Abstract
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme family of phospholipase A2 produced by the inflammatory cell in atherosclerotic plaque. It is transported in the circulation, attached mainly to low-density lipoprotein-cholesterol (LDL-C). It hydrolyzes glycerophospholipids particularly fatty acids at the sn-2 position and produces numerous bioactive lipids; and leads to endothelial dysfunction, atherosclerotic plaque inflammation, and development of the necrotic core in plaques.
There are two kinds of phospholipase A2, namely: secretory phospholipase A2 (sPLA2) and Lp-PLA2. They are deemed as evolving predictors of cardiovascular disease (CVD) risk in hospital- and population-based studies, including healthy subjects, acute coronary syndromes (ACS) and patients with CVD. Unfortunately, Lp-PLA2 inhibitor (darapladib) and s-PLA2 inhibitor (varespladib methyl) failed to prove to lower the risk of composite CVD mortality, myocardial infarction and stroke in those with stable CVD and ACS.
Herein, we describe the explanation based on the existing data why there is still a discrepancy among them. So, it highlights the opinion that phospholipase A2 is merely the inflammatory biomarkers of CVD and playing an important role in atherosclerosis. Further, there is more spacious room to prove the causation.
Keywords: Lipoprotein-associated phospholipase A2, LDL-C, cardiovascular disease, acute coronary syndromes, myocardial infarction, atherosclerosis
1. INTRODUCTION
Cardiovascular disease (CVD) is a rising public health problem throughout Asia and the Middle East. Moreover, CVD is mainly among the most dominant and devastating illnesses [1]. Dyslipidemia is a vital risk factor in the initiation of atherosclerosis and related cardiovascular outcomes; consequently, efficacious therapeutic strategies which are significant in lowering the risk of CVD are needed [2]. Developing countries contribute around 80% of the global CVD death [2, 3], however, the CVD mortality has been declining in the last decade in developed countries. It is recognized that dyslipidemia is a significant CVD risk factor, in which the failure to achieve significantly targeted lipid levels leads to the residual risk of CVD [4].
The pathophysiology of CVD is underpinned by atherosclerosis. The process is initiated by trapping oxidized low-density lipoprotein-cholesterol (LDL-C) in the sub-intimal space of large and medium-sized arteries. Oxidized LDL-C (OxLDL) is a general term which includes various oxidative modifications to LDL lipid moieties and apolipoprotein B (ApoB), the structural protein of the LDL-C particle [5]. These modifications consist of restructuring of the phospholipid molecules with exposure of phosphorylcholine and adduction of aldehydes, namely malondialdehyde (MDA), to ApoB. The enzyme called phospholipase A2 modifies the oxidative changes in LDL-C.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is produced by inflammatory cells in atherosclerotic plaque and is categorized as an enzyme family of phospholipase-A2. It is transported in the circulation and is mainly attached to LDL-C. The Lp-PLA2 and another phospholipase A2 (such as secretory phospholipase A2 = sPLA2) proliferate the inflammatory reaction by generating precursors of a mass that are significantly related with CVD events in stable coronary heart diseases (CHD), acute coronary syndromes (ACS), and incident peripheral arterial diseases (PAD) [6-8]. In a meta-analysis, there is a continuous association between the activity of plasma Lp-PLA2 and CHD risk. There is also a relative increase in the risk of 1.10 (95% CI, 1.05 to 1.16) for each 1-SD increase in plasma Lp-PLA2 activity after being adjusted for the traditional CVD risk factors [9]. Thus, the existing evidence has inspired endeavors to develop phospholipase inhibitors: agents to prevent CVD.
Darapladib is a molecule and a potent oral Lp-PLA2 inhibitor, and varespladib methyl is another nonspecific pan-secretory phospholipase A2 inhibitor. In an atherosclerotic animal model, darapladib reduces the expression of Lp-PLA2 in atherosclerotic plaque, decreases the necrotic size within the plaque, and inhibits the lesion initiation in coronary arteries [10]. It reduces the Lp-PLA2 activity in human carotid plaque as well [11]. Another study demonstrates that varespladib methyl reduces the concentrations of sPLA2-IIA by more than 90%, also reducing the LDL-C and C-Reactive Protein (CRP) in those with stable coronary artery disease and ACS [12, 13].
Although these drugs have demonstrated their role in improving the atherosclerotic process, unfortunately, in subjects with stable CHD, darapladib does not considerably decrease the primary composite outcomes of cardiovascular mortality, myocardial infarction, or stroke [14]. Likewise, varespladib methyl does not have the clinical benefit of decreasing recurrent cardiovascular outcomes and, even further, considerably increases myocardial infarction [15]. This review article elaborates the role of Lp-PLA2 and sPLA2-IIA in atherosclerosis and CVD, as published in the literature over the last 25 years, until 2019, their biological activity, and whether there is an inconsistency in their clinical benefits.
2. ROLE OF PHOSPHOLIPASE A2 IN ATHEROSCLEROSIS
The phospholipase A2 superfamily of enzymes has been recognized to have a role in atherosclerosis, and at least two groups in this family of enzymes are deemed potential candidates for CVD prevention. Human biomarker studies, animal studies, imaging studies, and genome-wide atherosclerosis studies have presented the rationale for clinical outcome trials directed at inhibiting sPLA2-IIA and Lp-PLA2 [16]. One study suggests that plasma sPLA2 is associated with ACS via increased serum amyloid-A protein, representing an inflammatory response in ACS [6].
The phospholipase A2 superfamily enzymes are considered to have the capacity to hydrolyze fatty acids at the sn-2 position of glycerophospholipids and create plentiful of bioactive lipids (Fig. 1) [16]. Plasma levels and activity of two families of phospholipase A2 enzymes, namely, sPLA2-IIA and Lp-PLA2, have been assessed as biomarkers of CVD risk in hospital-based and population-based studies including apparently healthy subjects, subjects with ACS, and patients with established CHD [6, 9, 17, 18]. Moreover, a multivariate analysis reveals that high sPLA2-IIA mass and activity levels as an independent predictor of early atherosclerosis in metabolic syndrome (Mets) subjects [19]. Even the Lp-PLA2 concentrations in human plasma are associated with the CHD severity, and an apparent interaction between Lp-PLA2 and classical CVD risk factors are settled in predicting CHD [20].
Fig. (1).
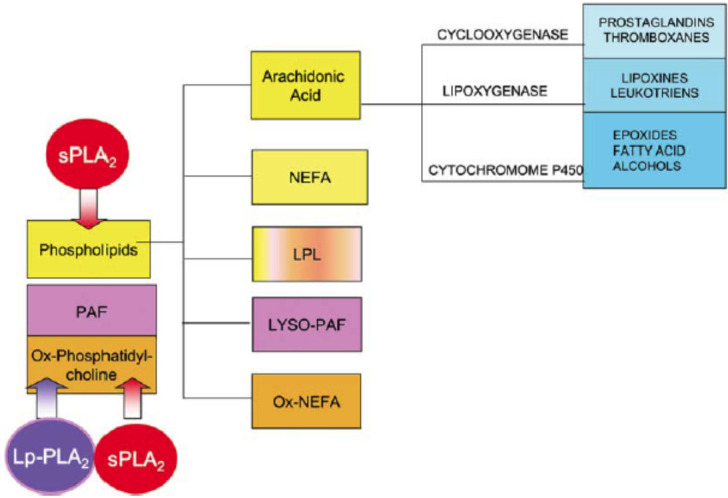
Phospholipase A2 enzymatic activity generates lipid products with biological properties and functions [16]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
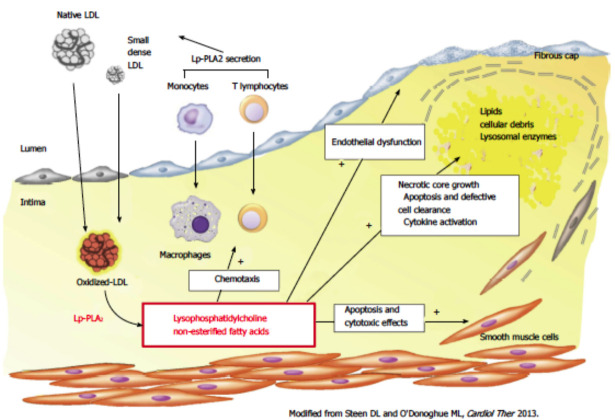
Lipoprotein-associated phospholipase A2, a vascular-specific inflammatory biomarker, is an enzyme produced by macrophages, T-lymphocytes, and monocytes, as well as the mast cells in atherosclerotic plaques, principally within the necrotic core and fibrotic cap of vulnerable plaques [21-24]. Lp-PLA2 hydrolyzes the modified and oxidized phospholipids on the LDL-C surface [24] and within the plaques to lead to endothelial dysfunction [25], plaque inflammation, and formation of the necrotic core in atherosclerotic plaques. It releases arachidonic acids, lysophosphatidylcholine, and oxidized fatty acids. This process is well-recognized as a trigger of the inflammatory reaction.
Further, Lp-PLA2 is hypothesized to take an essential role in oxidative modification of LDL-C and initiation of inflammatory reactions in the arterial intima [23, 26, 27]. The congregation of lysophosphatidylcholine and oxidized fatty acids in the sub-intimal space contributes to the development of the plaque lipid “core.” Furthermore, the macrophages, once they ingest these substrates, would consequently advance their conversion into foam cells. Additionally, lysophosphatidylcholine provokes the creation of reactive oxygen species such as superoxide by triggering the endothelial nicotinamide adenine dinucleotide phosphate oxidase and by activating nitric oxide synthase (eNOS) “uncoupling” [28]. The further process would drive the enzyme to become a superoxide and peroxynitrite producer. Consequently, this contributes to atherosclerosis and plaque destabilization. Serum levels of Lp-PLA2 change considerably during the early phase of ACS, and augmented Lp-PLA2 independently predicts CVD outcomes in patients with ACS after adjustment for potential confounders [29]. Elevated Lp-PLA2 concentrations in the elderly Chinese subjects were associated an increased risk of carotid atherosclerosis and myocardial infarction and CVD death [30].
Therefore, experimental pieces of evidence indicate that Lp-PLA2 is involved in multiple stages of atherosclerosis due to its pro-inflammatory and pro-oxidative effects (Fig. 2) and may be a useful biomarker to predict the clinical outcomes of patients with CHD independent of traditional CVD risk factors. The Lp-PLA2 activity and mass, each demonstrates the continuous associations with the risk of CHD, similar with non-high density lipoprotein cholesterol or systolic blood pressure [19]. In patients with TIA (transient ischemic attack) and first ischemic stroke, elevated Lp-PLA2 activity levels are associated with recurrent vascular events. Significant associations are also encountered between high hs-CRP and plasma Lp-PLA2 concentration and carotid stenosis (OR: 2.62, 95% CI: 0.93 - 7.38). This result suggests that the combination of hs-CRP and plasma Lp-PLA2 would be better predictors than either protein alone concerning carotid atherosclerosis [31].
Fig. (2).
Pathogenic role of lipoprotein-associated phospholipase A2 in atherosclerosis development [25]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Also, in the general population, high plasma Lp-PLA2 levels are related to the risk of stroke [32]. While a higher Lp-PLA2 activity is related to an enhanced risk for incident PAD, it is more likely that traditional CVD risk factors are more causative [7]. However, another study confirms no evidence of an association between Lp-PLA2 and incident PAD [33]. Additionally, Lp-PLA2 takes part by a critical role in microvascular dysfunction and oxidative stress, showing a positive relationship with metabolic disorders [34]. This result is translated to another study, indicating that higher plasma Lp-PLA2 concentrations are related to increasing the risk of mortality and the incident of diabetic retinopathy in diabetic subjects [35]. Further, Lp-PLA2 and sPLA2 are closely associated with insulin resistance and macroangiopathy in diabetic patients [36]. Accordingly; the phospholipase A2 is considered to modify the micro- and macroangiopathy complications in those with diabetes mellitus. Plasma Lp-PLA2 activity is also discovered to be higher in subjects with definite familial hypercholesterolemia (FH) than in non-definite FH subjects, independently of LDL-C concentrations and statin consumption. This confirms that FH patients present higher arterial inflammation, which may contribute to a higher CVD risk [37].
3. STRUCTURE OF LP-PLA2
Plasma Lp-PLA2 consists of a 43-kDa-monomer polypeptide, and the PLA2G7 gene encodes this enzyme [38]. Lp-PLA2 binds to two separate domains on the ApoB. Approximately 70% of this enzyme is associated with LDL-C and lipoprotein (a) once it is secreted in the plasma [39, 40], and the remainder is associated with high-density lipoprotein cholesterol (HDL-C). Previously, plasma Lp-PLA2 is also named as a platelet-activating factor acetylhydrolase (PAF-AH) (Fig. 3). This enzyme is a heterotetrameric enzyme consisting of catalytic 29- and 30-kDa and non-catalytic 45-kDa subunits (Fig. 4).
Fig. (3).
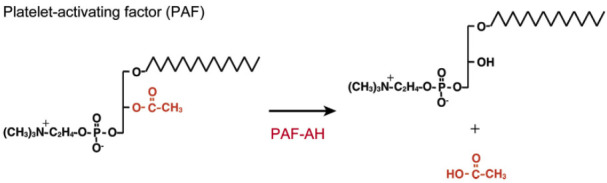
Enzymatic reaction of PAF – AH. PAF-AH: platelet-activating factor ~ acetyl hydrolase [40]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (4).
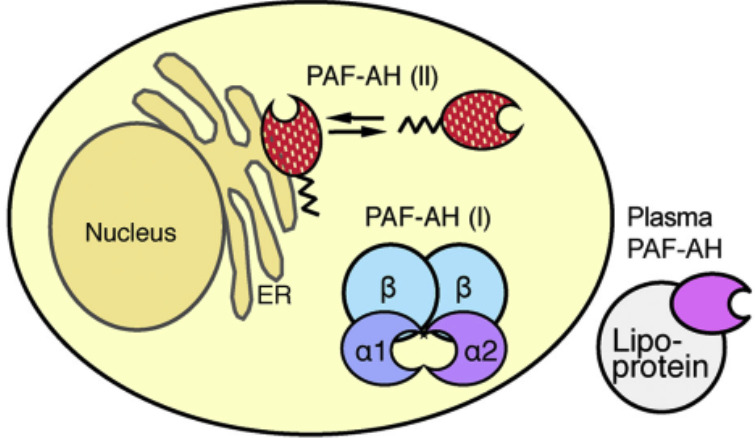
Structure of PAF-AH in mammals. PAH-AH or Lp-PLA2 is a heterotetrameric enzyme consisting of catalytic 29- and 30- kDa and non-catalytic 45-kDa subunits [40]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
It is initially a purified enzyme that hydrolyzes the acetyl group attached to the sn-2 position of the platelet-activating factor (PAF) [41]. Consequently, it is regarded as having anti-inflammatory and anti-atherogenic properties as well.
Beyond this, there is a dissociable and a non-dissociable form of the plasma Lp-PLA2 [42]. The activity of plasma Lp-PLA2 in vivo is affected by the transition between them, and it is considered to be the novel proposed mechanism. The amino acid sequence of human plasma PAF-AH involves 441 amino acid residues in length and contains a Gly-X-Ser-Gly (GXSXG) motif, which is also found in esterase, lipase, and other members of the α/ß hydrolase superfamily of enzymes [38, 43]. The crystal structure of Lp-PLA2 has a classic α/ß serine hydrolase fold containing a catalytic triad Ser273, Asp296, and His351 [43, 44]. Another triad (Trp115, Leu116, and Tyr205) is involved in the interaction with LDL-C [45].
4. GENETICS OF LP-PLA2
The plasma Lp-PLA2 is encoded by the PLA2G7 gene. This gene is positioned in chromosome 6p21.2 to 12 and entails 12 exons. Its cDNA has been cloned in 1995 [43] and contains an open reading frame codifying a precursor of 441 amino acids that is cleaved into 45.4 kDa mature protein [46]. Heritability studies revealed that approximately 62% of the Lp-PLA2 activity variation was deemed to be a genetic factor [47]. Traditional CVD risk factors and genetic difference contributed to variability in Lp-PLA2 activity and concentration [48].
The PLA2G7 gene is typified by the non-synonymous polymorphisms which could produce a loss of function in the enzymatic activity [24]. As stated by one study and a recent meta-analysis, Caucasian has a higher Lp-PLA2 activity level than Hispanics and African-Americans. It justifies the conclusion that Lp-PLA2 is genetically influenced [49, 50]. By contrast, atherosclerosis [51], stroke, [52] and dilated cardiomyopathy [53] are associated with a point mutation (rs76863441) close to the active site of the enzyme [54], which is found in 4% of Japanese people and implies undetectable plasma LpPLA2 activity. Likewise, natural insufficiency in LpPLA2 activity due to polymorphism in PLA2G7 gene (279F allele) protects from CHD in a Korean population [55].
Nevertheless, the association between A379V polymorphism and enzymatic levels and CVD outcomes was diverse amongst the studies, with some of them presenting the V379 allele as associated with increased enzyme levels [56, 57] whereas the other studies exhibited lower levels [58]. Also, a recent metanalysis result proposed that V279F polymorphism in PLA2G7 gene had a protective effect on CVD, while R92H polymorphism might contribute toward the enhanced risk of clinical atherosclerosis [59] (Figs. 5 and 6).
Fig. (5).
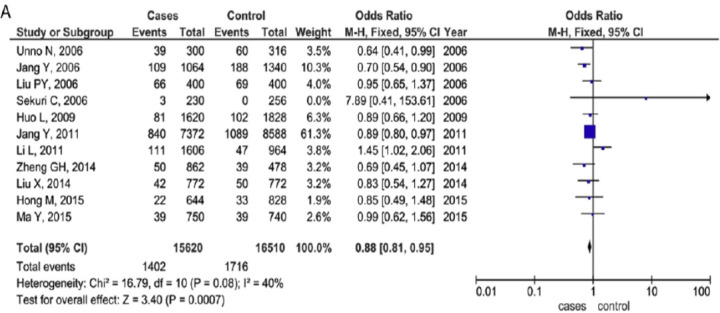
V279F polymorphism of PLA2G7 gene in Asian and Caucasian population [60]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (6).
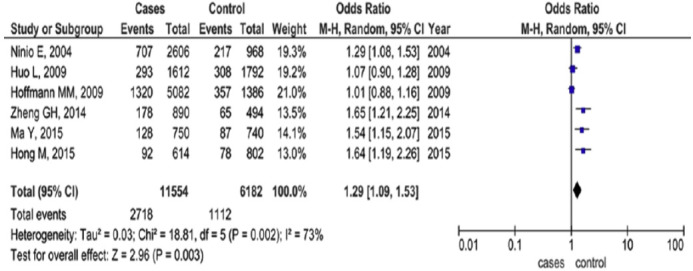
R92H polymorphism of PLA2G7 gene in Asian and Caucasian population [60]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Furthermore, Lp-PLA2 activity was proven to be 10% lower in female subjects in comparison with male, perhaps due to the higher estrogen concentration in women, down-regulating Lp-PLA2 activity and reducing LDL-C concentration. All this evidence contributes further to discrepancies in the theorized causative relationship between Lp-PLA2 and CVD.
5. EVIDENCE FROM RANDOMIZED CLINICAL TRIALS
Darapladib is a selective, effective and reversible oral agent inhibiting plasma Lp-PLA2 [60] that has also been shown to reduce Lp-PLA2 activity in human carotid plaque [11]. Furthermore, darapladib ceases the progression of the necrotic core of coronary plaques, compared to placebo. This evidence is shown in the Integrated Biomarker and Imaging Study 2 (IBIS-2) [61]. The mechanism of darapladib in halting the atherosclerotic progression has been verified in an animal study using the Sprague-Dawley rat model; this study signified that darapladib reduced the foam cells number, inducible nitric oxide synthase (i-NOS), and intracellular adhesion molecule-1 (ICAM-1) expression in the aorta at the early stages of atherosclerosis in type-2 diabetic model [62]. Consequently, there is a suggestion that altering the composition of atherosclerotic plaques to a less vulnerable state might decrease the risk of CVD outcomes.
The above hypothesis-generating statements have been further challenged to be translated using clinical endpoints. In the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial [14], darapladib, unfortunately, did not significantly show the clinical benefits of primary composite endpoints of cardiovascular mortality, myocardial infarction or stroke decrement, in those with stable CHD. This was a large, multicenter, randomized clinical study, randomly assigning 15,828 subjects with stable CHD. The median follow-up of the study was of 3.7 years. The absence of clinical effects of this drug on the primary CVD outcomes might be related to a minor impact on unstable atherosclerotic plaque than was expected by the earlier studies. The CVD risk of all patients in that trial had been minimized by standard concurrent therapy. Over a third of the patients had an LDL cholesterol concentration lower than 70 mg/dL at baseline; moreover, approximately 75% of the patients underwent revascularization procedures. Another point was that 96% of the patients in the study was consuming statins. Statins consumption has been revealed to decrease the concentration of plasma Lp-PLA2 by up to 35% [63]. The long-term inhibition of endogenous Lp-PLA2 activity with darapladib was not associated with a change in plaque progression, vulnerability indices after six months of therapy, or coronary endothelial function improvement [64]. These studies suggested that the endogenous Lp-PLA2 pathway might not have a direct, independent role in the progression of early atherosclerosis in humans [65].
Other explanations might come from the current meta-analysis [59], which disclosed a significant negative association between V279F polymorphism and clinical atherosclerosis. A rare, non-synonymous polymorphism (V279F) was commonly encountered in Japanese, Turk, Kyrgyz, and Azerbaijan populations. These were associated with a decreased enzymatic level of Lp-PLA2 in heterozygous subjects and complete loss of enzymatic levels in homozygous subjects [66]. All the above evidence may affected the results of those clinical outcome trials, in contrast to those of the previous mechanistic studies.
Another phospholipase inhibitor drug, varespladib methyl, is a nonspecific pan-sPLA2 inhibitor with positive effects on atherosclerotic plaques in animal studies. Previous studies confirmed that varespladib methyl decreased the concentration of sPLA2-IIA over 90% in addition to lowering LDL-cholesterol and C-reactive protein in stable CHD and ACS patients. The Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 Weeks (VISTA-16) trial was planned to evaluate the clinical benefits of varespladib methyl on cardiovascular risk in patients with ACS [15].
Although experimental and observational clinical studies suggest that pan-inhibition of plasma sPLA2 will provide beneficial CVD outcomes and significantly reduce the post-ACS inflammatory response [13, 67], the VISTA-16 trial shows evidence contrary to this. The VISTA-16 trial demonstrated that in those with current ACS, varespladib methyl did not decrease the risk of recurrent CVD events and even significantly enhanced the myocardial infarction risk. Hence, the authors concluded that sPLA2 inhibition with varespladib methyl could be harmful and is not a valuable strategy to reduce adverse CVD outcomes after ACS [15]. Possible factors in this negative result include insufficient penetration of varespladib methyl into vascular cells to hinder pro-inflammatory intracellular mediators. Otherwise, varespladib methyl might have abolished the effects of both pro-atherogenic (IIA and V) and anti-atherogenic sPLA2 isoforms. The above results with sPLA2 inhibition highlight how the identification of a plasma biomarker of CVD risk does not automatically suggest that pharmacologic inhibition of the biomarker will diminish the cardiovascular risk. The failure to prove any clinical advantage has been supported by a current report from Mendelian randomization studies and reveals that sPLA2 does not independently take a causal role in CVD [68]. Despite strong biological plausibility and convincing evidence from multiple studies and the failure of Mendelian randomization studies to prove the evidence of causation, this ideally should support the identification of the novel biomarkers most likely to be causally implicated in the pathobiology of CVD, shed light on convincing therapeutic targets and, finally, mitigate the huge costs expended in clinical trials [69].
None of the phospholipase inhibitors in those clinical trials have been distributed to the market yet. The failure of the Lp-PLA2 inhibitor darapladib currently suggests that this enzyme is just a biomarker of vascular inflammation rather than playing a key role in the causal pathway of CVD. These findings, together with the failure of the sPLA2 inhibitor varespladib methyl for the treatment of CVD, may indicate that more in-depth knowledge of these enzymes is needed.
CONCLUSION
Deciphering all the data together, Lp-PLA2 and sPLA2 are merely the biomarkers of vascular inflammation and play an important role in atherosclerosis. These may indicate that a more in-depth study is needed to prove the causation.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Yang Z.J., Liu J., Ge J.P., Chen L., Zhao Z.G., Yang W.Y. Prevalence of cardiovascular disease risk factor in the Chinese population: the 2007-2008 China National Diabetes and Metabolic Disorders Study. Eur. Heart J. 2012;33(2):213–220. doi: 10.1093/eurheartj/ehr205. [http://dx.doi.org/10.1093/eurheartj/ehr205]. [PMID: 21719451]. [DOI] [PubMed] [Google Scholar]
- 2.Alshamiri M., Ghanaim M.M.A., Barter P., et al. Expert opinion on the applicability of dyslipidemia guidelines in Asia and the Middle East. Int. J. Gen. Med. 2018;11:313–322. doi: 10.2147/IJGM.S160555. [http://dx.doi.org/10.2147/IJGM.S160555]. [PMID: 30050317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsheikh-Ali A.A., Omar M.I., Raal F.J., et al. Cardiovascular risk factor burden in Africa and the Middle East: the Africa Middle East Cardiovascular Epidemiological (ACE) study. PLoS One. 2014;9(8):e102830. doi: 10.1371/journal.pone.0102830. [http://dx.doi.org/10.1371/journal.pone.0102830]. [PMID: 25090638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao F., Zhou Y.J., Hu D.Y., et al. Contemporary management and attainment of cholesterol targets for patients with dyslipidemia in China. PLoS One. 2013;8(4):e47681. doi: 10.1371/journal.pone.0047681. [http://dx.doi.org/10.1371/journal.pone.0047681]. [PMID: 23593110]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartley A, Dorian H, Khamis R. Oxidized and anti-oxidized LDL antibodies in atherosclerosis - Novel insights and future direction in diagnosis and therapy. Trends Cardiovasc Med. 2018;S1050-1738(18):30083–30085. doi: 10.1016/j.tcm.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Santoso A., Kaniawati M., Bakri S., Yusuf I. Secretory phospholipase A2 is associated with the odds of acute coronary syndromes through elevation of serum amyloid-A protein. Int. J. Angiol. 2013;22(1):49–54. doi: 10.1055/s-0033-1334093. [http://dx.doi.org/10.1055/s-0033-1334093]. [PMID: 24436584]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg P.K., Norby F.L., Polfus L.M., et al. Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease: Findings from The Atherosclerosis Risk in Communities study (ARIC). Atherosclerosis. 2018;268:12–18. doi: 10.1016/j.atherosclerosis.2017.11.007. [http://dx.doi.org/10.1016/j.atherosclerosis.2017.11.007]. [PMID: 29169030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D., Zhao L., Yu J., et al. Lipoprotein-associated phospholipase A2 in coronary heart disease: Review and meta-analysis. Clin. Chim. Acta. 2017;465:22–29. doi: 10.1016/j.cca.2016.12.006. [http://dx.doi.org/10.1016/j.cca.2016.12.006]. [PMID: 27956130]. [DOI] [PubMed] [Google Scholar]
- 9.Thompson A., Gao P., Orfei L., et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [http://dx.doi.org/10.1016/S0140-6736(10)60319-4]. [PMID: 20435228]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilensky R.L., Shi Y., Mohler E.R., III, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat. Med. 2008;14(10):1059–1066. doi: 10.1038/nm.1870. [http://dx.doi.org/10.1038/nm.1870]. [PMID: 18806801]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson J.L., Shi Y., Snipes R., et al. Effect of darapladib treatment on endarterectomy carotid plaque lipoprotein-associated phospholipase A2 activity: a randomized, controlled trial. PLoS One. 2014;9(2):e89034. doi: 10.1371/journal.pone.0089034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenson R.S., Hislop C., McConnell D., et al. PLASMA Investigators. Effects of 1-H-indole-3-glyxoamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomized, placebo-controlled trial. Lancet. 2009;373(9664):649–658. doi: 10.1016/S0140-6736(09)60403-7. [DOI] [PubMed] [Google Scholar]
- 13.Rosenson R.S., Hislop C., Elliott M., Stasiv Y., Goulder M., Waters D. Effects of varespladib methyl on biomarkers and major cardiovascular events in acute coronary syndrome patients. J. Am. Coll. Cardiol. 2010;56(14):1079–1088. doi: 10.1016/j.jacc.2010.06.015. [http://dx.doi.org/10.1016/j.jacc.2010.06.015]. [PMID: 20863951]. [DOI] [PubMed] [Google Scholar]
- 14.White H.D., Held C., Stewart R., et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 2014;370(18):1702–1711. doi: 10.1056/NEJMoa1315878. [http://dx.doi.org/10.1056/NEJMoa1315878]. [PMID: 24678955]. [DOI] [PubMed] [Google Scholar]
- 15.Nicholls S.J., Kastelein J.J.P., Schwartz G.G., et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311(3):252–262. doi: 10.1001/jama.2013.282836. [http://dx.doi.org/10.1001/jama.2013.282836]. [PMID: 24247616]. [DOI] [PubMed] [Google Scholar]
- 16.Rosenson R.S., Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur. Heart J. 2012;33(23):2899–2909. doi: 10.1093/eurheartj/ehs148. [http://dx.doi.org/10.1093/eurheartj/ehs148]. [PMID: 22802388]. [DOI] [PubMed] [Google Scholar]
- 17.Rana J.S., Arsenault B.J., Després J-P., et al. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur. Heart J. 2011;32(3):336–344. doi: 10.1093/eurheartj/ehp010. [http://dx.doi.org/10.1093/eurheartj/ehp010]. [PMID: 19224930]. [DOI] [PubMed] [Google Scholar]
- 18.Hatoum I.J., Cook N.R., Nelson J.J., Rexrode K.M., Rimm E.B. Lipoprotein-associated phospholipase A2 activity improves risk discrimination of incident coronary heart disease among women. Am. Heart J. 2011;161(3):516–522. doi: 10.1016/j.ahj.2010.11.007. [http://dx.doi.org/10.1016/j.ahj.2010.11.007]. [PMID: 21392606]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun C.Q., Zhong C.Y., Sun W.W., et al. Elevated Type II Secretory Phospholipase A2 Increases the Risk of Early Atherosclerosis in Patients with Newly Diagnosed Metabolic Syndrome. Sci. Rep. 2016;6:34929. doi: 10.1038/srep34929. [http://dx.doi.org/10.1038/srep34929]. [PMID: 27941821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge P.C., Chen Z.H., Pan R.Y., et al. Synergistic Effect of Lipoprotein-Associated Phospholipase A2 with Classical Risk Factors on Coronary Heart Disease: A Multi-Ethnic Study in China. Cell. Physiol. Biochem. 2016;40(5):953–968. doi: 10.1159/000453153. [http://dx.doi.org/10.1159/000453153]. [PMID: 27941334]. [DOI] [PubMed] [Google Scholar]
- 21.Winkler K., Winkelmann B.R., Scharnagl H., et al. Platelet-activating factor acetylhydrolase activity indicates angiographic coronary artery disease independently of systemic inflammation and other risk factors: the Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2005;111(8):980–987. doi: 10.1161/01.CIR.0000156457.35971.C8. [http://dx.doi.org/10.1161/01.CIR.0000156457.35971.C8]. [PMID: 15710755]. [DOI] [PubMed] [Google Scholar]
- 22.Brilakis E.S., McConnell J.P., Lennon R.J., Elesber A.A., Meyer J.G., Berger P.B. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur. Heart J. 2005;26(2):137–144. doi: 10.1093/eurheartj/ehi010. [http://dx.doi.org/10.1093/eurheartj/ehi010]. [PMID: 15618069]. [DOI] [PubMed] [Google Scholar]
- 23.Kolodgie F.D., Burke A.P., Skorija K.S., et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26(11):2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [http://dx.doi.org/10.1161/01.ATV.0000244681.72738.bc]. [PMID: 16960105]. [DOI] [PubMed] [Google Scholar]
- 24.Maiolino G., Bisogni V., Rossitto G., Rossi G.P. Lipoprotein-associated phospholipase A2 prognostic role in atherosclerotic complications. World J. Cardiol. 2015;7(10):609–620. doi: 10.4330/wjc.v7.i10.609. [http://dx.doi.org/10.4330/wjc.v7.i10.609]. [PMID: 26516415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kheirandish-Gozal L., Philby M.F., Qiao Z., Khalyfa A., Gozal D. Endothelial dysfunction in children with obstructive sleep apnea is associated with elevated lipoprotein-associated phospholipase a2 plasma activity levels. J. Am. Heart Assoc. 2017;6(2):e004923. doi: 10.1161/JAHA.116.004923. [http://dx.doi.org/10.1161/JAHA.116.004923]. [PMID: 28183716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang E.H., McConnell J.P., Lennon R.J., et al. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler. Thromb. Vasc. Biol. 2006;26(1):106–111. doi: 10.1161/01.ATV.0000191655.87296.ab. [http://dx.doi.org/10.1161/01.ATV.0000191655. 87296.ab]. [PMID: 16239595]. [DOI] [PubMed] [Google Scholar]
- 27.Tousoulis D., Papageorgiou N., Androulakis E., Stefanadis C. Lp-PLA2--a novel marker of atherosclerosis: to treat or not to treat? Int. J. Cardiol. 2013;165(2):213–216. doi: 10.1016/j.ijcard.2012.09.210. [http://dx.doi.org/10.1016/j.ijcard.2012.09.210]. [PMID: 23103134]. [DOI] [PubMed] [Google Scholar]
- 28.Fleming I., Mohamed A., Galle J., et al. Oxidized low-density lipoprotein increases superoxide production by endothelial nitric oxide synthase by inhibiting PKCalpha. Cardiovasc. Res. 2005;65(4):897–906. doi: 10.1016/j.cardiores.2004.11.003. [http://dx.doi.org/10.1016/j.cardiores.2004.11.003]. [PMID: 15721870]. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Wang H., Tian J., Chen B., Du F. Change in lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndrome. Medicine (Baltimore) 2018;97(28):e11517. doi: 10.1097/MD.0000000000011517. [http://dx.doi.org/10.1097/MD.0000000000011517]. [PMID: 29995820]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C., Fang X., Hua Y., et al. Lipoprotein-associated phospholipase A2 and risk of carotid atherosclerosis and cardiovascular events in community-based older adults in China. Angiology. 2018;69(1):49–58. doi: 10.1177/0003319717704554. [http://dx.doi.org/10.1177/0003319717704554]. [PMID: 28429599]. [DOI] [PubMed] [Google Scholar]
- 31.Liu H., Yao Y., Wang Y., et al. Association between high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2 and carotid atherosclerosis: A cross-sectional study. J. Cell. Mol. Med. 2018;22(10):5145–5150. doi: 10.1111/jcmm.13803. [http://dx.doi.org/10.1111/jcmm.13803]. [PMID: 30094934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Y, Jia H, Li S, et al. The associations of stroke, transient ischemic attack, and stroke-related recurrent vascular events with Lipoprotein-associated phospholipase A2. A systematic review and meta-analysis. Medicine. 2017;96(51):e9413.. doi: 10.1097/MD.0000000000009413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg P.K., Jorgensen N.W., McClelland R.L., et al. Lipoprotein-associated phospholipase A2 and risk of incident peripheral arterial disease in a multi-ethnic cohort: The Multi-Ethnic Study of Atherosclerosis. Vasc. Med. 2017;22(1):5–12. doi: 10.1177/1358863X16671424. [http://dx.doi.org/10.1177/1358863X16671424]. [PMID: 28215109]. [DOI] [PubMed] [Google Scholar]
- 34.De Stefano A., Mannucci L., Tamburi F., et al. Lp-PLA2, a new biomarker of vascular disorders in metabolic diseases. Int. J. Immunopathol. Pharmacol. 2019;•••:332058738419827154. doi: 10.1177/2058738419827154. [http://dx.doi.org/10.1177/2058738419827154]. [PMID: 30706739]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui M.K., Kennedy G., Carr F., et al. Lp-PLA2 activity is associated with increased risk of diabetic retinopathy: a longitudinal disease progression study. Diabetologia. 2018;61(6):1344–1353. doi: 10.1007/s00125-018-4601-7. [http://dx.doi.org/10.1007/s00125-018-4601-7]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin X.H., Xu M.T., Tang J.Y., et al. Effect of intensive insulin treatment on plasma levels of lipoprotein-associated phospholipase A2 and secretory phospholipase A2 in patients with newly diagnosed type 2 diabetes. Lipids Health Dis. 2016;15(1):203. doi: 10.1186/s12944-016-0368-3. [http://dx.doi.org/10.1186/s12944-016-0368-3]. [PMID: 27881128]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattina A., Rosenbaum D., Bittar R., et al. Lipoprotein-associated phospholipase A2 activity is increased in patients with definite familial hypercholesterolemia compared with other forms of hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2018;28(5):517–523. doi: 10.1016/j.numecd.2018.01.012. [http://dx.doi.org/10.1016/j.numecd.2018.01.012]. [PMID: 29525223]. [DOI] [PubMed] [Google Scholar]
- 38.Tjoelker L.W., Wilder C., Eberhardt C., et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374(6522):549–553. doi: 10.1038/374549a0. [http://dx.doi.org/10.1038/374549a0]. [PMID: 7700381]. [DOI] [PubMed] [Google Scholar]
- 39.Kono N., Arai H. Platelet-activating factor acetylhydrolase: an overview and update. BBA – Molecular and Cell Biology of Lipids; 2018. [DOI] [PubMed] [Google Scholar]
- 40.Rosenson R.S. Lp-PLA(2) and risk of atherosclerotic vascular disease. Lancet. 2010;375(9725):1498–1500. doi: 10.1016/S0140-6736(10)60488-6. [http://dx.doi.org/10.1016/S0140-6736(10)60488-6]. [PMID: 20435213]. [DOI] [PubMed] [Google Scholar]
- 41.Rosenson R.S. Physiochemically modified apolipoprotein B-containing lipoproteins and the risk of cardiovascular disease. J. Intern. Med. 2010;268(4):316–319. doi: 10.1111/j.1365-2796.2010.02272.x. [http://dx.doi.org/10.1111/j.1365-2796.2010.02272.x]. [PMID: 21050285]. [DOI] [PubMed] [Google Scholar]
- 42.Tselepis A.D., Dentan C., Karabina S.A., Chapman M.J., Ninio E. PAF-degrading acetylhydrolase is preferentially associated with dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler. Thromb. Vasc. Biol. 1995;15(10):1764–1773. doi: 10.1161/01.atv.15.10.1764. [PMID: 7583554]. [http://dx.doi.org/10.1161/01.ATV.15.10.1764]. [PMID: 7583554]. [DOI] [PubMed] [Google Scholar]
- 43.Tjoelker L.W., Eberhardt C., Unger J., et al. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J. Biol. Chem. 1995;270(43):25481–25487. doi: 10.1074/jbc.270.43.25481. [http://dx.doi.org/10.1074/jbc.270.43.25481]. [PMID: 7592717]. [DOI] [PubMed] [Google Scholar]
- 44.Samanta U., Bahnson B.J. Crystal structure of human plasma platelet-activating factor acetylhydrolase: structural implication to lipoprotein binding and catalysis. J. Biol. Chem. 2008;283(46):31617–31624. doi: 10.1074/jbc.M804750200. [http://dx.doi.org/10.1074/jbc.M804750200]. [PMID: 18784071]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J., Hsu Y.H., Li S., Woods V.L., Dennis E.A. Lipoprotein-associated phospholipase A(2) interacts with phospholipid vesicles via a surface-disposed hydrophobic α-helix. Biochemistry. 2011;50(23):5314–5321. doi: 10.1021/bi101916w. [http://dx.doi.org/10.1021/bi101916w]. [PMID: 21553808]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tselepis A.D., John Chapman M. Inflammation, bioactive lipids, and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler. Suppl. 2002;3:57–68. doi: 10.1016/s1567-5688(02)00045-4. [http://dx.doi.org/10.1016/S1567-5688(02)00045-4]. [DOI] [PubMed] [Google Scholar]
- 47.Guerra R., Zhao B.R., Mooser V., Stafforini D., Johnston J.M., Cohen J.C. Determinants of plasma-activating acetylhydrolase: heritability and relationship to plasma proteins. J. Lipid Res. 1997;38:2281–2288. [PMID: 9392426]. [PubMed] [Google Scholar]
- 48.Schnabel R., Dupuis J., Larson M.G., et al. Clinical and genetic factors associated with lipoprotein-associated phospholipase A2 in the Framingham Heart Study. Atherosclerosis. 2009;204(2):601–607. doi: 10.1016/j.atherosclerosis.2008.10.030. [http://dx.doi.org/10.1016/j.atherosclerosis.2008.10.030]. [PMID: 19135199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brilakis E.S., Khera A., McGuire D.K., et al. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: observations from the Dallas Heart Study. Atherosclerosis. 2008;199(1):110–115. doi: 10.1016/j.atherosclerosis.2007.10.010. [PMID: 18061193]. [http://dx.doi.org/10.1016/j.atherosclerosis.2007.10.010]. [PMID: 18061193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregson J., Stirnadel-Farrant H.A., Doobaree I.U., Koro C. Variation of lipoprotein associated phospholipase A2 across demographic characteristics and cardiovascular risk factors: a systematic review of the literature. Atherosclerosis. 2012;225(1):11–21. doi: 10.1016/j.atherosclerosis.2012.06.020. [http://dx.doi.org/10.1016/j.atherosclerosis.2012.06.020]. [PMID: 22784637]. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y., Yoshida H., Ichihara S., Imaizumi T., Satoh K., Yokota M. Correlations between plasma platelet-activating factor acetylhydrolase (PAF-AH) activity and PAF-AH genotype, age, and atherosclerosis in a Japanese population. Atherosclerosis. 2000;150(1):209–216. doi: 10.1016/s0021-9150(99)00385-8. [http://dx.doi.org/10.1016/S0021-9150(99)00385-8]. [PMID: 10781653]. [DOI] [PubMed] [Google Scholar]
- 52.Hiramoto M., Yoshida H., Imaizumi T., Yoshimizu N., Satoh K. A mutation in plasma platelet-activating factor acetylhydrolase (Val279-->Phe) is a genetic risk factor for stroke. Stroke. 1997;28(12):2417–2420. doi: 10.1161/01.str.28.12.2417. [http://dx.doi.org/10.1161/01.STR.28.12.2417]. [PMID: 9412624]. [DOI] [PubMed] [Google Scholar]
- 53.Ichihara S., Yamada Y., Yokota M. Association of a G994-->T missense mutation in the plasma platelet-activating factor acetylhydrolase gene with genetic susceptibility to nonfamilial dilated cardiomyopathy in Japanese. Circulation. 1998;98(18):1881–1885. doi: 10.1161/01.cir.98.18.1881. [http://dx.doi.org/10.1161/01.CIR.98.18.1881]. [PMID: 9799208]. [DOI] [PubMed] [Google Scholar]
- 54.Stafforini D.M., Satoh K., Atkinson D.L., et al. Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J. Clin. Invest. 1996;97(12):2784–2791. doi: 10.1172/JCI118733. [http://dx.doi.org/10.1172/JCI118733]. [PMID: 8675689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang Y., Waterworth D., Lee J.E., et al. Carriage of the V279F null allele within the gene encoding Lp-PLA2 is protective from coronary artery disease in South Korean males. PLoS One. 2011;6(4):e18208. doi: 10.1371/journal.pone.0018208. [http://dx.doi.org/10.1371/journal.pone.0018208]. [PMID: 21490708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ninio E., Tregouet D., Carrier J.L., et al. Platelet-activating factor-acetylhydrolase and PAF-receptor gene haplotypes in relation to future cardiovascular event in patients with coronary artery disease. Hum. Mol. Genet. 2004;13(13):1341–1351. doi: 10.1093/hmg/ddh145. [http://dx.doi.org/10.1093/hmg/ddh145]. [PMID: 15115767]. [DOI] [PubMed] [Google Scholar]
- 57.Wootton P.T., Stephens J.W., Hurel S.J., et al. Lp-PLA2 activity and PLA2G7 A379V genotype in patients with diabetes mellitus. Atherosclerosis. 2006;189(1):149–156. doi: 10.1016/j.atherosclerosis.2005.12.009. [http://dx.doi.org/10.1016/j.atherosclerosis.2005.12.009]. [PMID: 16438975]. [DOI] [PubMed] [Google Scholar]
- 58.Liu P.Y., Li Y.H., Wu H.L., et al. Platelet-activating factor-acetylhydrolase A379V (exon 11) gene polymorphism is an independent and functional risk factor for premature myocardial infarction. J. Thromb. Haemost. 2006;4(5):1023–1028. doi: 10.1111/j.1538-7836.2006.01895.x. [http://dx.doi.org/10.1111/j.1538-7836.2006.01895.x]. [PMID: 16689754]. [DOI] [PubMed] [Google Scholar]
- 59.Santoso A., Maulana R., Alzahra F., Maghfirah I., Putrinarita A.D., Heriansyah T. Associations between four types of single-nucleotide polymorphisms in PLA2G7 gene and clinical atherosclerosis: a meta-analysis. Am. J. Cardiovasc. Dis. 2017;7(6):122–133. [PMID: 29348973]. [PMC free article] [PubMed] [Google Scholar]
- 60.Blackie J.A., Bloomer J.C., Brown M.J., et al. The identification of clinical candidate SB-480848: a potent inhibitor of lipoprotein-associated phospholipase A2. Bioorg. Med. Chem. Lett. 2003;13(6):1067–1070. doi: 10.1016/s0960-894x(03)00058-1. [http://dx.doi.org/10.1016/S0960-894X(03)00058-1]. [PMID: 12643913]. [DOI] [PubMed] [Google Scholar]
- 61.Serruys P.W., García-García H.M., Buszman P., et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118(11):1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [http://dx.doi.org/10.1161/CIRCULATIONAHA.108.771899]. [PMID: 18765397]. [DOI] [PubMed] [Google Scholar]
- 62.Wihastuti T.A., Heriansyah T., Hanifa H., et al. Darapladib inhibits atherosclerosis development in type 2 diabetes mellitus Sprague-Dawley rat model. Endocr. Regul. 2018;52(2):69–75. doi: 10.2478/enr-2018-0008. [http://dx.doi.org/10.2478/enr-2018-0008]. [PMID: 29715185]. [DOI] [PubMed] [Google Scholar]
- 63.Ridker P.M., MacFadyen J.G., Wolfert R.L., Koenig W. Relationship of lipoprotein-associated phospholipase A2 mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin. Chem. 2012;58(5):877–886. doi: 10.1373/clinchem.2011.180281. [http://dx.doi.org/10.1373/clinchem.2011.180281]. [PMID: 22419750]. [DOI] [PubMed] [Google Scholar]
- 64.Prasad M., Lennon R., Barsness G.W., et al. Chronic inhibition of lipoprotein-associated phospholipase A2 does not improve coronary endothelial function: A prospective, randomized-controlled trial. Int. J. Cardiol. 2018;253:7–13. doi: 10.1016/j.ijcard.2017.09.171. [http://dx.doi.org/10.1016/j.ijcard.2017.09.171]. [PMID: 29306475]. [DOI] [PubMed] [Google Scholar]
- 65.Choi W.G., Prasad M., Lennon R., et al. Long-term darapladib use does not affect coronary plaque composition assessed using multimodality intravascular imaging modalities: a randomized-controlled study. Coron. Artery Dis. 2018;29(2):104–113. doi: 10.1097/MCA.0000000000000573. [http://dx.doi.org/10.1097/MCA.000000000000573]. [PMID: 29135482]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millwood I.Y., Bennett D.A., Walters R.G., et al. A phenome-wide association study of a lipoprotein-associated phospholipase A2 loss-of-function variant in 90 000 Chinese adults. Int. J. Epidemiol. 2016;45(5):1588–1599. doi: 10.1093/ije/dyw087. [http://dx.doi.org/10.1093/ije/dyw087]. [PMID: 27301456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenson R.S., Fraser H., Goulder M.A., Hislop C. Anti-inflammatory effects of varespladib methyl in diabetic patients with acute coronary syndrome. Cardiovasc. Drugs Ther. 2011;25(6):539–544. doi: 10.1007/s10557-011-6344-2. [http://dx.doi.org/10.1007/s10557-011-6344-2]. [PMID: 21989792]. [DOI] [PubMed] [Google Scholar]
- 68.Holmes M.V., Simon T., Exeter H.J., et al. Secretory phospholipase A(2)-IIA and cardiovascular disease: a mendelian randomization study. J. Am. Coll. Cardiol. 2013;62(21):1966–1976. doi: 10.1016/j.jacc.2013.06.044. [http://dx.doi.org/10.1016/j.jacc.2013.06.044]. [PMID: 23916927]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talmud P.J., Holmes M.V. Deciphering the Causal Role of sPLA2s and Lp-PLA2 in Coronary Heart Disease. Arterioscler. Thromb. Vasc. Biol. 2015;35(11):2281–2289. doi: 10.1161/ATVBAHA.115.305234. [http://dx.doi.org/10.1161/ATVBAHA.115.305234]. [PMID: 26338298]. [DOI] [PubMed] [Google Scholar]