Abstract
Background:
Preexisting and new-onset atrial fibrillation (AF) in patients undergoing transcatheter aortic valve replacement (TAVR) are associated with poor outcomes.
Objectives:
This study evaluates impact of new-onset and preexisting AF on TAVR long-term outcomes compared to patients without AF.
Methods:
We identified 72,660 patients age ≥65 who underwent non-apical TAVR between 2014 and 2016 using Medicare inpatient claims. History of AF was defined by diagnoses on claims during the three years preceding the TAVR, and new-onset AF was defined as occurrence of AF during the TAVR admission or within 30 days after TAVR in a patient without prior history of AF. Outcomes included all-cause mortality, and readmission for bleeding, stroke, and heart failure (HF).
Results:
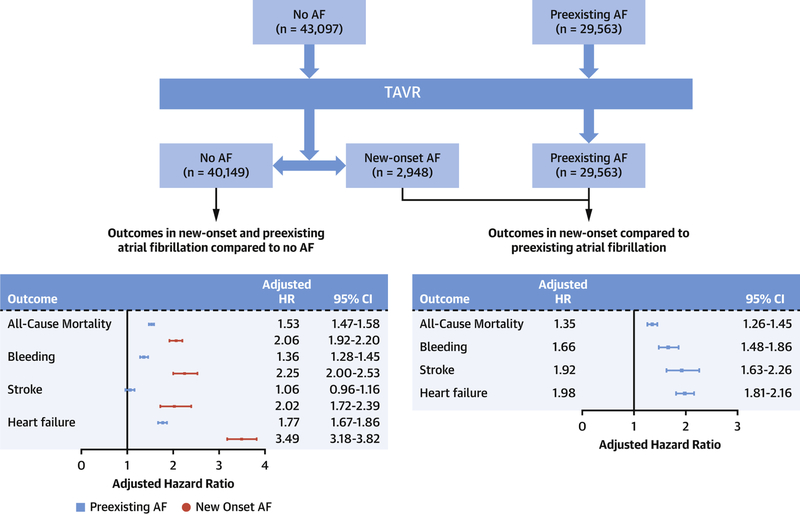
Overall, 40.7% had preexisting AF (n=29,563) and 6.8% experienced new-onset AF (n=2,948) after TAVR. Mean age was 81.3, 82.4 and 83.8 years in patients with no AF, preexisting and new-onset AF respectively. Preexisting AF patients had the highest burden of comorbidities. After follow-up of 73,732 person-years, mortality was higher with new-onset AF compared to preexisting and no AF (29.7, 22.6 and 12.8 per 100 person-years respectively, p<0.001). After adjusting for patient characteristics and hospital TAVR volume, new-onset AF remained associated with higher mortality compared to no AF (adjusted hazard ratio (aHR) 2.068, 95% (confidence interval) CI 1.92–2.20, p<0.01) and preexisting AF (aHR 1.35, 95% CI 1.26–1.45, p<0.01). In competing risk analysis, new-onset AF was associated with higher risk of bleeding (subdistribution HR (sHR) 1.66, 95% CI 1.48–1.86, p<0.01), stroke (sHR 1.92, 95% CI 1.63–2.26, p<0.01), and HF (sHR 1.98, 95% CI 1.81–2.16, p<0.01) compared to preexisting AF.
Conclusion:
In patients undergoing TAVR, new-onset AF is associated with increased risk of mortality, bleeding, stroke and HF hospitalizations compared to preexisting AF or no AF.
Keywords: Transcatheter aortic valve replacement, atrial fibrillation, mortality, stroke, heart failure, bleeding
CONDENSED ABSTRACT
We identified 72,660 patients age ≥65 who underwent transcatheter aortic valve replacement (TAVR) through non-apical approach between 2014 and 2016 using Medicare inpatient claims. Incidence of new-onset atrial fibrillation (AF) after TAVR was 6.8%. After adjusting for patient characteristics and hospital TAVR volume, new-onset AF was associated with significantly higher mortality compared to no AF (adjusted hazard ratio (aHR) 2.06, 95% (confidence interval) CI 1.92–2.20, p<0.01) and preexisting AF (aHR 1.35, 95% CI 1.26–1.45, p<0.01). Similarly, new-onset AF after TAVR was associated with higher risk of bleeding, stroke and HF admissions compared to preexisting AF and no AF.
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) has become the preferred therapy for patients with severe symptomatic aortic stenosis (AS) and a prohibitive or high risk of poor outcomes with surgical aortic valve replacement (SAVR) (1,2). More recently, trials have shown safety and efficacy of TAVR in intermediate and low risk patients compared with SAVR (3,4). With the advancement in technology and an enhanced learning curve, the number of TAVR procedures has continued to grow, reaching more than 50,000 procedures in the United States according to the 2016 annual report of the Society of Thoracic Surgeons / American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry (5).
The prevalence of atrial fibrillation (AF) in patients undergoing TAVR is substantial, averaging 50% when combining both preexisting and new-onset post-procedural AF. Because of their multiple comorbidities, TAVR patients are likely to be at a high thromboembolic as well as bleeding risk, making appropriate management of AF in those patients challenging. Studies have linked preexisting AF to worse outcomes after TAVR (6–8). Furthermore, a recent analysis from the STS/ACC TVT Registry showed an increased risk of 1-year mortality and re-hospitalization with new-onset post-procedural AF compared to sinus rhythm (9). However, studies comparing TAVR long-term outcomes in patients with new-onset AF, preexisting AF, and sinus rhythm are scarce and lacking.
The current study aimed to evaluate the impact of new-onset AF compared with preexisting AF and sinus rhythm on long-term mortality, stroke, bleeding and heart failure (HF) in TAVR patients using claims data for Medicare beneficiaries.
METHODS
The study cohort was derived from 100% Medicare Provider and Analysis Review (MEDPAR) Part A files for the years of 2011 to 2016, which were obtained from the Center for Medicare and Medicaid Services (CMS). MEDPAR part A files include all hospital discharges for Medicare beneficiaries discharged from hospitals nationwide. We identified patients who underwent TAVR through non-apical approach during 2014–2016 using ICD-9 and ICD-10 procedure codes (35.05, 02RF37Z, 02RF38Z, 02RF3JZ, 02RF3KZ, or X2RF332) during the study period. Dates of beneficiary Medicare enrollment and death were obtained from the 100% Beneficiary Summary file for the same years. Patients were excluded if they had been enrolled in Medicare fee-for-service for less than 36 months prior to the TAVR or if they underwent TAVR through apical approach.
The study cohort was divided into three groups based on presence of AF diagnosis. Patients with any hospital admission with the ICD-9 code of 427.31, and ICD-10 codes I48.0, I48.1, I48.2 or I48.91 (as a diagnosis in any position) in the 3 years prior to the TAVR admission were classified as patients with preexisting AF. These codes have been validated in prior studies with reported sensitivity and positive predictive value of >90% (10,11). Patients with no preexisting AF who had a new diagnosis with these codes during the admission or within 30 days after the TAVR procedure were defined as patients with new onset AF. Patients with neither diagnosis were considered patients with no AF. We used ICD-9 codes for the period until September 2015 and ICD-10 codes for the period after September 2015. Patients’ characteristics and comorbidities were derived from Medicare enrollment data and inpatient claims during the year prior to TAVR admission. Patient characteristics included patient age, sex, race (from the discharge record); dual eligibility for Medicaid (from the Beneficiary Summary file), and preexisting clinical conditions defined using ICD9/ICD10 codes. Comorbid diseases included 30 conditions defined by Elixhauser et al (12) and additional conditions relevant to patient outcomes (Supplementary Table 1).
The main outcome of the study was all-cause mortality. Secondary outcomes included subsequent hospital admissions with a primary diagnosis of stroke, bleeding, or HF after the discharge from the TAVR procedure. Supplementary Table 1 includes all ICD-9 and 10 codes used to define the secondary outcome. Patients were censored due to death, Medicare disenrollment, or end of the study period (December 31, 2016). The Institutional Review Board of the University of Iowa approved this study with waiver for individual informed consent given the retrospective nature of the study.
Patient characteristics were compared between the three groups. Categorical data were compared using Chi Square test. Continuous data were described as mean and standard deviation or median and interquartile range as appropriate and compared with analysis of variance or Mann Whitney test. To assess the primary outcome, a multivariable Cox proportional hazards regression with a time-dependent covariate for new onset AF was performed. The model adjusted for age, sex, preexisting comorbidities, and hospital TAVR volume then performed a stepwise backward selection process guided by the lowest Akaike information criterion (AIC) to determine the best model fit combined with insight from clinical experience and previous literature (13). Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated and reported. Kaplan-Meier (KM) curves with 95% CIs were generated to determine the cumulative proportion of patients with events as a function over time and compared using log-rank or Generalized Wilcoxon statistic, as appropriate. To assess if the proportional hazards assumption is not violated, Kolmogorov-type supremum test and graphical inspection of Schoenfeld residuals plotted against time were performed. Because of the limitations of standard KM to account for the time-dependent variable of new-onset AF (14), we also performed Mantel-Byar test (15) to compare all-cause mortality between new-onset AF and preexisting AF, and between new onset AF and no AF. Results of the tests were plotted using Simon and Makuch plot, which accounts for the time-dependency of new onset AF (16).
For secondary outcomes, to assess the importance of competing events of death, we measured the explained variation and predictive accuracy of the Cox model (17). Because all- cause mortality was a competing risk, multivariable survival analysis was performed by competing risk regression analysis using the Fine-Gray proportional sub hazards model, and subdistribution hazard ratios (sHRs) were calculated, along with 95% CIs (18). P value of 0.05 was chosen for statistical significance. Analysis was done with SAS version 9.4 (SAS Institute, Cary, North Carolina) and R 3.4.3 (R foundation for Statistical Computing, Vienna, Austria).
RESULTS
Our final cohort included 72,660 Medicare patients who underwent TAVR from 2014 to 2016. The three groups of no AF, preexisting AF and new-onset AF included 40,149, 29,563 and 2,948 patients respectively. Overall, the incidence of new-onset AF within 30-days after TAVR procedure was 6.8%. Incidence of AF decreased from 10.8% in 2014 to 4.9% in 2016, p<0.01. Table 1 shows baseline demographic characteristics and comorbidities for the three groups. Patients with new-onset were older than patients with preexisting AF, and patients in both groups were older than those with no AF. Patients with preexisting AF had the highest prevalence of prior coronary artery disease, kidney disease, chronic lung disease and congestive HF. Similarly; CHA2DS2VASc score was highest in the preexisting AF patients. Table 2 shows characteristics of TAVR admissions in the three groups. Lengths of hospital and of intensive care unit stay were longer in patients with new-onset AF compared to preexisting AF. The incidence of new PPM placement during TAVR admission was 25.3, 24.9 and 28.2% in patients with no, preexisting and new onset AF respectively (P<0.001). Patients with no AF had the highest percentage of next day discharge as well as early discharge (<72 hours) while new-onset AF patients had the lowest percentages of both. Median follow-up was 305 days (IQR 128–570) with a total of 73,732 person-years.
Table 1.
Baseline characteristics of the study population
| Variable | Overall N=72,660 | No AF N=40,149 | Preexisting AF N=29,563 | New-onset AF N=2,948 | P value |
|---|---|---|---|---|---|
| Age, years, mean (SD) | 81.9 (8.1) | 81.3 (8.3) | 82.4 (7.8) | 83.8 (7.5) | <0.001 |
| Male % | 53.0 | 51.8 | 55.1 | 49.6 | <0.001 |
| Other | 3.7 | 4.3 | 2.9 | 3.2 | |
| Diabetes | 37.3 | 37.6 | 37.4 | 33.7 | <0.001 |
| Hypertension | 88.1 | 88.5 | 87.5 | 87.3 | <0.001 |
| Coronary artery disease | 24.0 | 19.8 | 30.4 | 15.3 | <0.001 |
| Prior revascularization | 7.6 | 7.1 | 8.6 | 5.0 | <0.001 |
| Congestive heart failure | 74.6 | 69.9 | 80.6 | 77.2 | <0.001 |
| Chronic pulmonary disease | 31.1 | 29.0 | 33.7 | 31.8 | <0.001 |
| Obesity | 16.8 | 17.4 | 16.0 | 16.4 | <0.001 |
| Prior stroke | 4.3 | 4.2 | 4.7 | 2.6 | <0.001 |
| Chronic kidney disease | 36.5 | 33.5 | 40.4 | 37.3 | <0.001 |
| Liver disease | 2.6 | 2.8 | 2.2 | 2.3 | <0.001 |
| Peripheral vascular disease | 26.5 | 26.0 | 27.0 | 29.3 | <0.001 |
| Coagulopathy | 17.8 | 16.7 | 19.0 | 21.5 | <0.001 |
| Prior bleeding | 13.8 | 13.0 | 15.6 | 8.3 | <0.001 |
| Prior GI bleeding | 12.2 | 11.7 | 13.4 | 7.5 | <0.001 |
| Smoking | 7.0 | 6.3 | 8.1 | 5.4 | <0.001 |
| Alcohol abuse | 1.0 | 1.0 | 0.9 | 1.2 | 0.11 |
| Deficiency Anemia | 24.8 | 23.6 | 26.4 | 24.3 | <0.001 |
| Connective tissue disease | 5.0 | 5.3 | 4.6 | 4.8 | 0.0003 |
| Depression | 8.0 | 8.2 | 7.7 | 7.2 | 0.01 |
| Hypothyroidism | 21.8 | 20.9 | 23.1 | 22.3 | <0.001 |
| Lymphoma | 1.2 | 1.2 | 1.2 | 1.2 | 0.82 |
| Electrolyte disorders | 20.9 | 18.5 | 23.3 | 29.0 | <0.001 |
| Psychoses | 1.1 | 1.1 | 1.1 | 1.1 | 0.97 |
| Pulmonary hypertension | 10.7 | 7.8 | 14.0 | 16.1 | <0.001 |
| Tumor without metastasis | 2.2 | 2.3 | 2.0 | 2.6 | 0.01 |
| Weight loss | 4.0 | 3.2 | 4.9 | 5.5 | <0.001 |
| Prior Pacemaker | 7.0 | 3.4 | 12.3 | 2.5 | <0.001 |
| Prior ICD | 2.3 | 1.2 | 4.0 | 1.2 | <0.001 |
| ≥7 | 5.04 | 4.4 | 6.0 | 3.43 |
Table 2.
Hospital admission characteristics of study population
| Variable | Overall N=72,660 | No AF N=40,149 | Preexisting AF N=29,563 | New-onset AF N=2,948 | P value |
|---|---|---|---|---|---|
| Length of hospital stay (days) Median (IQR) | 4 (2–7) | 3 (2–6) | 4 (3–8) | 6 (3–10) | <0.001 |
| ICU stay (days) mean (SD) | 2.3 (4.2) | 2.0 (3.6) | 2.6 (4.8) | 3.3 (5.1) | <0.001 |
| Next day discharge | 7.7 | 9.1 | 6.2 | 3.9 | <0.001 |
| Early discharge (≤72 h) | 47.1 | 53.8 | 40.1 | 26.2 | <0.001 |
| Acute kidney injury | 13.5 | 11.0 | 16.0 | 20.7 | <0.001 |
| Respiratory complications | 1.8 | 1.6 | 2.1 | 2.5 | <0.001 |
| Transfusion | 11.7 | 10.4 | 13.0 | 16.1 | <0.001 |
| Vascular complication | 1.6 | 1.3 | 1.8 | 2.2 | <0.001 |
| In-hospital stroke | 2.0 | 1.8 | 2.0 | 4.0 | <0.001 |
| Inpatient rehabilitation | 3.8 | 2.9 | 4.0 | 12.7 | |
| High volume center | 53.6 | 53.2 | 54.9 | 53.1 | 0.001 |
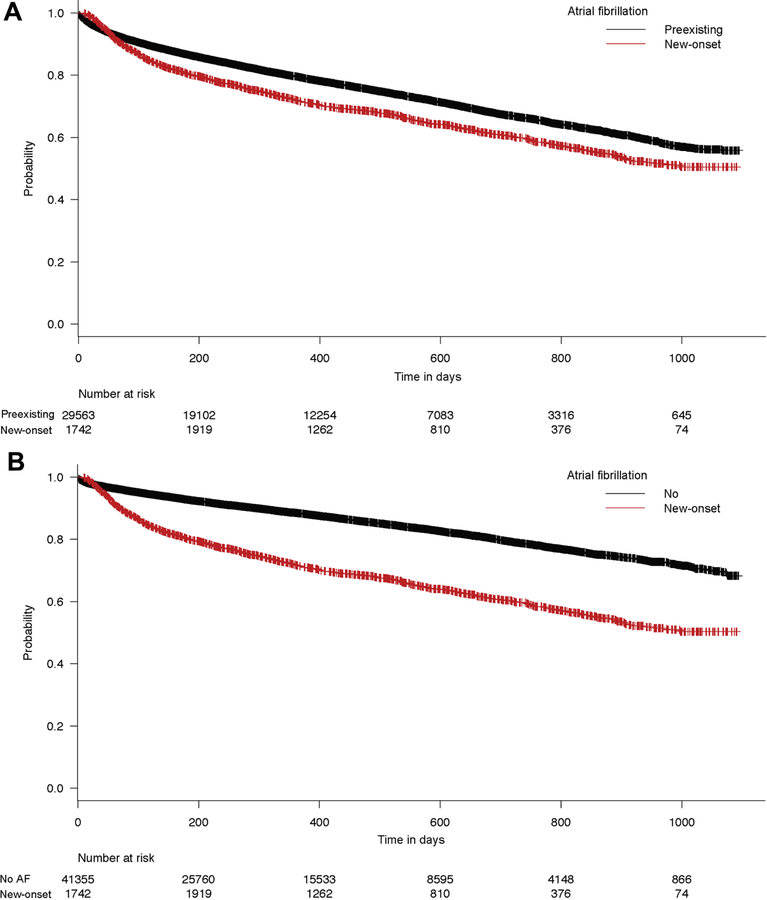
The primary outcome, all-cause mortality, occurred in 32% of patients with new-onset AF compared to 23.3% in patients with preexisting AF and 12.8% in patients with no AF which resulted in an event rate of 29.7, 22.6 and 12.8 deaths per 100 person-years in the three groups respectively (p<0.0001) (Table 3). Figure 1 shows the Kaplan Meier curves for survival probability in the three groups. After adjusting for patients’ characteristics and hospital volume, new-onset AF was associated with higher risk for mortality compared to patients with preexisting AF and patients without AF (adjusted Hazards Ratio (aHR) 1.35, 95% CI 1.26–1.45, p<0.001 compared to preexisting AF, and aHR 2.06, 95% CI 1.92–2.20, p<0.0001 compared to no AF). Similarly, pre-existing AF was associated with higher risk of mortality compared to patients with no AF (aHR 1.53, 95% CI 1.47–1.58, p<0.0001). Figure 2A and B show the Simon Makuch curve for Mantel Byar test comparing new onset AF to preexisting AF and no AF respectively.
Table 3.
primary and secondary outcomes of the study
| Variable | No AF N=40,149 | Preexisting AF N=29,563 | New-onset AF N=2,948 | P value |
|---|---|---|---|---|
| 30-day HF readmission | 1.5 | 3.3 | 9.4 | <0.001 |
| 30-day stroke admission | 2.4 | 2.3 | 6.4 | <0.001 |
| 30-day bleed admission | 1.0 | 1.2 | 3.8 | <0.001 |
| 30-day mortality | 2.6 | 4.6 | 2.8 | <0.001 |
| New PPM in same TAVR admission | 25.3 | 24.9 | 28.2 | 0.0004 |
| Long-term follow up event rates, per 100 person-year | ||||
| HF readmission | 6.3 | 13.6 | 25.2 | <0.0001 |
| Any bleeding | 3.1 | 7.7 | 12.4 | <0.0001 |
| Stroke | 2.6 | 2.9 | 5.7 | <0.0001 |
| All-cause mortality | 12.8 | 22.6 | 29.7 | <0.0001 |
Figure 1:
Kaplan Meier curves for the primary outcome of all-cause mortality after TAVR in the three study groups. Log-rank test p <0.0001
Figure 2:
Simon Makuch Curves with time dependent covariate for the primary outcome of all- cause mortality after TAVR. A) Primary outcome in patients with new-onset atrial fibrillation compared to patients with preexisting atrial fibrillation, Mantel Byar test p<0.0001. B): Primary outcome in patients with new-onset atrial fibrillation compared to patients without atrial fibrillation, Mantel Byar test p<0.0001.
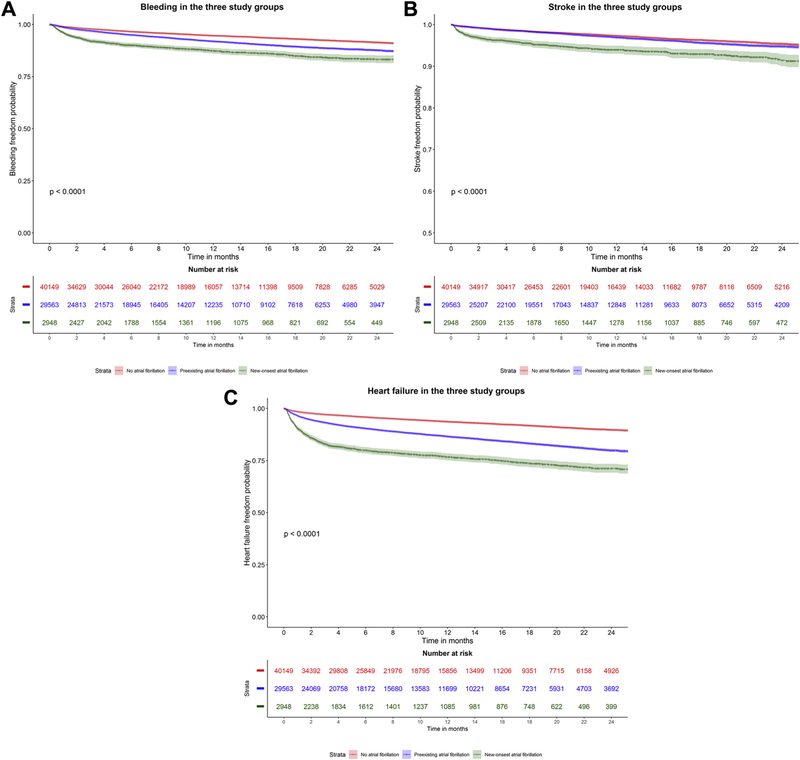
In the competing risk survival analysis for secondary outcomes, after adjusting for patients’ characteristics, new-onset AF was associated with higher risk of bleeding (subdistribution HR (sHR) 1.66, 95% CI 1.48–1.86, p<0.01), stroke (sHR 1.92, 95% CI 1.63–2.26, p<0.01), and HF admissions (sHR 1.98, 95% CI 1.81–2.16, p<0.01) compared to preexisting AF. Preexisting AF was associated with significantly higher risk of bleeding (sHR 1.36, 95% CI 1.28–1.45, p<0.0001), and HF hospitalizations (sHR 1.77, 95% CI 1.67–1.86, p<0.0001) compared to no AF. Figure 3A–C show the Kaplan Meier curves for the secondary outcomes in the three groups. Supplementary Tables 2–5 show the full-adjusted model for the study outcomes. Central illustration shows risk estimates for study outcomes comparing the three study groups.
Figure 3:
Kaplan Meier curves for the study secondary outcomes in the three groups. A) Bleeding after TAVR in the three study groups, B) Stroke after TAVR in the three study groups C) Heart failure admission after TAVR in the three study groups. Log-rank test p <0.0001 for all.
DISCUSSION
In this study from real world data using Medicare database, we demonstrated several important findings. First, the incidence of new onset of AF in patients undergoing non-apical TAVR is approximately 6.8% and has declined to 4.9% in the last year of the study, while prevalence of preexisting AF is 40.7%. Second, while patients with preexisting AF are at higher risk of mortality compared to patients with no AF, new onset AF carries a significantly worse prognosis and is associated with a much higher risk of mortality. This was despite that new-onset AF patients had a lower burden of most comorbidities at baseline. Last, patients who undergo TAVR and develop new-onset AF have higher risk of stroke, bleeding, and HF hospitalizations at follow up compared to patients with preexisting AF.
The incidence of new onset AF in TAVR patients in our study is comparable to the incidence reported in previous trials and observational studies (7.2%−8.6%) (1,9,19). Similarly, prevalence of preexisting AF in prior studies ranged from 34% to 49% which is consistent with our findings (8,19–21). We noticed a steady decline in the incidence of AF in the three years of the study period. A plausible explanation is that our study included more recent years, where the TAVR procedure is expanding to including relatively lower risk patients (22).
Several prior studies have shown that preexisting AF in TAVR patients is associated with worse outcomes including mortality compared to patients in sinus rhythm (6–8). In a prior study from TVT registry, preexisting AF was associated with approximately 37% increase in risk of mortality (23). On the other hand, the impact of new-onset AF on mortality after TAVR is debatable. A few prior studies failed to show an association between new-onset AF and mortality (6,7) while a study from the SOURCE XT registry showed that both preexisting and new-onset AF were associated with higher all-cause mortality compared to sinus rhythm (19). However, the study was underpowered to detect differences in adverse outcomes between types of AF. Similar findings were shown in a recent metaanalysis of eleven studies (24). A recent report from the STS/ACC TVT registry found that new-onset AF after TAVR is associated with higher mortality, bleeding and stroke rates compared to sinus rhythm. However, patients with preexisting AF were excluded from that study making the comparison between both types of AF not feasible (9). Regarding other outcomes, similar to our findings, studies have shown that preexisting AF is associated with increased risk of HF hospitalizations, but they failed to show a difference in risk of HF between both AF types (19,23).
The reason why new-onset AF is associated with such worse outcomes is not clearly identified. However, it is important to note that in one study, when monitoring was performed in TAVR patients before the procedure, only 30% of the patients who developed new-onset AF after TAVR had a missed diagnosis of AF (silent AF) prior to TAVR (25). This suggests that in majority of patients with new-onset AF, the new arrhythmia is related to the procedure injury itself. Several postulated mechanisms include acute peri-operative injury and oxidative atrial stress (26), magnitude of systemic inflammatory response after the procedure (27), acute left atrial volume overload and stretch (26), or lack of negative atrial remodeling after the procedure (28). Our analysis showed a low in-hospital mortality rate in new-onset AF compared with preexisting AF, however after a median of 10 days, we observed an exponential rise in the rates of mortality in new-onset AF group with subsequent crossing of the survival Kaplan-Meyer curves compared with both preexisting AF and no AF. A plausible explanation is that mortality might be related to complications of new-onset AF such as heart failure or stroke rather than to the TAVR procedure itself.
Prior studies have shown that post-operative AF after cardiac and lung surgery are associated with increased mortality (29–31). Similarly, our results suggest that patients who develop new-onset AF after TAVR represent a distinct population with a high risk for poor longterm outcomes that need to be appropriately managed to mitigate the associated increase in mortality risk. The recent STS/ACC TVT registry showed that less than 30% of patients with post-TAVR new-onset AF were discharged on anticoagulation, which was demonstrated to be one of the indicators of poor survival in that study cohort (9). However, the lack of full information regarding the use of anticoagulation precluded a more robust analysis to determine if rates of mortality may be reduced with such therapy.
It is worth mentioning that new onset AF was associated with the highest bleeding rates compared to preexisting AF and sinus rhythm in our study. This is similar to the outcomes reported from the SOURCE XT study (19). The high bleeding risk could be due to the new-onset AF patients being naïve to anticoagulation compared to patients with preexisting AF who are usually on chronic anticoagulation before the procedure. The optimum antithrombotic management of TAVR patients remains controversial and whether the direct oral anticoagulants could offer a safer or more effective option in TAVR patients with AF is yet unknown.
Limitations
Our study has several limitations. First, we defined preexisting AF by a look back period of three years. It is possible that some patients were misclassified as having no AF because they had prior AF episodes more than three years before the TAVR date. Similarly, we depended in our diagnosis of new-onset AF on ICD codes in subsequent admissions, it is possible that some patients were misclassified as having no AF because their new onset AF occurred in an outpatient setting or was miscoded. However, we believe that both misclassification events would have inflated the risk in the no AF group, and thus would have biased our results to the null. Second, despite performing multivariate cox regression model to adjust for many factors, there is always the possibility of residual confounding from unmeasured factors in analysis of administrative observation data. Third, we did not differentiate between paroxysmal and permanent AF, and outcomes could be different between both types. Fourth, we lacked information about medications, this is especially important for anticoagulation, as it is known to affect outcomes of AF patients. Last, it is possible that the longer hospital stay in new-onset AF patients was the cause to detect the arrhythmia given prolonged monitoring time and not a consequent to it.
CONCLUSIONS
In conclusion, in patients undergoing TAVR, new-onset AF is associated with significantly higher risk of mortality, bleeding, stroke and HF hospitalizations compared to patients with preexisting AF as well as patients with no AF.
Supplementary Material
Central illustration:
Study flow sheet and adjusted hazard ratios for study outcomes.
Panel A: Study flow sheet.
Panel B: Adjusted hazard ratios of study outcomes comparing new-onset and preexisting atrial fibrillation to patients with no atrial fibrillation. Panel C: Adjusted hazard ratios of study outcomes comparing new-onset atrial fibrillation to patients with preexisting atrial fibrillation.
PERSPECTIVES.
WHAT IS KNOWN?
Preexisting and new-onset atrial fibrillation in patients undergoing transcatheter aortic valve replacement is associated with poor short-term outcomes.
WHAT IS NEW?
New-onset atrial fibrillation after transcatheter aortic valve replacement is associated with worse long-term outcomes compared to patients with preexisting atrial fibrillation and patients with no AF.
WHAT IS NEXT?
Further research is required to determine the optimum prevention and management of new-onset atrial fibrillation after transcatheter aortic valve replacement to improve short and long-term outcomes
Funding:
This study is supported by funding from the National Institute on Aging (NIA; R01AG055663–01), and by the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs.
ABBREVIATIONS AND ACRONYMS
- AF
Atrial fibrillation
- AS
Aortic stenosis
- CMS
Center for Medicare and Medicaid Services
- HF
Heart failure
- PPM
Permanent pacemaker
- SAVR
Surgical Aortic Valve Replacement
- TAVR
Transcatheter Aortic Valve Replacement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Horwitz receives grant support from Edwards Lifesciences and Boston Scientific.
The remaining authors do not have any conflicts of interest or financial disclosures.
Tweet/Handle: @AmgadMentias; Work in @JACCJournals showing post-TAVR new-onset AF incidence declining but is associated with worse outcomes compared to preexisting and no AF
REFERENCES
- 1.Smith CR., Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364(23):2187–98. Doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA., Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. J Am Coll Cardiol 2017;70(2):252–89. Doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Leon MB., Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374(17):1609–20. Doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ., Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med 2019:NEJMoa1814052 Doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 5.Grover FL., Vemulapalli S, Carroll JD, et al. 2016 Annual Report ofThe Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol 2017;69(10):1215–30. Doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Yankelson L, Steinvil A, Gershovitz L, et al. Atrial Fibrillation, Stroke, and Mortality Rates After Transcatheter Aortic Valve Implantation. Am J Cardiol 2014;114(12):1861–6. Doi: 10.1016/j.amjcard.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Barbash IM., Minha S, Ben-Dor I, et al. Predictors and clinical implications of atrial fibrillation in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2015;85(3):468–77. Doi: 10.1002/ccd.25708. [DOI] [PubMed] [Google Scholar]
- 8.Stortecky S, Buellesfeld L, Wenaweser P, et al. Atrial Fibrillation and Aortic Stenosis. Circ Cardiovasc Interv 2013;6(1):77–84. Doi: 10.1161/CIRCINTERVENTIONS.112.000124. [DOI] [PubMed] [Google Scholar]
- 9.Vora AN., Dai D, Matsuoka R, et al. Incidence, Management, and Associated Clinical Outcomes of New-Onset Atrial Fibrillation Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2018;11(17):1746–56. Doi: 10.1016/j.jcin.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 10.Jensen PN., Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21:141–7. Doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokotailo RA., Hill MD Coding of Stroke and Stroke Risk Factors Using International Classification of Diseases , Revisions 9 and 10. Stroke 2005;36(8):1776–81. Doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, Coffey RM Comorbidity measures for use with administrative data. Med Care 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 13.Heinze G, Wallisch C, Dunkler D Variable selection – A review and recommendations for the practicing statistician. Biometrical J 2018. Doi: 10.1002/bimj.201700067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snapinn SM., Jiang Q, Iglewicz B Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 2005. Doi: 10.1198/000313005X70371. [DOI] [Google Scholar]
- 15.Mantel N, Byar DP Evaluation of response-time data involving transient states: An illustration using heart-transplant data. J Am Stat Assoc 1974. Doi: 10.1080/01621459.1974.10480131. [DOI] [Google Scholar]
- 16.Simon R, Makuch RW A non‐parametric graphical representation of the relationship between survival and the occurrence of an event: Application to responder versus non‐ responder bias. Stat Med 1984. Doi: 10.1002/sim.4780030106. [DOI] [PubMed] [Google Scholar]
- 17.Schemper M, Henderson R Predictive accuracy and explained variation in Cox regression. Biometrics 2000. Doi: 10.1111/j.0006-341X.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 18.Fine JP., Gray RJ A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999. Doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 19.Tarantini G, Mojoli M, Windecker S, et al. Prevalence and Impact of Atrial Fibrillation in Patients With Severe Aortic Stenosis Undergoing Transcatheter AorticValve Replacement: An Analysis From the SOURCE XT ProspectiveMulticenter Registry. JACC Cardiovasc Interv 2016;9(9):937–46. Doi: 10.1016/j.jcin.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Maan A, Heist EK, Passeri J, et al. Impact of Atrial Fibrillation on Outcomes in Patients Who Underwent Transcatheter Aortic Valve Replacement. Am J Cardiol 2015;115(2):220–6. Doi: 10.1016/j.amjcard.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Popma JJ., Adams DH, Reardon MJ, et al. Transcatheter Aortic Valve Replacement Using a Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis at Extreme Risk for Surgery. J Am Coll Cardiol 2014;63(19):1972–81. Doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 22.Mori M, Bin Mahmood SU, Geirsson A, et al. Trends in volume and risk profiles of patients undergoing isolated surgical and transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2018. Doi: 10.1002/ccd.27855. [DOI] [PubMed] [Google Scholar]
- 23.Holmes DR., Brennan JM, Rumsfeld JS, et al. Clinical Outcomes at 1 Year Following Transcatheter Aortic Valve Replacement. JAMA 2015;313(10):1019 Doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 24.Mojoli M, Gersh BJ, Barioli A, et al. Impact of atrial fibrillation on outcomes of patients treated by transcatheter aortic valve implantation: A systematic review and meta-analysis. Am Heart J 2017;192:64–75. Doi: 10.1016/j.ahj.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Urena M, Hayek S, Cheema AN, et al. Arrhythmia Burden in Elderly Patients With Severe Aortic Stenosis as Determined by Continuous Electrocardiographic Recording. Circulation 2015;131(5):469–77. Doi: 10.1161/CIRCULATIONAHA.114.011929. [DOI] [PubMed] [Google Scholar]
- 26.Tarantini G, Mojoli M, Urena M, Vahanian A Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J 2016;38(17):ehw456 Doi: 10.1093/eurheartj/ehw456. [DOI] [PubMed] [Google Scholar]
- 27.Stähli BE., Grünenfelder J, Jacobs S, et al. Assessment of inflammatory response to transfemoral transcatheter aortic valve implantation compared to transapical and surgical procedures: a pilot study. J Invasive Cardiol 2012;24(8):407–11. [PubMed] [Google Scholar]
- 28.Truong VT., Chung E, Nagueh S, et al. Effect of transcatheter aortic valve replacement on left atrial function. Echocardiography 2018;35(11):1713–20. Doi: 10.1111/echo.14109. [DOI] [PubMed] [Google Scholar]
- 29.Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. Executive summary. J Thorac Cardiovasc Surg 2014. Doi: 10.1016/j.jtcvs.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg JW., Lancaster TS, Schuessler RB, Melby SJ Postoperative atrial fibrillation following cardiac surgery: A persistent complication. Eur J Cardio-Thoracic Surg 2017. Doi: 10.1093/ejcts/ezx039. [DOI] [PubMed] [Google Scholar]
- 31.Saad M, Elgendy IY, Mentias A, et al. Incidence, Predictors, and Outcomes of Early Atrial Arrhythmias After Lung Transplant: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol 2017;3(7). Doi: 10.1016/j.jacep.2016.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.