Abstract
BACKGROUND
Some people rapidly develop iron deficiency anemia following blood donation, while others can repeatedly donate without becoming anemic.
METHODS
Two cohorts of blood donors were studied. Participants (775) selected from a 2-year longitudinal study were classified into six analysis groups based on sex, donation intensity, and low hemoglobin deferral. Associations with iron supplement use, cigarette smoking, and four genetic variants of iron metabolism were examined at enrollment and with longitudinal regression models. An unbiased assessment of genetic variability and ability to repeatedly donate blood without experiencing low hemoglobin deferral was conducted on participants (13,403) in a cross-sectional study who were examined by genome wide association (GWA).
RESULTS
Behaviors and genetic variants were associated with differences in hemoglobin and ferritin change following repeated donation. At least weekly iron supplement use was associated with improved status in first-time donors, while daily use was associated with improved status in high-intensity donors. Cigarette smoking was associated with 0.5 g/dL increased hemoglobin in high-intensity donors. A736V in TMPRSS6 was associated with a rapid drop in hemoglobin and ferritin in first-time females following repeated donation. Conversely, the protective TMPRSS6 genotype was not enriched among high-intensity donors. H63D in HFE was associated with increased hemoglobin in female high-intensity donors. However, no differences in genotype between first-time and high-intensity donors were found in GWA analyses.
CONCLUSION
Behavioral and genetic modifiers contributed to first-time donor hemoglobin and iron status, while iron supplement use was more important than underlying genetics in high-intensity donors.
Blood donors give 525 mL whole blood at each donation. Donation is allowed every 56 days in the United States regardless of sex or age, so the highest intensity donors can give blood 6.5 times per year. Each donation removes 200–250 mg of iron. Therefore, frequent blood donors are susceptible to iron deficiency and iron deficiency anemia.1–4 There is considerable variability in individual responses to blood donation. Some donors rapidly become anemic, while others, both males and females, repeatedly donate without developing anemia, making blood donors a unique population for studies of iron metabolism and iron deficiency anemia.5,6 Several studies have found that high-intensity donors paradoxically have decreased risk for low hemoglobin deferral when compared to infrequent donors, suggesting that high-intensity donors may be a self-selected group that is genetically resistant to the development of anemia.5–7 The relative contributions of genetic polymorphisms and behavioral variation, such as iron supplement use or cigarette smoking,6,8 are incompletely understood.
Several studies have examined the association of genetic factors with iron deficiency and anemia in blood donors.9–12 Hepcidin decreases iron absorption from the gastrointestinal tract,13 and genetic modifiers of its production may influence iron recovery following blood donation.6 The HFE C282Y (rs1800562) and H63D (rs1799945) mutations decrease hepcidin production and increase risk for hemochromatosis.13,14 TMPRSS6 encodes matriptase-2, a membrane associated serine protease that degrades hemojuvelin, thereby decreasing hepcidin production.15 Several genome wide association (GWA) studies have identified polymorphisms in TMPRSS6 associated with changes in red blood cell indices and iron status.16,18 An alanine to valine change at position 736, (A736V; rs855791) has the largest negative effect. Finally, the G277S (rs1799899) mutation in Transferrin is associated with iron deficiency in menstruating women.19
We evaluated blood donors participating in the Retrovirus Epidemiology Donor Study-II (REDS-II) Iron Status Evaluation (RISE) Study3 and the REDS-III Red Blood Cell Omics (RBC-Omics) study20 to assess relationships between donor genetics and behaviors on hemoglobin and iron status. Longitudinal analyses were performed in the RISE cohort to define the impact of iron supplements, cigarette smoking, H63D, C282Y, A736V, and G277S. An unbiased cross-sectional GWA approach was used to identify genetic factors associated with high- intensity blood donors in the RBC-Omics cohort.
METHODS
Longitudinal population
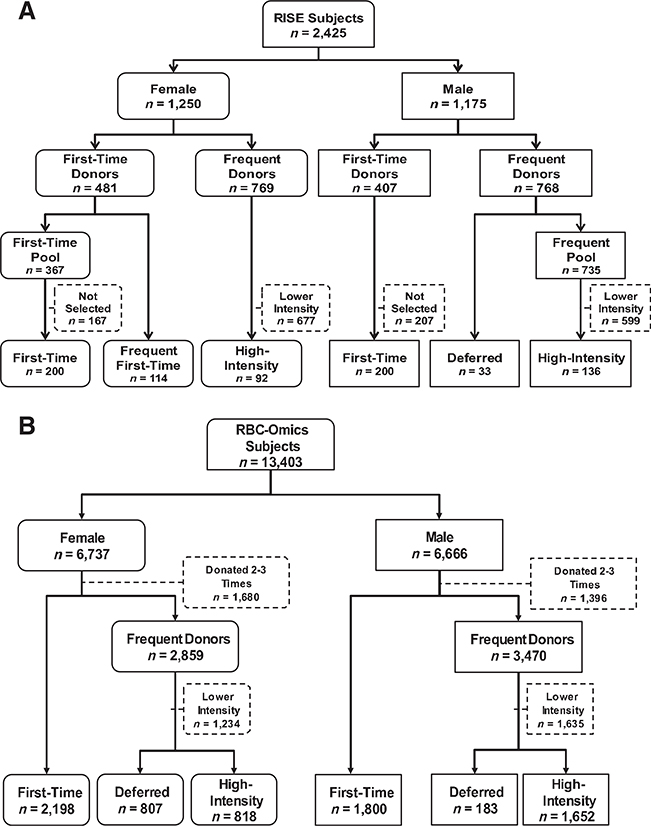
The National Institutes of Health (NIH) National Heart Lung and Blood Institute (NHLBI) REDS-II RISE Study was a multicenter, 2-year study of iron and hemoglobin in 2425 blood donors.3 We selected 775 RISE participants, stratified by sex, and created six analysis groups based on donation and low hemoglobin deferral history (Figure 1). Groups 1 (200 first-time females) and 2 (200 first-time males) were randomly selected first-time or reactivated donors (no donations in previous 2 years). Reactivated donors had the same hemoglobin and iron status as first-time donors and, therefore, were combined with first-time donors.3 Group 3 (114 frequent first-time females) was first-time or reactivated females donating at least 4 times during RISE. These donors were examined previously21 and, therefore, were not included in first-time females (Figure 1). They were studied here as a separate donor cohort because they represented a group of first-time or reactivated females who could possibly become high-intensity donors. Group 4 (33 deferred males) was males with low hemoglobin deferral during the RISE study period. They were studied because they had a higher susceptibility to anemia than other male donors. Groups 5 (92 high-intensity females) and 6 (136 high-intensity males) gave at least eight whole blood donations during RISE without low hemoglobin deferral. They were studied because they represented self-selected groups of donors with potential genetic resistance to development of anemia following repeated blood donation. The NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) repository of RISE samples contains plasma and buffy-coat/packed RBC aliquots. Participants provided informed consent for plasma and DNA testing related to hemoglobin and iron metabolism. Ferritin, complete blood count, C282Y, H63D, G277S, iron supplement use and cigarette smoking history were available.3 DNA from enrollment whole blood samples was tested for A736V using real-time PCR. Two primer pairs simultaneously amplified DNA. If they amplified equally (Cycle threshold difference [ACt] <2 cycles), the sample was classified as heterozygous; if one primer pair amplified more than the other (ACt >5 cycles), the sample was classified as homozygous.
Fig. 1.
Flow diagrams depicting selection of blood donors for participation in the study and segregation into analysis groups.
(A) Participants in the longitudinal study were selected from 2425 subjects enrolled in RISE. The flow diagram outlines the numbers of male and female subjects in RISE, the numbers of first-time/reactivated donors and the numbers of frequent donors, and how they were sorted into the final six analysis groups. (B) Participants in the cross-sectional study were selected from 13,403 subjects enrolled in RBC- Omics. The flow diagram outlines the numbers of male and female subjects in RBC-Omics, the numbers of first-time/reactivated donors and the numbers of frequent donors, and how they were sorted into the final six analysis groups.
Cross-sectional population
The NHLBI REDS-III RBC-Omics Study was a multi-center study enrolling 13,403 blood donors.20 This cohort was gen- otyped using a customized Affymetrix Axiom Array with approximately 879,000 single nucleotide polymorphisms (SNPs).22 Participants were stratified by sex and categorized into six groups that approximated the longitudinal groups (Figure 1B), differing by selection of females with a previous low hemoglobin deferral, instead of frequent first-time females, who could not be defined because of the cross-sectional study design. Groups 1 (2198 females) and 2 (1800 males) consisted of first-time or reactivated donors. Groups 3 (807 females) and 4 (183 males) had at least one low hemoglobin deferral in the previous 2 years. Groups 5 (818 females) and 6 (1652 males) had at least 8 whole blood donations within the prior 2 years. Iron supplement and cigarette use were obtained by survey.20 Ferritin and CBC were performed as described.20,23
RESULTS
RISE participants with longitudinal analyses
Demographics
The demographics of the longitudinal cohort are presented in Table 1. Of interest, high-intensity males weighed 19 pounds less (p < 0.0001) than first-time males. First-time females and males were about 20 years younger than their high-intensity counterparts (p < 0.0001 for both), First-time males were twice as likely as high-intensity males to have smoked in the previous 90 days (16.5% vs. 7.4%, p = 0.01). Deferred males averaged 8.2 whole blood donations over 2 years before enrollment, while high-intensity females and males averaged 8.1 and 9.0, respectively. Table 2 provides demographics for the cross-sectional cohort as described below.
TABLE 1.
Demographics and enrollment laboratory values for the longitudinal cohort
| FT females n = 200 | Frequent FT females n = 114 | HI females n = 92 | FT males n = 200 | Deferred males n = 33 | HI males n = 136 | ||
|---|---|---|---|---|---|---|---|
| White race | n (Col %) | 153 (75.5%) | 110 (96.5%) | 86 (93.5%) | 165 (82.5%) | 32 (97.0%) | 121 (89.0%) |
| Black race | n (Col %) | 17 (8.5%) | 1 (0.9%) | 1 (1.1%) | 13 (6.5%) | 0 (0.0%) | 0 (0.0%) |
| Asian/other/missing race | n (Col %) | 21 (10.5%) | 1 (0.9%) | 2 (2.2%) | 16 (8.0%) | 1 (3.0%) | 10 (7.4%) |
| Hispanic ethnicity | n (Col %) | 9 (4.5%) | 2 (1.8%) | 3 (3.3%) | 6 (3.0%) | 0 (0.0%) | 5 (3.7%) |
| Age (years) | Mean | 35.9 | 48.2 | 54.7 | 39.2 | 59.2 | 54.9 |
| Weight (lbs) | Mean | 158.9 | 166.3 | 155.6 | 198.9 | 186.4 | 179.9 |
| Body mass index | Mean | 26.5 | 27.7 | 26.4 | 27.7 | 26.7 | 26.6 |
| Any iron supplement | n (%) | 76 (39.2%) | 55 (48.2%) | 63 (68.5%) | 49 (24.5%) | 11 (33.3%) | 49 (36.3%) |
| Iron daily | n (%) | 45 (23.2%) | 35 (30.7%) | 53 (57.6%) | 33 (16.5%) | 8 (24.2%) | 36 (26.7%) |
| Iron at least weekly (<Daily) | n (%) | 31 (16.0%) | 20 (17.5%) | 10 (10.9%) | 16 (8.0%) | 3 (9.1%) | 13 (9.6%) |
| No iron or <weekly | n (%) | 118 (60.8%) | 59 (51.8%) | 29 (31.5%) | 151 (75.5%) | 22 (66.7%) | 86 (63.7%) |
| Any smoking - ever | n (%) | 72 (36.0%) | 48 (42.1%) | 38 (41.3%) | 78 (39.0%) | 15 (45.5%) | 54 (39.7%) |
| Any smoking - last 90 days | n (%) | 40 (20.0%) | 11 (9.6%) | 12 (13.0%) | 33 (16.5%) | 1 (3.0%) | 10 (7.4%) |
| Menstruating | n (%) | 149 (74.9%) | 60 (52.6%) | 26 (28.3%) | - | - | - |
| # Study donations | Mean | 2.19 | 5.04 | 7.90 | 3.08 | 6.88 | 7.84 |
| Any Hgb deferral | n (%) | 37 (18.5) | 48 (42.1) | 0 (0.0) | 2 (1.0) | 32 (97.0) | 0 (0.0) |
| Hemoglobin (g/dL) | Mean | 13.4 | 13.5 | 13.8 | 15.0 | 13.3 | 14.5 |
| Ferritin (ng/mL) | Mean | 44.7 | 62.4 | 26.0 | 140.9 | 13.0 | 28.4 |
| sTfR (μg/mL) | Mean | 2.8 | 2.9 | 3.3 | 2.9 | 4.5 | 3.5 |
| log(sTfR/ferritin) | Mean | 1.9 | 1.8 | 2.2 | 1.4 | 2.6 | 2.2 |
| Ferritin <12 ng/mL | n (%) | 18 (9.0%) | 3 (2.6%) | 16 (17.4%) | 0 (0.0%) | 20 (60.6%) | 23 (16.9%) |
| log(sTfR/ferritin)≥2.07 | n (%) | 59 (29.5%) | 23 (20.2%) | 59 (64.1%) | 4 (2.0%) | 31 (93.9%) | 82 (60.3%) |
| CHr (pg) | Mean | 32.4 | 33.1 | 32.5 | 33.2 | 30.6 | 32.4 |
| Percent low CHr | Mean | 7.2 | 4.7 | 5.8 | 4.9 | 20.7 | 7.2 |
TABLE 2.
Demographics and enrollment laboratory values for the cross-sectional cohort
| FT females | Frequent FT females | HI females | FT males | Deferred males | HI males | ||
|---|---|---|---|---|---|---|---|
| White race | n (Col %) | 923 (42%) | 603 (74.7%) | 781 (95.5%) | 751 (41.7%) | 140 (76.5%) | 1536 (93%) |
| Black race | n (Col %) | 399 (18.2%) | 75 (9.3%) | 6 (0.7%) | 280 (15.6%) | 25 (13.7%) | 32 (1.9%) |
| Asian race | n (Col %) | 406 (18.5%) | 53 (6.6%) | 8 (1%) | 456 (25.3%) | 8 (4.4%) | 37 (2.2%) |
| Other/unknown race | n (Col %) | 172 (7.8%) | 29 (3.6%) | 16 (2%) | 134 (7.4%) | 7 (3.8%) | 30 (1.8%) |
| Hispanic ethnicity | n (Col %) | 298 (13.6%) | 47 (5.8%) | 7 (0.9%) | 179 (9.9%) | 3 (1.6%) | 17 (1%) |
| Age (Years) | Mean | 35.9 | 51.3 | 58.6 | 37.1 | 57.2 | 57.9 |
| Weight (lbs) | Mean | 160.8 | 166.4 | 164.7 | 190.2 | 196.1 | 201.3 |
| Body mass index | Mean | 27.1 | 27.9 | 27.7 | 27.5 | 28.1 | 28.8 |
| Any iron supplement | n (%) | 716 (32.6%) | 469 (58.1%) | 509 (62.2%) | 404 (22.4%) | 86 (47%) | 677 (41%) |
| Iron daily | n (%) | 408 (18.6%) | 281 (34.8%) | 392 (47.9%) | 227 (12.6%) | 59 (32.2%) | 546 (33.1%) |
| Iron at least weekly (<daily) | n (%) | 224 (10.2%) | 130 (16.1%) | 92 (11.2%) | 129 (7.2%) | 17 (9.3%) | 103 (6.2%) |
| No iron or <weekly | n (%) | 1556 (70.8%) | 381 (47.2%) | 319 (39%) | 1437 (79.8%) | 103 (56.3%) | 984 (59.6%) |
| Current smoking: Yes vs No | n (%) | 242 (11%) | 28 (3.5%) | 55 (6.7%) | 224 (12.4%) | 6 (3.3%) | 78 (4.7%) |
| Menstruating: Yes vs. No | n (%) | 303 (13.8%) | 335 (41.5%) | 518 (63.3%) | - | - | - |
| Venous Hgb (g/dL) | Mean | 13.3 | 12.8 | 13.5 | 15 | 13.5 | 14.3 |
| Fingerstick Hgb (g/dL) | Mean | 13.7 | 13.4 | 14 | 15.2 | 13.9 | 14.6 |
| Ferritin (ng/mL) | Mean | 49.6 | 20.1 | 21 | 131.7 | 25.4 | 26.3 |
High-intensity females had higher enrollment hemoglobin than first-time females despite a higher frequency of iron deficiency
High-intensity females had higher hemoglobin (13.8 g/dL) than first-time (13.4 g/dL; p < 0.0001) or frequent first-time females (13.5 g/dL; p = 0.006; Table 1). Nevertheless, many high-intensity females had iron deficiency; 17.4% had ferritin <12 ng/mL, indicating absent bone marrow iron stores,24 and 64.1% had log(soluble transferrin receptor [sTfR]/ferri- tin) >2.07, a marker for iron deficient erythropoiesis.3 About one-half as many first-time females had iron deficiency; 9.0% had ferritin <12 ng/mL (p = 0.038) and 29.5% had log(sTfR/ferritin) >2.07 (p < 0.0001), as did frequent first-time females; ferritin <12 ng/mL (2.6%; p = 0.0003), log(sTfR/ferritin) >2.07 (20.2%; p < 0.0001). Despite having iron deficiency, high-intensity females produced red cells with normal reticulocyte hemoglobin content (CHr), a measure of iron available for hemoglobin synthesis over the previous 4 days.25 Mean CHr (32.5 pg) and percentage of reticulocytes with low hemoglobin content (<17 pg; 5.8%) in high-intensity females were similar to those of first-time females (32.4 pg; 7.2%). The ability of high-intensity females to maintain normal hemoglobin despite having iron deficiency indicates they effectively absorbed iron from the gastrointestinal tract to replace hemoglobin lost from each donation.
Deferred males had iron deficiency anemia while high-intensity males had iron deficiency without anemia
Despite qualifying for blood donation at enrollment, the average hemoglobin in deferred males over the course of the study was 13.3 g/dL (Table 1), which was well below first-time males (15.0 g/dL), high-intensity males (14.5 g/dL; p < 0.0001 for both), and the fifth percentile (13.7 g/dL) for Caucasian men <60 years old.26 They also frequently had severe iron deficiency. Ferritin was <12 ng/mL in 60.6% and 93.9% had log(sTfR/ferritin) >2.07. Their severe iron deficiency impacted hemoglobin synthesis. Mean CHr was 30.6 pg, and 20.7% of reticulocytes had low hemoglobin content (Table 1). Although iron deficiency was frequent in high-intensity males—16.9% had ferritin <12 ng/mL, 60.3% had log(sTfR/ferritin) >2.07— they maintained normal hemoglobin synthesis with CHr of 32.4 pg with 7.2% of reticulocytes having low hemoglobin content. These findings show that high-intensity males, in contrast to deferred males, effectively absorbed iron from the gastrointestinal tract and incorporated it into hemoglobin following donation.
Hemoglobin decreased more in first-time than in high-intensity donors with repeated donation
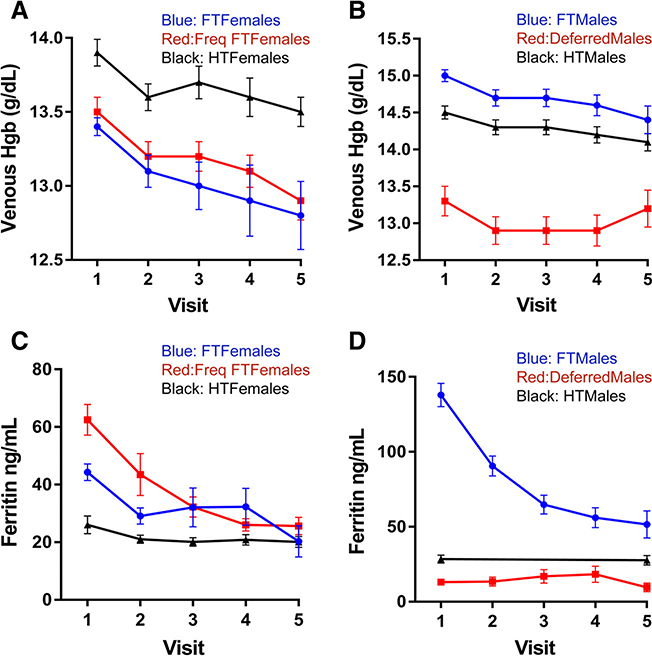
Hemoglobin decreased with repeated donation in all groups but differed in magnitude. High-intensity females had a smaller decrease (0.3 g/dL) over five donations than first-time (0.6 g/dL) or frequent females (0.6 g/dL; Figure 2A). Similarly, high-intensity males had a smaller decrease (0.3 g/dL) over five donations than first-time males (0.6 g/dL) (Figure 2B). These findings suggest that following each donation, high-intensity donors absorb more iron from the gastrointestinal tract and incorporate it into hemoglobin than first-time donors.
Fig. 2.
Change in hemoglobin and ferritin over multiple donations in different analysis groups. (A) Venous hemoglobin in female groups; (B) venous hemoglobin in male groups; (C) ferritin in female groups; and (D) ferritin in male groups. In A and C, blue lines are first- time/reactivated (FT) females; red lines are frequent first-time (Freq FT) females; and black lines are high-intensity (HT) females. In B and D, blue lines are first-time/reactivated (FT) males; red lines are deferred males; and black lines high-intensity (HT) males error bars represent standard error of the mean. [Color figure can be viewed at wileyonlinelibrary.com]
Iron stores decreased more in first-time than in high-intensity donors with repeated donation
Ferritin in first-time females, frequent first-time females (Figure 2C), and first-time males (Figure 2D) dropped substantially over three initial donations, as previously described,1,8 confirming first-time blood donors do not absorb sufficient iron to replace that lost from repeated donation. High-intensity females (Figure 2C) and males (Figure 2D) had similar average ferritin of 26–28 ng/mL at enrollment, which decreased to 20–22 ng/mL demonstrating worsening iron deficiency with continued donation.
The frequency of C282Y, H63D, and A736V was similar between enrollment groups
Although high-intensity donors were capable of maintaining hemoglobin production, they did not have increased frequency of the C282Y, H63D, or A736V polymorphisms that are associated with decreased hepcidin production and increased iron absorption from the gastrointestinal tract (Table 3).
TABLE 3.
Genotype frequencies for the longitudinal and cross-sectional cohorts
| FT females n = 200 | Frequent FT females n = 114 | HI females n = 92 | FT males n = 200 | Deferred males n = 33 | HI males n = 136 | |||
| Longitudinal cohort | HFE C282Y | Wild type | 180 (90%) | 185 (93%) | 103 (90%) | 27 (82%) | 80 (87%) | 124 (91%) |
| Heterozygous | 20 (10%) | 14 (7%) | 11 (10%) | 6 (18%) | 12 (13%) | 12 (9%) | ||
| Homozygous | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| HFE H63D | Wild type | 142 (71%) | 141 (71%) | 81 (71%) | 25 (76%) | 67 (73%) | 104 (76%) | |
| Heterozygous | 53 (27%) | 58 (29%) | 32 (28%) | 8 (24%) | 23 (25%) | 31 (23%) | ||
| Homozygous | 5 (3%) | 1 (1%) | 1 (1%) | 0 (0%) | 2 (2%) | 1 (1%) | ||
| TMPRSS6 A736V | Wild type | 81 (41%) | 33 (29%) | 27 (29%) | 79 (40%) | 9 (27%) | 40 (29%) | |
| Heterozygous | 86 (43%) | 62 (54%) | 49 (53%) | 82 (41%) | 14 (42%) | 61 (45%) | ||
| Homozygous | 33 (17%) | 19 (17%) | 16 (17%) | 39 (20%) | 10 (30%) | 35 (26%) | ||
| FT females n = 2055 | Frequent FT females n = 762 | HI females n = 788 | FT males n = 1713 | Deferred males n = 180 | HI males n = 1575 | |||
| Cross-sectional cohort | HFE C282Y | Wild type | 1923 (87.5%) | 687 (85.1%) | 693 (84.7%) | 1600 (88.9%) | 162 (88.5%) | 1411 (85.4%) |
| Heterozygous | 118 (5.4%) | 73 (9%) | 91 (11.1%) | 91 (5.1%) | 18 (9.8%) | 156 (9.4%) | ||
| Homozygous | 14 (0.6%) | 2 (0.2%) | 4 (0.5%) | 22 (1.2%) | 0 (0%) | 8 (0.5%) | ||
| HFE H63D | Wild type | 1645 (74.8%) | 571 (70.8%) | 587 (71.8%) | 1366 (75.9%) | 131 (71.6%) | 1146 (69.4%) | |
| Heterozygous | 383 (17.4%) | 175 (21.7%) | 177 (21.6%) | 319 (17.7%) | 44 (24%) | 396 (24%) | ||
| Homozygous | 27 (1.2%) | 16 (2%) | 24 (2.9%) | 28 (1.6%) | 5 (2.7%) | 33 (2%) | ||
| TMPRSS6 A736V | Wild type | 759 (34.5%) | 289 (35.8%) | 272 (33.3%) | 621 (34.5%) | 60 (32.8%) | 553 (33.5%) | |
| Heterozygous | 923 (42%) | 347 (43%) | 397 (48.5%) | 778 (43.2%) | 83 (45.4%) | 748 (45.3%) | ||
| Homozygous | 373 (17%) | 126 (15.6%) | 119 (14.5%) | 314 (17.4%) | 37 (20.2%) | 274 (16.6%) | ||
Longitudinal regression models of genetic and behavioral factors on hemoglobin and iron status
Longitudinal hemoglobin (Table 4) and ferritin (Table 5) models were developed to assess associations with genetic heterogeneity at A736V, C282Y, and H63D, and with behavioral effects of iron supplements and cigarette smoking. The hemoglobin and ferritin models included data for up to seven and five donation visits, respectively. Truncation of visit data was needed to insure model fit. The number of hemoglobin values available for modeling at each visit is presented in the Supplementary Table, Appendix S1, available as supporting information in the online version of this paper. Initial analyses indicated G277S was not associated with hemoglobin or ferritin in any group and was dropped from final analyses. There was no relationship between these covariates and hemoglobin or ferritin in deferred males, perhaps reflecting the low number of subjects for analysis in this group.
TABLE 4.
Longitudinal regression model of hemoglobin*
| First time (FT) Females |
Frequent FT Females |
High intensity Females |
First time (FT) Males |
Deferred Males |
High intensity Males |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | n = 116 Estimate | d = 334 p value | n = 113 Estimate | d = 607 p value | n = 92 Estimate | d = 576 p value | n = 131 Estimate | d = 502 p value | n = 33 Estimate | d = 217 p value | n = 135 Estimate | d = 804 p value |
| TMPRSS6 Genotype | - | <.0001 | - | 0.0178 | - | 0.9568 | - | 0.4983 | - | 0.2216 | 0.1142 | - |
| AA vs VV | 1.00 | <.0001 | 0.50 | 0.0047 | 0.03 | 0.8176 | 0.13 | 0.4851 | −0.51 | 0.1064 | 0.23 | 0.0884 |
| AV vs VV | 0.77 | <.0001 | 0.32 | 0.0422 | 0.04 | 0.7704 | −0.04 | 0.7844 | −0.07 | 0.8133 | 0.25 | 0.0492 |
| AA vs AV | 0.23 | 0.0710 | 0.18 | 0.1760 | −0.01 | 0.9511 | 0.17 | 0.2413 | −0.44 | 0.1539 | −0.02 | 0.8472 |
| C282Y wild type vs Ho/He | −0.70 | 0.0010 | −0.13 | 0.4773 | 0.16 | 0.2519 | −0.31 | 0.2135 | −0.17 | 0.5660 | 0.21 | 0.2672 |
| H63D wild type vs Ho/He | −0.14 | 0.2801 | 0.17 | 0.1791 | −0.43 | 0.0002 | 0.07 | 0.6045 | 0.08 | 0.7923 | −0.23 | 0.0565 |
| Race/ethnicity | - | 0.4582 | - | 0.7688 | - | 0.6768 | - | 0.1426 | - | - | - | 0.0009 |
| Asian vs White | <0.01 | 0.9960 | - | - | 0.12 | 0.7241 | −1.06 | 0.0373 | - | - | −0.27 | 0.2774 |
| Black vs White | −0.32 | 0.4163 | −0.56 | 0.4028 | 0.29 | 0.5262 | −0.05 | 0.8953 | - | - | - | - |
| Hispanic vs White | −0.35 | 0.2500 | −0.14 | 0.7594 | 0.32 | 0.2863 | −0.65 | 0.0813 | - | - | −1.07 | <.0001 |
| Other vs White | −1.11 | 0.1602 | −0.42 | 0.5451 | - | - | −0.09 | 0.8399 | - | - | −0.14 | 0.6280 |
| Length of time since prev. visit | <0.01 | 0.8331 | <0.01 | 0.0045 | <0.01 | 0.2483 | <0.01 | 0.5443 | <0.01 | 0.5595 | <−0.01 | 0.0339 |
| # RBC donations in past 12m | −0.26 | <.0001 | −0.03 | 0.5249 | −0.08 | 0.1766 | −0.08 | 0.1170 | −0.20 | 0.0405 | −0.12 | 0.0149 |
| Age (yrs) at baseline visit | −0.01 | 0.0214 | 0.01 | 0.0201 | <−0.01 | 0.7541 | −0.01 | 0.0006 | 0.02 | 0.0511 | −0.01 | 0.0475 |
| Weight (lbs) at baseline visit | <0.01 | 0.0378 | <−0.01 | 0.6709 | <−0.01 | 0.2158 | <−0.01 | 0.8570 | −0.01 | 0.1215 | <−0.01 | 0.0800 |
| Iron supplementation | - | 0.0094 | - | 0.0303 | - | 0.3383 | - | 0.0030 | - | 0.1482 | - | 0.0012 |
| Daily vs. none | 0.07 | 0.5661 | −0.28 | 0.0329 | −0.11 | 0.3625 | −0.30 | 0.0571 | −0.54 | 0.0703 | 0.42 | 0.0006 |
| Weekly vs. none | 0.62 | 0.0024 | 0.16 | 0.3341 | −0.26 | 0.1465 | 0.68 | 0.0086 | −0.46 | 0.2834 | 0.34 | 0.0623 |
| Daily vs. weekly | −0.55 | 0.0081 | −0.45 | 0.0170 | 0.16 | 0.3189 | −0.98 | 0.0007 | −0.08 | 0.8679 | 0.08 | 0.7025 |
| Currently smoking: Yes vs. No | 0.15 | 0.3250 | 0.25 | 0.1852 | 0.48 | 0.0015 | −0.22 | 0.2495 | −0.63 | 0.4169 | 0.53 | 0.0075 |
| Still menstrating: Yes vs No | −0.59 | 0.0005 | −0.08 | 0.6142 | 0.04 | 0.8093 | n/a | n/a | n/a | n/a | n/a | n/a |
Longitudinal multiple regression models of Venous Hemoglobin (g/dL) on 8 covariates and 3 genotypes for donors with 2 or more visits at which there was a donation or hemoglobin deferral.
Separate regression models were estimated, one for each blood donor analysis group. Results for the first seven hemoglobin donations or deferrals were included in the model. The number of donors (n) and the number of donations (d) are included in each model is reported in the column headings. Parameter estimates rounded to 2 decimal places; p values are presented to 4 digits. The C282 and H63D genotypes were modeled as binary variables, with homozygous (Ho) and heterozygous (He) results combined so that these models would converge to a solution. Some levels of race are missing in certain analysis groups. Race was excluded from the model of deferred males due to a lack of representative data in this smaller group. For multiple-level categorical variables, an overall p value is presented along with the p values for differences in individual levels.
TABLE 5.
Longitudinal regression model of ferritin*
| First time (FT) Females |
Frequent FT Females |
High intensity Females |
First time (FT) Males |
Deferred Males |
High intensity Males |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | n = 116 Estimate | d = 287 p value | n = 113 Estimate | d = 495 p value | n = 92 Estimate | d = 315 p value | n = 131 Estimate | d = 420 p value | n = 33 Estimate | d = 125 p value | n = 135 Estimate | d = 243 p value |
| TMPRSS6 Genotype | - | 0.0066 | - | 0.1089 | - | 0.5893 | - | 0.0131 | - | 0.1814 | - | 0.5361 |
| AA vs VV | 0.27 | 0.0016 | 0.11 | 0.0391 | −0.05 | 0.3368 | 0.07 | 0.1619 | −0.14 | 0.1229 | 0.05 | 0.3618 |
| AV vs VV | 0.21 | 0.0140 | 0.08 | 0.0909 | −0.02 | 0.6720 | −0.05 | 0.2833 | 0.01 | 0.9014 | <0.01 | 0.9888 |
| AA vs AV | 0.06 | 0.2668 | 0.03 | 0.4750 | −0.03 | 0.4533 | 0.12 | 0.0033 | −0.15 | 0.0881 | 0.05 | 0.3124 |
| C282Y wild type vs Ho/He | −0.04 | 0.6581 | −0.13 | 0.0212 | 0.12 | 0.0217 | 0.07 | 0.3661 | −0.18 | 0.0622 | −0.07 | 0.3635 |
| H63D wild type vs Ho/He | 0.04 | 0.4562 | 0.09 | 0.0178 | 0.05 | 0.2434 | −0.01 | 0.7100 | −0.10 | 0.2399 | −0.06 | 0.2517 |
| Race/ethnicity | - | 0.3642 | - | 0.2922 | - | 0.5141 | - | 0.0546 | - | - | - | 0.4585 |
| Asian vs White | 0.10 | 0.3455 | - | - | 0.06 | 0.6073 | 0.02 | 0.8659 | - | - | 0.07 | 0.5168 |
| Black vs White | 0.10 | 0.4900 | 0.03 | 0.8583 | 0.15 | 0.2986 | −0.11 | 0.2688 | - | - | - | - |
| Hispanic vs White | 0.17 | 0.1556 | −0.26 | 0.0560 | 0.15 | 0.2813 | 0.03 | 0.7930 | - | - | −0.11 | 0.3282 |
| Other vs White | −0.33 | 0.3503 | 0.02 | 0.9383 | - | - | 0.35 | 0.0056 | - | - | 0.13 | 0.2819 |
| Length of time since prev. visit | <0.01 | <.0001 | <0.01 | <.0001 | <0.01 | 0.0020 | <0.01 | <.0001 | <0.01 | 0.0291 | <0.01 | 0.0002 |
| # RBC donations in past 12m | −0.09 | 0.0013 | −0.06 | 0.0004 | −0.03 | 0.1683 | −0.07 | <.0001 | −0.03 | 0.2130 | −0.07 | <.0001 |
| Age (yrs) at baseline visit | 0.01 | 0.0084 | <0.01 | 0.0078 | <−0.01 | 0.6956 | <0.01 | 0.6931 | 0.02 | <.0001 | <0.01 | 0.0256 |
| Weight (lbs) at baseline visit | <0.01 | 0.2670 | <0.01 | <.0001 | <0.01 | 0.0929 | <0.01 | <.0001 | <−0.01 | 0.4543 | <0.01 | 0.4377 |
| Missing ferritin data count | −0.01 | 0.7530 | −0.01 | 0.6046 | 0.01 | 0.0930 | −0.06 | 0.1218 | 0.02 | 0.1339 | <−0.01 | 0.3039 |
| Iron supplementation | - | 0.0923 | - | 0.3874 | - | 0.0006 | - | 0.0005 | - | 0.3249 | - | 0.0020 |
| Daily vs none | 0.05 | 0.3532 | 0.05 | 0.2416 | 0.16 | 0.0002 | −0.14 | 0.0017 | 0.09 | 0.2781 | 0.18 | 0.0008 |
| Weekly vs none | 0.17 | 0.0323 | 0.05 | 0.3159 | 0.07 | 0.2583 | 0.16 | 0.0422 | −0.10 | 0.4053 | 0.12 | 0.0965 |
| Daily vs weekly | −0.12 | 0.1748 | <−0.01 | 0.9287 | 0.09 | 0.0979 | −0.30 | 0.0005 | 0.19 | 0.1567 | 0.05 | 0.5105 |
| Currently smoking: Yes vs No | −0.02 | 0.6811 | 0.12 | 0.0436 | 0.06 | 0.2775 | 0.13 | 0.0208 | −0.15 | 0.4979 | 0.12 | 0.1749 |
| Still menstrating: Yes vs No | −0.03 | 0.7099 | −0.06 | 0.2073 | −0.07 | 0.1951 | n/a | n/a | n/a | n/a | n/a | n/a |
Longitudinal multiple regression models of log10 ferritin (ng/mL) on 9 covariates and 3 genotypes for donors with two or more visits at which there was a donation or hemoglobin deferral.
Separate regression models were estimated, one for each blood donor analysis group. Results for the first five hemoglobin donations or deferrals were included in the model. The number of donors (n) and the number of donations (d) are included in each model is reported in the column headings. Parameter estimates rounded to two decimal places; p values are presented to four digits. The C282 and H63D genotypes were modeled as binary variables, with homozygous (Ho) and heterozygous (He) results combined so that these models would converge to a solution. Some levels of race are missing in certain analysis groups. Race was excluded from the model of deferred males due to a lack of representative data in this smaller group. For multiple-level categorical variables, an overall p value is presented along with the p values for differences in individual levels.
TMPRSS6 A736V altered longitudinal change in hemoglobin and ferritin in first-time donors
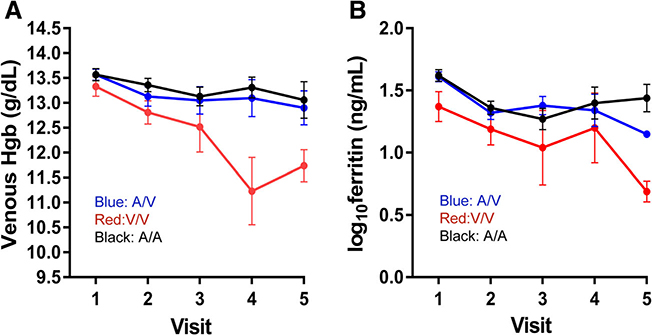
First-time females homozygous for valine (VV) had rapidly decreasing hemoglobin and ferritin with repeated donation when compared to those homozygous (AA) or heterozygous (AV) for alanine (Figure 3A and B). These differences were quantified in the longitudinal models, where hemoglobin was 1.00 and 0.77 g/dL higher in AA (p < 0.0001) and AV (p = 0.0001), respectively, than in VV (Table 4). Log10ferritin was 0.27 and 0.21 ng/mL higher in AA (p = 0.0016) and AV (p = 0.0140), respectively, than in VV (Table 5). A similar effect was observed in frequent first-time females where hemoglobin was 0.50 (p = 0.0047) and 0.32 g/dL (p = 0.0422) higher in AA and AV, respectively, than in VV (Table 4). However, an association with ferritin was not observed (Table 5). In first-time males, a longitudinal association with ferritin was observed. Those with AA had 0.12 ng/mL higher log10ferritin than those with AV (p = 0.0033). However, an association with hemoglobin was not observed, likely reflecting the higher baseline iron stores in males compared to females. These data suggest that changes in TMPRSS6 activity induced by the A736V polymorphism substantially alter iron absorption from the gastrointestinal tract in first-time donors following blood donation.
Fig. 3.
Change in hemoglobin and ferritin in female analysis groups by TMPRSS6 genotype. (A) Venous hemoglobin; and (B) log10 ferritin with continued donation for first-time/reactivated female donors segregated by TMPRSS6 genotype. Black lines are A/A; blue lines are A/V; red lines are V/V. Error bars represent standard error of the mean. [Color figure can be viewed at wileyonlinelibrary.com]
The TMPRSS6 A736V did not affect longitudinal change in hemoglobin and ferritin in high-intensity donors
A736V was not associated with longitudinal change in hemoglobin (Table 4) or ferritin (Table 5) in high-intensity donors. Given the differences observed in first-time donors, this was a somewhat surprising finding. However, it is consistent with the equal prevalence of this polymorphism in first-time and high-intensity donors (Table 3) and suggests that it does not contribute to the resistance of high-intensity donors to iron deficiency anemia.
HFE C282Y had mixed associations with longitudinal change in hemoglobin and ferritin
Analyses of C282Y combined those homozygous and heterozygous for the mutation into a single group because of the low numbers of homozygotes (Tables 4 and 5). An association with increased hemoglobin (0.70 g/dL; p = 0.0010) was observed in first-time females, but not in other groups. An association with increased log10ferritin was observed in high-intensity females (0.12 ng/mL; p = 0.0217), but an association with decreased log10ferritin was observed in frequent first-time females (−0.13 ng/mL: p = 0.0212). These mixed associations of C282Y were likely observed because of the small and variable numbers of homozygotes in the groups, which is associated with high baseline ferritin in blood donors.9
HFE H63D was associated with increased hemoglobin in high-intensity, but not first-time, donors
H63D was not associated with longitudinal change in hemoglobin in any first-time group. It was associated with 0.43 g/dL increased hemoglobin in high-intensity females (p = 0.0002) and a non-significant 0.23 g/dL increase in high-intensity males (p = 0.0565). (Table 4). These findings suggest that the H63D polymorphism may contribute to the resistance of high- intensity donors to iron deficiency anemia.
First-time and high-intensity donors benefited from different frequencies of iron supplement use
The frequency of iron supplement use (multiple vitamins with iron or iron pills) was recorded by participants as daily, at least weekly, or none. Longitudinal analyses found that at least weekly, but not daily, iron supplementation was most beneficial for first-time donors, while daily use was most beneficial for high-intensity donors. For example, at least weekly use was associated with higher hemoglobin in first-time females (0.55 g/dL; p = 0.0081), frequent first-time females (0.45 g/dL; p = 0.0170), and first time males (0.98 g/dL; p = 0.0007), while daily use by these groups was not associated with increased hemoglobin (Table 4). In contrast, longitudinal analyses found that daily iron use was most beneficial for high-intensity donors. For example, daily iron supplements were associated with higher log10ferritin in high-intensity females (0.16ng/mL, p = 0.0002) and males (0.18 ng/mL, p = 0.0008), while weekly use had no effect (Table 5).
Smoking was associated with increased ferritin in first-time donors and increased hemoglobin in high-intensity donors
Smoking was not associated with hemoglobin in first-time donors (Table 4). It was associated with increased log10ferritin by 0.12 (p = 0.0436) and 0.13 (p = 0.0208) ng/mL in frequent first-time females and first-time males, respectively (Table 5). By contrast, smoking was associated with increased hemoglobin by 0.48 g/dL (p = 0.0015) in high-intensity females and by 0.53 g/dL (p = 0.0075) in high-intensity males (Table 4).
RBC-Omics participants with cross-sectional analyses
Demographics
The REDS-III RBC-Omics cohort had a high number of high- intensity donors who had been genotyped using a transfusion medicine array,20,22 and therefore, were a unique population to identify genetic polymorphisms associated with the ability to repeatedly donate blood without developing anemia. The demographics of the cross-sectional cohort are presented in Table 2. High-intensity males weighed more than first-time male donors, an observation different from the longitudinal cohort. Similar to the longitudinal cohort, first-time females and males were about 20 years younger than high-intensity females and males, respectively (p < 0.0001 for both). First-time donors were two-to-three times more likely to smoke than high-intensity donors (11% vs. 6.7%, p = 0.0003 females; 12.4% vs. 4.7%, p < 0.0001 males). The baseline hemoglobin and iron status of the cross-sectional groups were similar to those of the longitudinal groups.
The frequency of C282Y, H63D, and A736V was similar between cross-sectional groups
As observed in the longitudinal cohort, C282Y, H63D, or A736V frequency was similar between first-time and high- intensity donors (Table 3).
Whole genome analyses did not identify genotype differences between first-time and high-intensity donors
Whole genome analyses comparing first-time and high- intensity donors were performed as a non-biased test for identification of polymorphisms that may provide genetic resistance to iron deficiency anemia. The genotype of donors was examined in several analyses including; 1) high-intensity donors versus first-time Caucasian donors (n = 1674); 2) high-intensity donors (n = 1321 males, 655 females) versus first-time Caucasian donors by sex (n = 751 males, 923 females); 3) high-intensity donors not taking iron supplements (n = 749 males, 221 females) versus first-time Caucasian donors; and 4) high-intensity donors versus Caucasian donors with a low hemoglobin deferral (n = 159 males, 742 females). There were no genome wide significant differences in genotype of the high-intensity donors versus comparator groups in any of the four analyses at GWAS required significance levels of p < 5 × 10−8. These findings suggest that donor genetics do not substantially contribute to the resistance of high-intensity donors to iron deficiency anemia.
DISCUSSION
Donor sex, donation frequency, and low hemoglobin deferral history were used to categorize blood donors into groups to examine baseline and longitudinal hemoglobin and iron status. Analyses of the different groups identified distinct differences that would not have been recognized in analyses of unselected groups. Differences were large and sometimes counterintuitive. Baseline hemoglobin was greater in high- intensity females than in first-time females, despite an increased prevalence of iron deficiency. Hemoglobin and ferritin were higher in high-intensity males than deferred males, despite having similar donation history. Hemoglobin and ferritin declined with repeated donation in first-time donors but remained steady in high-intensity donors. Finally, high-intensity males and females had nearly identical hemoglobin and iron status at baseline and longitudinally. This is unusual because women have naturally lower hemoglobin and iron stores than men, which are partially accounted for by hormonal differences and iron loss from menstruation and pregnancy.26 Thus, repeated blood donation overwhelms natural biologic differences in hemoglobin and iron stores between males and females.
TMPRSS6 A736V, HFE C282Y, HFE H63D, and transferrin G277S, as well as iron supplement use and cigarette smoking, were characterized to define associations with changes in hemoglobin and iron occurring with repeated donation. A736V was associated with differences within first-time donors, while H63D showed differences within high-intensity donors. Thus, underlying genetics do effect responses to blood donation. Nevertheless, cross-sectional GWA analyses did not identify differences in genotype between high-intensity donors and multiple categories of first-time donors. This suggests that behaviors, such as iron supplementation, rather than genetics, are primarily responsible for the ability of some high-intensity donors to repeatedly donate blood without experiencing low hemoglobin deferral. As such, there were disparate associations of behaviors between first-time and high-intensity donors. Daily iron supplement use was most strongly associated with mitigation of iron deficiency anemia in high-intensity donors, while at least weekly, but not daily, use showed the strongest association in first-time donors. Cigarette smoking was associated with increased hemoglobin in high-intensity donors, but not in first-time donors. Also of interest, donor age was not strongly associated with hemoglobin or ferritin recovery in any analysis group. However, limited numbers of donors over the age of 70 were enrolled here and further studies are needed to define recovery in elderly donors.
TMPRSS6 A736V was associated with large differences in hemoglobin decline with repeated donation within first-time and frequent first-time females. It was not associated with hemoglobin decline in first-time males but was associated with ferritin decline in first-time females and males, suggesting it influences repletion of iron stores following donation in both sexes. These findings are supported by a study in which none of 50 blood donors was VV, leading the authors to suggest that A736V influences the ability of donors to tolerate repeated blood donation without developing iron deficiency.27 Nevertheless, the frequency of the A736V was similar in first-time and high-intensity groups, and it had no observed association with hemoglobin or ferritin within high-intensity donors in longitudinal models. The variable effects of A736V among first-time and high-intensity donors may explain why it did not impact ferritin in women in a large cohort of donors with a range of donation intensity10 and is consistent with its variable association with hemoglobin and ferritin found in other studies.11,12
There is evidence for higher hemoglobin or ferritin in blood donors with H63D and C282Y, but the findings sometimes vary by sex and number of mutated alleles.8,10,12.Here, we found that H63D was more strongly associated with increased ferritin and hemoglobin in high-intensity donors than C282Y. This was somewhat surprising, since C282Y is more strongly associated with hemochromatosis.28 However, previous studies have found a protective association of H63D, but not C282Y, on iron status in frequent donors.29 For example, H63D is fourfold more prevalent in frequent Black donors than in first-time Black donors.9 And, in a population of high-intensity donors, those with H63D had decreased hepcidin:ferritin ratio compared to those without this mutation.6 These findings suggest that H63D may impact dietary iron absorption in iron deficient individuals. Further studies are needed to confirm this notion and understand the biochemistry underlying the effect.
Blood donors are a robust population for studies of the treatment of iron deficiency, since many are iron deficient, yet they continue to give blood. Previously, we found that 19 mg daily iron is equal to 38 mg for mitigation of iron deficiency in frequent donors,30 suggesting that low dose iron may be effective for treatment of clinical iron deficiency. Others have suggested that iron deficiency may be best treated with every other day dosing.31,32 Comparison of daily versus less than daily, but at least weekly, use of iron supplements found that less than daily supplementation was associated with higher hemoglobin in all groups except for the high-intensity donors, where daily iron was most effective. Thus, lower intensity blood donors may be best treated with less than daily low dose iron. Definitive conclusions cannot be made, however, as the data are limited by the accuracy of self-reported iron use. Regardless of the dosing frequency, essentially all donors will benefit from oral iron supplements.33,34 It is important to emphasize iron is most effective in the 4 to 8 weeks immediately following donation, when its absorption is greatest.35
Cigarette smoking is associated with increased hemoglobin in the general public,36 first-time blood donors,37 and high-intensity blood donors.6 The effect in first-time donors varies from 0.26 to 0.59 g/dL depending on smoking intensity.37 For unclear reasons, an effect of cigarette smoking on hemoglobin was not observed in first-time females or males here. However, it was associated with an increase of approximately 0.5 g/dL in high-intensity donors. Thus, smoking is a behavior that may contribute to the ability to repeatedly donate without low hemoglobin deferral. However, only 10% of high-intensity donors smoked.
In summary, there are a variety of genetic and behavioral factors altering changes in hemoglobin and iron status following repeated blood donation. Study of blood donors is informative in understanding how polymorphisms or mutations in proteins regulating hepcidin production alter iron absorption from the gastrointestinal tract. It appears that underlying genetics of the donor modulate recovery from blood donation in first-time donors, while use of iron supplements is more important than underlying genetics for successful repeated blood donation by high-intensity donors.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), which was supported by NHLBI contracts NHLBI HHSN2682011–00001I, –00002I, –00003I, –00004I, –00005I, –00006I, –00007I, –00008I, and –00009I. The authors would like to express their deep gratitude to the RBC-Omics research staff at the participating blood centers and testing lab for its exceptional performance and contribution to this project.
The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), domestic component, is the responsibility of the following persons:
Hubs:
A.E. Mast and J.L. Gottschall, Versiti, Milwaukee, Wisconsin.
D. J. Triulzi and J.E. Kiss, The Institute for Transfusion Medicine (now Vitalant Northeast Division), Pittsburgh, Pennsylvania.
E. L. Murphy and E.M. St. Lezin, University of California, San Francisco (UCSF), and Laboratory Medicine,
Department of Veterans Affairs Medical Center, San Francisco, California.
E.L. Snyder, Yale University School of Medicine, New Haven, Connecticut R.G. Cable, American Red Cross Blood Services, Farmington, Connecticut.
Data coordinating center:
D.J. Brambilla and M.T. Sullivan, Research Triangle International, Rockville, Maryland.
Central laboratory:
M.P. Busch and P.J. Norris, Blood Systems Research Institute, San Francisco, California.
Publication committee chairman:
R. Y. Dodd, American Red Cross, Holland Laboratory, Rockville, Maryland.
Steering committee chairman:
S. H. Kleinman, University of British Columbia, Victoria, British Columbia, Canada.
National Heart, Lung, and Blood Institute, National Institutes of Health:
S.A. Glynn, K.B. Malkin Bethesda, Maryland.
Footnotes
CONFLICT OF INTEREST
BRS serves on the advisory board of HemaStrat. AEM receives research grant funding from Novo Nordisk. The other authors have declared no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Appendix S1: Supporting Information.
REFERENCES
- 1.Finch CA, Cook JD, Labbe RF, Culala M. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood. 1977;50: 441–447. [PubMed] [Google Scholar]
- 2.Simon TL, Garry PJ, Hooper EM. Iron stores in blood donors. JAMA. 1981;245:2038–2043. [PubMed] [Google Scholar]
- 3.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II donor iron status evaluation (RISE) study. Transfusion. 2011;51:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di AE, Thompson SG, Kaptoge S, et al. Efficiency and safety of varying the frequency of whole blood donation (INTERVAL): a randomised trial of 45 000 donors. Lancet. 2017;390:2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mast AE, Schlumpf KS, Wright DJ, et al. Demographic correlates of low hemoglobin deferral among prospective whole blood donors. Transfusion. 2010;50:1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mast AE, Foster TM, Pinder HL, et al. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion. 2008;48:2197–2204. [DOI] [PubMed] [Google Scholar]
- 7.Spencer BR, Johnson B, Wright DJ, et al. Potential impact on blood availability and donor iron status of changes to donor hemoglobin cutoff and interdonation intervals. Transfusion. 2016;56:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II donor iron status evaluation (RISE) study. Transfusion. 2012;52:702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mast AE, Lee TH, Schlumpf KS, et al. The impact of HFE mutations on haemoglobin and iron status in individuals experiencing repeated iron loss through blood donation*. Br J Haematol. 2012; 156:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorensen E, Rigas AS, Thorner LW, et al. Genetic factors influencing ferritin levels in 14,126 blood donors: Results from the Danish blood donor study. Transfusion. 2016;56:622–627. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Flower R, Hyland C, Saiepour N, Faddy H. Genetic factors associated with iron storage in Australian blood donors. Blood Transfus. 2018;16:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen E, Rigas AS, Didriksen M, et al. Genetic factors influencing hemoglobin levels in 15,567 blood donors: results from the Danish blood donor study. Transfusion. 2019;59:226–231. [DOI] [PubMed] [Google Scholar]
- 13.Nicolas G, Bennoun M, Devaux I, et al. From the cover: Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth E, Ganz T. Regulation of iron metabolism by Hepcidin. Ann Rev Nutr. 2006;26:323–342. [DOI] [PubMed] [Google Scholar]
- 15.Silvestri L, Pagani A, Nai A, de Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers JC, Zhang W, Li Y, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41:1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benyamin B, Ferreira MA, Willemsen G, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesh SK, Zakai NA, van Rooij FJ, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. 2009;41:1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PL, Halloran C, Trevino R, Felitti V, Beutler E. Human transferrin G277S mutation: a risk factor for iron deficiency anaemia. Br J Haematol. 2001;115:329–333. [DOI] [PubMed] [Google Scholar]
- 20.Endres-Dighe SM, Guo Y, Kanias T, et al. Blood, sweat, and tears: red blood cell-omics study objectives, design, and recruitment activities. Transfusion. 2019;59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mast AE, Schlumpf KS, Wright DJ, et al. Hepcidin level predicts hemoglobin concentration in individuals undergoing repeated phlebotomy. Haematologica. 2013;98:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Busch MP, Seielstad M, et al. Development and evaluation of a transfusion medicine genome wide genotyping array. Transfusion. 2019;59:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanias T, Stone M, Page GP, et al. Frequent blood donations alter susceptibility of red blood cells to storage-and stress-induced hemolysis. Transfusion. 2019;59:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali MA, Luxton AW, Walker WH. Serum ferritin concentration and bone marrow iron stores: a prospective study. Can Med Assoc J. 1978;118:945–946. [PMC free article] [PubMed] [Google Scholar]
- 25.Mast AE, Blinder MA, Dietzen DJ. Reticulocyte hemoglobin content. Am J Hematol. 2008;83:307–310. [DOI] [PubMed] [Google Scholar]
- 26.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poggiali E, Andreozzi F, Nava I, Consonni D, Graziadei G, Cappellini MD. The role of TMPRSS6 polymorphisms in iron deficiency anemia partially responsive to oral iron treatment. Am J Hematol. 2015;90:306–309. [DOI] [PubMed] [Google Scholar]
- 28.Gottschalk R, Seidl C, Schilling S, et al. Iron-overload and genotypic expression of HFE mutations H63D/C282Y and transferrin receptor Hin6I and BanI polymorphism in german patients with hereditary haemochromatosis. Eur J Immunogenet. 2000;27:129–134. [DOI] [PubMed] [Google Scholar]
- 29.Konig D, Mattler S, Eichler H, Kluter H, Bugert P. Prevalence of the H63D and C282Y mutations in the HFE gene in 3,015 blood donors from southwestern Germany. Trans Med Hemother. 2003;30:66–70. [Google Scholar]
- 30.Bialkowski W, Kiss JE, Wright DJ, et al. Estimates of total body iron indicate 19 mg and 38 mg oral iron are equivalent for the mitigation of iron deficiency in individuals experiencing Crepeated phlebotomy. Am J Hematol. 2017;92:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126:1981–1989. [DOI] [PubMed] [Google Scholar]
- 32.Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533. [DOI] [PubMed] [Google Scholar]
- 33.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer BR, Guo Y, Cable RG, et al. Iron status and risk factors for iron depletion in a racially/ethnically diverse blood donor population. Transfusion. 2019;59:3146–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cable RG, Brambilla D, Glynn SA, et al. Effect of iron supplementation on iron stores and total body iron after whole blood donation. Transfusion. 2016;56:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordenberg D, Yip R, Binkin NJ. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990;264:1556–1559. [PubMed] [Google Scholar]
- 37.Mast AE, Steele WR, Johnson B, et al. Population-based screening for anemia using first-time blood donors. Am J Hematol. 2012;87:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.