Quantifying facility-level variation in cardiac stress test utilization is important for healthcare systems seeking to improve the efficiency and quality of cardiovascular care. Limited registry and payer data suggest such variation may be wide (1,2) but benchmark data from a single, large healthcare system are lacking. Therefore, our aim was to quantify variation in cardiac stress test utilization across the Veterans Health Administration (VA) in patients with established ischemic heart disease (IHD).
We used VA datasets to identify adults with IHD (myocardial infarction, percutaneous coronary intervention and/or coronary artery bypass grafting) with a primary care clinic visit at the VA in fiscal year 2014. Patients with metastatic cancer, receiving hospice care or with missing date of birth or gender information were excluded. VA datasets were used to obtain patient characteristics and medication use and to calculate the Diagnostic Cost Group Relative Risk Score (DCG RRS), a measure of overall illness burden. Facility-level cardiac stress test utilization was defined as the number of cardiac stress tests (exercise treadmill testing [ETT], stress echocardiography [SE), and stress myocardial perfusion imaging [MPI]) performed per 100 IHD patients per year at each of 130 VA medical centers. Utilization was mapped to patient county of residence to assess geographic variation and the influence of rurality. To quantify facility-level variation, we calculated median rate ratios (MRRs) with multivariable hierarchical regression models that adjusted for clustering of patients within facilities, treated patient-level characteristics as fixed effects and treated individual facilities as a random effect. Unadjusted and adjusted MRRs were calculated for: 1) overall cardiac stress test utilization and 2) non-imaging (i.e., ETT) versus imaging-based tests (i.e., SE, SPECT stress MPI and PET stress MPI). The Michael E. DeBakey VA Medical Center granted institutional review board approval.
The study cohort included 994,929 patients. Patients were predominantly non-Hispanic white males and the mean age was 72 years. Hypertension was highly prevalent (82%) and diabetes was present in 46%. 83% of patients were on statins and 58% on beta-blockers. The mean DCG RRS was 1.9. Unadjusted facility-level cardiac stress test utilization ranged from 2.1–31.7 studies/100 patients with a mean (standard deviation) of 15.4 (5.5). Figure 1 shows marked variation in unadjusted utilization at the county level between and often within states. The unadjusted MRR for overall cardiac stress test utilization was 1.69 (95% confidence interval [CI]: 1.58–1.79). Adjusting for patient-level characteristics (age, gender, race, hypertension, diabetes, and DCG RRS), the MRR decreased to 1.40 (95% CI: 1.34–1.46). Facility-level variation was similar between ETT (adjusted MRR 1.50, 95% CI 1.42–1.57) and imaging-based tests (adjusted MRR 1.44, 95% CI: 1.37–1.50). Additional adjustment for rurality did not alter the above MRRs.
Figure 1. County-level cardiac stress utilization rates.
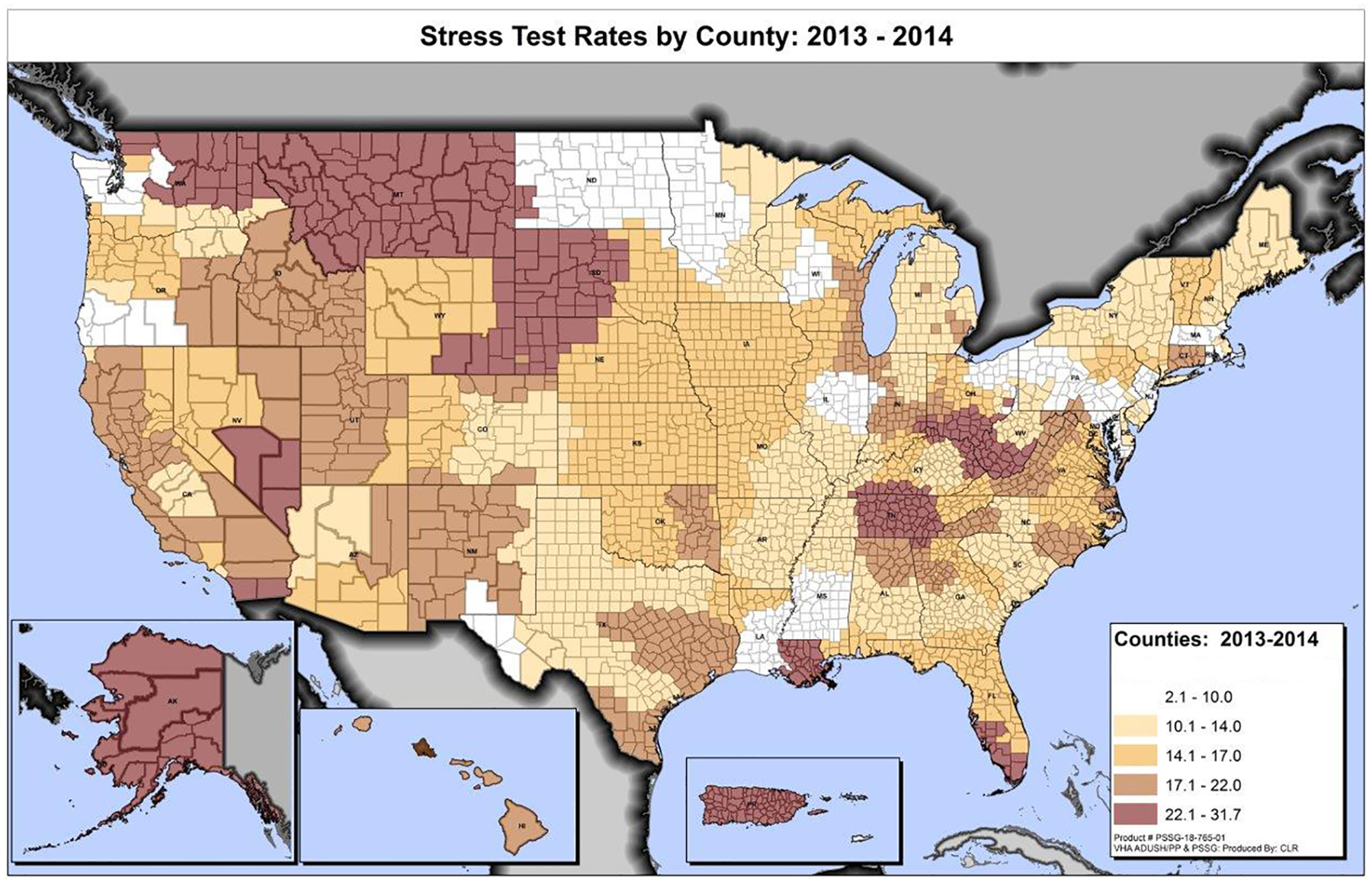
Rates represent unadjusted number of stress tests performed/100 patients with ischemic heart disease/county/year.
Our data suggest that even after adjustment for patient mix, there is significant unexplained residual variation in cardiac stress test utilization in Veterans with IHD. These are the first benchmark data from a large, nationwide healthcare system. A number of provider- and facility-level variables could explain our findings. Providers in facilities with higher utilization rates may be overusing or misusing these tests. Given that self-referral and ‘defensive medicine’ motives are largely attenuated within the VA (3), overuse or misuse may reflect insufficient awareness about appropriate use criteria or perhaps ‘carryover’ from practice outside the VA. Limited data suggest the proportion of ‘rarely appropriate’ stress tests within and outside the VA are comparable (3,4), but a system-wide VA analysis remains undone. In addition, facility-level variables such as on-site availability of invasive cardiac catheterization (5) and cardiac CT angiography could have influenced referrals for cardiac stress tests. Future studies should include these variables, as well as patient-level symptom data, to better contextualize our findings.
In conclusion, we found 40% residual facility-level variation in cardiac stress test utilization within the VA. Further contextualization with relevant patient/provider/facility-level variables, appropriateness, and patient outcomes could meaningfully improve efficiencies in and the quality of Veterans’ cardiovascular care.
Financial Support
Dr. Shah is supported by an Agency for Healthcare Research and Quality award (5K12HS022998). Dr. Winchester is supported by a Career Development Award from the Department of Veterans Affairs Health Service Research & Development Service (CDA0-020-16W). Dr. Virani is supported by an American Heart Association Beginning Grant-in-Aid (14BGIA20460366), an American Diabetes Association Clinical Science and Epidemiology award (1-14-CE-44), and an Investigator-Initiated Research grant from the Department of Veterans Affairs Health Service Research & Development Service (IIR 16-072). Dr. Waldo receives research support to the Denver Research Institute from Abiomed, Cardiovascular Systems Inc., and Merck Pharmaceuticals. Dr. Petersen is supported by an Investigator-Initiated Research grant from the Department of Veterans Affairs Health Service Research & Development Service (IIR 15-438). This work was also supported by a grant from the Houston Veterans Affairs Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs or the US government.
Abbreviations List
- DCG RRS
Diagnostic Cost Group Relative Risk Score
- ETT
exercise treadmill testing
- IHD
ischemic heart disease
- MPI
myocardial perfusion imaging
- MRR
median rate ratio
- PET
positron emission tomography
- SE
stress echocardiography
- SPECT
single photon emission computed tomography
- VA
Veterans Health Administration
Footnotes
Disclosures
None of the authors have any relationships with industry to disclose.
References
- 1.Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA : the journal of the American Medical Association 2008;300:1765–73. [DOI] [PubMed] [Google Scholar]
- 2.Federspiel JJ, Mudrick DW, Shah BR et al. Patterns and predictors of stress testing modality after percutaneous coronary stenting: data from the NCDR((R)). JACC Cardiovascular imaging 2012;5:969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winchester DE, Meral R, Ryals S, Beyth RJ, Shaw LJ. Appropriate use of myocardial perfusion imaging in a veteran population: profit motives and professional liability concerns. JAMA Intern Med 2013;173:1381–3. [DOI] [PubMed] [Google Scholar]
- 4.Ladapo JA, Blecker S, O’Donnell M, Jumkhawala SA, Douglas PS. Appropriate Use of Cardiac Stress Testing with Imaging: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0161153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen LA, Normand SL, Leape LL, McNeil BJ. Regionalization and the underuse of angiography in the Veterans Affairs Health Care System as compared with a fee-for-service system. The New England journal of medicine 2003;348:2209–17. [DOI] [PubMed] [Google Scholar]


