduced SV and CO often reported in females. It is to be noticed that the smaller body size of women may not entirely account for their lower SV, as stroke index (SI, i.e. SV normalized by body surface area) has also been demonstrated to be lower in females with respect to males [21], even though this difference is obviously less evident when this parameter is normalized for body surface area. However, this difference has not been unanimously reported and others did not find any difference in SI regardless of the sex [20]. Moreover, sex-related differences in SV tend to disappear when SV is normalized for lean body mass. This suggests that the different body composition between genders, with women having a higher percentage of body fat, may account for the small - if any - reduction in SV found in females with respect to males [19].
Previous studies have demonstrated that in normal subjects SV increases during dynamic exercise because of the left ventricle end-diastolic volume increment (due to increase in pre-load) and the left ventricle end-systolic volume reduction (due to improved inotropism), which concur in increasing ejection fraction (EF) [53-55]. It has been suggested that women have a blunted increase in EF in comparison with men [54, 56, 57]. On the other hand, in contrast to these findings, during various intensities of exercise expressed as a percentage of VO2max, Sullivan and co-workers have reported no gender-related difference in the magnitude of changes of left ventricular end-diastolic and end-systolic volumes [52]. These conflicting results pose the question of whether females’ capacity to enhance myocardial contractility and EF is reduced in comparison with males. To the best of our knowledge, no conclusive answer to this question has been provided to date.
Another interesting phenomenon occurring during dynamic exercise is the possibility to increase SV throughout exercise. In elite male athletes, it has been reported that SV does not plateau during incremental exercise till exhaustion. Rather, it increases progressively to maximum workload without flattening out. This is in sharp contrast with what usually observed in sedentary and moderately fit subjects of both genders, where a SV plateau has usually reported at submaximal workloads [13, 15, 58-60]. The possibility that this phenomenon is present also in elite, female athletes is contentious. A recent study by Wang and co-workers [61] reported that even in highly trained females SV does not plateau during incremental exercise. However, it should be highlighted that, to the best of our knowledge, the study by Wang et al. is to date the only one focusing on this topic. Hence, further research is warranted to better clarify the real capacity of highly trained females to increase SV throughout incremental exercise.
Another gender-related difference in the hemodynamic adjustment to exercise is the lower a-vO2D of women as compared to men. The a-vO2D depends on CaO2 [11-15], which in turn results from the oxygen carried by hemoglobin. Since on average women have lower hemoglobin concentration and relatively less blood volume than men, women also have a lower O2 supply for the same quantity of blood flow than men [62]. This can explain why women show a reduced maximum a-vO2D [21]. Notwithstanding the lower a-vO2D, women do not appear to have different slope of the VO2/CO relationship during submaximal exercise as compared to men [12, 52]. This suggests that cardiovascular control during exercise is similar between genders.
In the light of the fact that women have a lower maximum CO, and also considering that CO is a major determinant of systemic O2 transport in humans, it is not surprising that women usually show lower values of VO2max in comparison with age and fitness-matched men [19, 20, 47, 50, 63]. This sex-related difference remains significant even when VO2max is expressed in relation to body mass, although sex-related differences tend to disappear when this parameter is normalized for fat-free body mass [12, 20].
Regarding hemodynamics during recovery from dynamic exercise, women have often been reported to exhibit a reduced capacity to vasoconstrict the arteriolar bed as compared to men. It has been in fact demonstrated that the decrease in blood pressure during recovery after dynamic effort is greater in females than in males. This difference was attributed to a greater decrease in SV and a lower raise in SVR in women during recovery, which usually persisted for 5 minutes after the end of exercise [41]. It appears that women have greater reduction in CO without an adequate arteriolar constriction (i.e. SVR increment) during recovery. This is true especially when recovery is performed inactive [41]. The lower SVR level (i.e. a reduced afterload) of women after exercise has at least two possible explanations: a) female sex hormones, which exert a relaxing effect on peripheral resistance vessels, influence vaso-reactivity, and may induce post-exercise vasodilation [64-66]; and b), greater parasympathetic activity towards the heart as well as less sympathetic input to vascular regulation in women than in men [67-70]. This can also explain the higher occurrence of post-exercise hypotension in women [40, 42]. The observation that the capacity to vasoconstrict the arteriolar bed and to counteract hypotension is less in females compared with males has the implication for adequately monitoring of blood pressure after exercise in women. Moreover, active recovery from exercise should reduce the risk of post-exercise hypotension and fainting by activating muscle pump [71, 72]. However, recent findings reported that cardiovascular responses after an ultra-marathon were similar between the two sexes. The authors suggested that the training status rather than sex was the most important factor associated with signs of cardiovascular impairment after effort [73].
In summary, although conflicting results are reported by the scientific literature, differences between sexes in the hemodynamic adjustments during and after dynamic exercise tend to disappear when parameters are normalized by body mass and/or composition and when the training status is taken into consideration. Thus, normalized parameters should be used instead of absolute values. The only parameter which is unanimously considered lower in females is a-vO2D ought to the lower hemoglobin concentration of women in comparison with men. Table 1 shows the main differences in hemodynamic parameters and their sex-related differences at peak dynamic exercise.
Table 1. List of the main cardiovascular parameters and their sex-related difference at peak dynamic exercise.
| Parameter | Sex-related Differences |
|---|---|
| Heart Rate | No-difference between sexes |
| Stroke Volume | Usually higher in men |
| Stroke Index | Slightly higher in men or similar between sexes |
| Stroke Volume normalized by lean body mass | No-difference between sexes |
| Cardiac Output | Usually higher in men |
| Cardiac Index | Slightly higher in men or similar between sexes |
| Ejection Fraction | Slightly higher in men or similar between sexes |
| Arterio-venous oxygen difference | Usually higher in men |
| Systemic Vascular Resistance | Usually lower in women |
5. GENDER-RELATED DIFFERENCES IN CENTRAL COMMAND, EXERCISE PRESSURE REFLEX, AND BAROREFLEX
Very few studies have dealt with gender-related differences in the three main neural reflexes which adjust the cardiovascular system during exercise. To the best of our knowledge, there is only one study addressing the potential differences between males and females in central command activation [74]. In this research conducted during static handgrip, authors found sex-related different patterns of activation in insular gyral responses. This part of the brain is involved in autonomic control and cardiovascular regulation during exercise. Authors found different lateralization between sexes. In detail, the anterior-most gyri had a prominent sex difference, with females showing a greater right-sided activation, while males exhibited a greater left-sided activation. They concluded that these sex-specific functional brain patterns may contribute over time to variations in cardiovascular disease between the two sexes. However, this investigation has never been replied. Moreover, static is quite different from dynamic exercise. Thus, further studies in this area are warranted. In particular, given the importance of central command in the sympathetic activation during exercise [23], it would be useful to investigate on central command during dynamic efforts with large muscle mass, such as running or cycling, to ascertain the role of this reflex in the supposed blunted sympathetic response of women [46].
The influence of gender on exercise pressor reflex was addressed in a number of studies [75-79]. From these investigations it can be gleaned that the metabolic part of the exercise pressor reflex (i.e. the metaboreflex) is attenuated in women as compared to men, thus resulting in reduced increase in blood pressure, sympathetic nerve activity, and central venous pressure [75, 76]. This effect appears independent of muscle mass, workload, and level of training [75]. Moreover, the administration of oral contraceptives was found to attenuate sex-related differences in the metaboreflex [77, 78]. Similarly, the mechanic arm of the exercise pressor reflex (i.e. the mechanoreflex) appears to be attenuated in women more than in men [79].
As far as baroreflex is concerned, several human studies have reported that resting cardiac baroreflex sensitivity is reduced in females in comparison with males, whereas others did not discover any difference [40, 80-84]. In particular, some authors suggested that young women have greater depressor responses to simulated carotid hypertension than aged-matched men [38, 39]. However, conclusive proof is still lacking and further research is strongly needed to better elucidate this point.
Baroreflex resetting during exercise does not appear to be influenced by sex or by physiological fluctuation in ovarian hormones [84, 85], although some investigations reported that women have greater baroreflex-mediated control upon HR in response to hypertension during exercise. This effect was present throughout the menstrual cycle and it appeared to be mediated by a shift of the operating point of the carotid-cardiac function curve, which was located away from the centering point towards the threshold of the full baroreflex function curve [86, 87]. More recently it was found that, during post-exercise muscle ischemia, females exhibited an attenuated blood pressure response in comparison with males. Moreover, baroreflex control upon circulation remained more elevated and lasted longer in women than in men. Taken together, all these findings appear to suggest a sex-related difference in baroreflex activity during contemporary activation of the muscle metaboreflex and a sex-related difference in baroreflex sensitivity time course [88, 89]. The elevated baroreflex control in women, together with the reduced metabo-mechanoreflex activity, may explain why some studies have reported lesser sympathetic activity in women than in men [67-69]. Moreover, as previously pointed out, gender-related differences in central command activation may play a role in the different autonomic activity between sexes. However, the specific role of the three cardiovascular reflexes in the different autonomic activity between sexes has never been tested and this hypothesis remains speculative. Fig. (1) is a schematic representation of gender-related mechanisms affecting sympathetic activation during dynamic exercise.
Fig. (1).
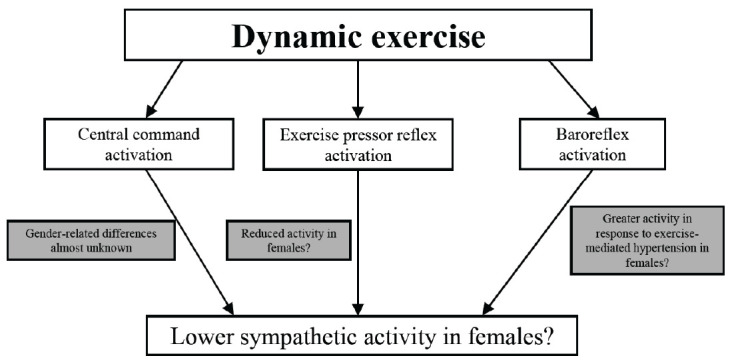
Putative mechanisms explaining the lower sympathetic activity in females in comparison with males during dynamic exercise. See text for more details. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In summary, very few research was conducted to investigate the gender-related differences in the main reflexes controlling the cardiovascular adjustments to dynamic exercise. While metaboreflex, mechanoreflex, and baroreflex seem to be influenced by sex, central command has been almost completely overlooked by investigators. Specific studies in this area are warranted.
6. SEX-RELATED DIFFERENCES IN HEART DIMENSIONS AND CARDIOVASCULAR ADAPTATIONS TO EXERCISE
Regular training enhances heart capacity to pump blood into circulation by inducing cardiac hypertrophy and increasing cardiac chamber size. Furthermore, diastolic filling is also enhanced. As a consequence, athletes can rely on a higher SV reserve.
One of the largest studies on cardiovascular adaptations to exercise in female athletes was performed by Pelliccia and co-workers [89]. By employing echocardiography, they measured cardiac structure and function in 600 females of 27 different sport activities. Authors compared results with those obtained in 735 males of similar age and training status and in 65 sedentary age-matched subjects. Trained women had 6% and 14% larger LVWT and LVCS than sedentary women. Compared with men, trained women exhibited smaller LVWT, LVCS, and LVM. These results were substantially confirmed by Sharma et al. and Rowland and Roti [90, 91]. Although in females the nature of physiologic adaptation is similar to that observed in male athletes, cardiac hypertrophy (defined as wall thickness >11 mm) in females is rare in comparison with males. A LVWT value greater than 12 mm in trained women should be viewed with caution and should be carefully evaluated to exclude hypertrophic cardiomiopathy [92, 93]. These reported sex-related differences in cardiac adaptations may be partially explained by the higher circulating concentrations of endogenous anabolic hormones in males, which promote increased skeletal muscle mass and allow training at greater intensity. In contrast to hypertrophic cardiomiopathy, physiological cardiac hypertrophy is considered beneficial [94, 95]. It was reported that male cardiac tissue possesses higher hypertrophic potential if compared to women [94]. However, specific sex-specific differences in exercise-induced physiological hypertrophy has been not unanimously reported [96].
Although these early studies have demonstrated smaller cardiac dimension in trained women compared with men, recent research has sought to eliminate inter-sex differences in body composition and size [97]. Authors found that, when scaled to body surface area, male had greater LVM in comparison with females; in contrast, when scaled to lean body mass, there was no significant difference between sexes. Similarly, when allometrically scaled to lean body mass, there were no significant gender-related differences in left ventricular or atrial volumes. They concluded that gender-related differences in ventricular dimensions are less marked, if not absent, when indexing using lean body mass allometrically. Thus, whether these differences in cardiac adaptations can be accounted for body size and composition or whether a sex-specific difference in physiological remodeling exists is still the matter of debate.
It is noteworthy that a recent study comparing female endurance athletes with non-athletes found that half of the variability in maximum VO2max could be explained by left ventricular end-diastolic volume [98]. This result underscores the importance of diastolic capacity and function in the cardiac adaptations to training in women.
In summary, studies focusing on cardiovascular adaptations to exercise reported similar physiologic responses between sexes. However, it should be underscored that wall thickness >11 mm is rare in females. Although early studies demonstrated smaller cardiac dimension in women than men, this difference seems to disappear when body size and composition were considered. These observations could be useful in evaluating female endurance athletes with suspected cardiac disease.
CONCLUSION AND FUTURE DIRECTIONS
Gender may differ in physiological cardiovascular adjustments and adaptations to dynamic exercise. Genetic, endocrine, and body composition features may be the key determinants of these differences, and they should be considered when conducting research in this field and during diagnostic and therapeutic procedures. However, their detailed mechanisms are still not well understood.
The most striking sex-related difference in cardiovascular adjustments is reported for vascular tone, with females exhibiting a reduced vasoconstriction and a lower vascular resistance in comparison with males. This seems true at rest as well as during recovery from dynamic exercise, while whether this different vasomotor capacity is present also during dynamic exercise is contentious. One potential confounding factor is the fluctuation in estrogens throughout menstrual cycle, which can impact blood volume, SVR, and ventricular functions [99]. Hence, specific studies focusing on gender-related differences in arteriolar tone during dynamic efforts should be conducted. Moreover, the potential role played by autonomic activity and hormone fluctuations should be investigated.
It is surprising that the role played by sex in the three main cardiovascular reflexes operating during exercise has been poorly investigated. This despite several clues point towards a clear influence of sex on metaboreflex, mechanoreflex, and baroreflex activity. In particular, central command has been almost completely overlooked by scientists. This area of research deserves much consideration in the future.
Significant differences appear to exist also in the cardiovascular adaptations to physical training, with trained women showing smaller cardiac volume and wall thickness compared with male athletes. However, scarce information exists in this area of research and it is not clear whether reported differences between sexes could be dependent also on subjects’ training status. Future studies are needed to better clarify this point.
Finally, inasmuch as differences between genders in many cardiovascular variables are small or absent when parameters are indexed for body surface area and composition, indexed parameters should be used instead of absolute values in future studies. Sex appears to be less important than body size and composition in explaining differences in cardiac dimensions between genders.
ACKNOWLEDGEMENTS
Authors wish to thank Dr. Roberto Mura for his editorial assistance.
LIST OF ABBREVIATIONS
- a-vO2D
arterio-venous Oxygen Difference
- CaO2
Arterial O2 Content
- CO
Cardiac Output
- EF
Ejection Fraction
- Hb
Hemoglobin
- HbO2Sat
Percentage of Saturated Hemoglobin
- HR
Heart Rate
- LVCS
Left Ventricular Cavity Size
- LVM
Left Ventricular Mass
- LVWT
Left Ventricular Wall Thickness
- MAP
Mean Blood Pressure
- SI
Stroke Index
- SV
Stroke Volume
- SVR
Systemic Vascular Resistance
- VO2
Oxygen Uptake
- VO2max
Maximum Oxygen Uptake
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Li Y., Kloner R.A. Is there a gender difference in infarct size and arrhythmias following experimental coronary occlusion and reperfusion? J. Thromb. Thrombolysis. 1995;2(3):221–225. doi: 10.1007/BF01062713. [DOI] [PubMed] [Google Scholar]
- 2.Leinwand L.A. Sex is a potent modifier of the cardiovascular system. J. Clin. Invest. 2003;112:302–304. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos R.L., Marin E.B., Gonçalves W.L., Bissoli N.S., Abreu G.R., Moyses M.R. Sex differences in the coronary vasodilation induced by 17 β-oestradiol in the isolated perfused heart from spontaneously hypertensive rats. Acta Physiol. (Oxf.) 2010;200:203–210. doi: 10.1111/j.1748-1716.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- 4.Lagranha C.J., Deschamps A., Aponte A., Steenbergen C., Murphy E. Sex differences in the phosphorylation of mithocondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ. Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy E. Estrogen signaling and cardiovascular disease. Clin. Res. 2011;109:687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolar F., Ostalad B. Sex differences in cardiovascular function. Acta Physiol. (Oxf.) 2013;207:584–587. doi: 10.1111/apha.12057. [DOI] [PubMed] [Google Scholar]
- 7.Marongiu E., Crisafulli A. Gender differences in cardiovascular functions during exercise: A brief review. Sport Sci. Health. 2015;11:235–241. [Google Scholar]
- 8.Jochmann N., Stangl K., Garbe E., Baumann G., Stangl V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur. Heart J. 2005;26(16):1585–1595. doi: 10.1093/eurheartj/ehi397. [DOI] [PubMed] [Google Scholar]
- 9.Vongpatanasin W. Autonomic regulation of blood pressure in menopause. Semin. Reprod. Med. 2009;27(4):338–345. doi: 10.1055/s-0029-1225262. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi I., Komatsu E., Miyazawa K. Intersubject variability in cardiac output-O2 uptake relation of men during exercise. J. Appl. Physiol. 1986;61:2168–2174. doi: 10.1152/jappl.1986.61.6.2168. [DOI] [PubMed] [Google Scholar]
- 11.Stringer W., Hansen J.E., Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J. Appl. Physiol. 1997;82:908–912. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- 12.Proctor D.N., Beck K.C., Shen P.H., Eickhoff T.J., Halliwill J.R., Joyner M.J. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. J. Appl. Physiol. 1998;84:599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- 13.Crisafulli A., Melis F., Tocco F., et al. Anaerobic Threshold and the oxygen consumption/cardiac output relationship during exercise. Sport Sci. Health. 2005;2:75–80. [Google Scholar]
- 14.Crisafulli A., Tocco F., Pittau G., et al. Detection of lactate threshold by including haemodynamic and oxygen extraction data. Physiol. Meas. 2006;27:85–97. doi: 10.1088/0967-3334/27/1/008. [DOI] [PubMed] [Google Scholar]
- 15.Crisafulli A., Piras F., Chiappori P., et al. Estimating stroke volume from oxygen pulse during exercise. Physiol. Meas. 2007;28:1201–1212. doi: 10.1088/0967-3334/28/10/006. [DOI] [PubMed] [Google Scholar]
- 16.Shaskey D.J., Green G.A. Sports haematology. Sports Med. 2000;29:27–38. doi: 10.2165/00007256-200029010-00003. [DOI] [PubMed] [Google Scholar]
- 17.Stringer W., Wasserman K., Casaburi R., Pŏrszăsz J., Maehara K., French W. Lactic acidosis as a facilitator of oxyhemoglobin dissociation during exercise. J. Appl. Physiol. 1994;76:1462–1467. doi: 10.1152/jappl.1994.76.4.1462. [DOI] [PubMed] [Google Scholar]
- 18.Wittenberg B.A., Wittenberg J.B. Transport of oxygen in muscle. Annu. Rev. Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T., Spina R.J., Martin W.H., et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 20.Farinatti P., Monteiro W., Oliveira R., Crisafulli A. Cardiorespiratory responses and myocardial function within incremental exercise in healthy unmedicated older vs. young men and women. Aging Clin. Exp. Res. 2018;30(4):341–349. doi: 10.1007/s40520-017-0776-x. [DOI] [PubMed] [Google Scholar]
- 21.Hossack K.F., Bruce R.A. Maximal cardiac function in sedentary normal men and women: comparison of age-related changes. J. Appl. Physiol. 1982;53:799–804. doi: 10.1152/jappl.1982.53.4.799. [DOI] [PubMed] [Google Scholar]
- 22.Higginbotham M.B., Morris K.G., Williams R.S., McHale P.A., Coleman R.E., Cobb F.R. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ. Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 23.Nóbrega A.C.L., O’Leary D.S., Silva B.M., Marongiu E., Piepoli M.F., Crisafulli A. Neural regulation of cardiovascular response to exercise: Role of central command and peripheral afferents. BioMed Res. Int. 2014;•••:478965. doi: 10.1155/2014/478965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crisafulli A., Marongiu E., Ogho S. Cardiovascular reflexes activity and their interaction during exercise. BioMed Res. Int. 2015;•••:394183. doi: 10.1155/2015/394183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green A.L., Paterson D.J. Identification of the neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: Implications for exercise control. Exp. Physiol. 2008;93:1022–1028. doi: 10.1113/expphysiol.2007.039461. [DOI] [PubMed] [Google Scholar]
- 26.O’Leary D.S. Autonomic mechanisms of muscle metaboreflex control of heart rate. J. Appl. Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- 27.Kaur J., Spranger M.D., Hammond R.L., et al. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H524–H529. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crisafulli A. The impact of cardiovascular diseases on cardiovascular regulation during exercise in humans: Studies on metaboreflex activation elicited by the post-exercise muscle ischemia method. Curr. Cardiol. Rev. 2017;13(4):293–300. doi: 10.2174/1573403X13666170804165928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fadel P.J., Ogoh S., Watenapaugh D.E., et al. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- 30.Sheriff D.D. Baroreflex resetting during exercise: Mechanisms and meaning. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1406–H1407. doi: 10.1152/ajpheart.01275.2005. [DOI] [PubMed] [Google Scholar]
- 31.Raven P.B., Fadel P.J., Ogoh S. Arterial baroreflex resetting during exercise: A current perspective. Exp. Physiol. 2006;91:37–49. doi: 10.1113/expphysiol.2005.032250. [DOI] [PubMed] [Google Scholar]
- 32.Blomquist C.G., Salting B. Cardiovascular adaptations to physical training. Annu. Rev. Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 33.Maron B.J. Structural features of the athletes heart as defined by echocardiography. J. Am. Coll. Cardiol. 1986;7:190–203. doi: 10.1016/s0735-1097(86)80282-0. [DOI] [PubMed] [Google Scholar]
- 34.Rost R. The athlete’s heart: Historical perspective. Cardiol. Clin. 1992;10:197–207. [PubMed] [Google Scholar]
- 35.Sharma S. Athlete’s heart – effect of age, sex, ethnicity and sporting discipline. Exp. Physiol. 2003;88:665–669. doi: 10.1113/eph8802624. [DOI] [PubMed] [Google Scholar]
- 36.Bassareo P.P., Saba L., Solla P., Barbanti C., Marras A.R., Mercuro G. Factors influencing adaptation and performance at physical exercise in complex congenital heart diseases after surgical repair. BioMed Res. Int. 2014;2014:862372. doi: 10.1155/2014/862372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penna C., Tullio F., Merlino A., et al. Postconditioning cardioprotection against infarct size and post-ischemic systolic dysfunction is influenced by gender. Basic Res. Cardiol. 2009;104:390–402. doi: 10.1007/s00395-008-0762-8. [DOI] [PubMed] [Google Scholar]
- 38.Christou D.D., Jones P.P., Jordan J., Diedrich A., Robertson D., Seals D.R. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111(4):494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt J.A., Joyner M.J., Charkoudian N., Wallin B.G., Hart E.C. Sex differences in alpha-adrenergic support of blood pressure. Clin. Auton. Res. 2010;20(4):271–275. doi: 10.1007/s10286-010-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Convertino V.A. Gender differences in autonomic function associated with blood pressure regulation. Am. J. Physiol. 1998;275:1909–1920. doi: 10.1152/ajpregu.1998.275.6.R1909. [DOI] [PubMed] [Google Scholar]
- 41.Carter R., Watenpaugh D.E., Smith M.L. Selected contribution: Gender differences in cardiovascular regulation during recovery from exercise. J. Appl. Physiol. 2001;91:1902–1907. doi: 10.1152/jappl.2001.91.4.1902. [DOI] [PubMed] [Google Scholar]
- 42.Senitko A.N., Charchoudian N., Halliwill J.R. Influence of endurance exercise training status and gender on postexercise hypotension. J. Appl. Physiol. 2002;92:2368–2374. doi: 10.1152/japplphysiol.00020.2002. [DOI] [PubMed] [Google Scholar]
- 43.Reckelhoff J.F. Gender differences in regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 44.Ogola B.O., Zimmerman M.A., Clark G.L., et al. New insights into arterial stiffening: Does sex matter? Am. J. Physiol. Heart Circ. Physiol. 2018;315(5):H1073–H1087. doi: 10.1152/ajpheart.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimpka U, Ugwu A, Oshi D. Assessment of sex differences in systolic blood pressure responses to exercise in healthy, non-athletic young adults. JEP online. 2008;11:18–25.. [Google Scholar]
- 46.Wheatley C.M., Snyder E.M., Johnson B.D., Olson T.P. Sex differences in cardiovascular function during submaximal exercise in humans. Springerplus. 2014;3:445. doi: 10.1186/2193-1801-3-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Toole M.L. Gender differences in the cardiovascular response to exercise. Cardiovasc. Clin. 1989;19:17–33. [PubMed] [Google Scholar]
- 48.Maruf F.A., Ogochukwu U.N., Dim P.A., Alada A.R. Absence of sex differences in systolic blood pressure and heart rate responses to exercise in healthy young adults. Niger. J. Physiol. Sci. 2012;27:95–100. [PubMed] [Google Scholar]
- 49.Gardin J.M., Savage D.D., Ware J.H., Henry W.L. Effect of age, sex, and body surface area on echocardiographic left ventricular wall mass in normal subjects. Hypertension. 1987;9:36–39. doi: 10.1161/01.hyp.9.2_pt_2.ii36. [DOI] [PubMed] [Google Scholar]
- 50.Hutchinson P.L., Cureton K.J., Outz H., Wilson G. Relationship of cardiac size to maximal oxygen uptake and body size in men and women. Int. J. Sports Med. 1991;12:369–373. doi: 10.1055/s-2007-1024696. [DOI] [PubMed] [Google Scholar]
- 51.Fu Q., Levine B.D. Cardiovascular response to exercise in women. Med. Sci. Sports Exerc. 2005;37:1433–1435. doi: 10.1249/01.mss.0000174886.08219.85. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan M.J., Cobb F.R., Higginbotham M.B. Stroke volume increases by similar mechanisms during upright exercise in normal men and women. Am. J. Cardiol. 1991;67:1147–1154. doi: 10.1016/0002-9149(91)90472-w. [DOI] [PubMed] [Google Scholar]
- 53.Poliner L.R., Dehmer G.J., Lewis S.E., Parkey R.W., Blomqvist C.G., Willerson J.T. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation. 1980;62:528–534. doi: 10.1161/01.cir.62.3.528. [DOI] [PubMed] [Google Scholar]
- 54.Higginbotham M.B., Morris K.G., Coleman R.E., Cobb F.R. Sex-related differences in the normal cardiac response to upright exercise. Circulation. 1984;70:357–366. doi: 10.1161/01.cir.70.3.357. [DOI] [PubMed] [Google Scholar]
- 55.Steingart R.M., Wexler J., Slagle S., Scheuer J. Radionuclide ventriculographic responses to graded supine and upright exercise: Critical role of the Frank-Starling mechanism at submaximal exercise. Am. J. Cardiol. 1984;53:1671–1677. doi: 10.1016/0002-9149(84)90600-3. [DOI] [PubMed] [Google Scholar]
- 56.Adams K.F., Vincent L.M., McAllister S.M., El-Ashnmawy H., Sheps D.S. The influence of age and gender on left ventricular response to supine exercise in asymptomaticnormal subjects. Am. Heart J. 1987;113:732–742. doi: 10.1016/0002-8703(87)90714-9. [DOI] [PubMed] [Google Scholar]
- 57.Hanley P.C., Zinsmeister A.R., Clements I.P., Bove A.A., Brown M.L., Gibbson R.J. Gender related differences in cardiac response to supine exercise assessed by radionuclide angiography. J. Am. Coll. Cardiol. 1989;13:624–629. doi: 10.1016/0735-1097(89)90603-7. [DOI] [PubMed] [Google Scholar]
- 58.Gledhill N., Cox D., Jamnik R. Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med. Sci. Sports Exerc. 1994;26:1116–1121. [PubMed] [Google Scholar]
- 59.Zhou B., Conlee R.K., Jensen R., Fellingham G.W., George J.D., Fisher A.G. Stroke volume does not plateau during graded exercise in elite male distance runners. Med. Sci. Sports Exerc. 2001;33:1849–1854. doi: 10.1097/00005768-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Vella C.A., Robergs R.A. A review of the stroke volume response to upright exercise in healthy subjects. Br. J. Sports Med. 2005;39(4):190–195. doi: 10.1136/bjsm.2004.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang E., Solli G.S., Nyberg S.K., Hoff J., Helgerud J. Stroke volume does not plateau in female endurance athletes. Int. J. Sports Med. 2012;33:734–739. doi: 10.1055/s-0031-1301315. [DOI] [PubMed] [Google Scholar]
- 62.Astrand P.O., Cuddy T.E., Saltin B., Stenberg J. Cardiac output during submaximal and maximal work. J. Appl. Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- 63.Fleg J.L., O’Connor F., Gernstenblith G., et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J. Appl. Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald M.D., Tanaka H., Tran Z.V., Seals D.R. Age-related decline in maximal aerobic capacity in regularly exercising vs. sedentary females: A meta-analysis. J. Appl. Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 65.Charkoudian N. Influences of female reproductive hormones on sympathetic control of the circulation in humans. Clin. Auton. Res. 2001;11:295–301. doi: 10.1007/BF02332974. [DOI] [PubMed] [Google Scholar]
- 66.Wallin B.G., Hart E.C., Wehrwein E.A., Charkoudian N., Joyner M.J. Relationship between breathing and cardiovascular function at rest: sex-related differences. Acta Physiol. (Oxf.) 2010;200:193–200. doi: 10.1111/j.1748-1716.2010.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raemakers D., Ector H., Aubert A.E., Rubens A. Van de Werf. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur. Heart J. 1998;19:1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- 68.Barnett S.R., Morin R.J., Kiely D.K., et al. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension. 1999;33:301–306. doi: 10.1161/01.hyp.33.5.1195. [DOI] [PubMed] [Google Scholar]
- 69.Evans J.M., Zielgler M.G., Patwardhan A.R., et al. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamics indexes. J. Appl. Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 70.Fisher J.P., Adlan A.M., Shantsila A., Secher F.J., Sørensen H., Secher N.H. Muscle metaboreflex and autonomic regulation of heart rate in humans. J. Physiol. 2013;591:3777–3788. doi: 10.1113/jphysiol.2013.254722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carter R., III, Watenpaugh D.E., Wasmund W.L., Wasmund S.L., Smith M.L. Muscle pump and central command during recovery from exercise in humans. J. Appl. Physiol. 1999;87:1463–1469. doi: 10.1152/jappl.1999.87.4.1463. [DOI] [PubMed] [Google Scholar]
- 72.Crisafulli A., Melis F., Orrù V., Lener R., Lai C., Concu A. Hemodynamics during a postexertional asystolia in a healthy athlete: A case study. Med. Sci. Sports Exerc. 2000;32:4–9. doi: 10.1097/00005768-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Cote A.T., Phillips A.A., Foulds H.J., et al. Sex differences in cardiac function after prolonged strenuous exercise. Clin. J. Sport Med. 2015;25(3):276–283. doi: 10.1097/JSM.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 74.Macey P.M., Rieken N.S., Ogren J.A., Macey K.E., Kumar R., Harper R.M. Sex differences in insular cortex gyri responses to a brief static handgrip challenge. Biol. Sex Differ. 2017;8:13. doi: 10.1186/s13293-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ettinger S.M., Silber D.H., Collins B.G., et al. Influences of gender on sympathetic nerve responses to static exercise. J. Appl. Physiol. 1996;80(1):245–251. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- 76.Lalande S., Barron C.C., Shoemaker J.K. Sex difference in the influence of central blood volume mobilization on the exercise pressor response. Eur. J. Appl. Physiol. 2015;115(12):2653–2660. doi: 10.1007/s00421-015-3272-z. [DOI] [PubMed] [Google Scholar]
- 77.Minahan C., O’Neill H., Sikkema N., Joyce S., Larsen B., Sabapathy S. Oral contraceptives augment the exercise pressor reflex during isometric handgrip exercise. Physiol. Rep. 2018;6(5) doi: 10.14814/phy2.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parmar H.R., Sears J., Molgat-Seon Y., et al. Oral contraceptives modulate the muscle metaboreflex in healthy young women. Appl. Physiol. Nutr. Metab. 2018;43(5):460–466. doi: 10.1139/apnm-2017-0482. [DOI] [PubMed] [Google Scholar]
- 79.Ives S.J., McDaniel J., Witman M.A., Richardson R.S. Passive limb movement: evidence of mechanoreflex sex specificity. Am. J. Physiol. Heart Circ. Physiol. 2013;304(1):H154–H161. doi: 10.1152/ajpheart.00532.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdel-Rahman A.R., Merrill R.H., Wooles W.R. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J. Appl. Physiol. 1994;77(2):606–613. doi: 10.1152/jappl.1994.77.2.606. [DOI] [PubMed] [Google Scholar]
- 81.Laitinen T., Hartikainen J., Vanninen E., Niskanen L., Geelen G., Länsimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J. Appl. Physiol. 1998;84(2):576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 82.Cooke W.H., Ludwig D.A., Hogg P.S., Eckberg D.L., Convertino V.A. Does the menstrual cycle influence the sensitivity of vagally mediated baroreflexes? Clin. Sci. (Lond.) 2002;102(6):639–644. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 83.Tank J., Diedrich A., Szczech E., Luft F.C., Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension. 2005;45(6):1159–1164. doi: 10.1161/01.HYP.0000165695.98915.9a. [DOI] [PubMed] [Google Scholar]
- 84.Fisher J.P., Kim A., Hartwich D., Fadel P.J. New insights into the effects of age and sex on arterial baroreflex function at rest and during dynamic exercise in humans. Auton. Neurosci. 2012;172(1-2):13–22. doi: 10.1016/j.autneu.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kingsley JD, Tai YL, Marshall EM, et al. Autonomic modulation and baroreflex sensitivity after acute resistance exercise: Responses between sexes. J Sports Med Phys Fitness 2018 (at press). 2018 doi: 10.23736/S0022-4707.18.08864-3. [DOI] [PubMed] [Google Scholar]
- 86.Kim A., Deo S.H., Fisher J.P., Fadel P.J. Effect of sex and ovarian hormones on carotid baroreflex resetting and function during dynamic exercise in humans. J. Appl. Physiol. 2012;112(8):1361–1371. doi: 10.1152/japplphysiol.01308.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teixeira A.L., Ritti-Dias R., Antonino D., Bottaro M., Millar P.J., Vianna L.C. Sex differences in cardiac baroreflex sensitivity after isometric handgrip exercise. Med. Sci. Sports Exerc. 2018;50(4):770–777. doi: 10.1249/MSS.0000000000001487. [DOI] [PubMed] [Google Scholar]
- 88.Samora M, Teixeira AL, Sabino-Carvalho JL, Vianna LC. Spontaneous cardiac baroreflex sensitivity is enhanced during post-exercise ischemia in men but not in women. Eur J Appl Physiol. 2018 doi: 10.1007/s00421-018-4004-y. (at press). [DOI] [PubMed] [Google Scholar]
- 89.Pelliccia A., Maron B., Culasso F., Spataro A., Caselli G. Athlete’s heart in women. Echocardiographic characterisation of highly trained elite female athletes. JAMA. 1996;276:211–215. doi: 10.1001/jama.276.3.211. [DOI] [PubMed] [Google Scholar]
- 90.Sharma S., Maron B.J., Whyte G., Firoozi S., Elliott P.M., McKenna W.J. Physiologic limits of left ventricular hypertrophy in junior elite athletes: relevant to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002;40:1431–1436. doi: 10.1016/s0735-1097(02)02270-2. [DOI] [PubMed] [Google Scholar]
- 91.Rowland T., Roti M. Influence of sex on the “Athlete’s Heart” in trained cyclists. J. Sci. Med. Sport. 2010;13(5):475–478. doi: 10.1016/j.jsams.2009.10.488. [DOI] [PubMed] [Google Scholar]
- 92.Whyte G.P., George K., Sharma S., et al. The upper limit of physiological cardiac hypertrophy in elite male and female athletes: The British experience. Eur. J. Appl. Physiol. 2004;92(4-5):592–597. doi: 10.1007/s00421-004-1052-2. [DOI] [PubMed] [Google Scholar]
- 93.Whyte G.P., George K., Nevill A., Shave R., Sharma S., McKenna W.J. Left ventricular morphology and function in female athletes: A meta-analysis. Int. J. Sports Med. 2004;25(5):380–383. doi: 10.1055/s-2004-817827. [DOI] [PubMed] [Google Scholar]
- 94.Bernardo B.C., Weeks K.L., Pretorius L., McMullen J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010;128(1):191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Foryst-Ludwig A., Kintscher U. Sex differences in exercise-induced cardiac hypertrophy. Pflugers Arch. 2013;465(5):731–737. doi: 10.1007/s00424-013-1225-0. [DOI] [PubMed] [Google Scholar]
- 96.Petersen S.E., Wiesmann F., Hudsmith L.E., et al. Functional and structural vascular remodeling in elite rowers assessed by cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2006;48(4):790–797. doi: 10.1016/j.jacc.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 97.Giraldeau G., Kobayashi Y., Finocchiaro G., et al. Gender differences in ventricular remodeling and function in college athletes, insights from lean body mass scaling and deformation imaging. Am. J. Cardiol. 2015;116(10):1610–1616. doi: 10.1016/j.amjcard.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 98.Hedman K., Tamás Ĕ., Henriksson J., Brudin L., Nylander E. Female athlete’s heart: systolic and diastolic function related to circulatory dimensions. Scand. J. Med. Sci. Sports. 2015;25(3):372–381. doi: 10.1111/sms.12246. [DOI] [PubMed] [Google Scholar]
- 99.Green D.J., Hopkins N.D., Jones H., Thijssen D.H., Eijsvogels T.M., Yeap B.B. Sex differences in vascular endothelial function and health in humans: Impacts of exercise. Exp. Physiol. 2016;101(2):230–242. doi: 10.1113/EP085367. [DOI] [PubMed] [Google Scholar]


