Abstract
Sarcomas are rare tumors with limited treatment options. Although chemotherapy is standard for certain subtypes, overall survival has not improved in several decades. Bupivacaine has been shown to induce apoptosis and prevent cell growth in multiple different types of malignancies but has not been studied in sarcoma. The current study evaluated the effects of bupivacaine on multiple patient-derived sarcoma cells and a commercial sarcoma cell line. Multiple patient-derived sarcoma cell subtypes and a commercial synovial cell sarcoma cell line were exposed to bupivacaine for different durations and at different concentrations. The patient-derived cells included a high-grade conventional osteosarcoma, a high-grade undifferentiated pleomorphic sarcoma of bone, and a high-grade synovial sarcoma. Flow cytometry and an MTT assay were used to evaluate whether a treatment effect was observed. Treatment of all the subtypes of sarcomas in this study with bupivacaine demonstrated a time- and dose-dependent increase in apoptosis and decrease in cell viability. A cell viability assay demonstrated that the IC50 was between 0.04 and 0.05% and that the treatment effect occurred at clinically relevant doses in vitro. Bupivacaine was toxic to both the patient-derived cells and the commercial cell line at doses commonly used in the clinical setting. These findings provide a foundation for further in vivo studies to evaluate whether these effects will translate to the clinical setting. Although further research is necessary, bupivacaine shows promise as not only an adjunct for pain management but as a treatment modality for sarcoma.
Keywords: apoptosis, bupivacaine, osteosarcoma, pleomorphic, synovial sarcoma, sarcoma, anesthetic
Introduction
Sarcomas are malignant tumors of mesenchymal origin, arising from bones, muscles, cartilage, fat, nerves, blood vessels, fibrous tissues, or deep skin tissues. Soft tissue sarcomas (STS)are approximately three to four times as common as bone sarcomas (1). Osteosarcoma (OS) is the most common form of primary malignant bone tumor in adolescents and young adults and is extremely aggressive (2). High-grade OS requires surgery and systemic chemotherapy. The 5-year survival rate is less than 20% for patients with localized resectable primary tumors treated with surgery without chemotherapy (3).Unfortunately, overall survival has not improved significantly over the past several decades as no new effective drug regimen has been developed.
Undifferentiated pleomorphic sarcoma (UPS), previously known as malignant fibrous histiocytoma, is the most common sarcoma appearing in adult life (4). It is most commonly found in the soft tissues with a less frequent occurrence in the bone. Morphologically, these tumors are composed of fibroblasts, myofibroblasts and histiocytes (5). UPS has a high rate of local recurrence and metastasis. It is recommended to treat UPS arising in the bone with a combination of surgery and chemotherapy similar to OS (6-8).
Synovial sarcoma (SS) is the fourth most common type of STS and accounts for 5-10% of all STSs (9). SS occurs predominantly in younger adults with a median age of diagnosis of 35 years (10). Approximately 70% these tumors arise in the extremities, with significantly better long-term survival outcomes than those with non-extremity involvement (11,12). In patients with localized disease, 10-year survival varies from 8 to 88% depending on the tumor size and location (13). Standard treatment for SS is tumor resection and is frequently accompanied by radiotherapy and sometimes chemotherapy.
Local anesthetics (LAs) are widely available medications and relatively inexpensive. LAs are used for various reasons, including adjunctive pain management to decrease opioid use in cancer patients (14,15). They have also been shown to induce apoptosis and arrest cell growth in certain malignancies such as carcinoma of the thyroid and breast (16,17). Recently, we have demonstrated the inhibitory effect of bupivacaine on cartilage-forming tumor cells which was harvested from patients during tumor resection (18). In addition to this, the potential benefit of use of LAs during the surgical resection includes a decrease in the risk of metastasis, cancer recurrence, and improvement of overall survival (19-21). LAs may also indirectly influence the long-term outcomes in cancer patients undergoing surgery by modulation of the neuroendocrine stress response and attenuation of immune responses, which both may play a role in tumor metastasis and recurrence (22,23). Metastasis negatively impacts patient prognosis and significantly reduces survival outcomes. Most metastases from sarcoma develop in the lungs (80%), although bone (9.9%) and liver (4.5%) can also occur (24).
The purpose of this study was to investigate the effects of bupivacaine on various patient-derived sarcoma tumor cells. This study allows us to evaluate the treatment effects of a medication that is currently available, FDA approved, cost effective, and has an established side-effect profile.
Materials and methods
Cell culture and reagents
Different sarcoma types were evaluated in this study including: A high-grade conventional OS obtained from a 24-year-old female with a right distal femur tumor who had undergone neoadjuvant chemotherapy, a high-grade UPS from a 10-year-old male with a right distal femur tumor who had undergone neoadjuvant chemotherapy, and a high-grade SS from a 10-year-old female with a right forearm tumor. The SS was further classified as a monophasic, spindle cell type. There was only limited chemotherapy related tumor necrosis noted in the specimens that were obtained after the patients had undergone chemotherapy.
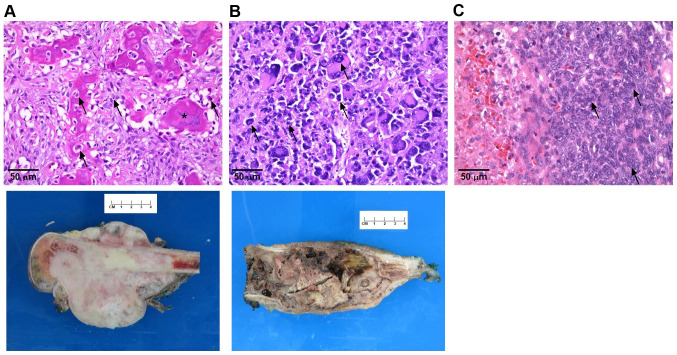
All specimens were harvested from patients during tumor resection. Human tissue collection protocols were reviewed and approved by the Loma Linda University Institutional Review Board (IRB, cat. no. 58238) in accordance to the provisions of the Declaration of Helsinki, and informed, written consent was obtained from all patients or their guardians. Diagnosis of the harvested tumors was confirmed by pathology (Fig. 1).
Figure 1.
Pathological diagnosis of sarcomas. Histopathology slides of the operative specimens shown on the top row showing features of (A) high-grade osteosarcoma from a right distal femur tumor resection. Arrows note abnormal cells with increased chromatin and mitoses whereas the asterisk demonstrates osteoid formation. The bottom image shows the gross pathology with a calcifying tumor growing outside the femur. (B) A high-grade undifferentiated pleomorphic sarcoma from a right distal femur resection where arrows demonstrate pleomorphic cells of different sizes with mitoses noted. The bottom image is a photograph of the gross pathology with replacement of the bone by tumor with an associated soft tissue component. (C) A high-grade SS from a right forearm tumor resection. Arrows demonstrate hyperchromatic nuclei that are tightly packed together consistent with monophasic SS. The diagnosis was confirmed with an SS18-SSX1 translocation. SS, synovial sarcoma.
The tumor cell suspension was prepared by cutting the tumors into small pieces. Collagenase was added and shaken at 37̊C for several hours until the tissues dispersed into single cells. The collagenase solution was then centrifuged, and the precipitate was washed. In addition, a human HTB-93 (SS) cell line was obtained from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) and supplemented with 10% fetal bovine serum, 100 U/ml penicillin G and streptomycin, and 1% nonessential amino acids. Cell cultures were maintained in a humidified incubator containing 5% CO2 at 37̊C. Patient-derived sarcoma cells were used in experiments after up to 4-5 passages in culture, in order to not significantly change their gene expressions. The doubling rate was constant for up to 5 passages and decreased after that. Preservative-free 0.5% bupivacaine (Marcaine, Hospira) was purchased from McKesson Medical-Surgical Inc.
Cell viability assay
Cells were seeded in triplicate at a density of 5,000 cells/well in flat bottom, 96-well plates. After cell confluence reached 80%, the cells were treated with 0.125, 0.25 and 0.5% of bupivacaine (4.33, 8.66 and 17.33 mM, respectively) for 60, 120, 240 and 480 min at 37̊C. These doses replicate the commonly used doses of bupivacaine in the clinical setting. The untreated group was exposed to phosphate-buffered saline (PBS) with a pH of 5.5 in order to control for the pH and microenvironment of the treated cells. After various time durations, the bupivacaine was removed and fresh media (10% DMEM) was added. Viability was assessed 48 h after treatment with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT reagent, Roche Diagnostics) according to the manufacturer's protocol. The plates were read, and the absorbance at 570 nm was measured on a microplate reader (model 3550; Bio-Rad). The results were expressed as a percentage of the untreated control (% of control). Each experiment was done in triplicate. The mean values for each individual tumor sample were averaged to yield a single mean for the separate tumors.
Colony forming assay
UPS, SS and HTB-93 cells were exposed to 0.125, 0.25 and 0.5% bupivacaine for 60 and 480 min. The bupivacaine was then replaced with fresh media and colony counting was performed to determine the colony forming potential of the adherent cells. Colonies were stained with 0.01% crystal violet (Sigma-Aldrich) and counted under microscopy on day 14. Cell clusters were considered colonies and therefore counted. Experiments were done in triplicate.
Microscopic observation of cell morphology
Cells were exposed to 0.125, 0.25 and 0.5% of bupivacaine for 1 h and 0.0156, 0.0312, 0.0625 and 0.125% (0.54, 1.08, 2.16 and 4.33 mM, respectively) for 24 h. Morphological changes in tumor cells were examined by phase-contrast photomicrograph at 24 h after exposure. Apoptotic cells were characterized by cell shrinkage and detachment from the plates.
Analysis of apoptosis
Cell death by apoptosis was analyzed using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis kit (BD Biosciences). Tumor cells were treated with either 0.25% or 0.5% bupivacaine for various durations. Initially, 1x105 cells were seeded in 6-well plates, and when confluence reached 80%, they were exposed to either 0.25% or 0.5% bupivacaine. After 24 h, both floating and attached cells were harvested and then washed. Flow cytometry with FITC-conjugated Annexin-V/propidium iodide (PI) double staining was used to assess the number of apoptotic cells. Samples were analyzed by flow cytometry (MACSQuant; Miltenyi Biotec). Using the FlowJo software (v10; TreeStar), measurements were displayed as 4 quadrants, in which the lower right quadrant represented the apoptosis rate during the early stages, the upper right quadrant indicated advanced apoptosis rate, the upper left quadrant represented dead cells, and the lower left quadrant represented living cells. The apoptotic rate was calculated as early apoptosis (Ann+/PI-) and late apoptosis (Ann+/PI+). This experiment was repeated 3 times.
Statistical analysis
Each assay was performed in triplicate and the results were expressed as a mean ± SEM. Statistical comparisons were performed using analysis of variance followed by the Bonferroni t-test and done with Prism v5.01 software (GraphPad Software). A P-value of <0.05 was considered to be statistically significant.
Results
Bupivacaine reduces the viability of sarcoma cells
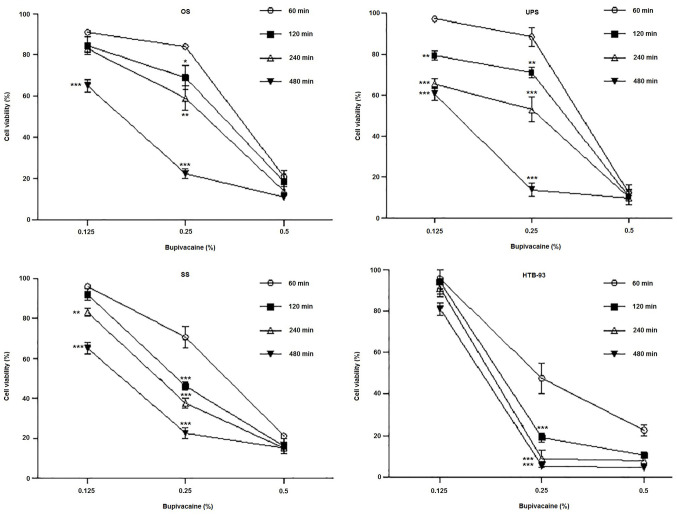
Cell viability data on the cultured tumor cells showed that bupivacaine had dose- and time-dependent cytotoxic effect across all tumor types at clinically relevant concentrations when compared with the controls. Exposure to bupivacaine resulted in a dose- and time-dependent decrease in OS cell viability (Fig. 2). Significantly decreased viability was observed after exposure to 0.5% of bupivacaine when compared to 0.125% at 60, 120, 240 and 480 min, and to 0.25% at 60, 120 and 240 min (P<0.001). As the duration of exposure increased, there was a corresponding decrease in cell viability. The time-dependent effect was more pronounced after treatment with 0.125 and 0.25% of bupivacaine, with a significant decrease occurring after 480 min of exposure compared to 60 min (P<0.001).
Figure 2.
Bupivacaine reduces the viability of sarcoma tumor cells. Patient-derived sarcoma tumor cells were treated with 0.125, 0.25 and 0.5% (4.33, 8.66 and 17.33 mM, respectively) of bupivacaine for various periods of time. MTT assay was performed after 48 h. The bars represent the mean values of three independent experiments with standard error as error bars. Each experiment was performed in triplicate. ***P<0.001, **P<0.01, *P<0.05 vs. control. SS, synovial sarcoma; UPS, undifferentiated pleomorphic sarcoma.
Analysis of cell viability data on UPS tumor cells revealed a similar cytotoxic effect after treatment with different doses of bupivacaine (Fig. 2). Cell death occurred more rapidly after treatment with 0.5% bupivacaine, with a significant reduction in cell viability observed after 60 min. (P<0.001). The difference in viability with 0.125 and 0.25% bupivacaine was significant at 120, 240 and 480 min compared to 60 min (P<0.01, P<0.001 and P<0.001, respectively).
All concentrations of bupivacaine caused a significant decrease in the viability of SS tumor cells (Fig. 2). The difference in cell viability with 0.125 and 0.25% bupivacaine compared to 0.5% bupivacaine was significant at all the time points (P<0.001). Moreover, the difference in cell viability with 0.125% bupivacaine was significant at 240 and 480 min compared to 60 min (P<0.05, P<0.001 respectively) but was not significant at 120 min (P>0.5). The difference in viability was significant at 120, 240 and 480 min compared to 60 min (P<0.001) in cells that were treated with 0.25% bupivacaine. The cytotoxicity of 0.25% bupivacaine on SS tumor cells at 60, 120 and 240 min was more pronounced compared to OS and UPS cells at the same doses (70% viability vs. 84 and 88%, 46% vs. 69 and 71% and 37% vs. 59 and 53%, respectively). The cell viability data on HTB-93 cells revealed a similar cytotoxic effect after treatment with different doses of bupivacaine (Fig. 2).
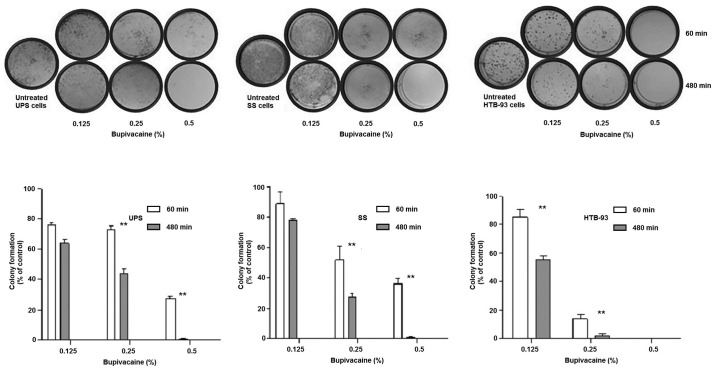
Bupivacaine was also found to disrupt colony forming ability in UPS and SS in a heterologous population as well in HTB-93 cells which are a homologous cell population when compared with the controls. The results showed that the colony formation ability of tumor cells was reduced after exposure to bupivacaine, in a time- and dose-dependent manner (Fig. 3). The UPS cells had a significant decrease in colony formation at both 0.25 and 0.5% bupivacaine between 60 and 480 min (P<0.01) with minimal colonies remaining after 480 min at 0.5%. SS demonstrated similar results. HTB-93 had a significant difference between 60 and 480 min at both 0.125 and 0.25%. No colonies remained after treatment with 0.5% at both 60 and 480 min.
Figure 3.
Bupivacaine reduces the colony formation ability of sarcoma tumor cells. Sarcoma cells (UPS, SS and HTB-93) were treated with 0.125, 0.25 and 0.5% (4.33, 8.66 and 17.33 mM, respectively) of bupivacaine for various time points. Colonies were stained with crystal violet after 14 days, and the number of colonies was counted. The bars represent the mean values of three independent experiments with standard error as error bars. Each experiment was performed in triplicate. **P<0.01 vs. control. SS, synovial sarcoma; UPS, undifferentiated pleomorphic sarcoma.
Bupivacaine induces abnormal morphologic changes in sarcoma cells
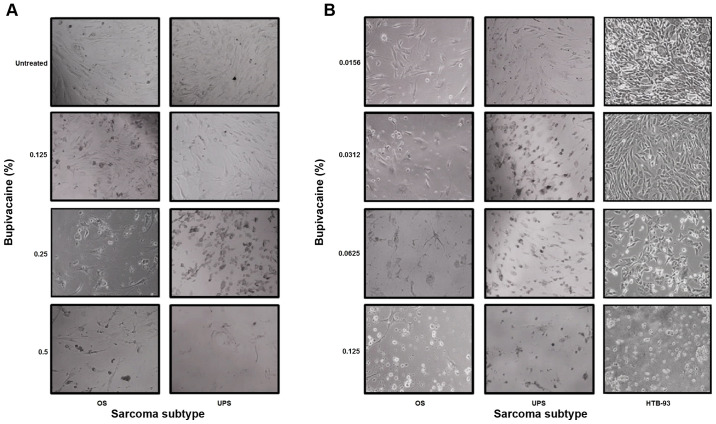
To verify the cytotoxicity of the drug, the morphological changes in OS, UPS and HTB-93 cells were examined by phase-contrast photomicrograph at 24 h after exposure to bupivacaine. Fig. 4A shows that OS and UPS cells exhibited abnormal morphological changes, which are associated with programmed cell death (apoptosis) and characterized by cellular shrinkage, turning round, floating and eventually death when compared to untreated tumor cells after 1 h in a dose-dependent manner. Similar results were observed for SS cells (data not shown). These results occurred at clinically relevant doses of bupivacaine.
Figure 4.
Bupivacaine induces abnormal morphological changes in sarcoma tumor cells. (A) OS and UPS tumor cells were treated with bupivacaine for 1 h at the following concentrations: 0.125, 0.25 and 0.5% (4.33, 8.66 and 17.33 mM, respectively). (B) OS, UPS and HTB-93 cells were treated with bupivacaine at concentrations of 0.0156, 0.0312, 0.0625 and 0.125% (0.54, 1.08, 2.16 and 4.33 mM, respectively) for 24 h. Morphologic changes of the tumor cells were examined by phase-contrast photomicrograph after 24 h. The treated cells underwent cellular shrinkage, turned round, floated and eventually death when compared to untreated tumor cells. SS, synovial sarcoma; UPS, undifferentiated pleomorphic sarcoma; OS, osteosarcoma.
OS, UPS and HTB-93 cells also exhibited abnormal morphological changes, when exposed to 0.0156, 0.0312, 0.0625 and 0.125% (0.54, 1.08, 2.16 and 4.33 mM) of bupivacaine after 24 h in a dose-dependent manner compared to untreated tumor cells (Fig. 4B). The cell viability assay showed that the IC50 was between 0.04 and 0.05% (data not shown). All together it shows that the cytotoxicity of bupivacaine occurs in a time- and dose-dependent manner.
Bupivacaine induces apoptosis in tumor cells
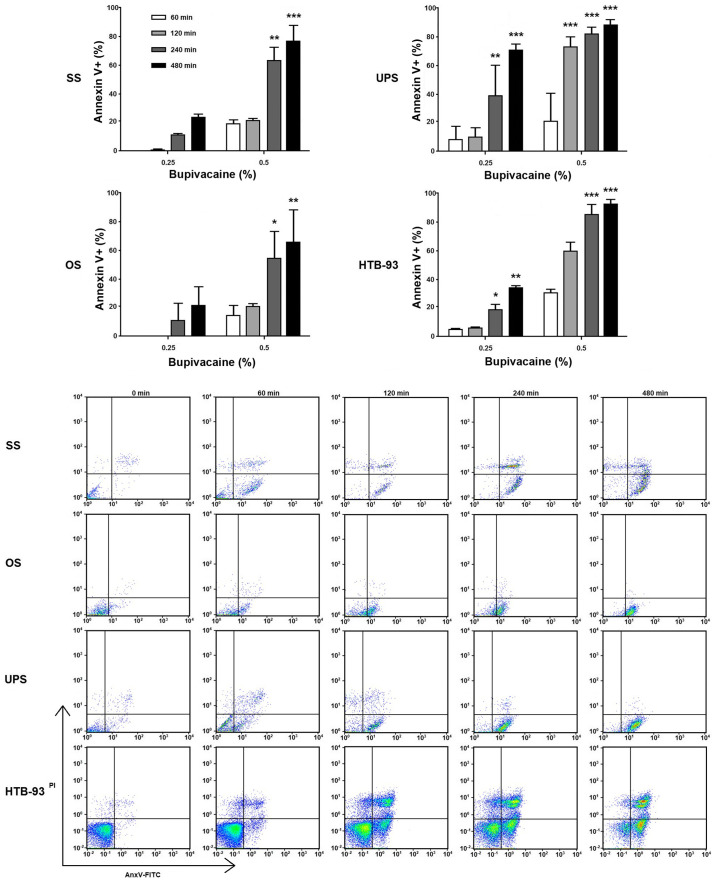
During apoptosis, translocation of phosphatidylserine (PS) from the internal leaflet of the cellular membrane to the external leaflet is a common feature and key step (25). To investigate the mechanism underlying the decreased viability, tumor cells were exposed to bupivacaine at various doses for different time durations. After 24 h, Annexin-V and PI staining was performed. Similar to the results obtained in the cell viability assay, an increase in apoptotic cells occurred across all tumor groups with increased bupivacaine concentration and duration of exposure when compared with the controls (Fig. 5). For the OS cells, an increase in apoptotic cells was seen after 240 and 480 min of exposure to 0.25%. After exposure to 0.5% bupivacaine an increase was seen at 60 and 120 min, with a significant increase occurring after 240 (P<0.01) and 480 min (P<0.001), and a range of 40-70% apoptotic cells present. In the UPS tumor cells, an increase in apoptosis was seen with both 0.25 and 0.5% of bupivacaine. A significant increase occurred after 240 and 480 min of exposure to 0.25% of bupivacaine, with a range of 70-90% apoptotic cells present (P<0.01 and P<0.001, respectively). A significant increase occurred after 120, 240 and 480 min of exposure to 0.5% of bupivacaine, with a range of 40-70% apoptotic cells present (P<0.001; Fig. 5). For the SS tumor cells, an increase in apoptotic cells was seen with both concentrations, with a significant difference occurring after 240 min (P<0.05) and 480 min (P<0.01) of exposure to 0.5% of bupivacaine. The percentage of apoptotic cells remained below 20 with 0.25 and 0.5% of bupivacaine at the remaining time points and did not represent a significant difference (P>0.05). The HTB-93 cells had a significant increase in the number of apoptotic cells at both concentrations. At 0.25%, a difference was noted at 240 (P<0.05) and 480 (P<0.01) min. After exposure to 0.5% bupivacaine, a significant difference was noted at 240 and 480 min (P<0.001).
Figure 5.
Bupivacaine induces apoptosis in patient-derived sarcoma tumor cells. Tumor cells were treated with 0.25 and 0.5% (8.66 and 17.33 mM, respectively) of bupivacaine for various time points (60, 120, 240 and 480 min). Cell death by apoptosis was analyzed using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis kit after 24 h. The pseudocolor plot graphs represent Annexin-V/propidium iodide (PI) double staining to assess the number of apoptotic cells from both the patient-derived tumor cells and HTB-93 after treatment with 0.5% of bupivacaine at the indicated time points. Each experiment was performed in triplicate. ***P<0.001, **P<0.01, *P<0.05 vs. control. SS, synovial sarcoma; UPS, undifferentiated pleomorphic sarcoma; OS, osteosarcoma.
Discussion
At clinically relevant concentrations, in vitro exposure to bupivacaine caused a decrease in cellular viability and an increase in the induction of apoptosis. These effects were seen in all tumor types evaluated in this study. The results from both the viability and apoptosis assays indicate that longer exposure to bupivacaine causes greater toxicity. Additionally, the cytotoxicity of 0.5% of bupivacaine was greater than that of 0.125 and 0.25% of bupivacaine at all time points for each sarcoma subtype. The data also suggests that apoptosis may play a role in the bupivacaine-induced cytotoxicity observed among the different sarcoma tumors.
The concentrations of bupivacaine (4.33, 8.66, and 17.33 mM) which were used in this study may raise the question whether these concentrations are applicable in a clinical context. Other studies regarding the anti-cancer properties of bupivacaine have used 1-5 mM and tumor cells were exposed for longer times (24-72 h) (26,27). However, in our study, cells were exposed to these concentrations for only 1-8 h. In addition, Fig. 4 shows that the inhibitory effect of bupivacaine can be reached at a lower concentration with a longer exposure time.
Bupivacaine is a common medication for pain control that can be used directly at the surgical site, with peripheral nerve blocks, and with epidural/spinal analgesia (28,29). A continuous infusion can also be performed using patient controlled analgesia or a continuous pump (30,31). During the biopsy of sarcoma, seeding of the biopsy tract can occur (32). Infiltrating the biopsy tract with bupivacaine could decrease the risk of contamination during the biopsy. Still, care should be taken not to develop separate planes in the tissue where viable tumor cells could extravasate. After resection, a catheter with a continuous pump of bupivacaine could be used. In theory, this would bathe the resection area in bupivacaine and hopefully decrease the risk of local recurrence.
A limitation of this study is that it was conducted in vitro, and therefore does not account for the dilutional effects of bleeding or the absorption and clearance of bupivacaine that would occur in vivo. The latter issue may be mitigated with the use of a continuous infusion pump, which would continually bathe the resected tumor bed as noted above. As with all in vitro studies, the findings cannot be extrapolated to in vivo situations. However, the controlled nature of in vitro studies allows for a reproducible and quantitative means of assessing cell viability. Using flow cytometry, we were able to show a dose- and time-dependent cytotoxic effect of bupivacaine on all of the sarcoma tumors that were analyzed.
A significant advantage to the use of bupivacaine is that it is cost-effective, commonly used and has an established side-effect profile. This would allow the medication to be used in clinical trials without the need for the development of a new medication. It also provides a method of pain control for the patient and has been used to perform opioid-free anesthesia in patients with breast cancer undergoing modified radical mastectomy (33,34). Similarly, bupivacaine is commonly used for pain control in orthopaedic surgery to decrease opioid consumption after surgery (35). Pain due to cancer is a significant problem and decreasing opioid use in this patient population can have multiple benefits while decreasing the complications associated with opioid use (36-38).
One concern regarding the use of bupivacaine is the risk of systemic toxicity (39). Inappropriate dosing or intravascular injection can result in complications such as cardiac arrhythmia and arrest. In the current study, bupivacaine caused cell death in vitro with an IC50 between 0.04 and 0.05%, whereas 0.25 and 0.5% bupivacaine are the typical concentrations used in the clinical setting. The results are promising that the effects noted in this study could occur in vivo at clinically appropriate doses. Another consideration would be to administer bupivacaine while utilizing isolated limb perfusion (40). Although the patient would still have to be monitored for both local and systemic toxicity, this may provide an option to treat sarcoma locally by directing the medication through the blood supply.
In this study, we used tumor cells which were harvested directly from patients as well as a commercial cell line. The heterogeneity of the resected sarcoma is present in harvested cells in addition to separate genetic factors for each patient. Still, this raises the question of whether the treatment effect only occurred due to these factors. The advantage of using the cell line in this study would be that the treatment effect is reproducible as the individual patient factors are absent. Sarcomas are a heterogeneous group of malignancies with over 100 subtypes described (41). This heterogeneity is further observed in the different subtypes of sarcoma which can lead to resistance to chemotherapy and a worse prognosis (42). The fact that each different subtype of sarcoma in this study was responsive to treatment with bupivacaine is promising. This demonstrates that not only the heterogeneity of the different subtypes as well as the heterogeneity of each individual patient was able to be effectively treated.
Future experiments might compare tumor cell viability on a larger number of sarcoma subtypes and with additional formulations of bupivacaine, including 0.75% and liposomal bupivacaine. Other LAs, such as lidocaine may also be considered. If lidocaine also demonstrated toxicity, other treatments, such as intravenous regional anesthesia with a Bier block could also be considered to deliver the medication through the circulation (43). Determining the mechanism of action of bupivacaine would also be useful as this may provide an opportunity to develop targeted therapies. An increased understanding of the biomarkers involved would be important as the mechanism causing cell death may differ between the sarcoma subtypes. Testing the treatment in vivo in an animal model would also determine whether the in vitro findings are translatable.
In conclusion, these findings have potential clinical relevance in the management of patients with sarcoma. Consideration should be given to using bupivacaine while performing biopsies to possibly eliminate contamination of the biopsy tract, and as an adjuvant treatment after tumor resection. Further studies are warranted to determine if these effects are demonstrated in vivo.
Acknowledgements
The authors would like to thank Mrs. Elisabeth Clarke, Clinical Research Coordinator, Department of Orthopaedic Surgery, Loma Linda University Medical Center, for her support of this research.
Funding
This study was funded by the Loma Linda University Cancer Center, School of Medicine Dean's Office and Department of Orthopaedic Surgery through the Grants to Promote Collaborative and Translational Research program (GCAT 2016; grant no. 681163).
Availability of data and materials
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Authors' contributions
LMZ and SM conceived and designed this study. SM, HRM and SO performed the testing on the cells and analyzed the data. LMZ, SM, WLF, NLW, and TGS acquired the cells used for the data in this study, interpreted the data, and drafted and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Loma Linda University Institutional Review Board (IRB #58238) in accordance to the provisions of the Declaration of Helsinki, and informed, written consent was obtained from all patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nystrom LM, Reimer NB, Reith JD, Dang L, Zlotecki RA, Scarborough MT, Gibbs CP Jr. Multidisciplinary management of soft tissue sarcoma. Sci World J. 2013;2013(852462) doi: 10.1155/2013/852462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475–487. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacci G, Ferrari S, Longhi A, Donati D, Manfrini M, Giacomini S, Briccoli A, Forni C, Galletti S. Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: Local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand. 2003;74:449–454. doi: 10.1080/00016470310017776. [DOI] [PubMed] [Google Scholar]
- 4.Pobirci DD, Bogdan F, Pobirci O, Petcu CA, Roşca E. Study of malignant fibrous histiocytoma: Clinical, statistic and histopatological interrelation. Rom J Morphol Embryol. 2011;52:385–388. [PubMed] [Google Scholar]
- 5.Chen S, Fritchie K, Wei S, Ali N, Curless K, Shen T, Brini AT, Latif F, Sumathi V, Siegal GP, Cheng L. Diagnostic utility of IDH1/2 mutations to distinguish dedifferentiated chondrosarcoma from undifferentiated pleomorphic sarcoma of bone. Hum Pathol. 2017;65:239–246. doi: 10.1016/j.humpath.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Jeon DG, Song WS, Kong CB, Kim JR, Lee SY. MFH of bone and osteosarcoma show similar survival and chemosensitivity. Clin Orthop Relat Res. 2011;469:584–590. doi: 10.1007/s11999-010-1428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natarajan MV, Mohanlal P, Bose JC. Limb salvage surgery complimented by customised mega prostheses for malignant fibrous histiocytomas of bone. J Orthop Surg (Hong Kong) 2007;15:352–356. doi: 10.1177/230949900701500322. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Zhang RM, Zheng YX. En bloc resection and prosthesis implantation to treat malignant fibrous histiocytoma of the humerus. Adv Clin Exp Med. 2017;26:781–787. doi: 10.17219/acem/63744. [DOI] [PubMed] [Google Scholar]
- 9.Rajwanshi A, Srinivas R, Upasana G. Malignant small round cell tumors. J Cytol. 2009;26:1–10. doi: 10.4103/0970-9371.54861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol. 2008;97:314–320. doi: 10.1002/jso.20974. [DOI] [PubMed] [Google Scholar]
- 11.Sultan I, Rodriguez-Galindo C, Saab R, Yasir S, Casanova M, Ferrari A. Comparing children and adults with synovial sarcoma in the surveillance, epidemiology, and end results program, 1983 to 2005: An analysis of 1268 patients. Cancer. 2009;115:3537–3547. doi: 10.1002/cncr.24424. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AH, Hefti F, Speth BM, Jundt G, Guillou L, Exner UG, von Hochstetter AR, Cserhati MD, Fuchs B, Mouhsine E, et al. Synovial sarcomas usually metastasize after >5 years: A multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22:458–467. doi: 10.1093/annonc/mdq394. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: The importance of size and location for survival. Clin Orthop Relat Res. 2004;419:155–161. [PubMed] [Google Scholar]
- 14.Paice JA, Ferrell B. The management of cancer pain. CA Cancer J Clin. 2011;61:157–182. doi: 10.3322/caac.20112. [DOI] [PubMed] [Google Scholar]
- 15.Borgeat A, Aguirre J. Update on local anesthetics. Curr Opin Anaesthesiol. 2010;23:466–471. doi: 10.1097/ACO.0b013e328339eef2. [DOI] [PubMed] [Google Scholar]
- 16.Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9(e89563) doi: 10.1371/journal.pone.0089563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YC, Liu CL, Chen MJ, Hsu YW, Chen SN, Lin CH, Chen CM, Yang FM, Hu MC. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. 2014;118:116–124. doi: 10.1213/ANE.0b013e3182a94479. [DOI] [PubMed] [Google Scholar]
- 18.Chapman GL, Zuckerman LM, Mirshahidi S. The in vitro effects of bupivacaine on cartilage-forming tumor cells. J Am Acad Orthop Surg. 2019;27:e337–e345. doi: 10.5435/JAAOS-D-17-00407. [DOI] [PubMed] [Google Scholar]
- 19.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings KC, Xu F, Cummings LC, Cooper GS. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: A population-based study. Anesthesiology. 2012;116:797–806. doi: 10.1097/ALN.0b013e31824674f6. [DOI] [PubMed] [Google Scholar]
- 21.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 22.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–1250. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 23.Aamri E, Basnawi A. Effects of anesthesia & anesthetic techniques on cellular immunity. J Anesth Crit Care Open Access. 2017;7(00283) [Google Scholar]
- 24.Vlenterie M, Litière S, Rizzo E, Marréaud S, Judson I, Gelderblom H, Le Cesne A, Wardelmann E, Messiou C, Gronchi A, van der Graaf TW. Outcome of chemotherapy in advanced synovial sarcoma patients: Review of 15 clinical trials from the European organisation for research and treatment of cancer soft tissue and bone sarcoma group; setting a new landmark for studies in this entity. Eur J Cancer. 2016;58:62–72. doi: 10.1016/j.ejca.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dan J, Gong X, Li D, Zhu G, Wang L, Li F. Inhibition of gastric cancer by local anesthetic bupivacaine through multiple mechanisms independent of sodium channel blockade. Biomed Pharmacother. 2018;103:823–828. doi: 10.1016/j.biopha.2018.04.106. [DOI] [PubMed] [Google Scholar]
- 27.Xuan W, Zhao H, Hankin J, Chen L, Yao S, Ma D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci Rep. 2016;6(26277) doi: 10.1038/srep26277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vishwanatha S, Kalappa S. Continuous femoral nerve blockade versus epidural analgesia for postoperative pain relief in knee surgeries: A randomized controlled study. Anesth Essays Res. 2017;11:599–605. doi: 10.4103/0259-1162.206852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam KW, Chen SY, Huang TW, Lin CC, Su CM, Li CL, Ho YS, Wang WY, Wu CH. Effect of wound infiltration with ropivacaine or bupivacaine analgesia in breast cancer surgery: A meta-analysis of randomized controlled trials. Int J Surg. 2015;22:79–85. doi: 10.1016/j.ijsu.2015.07.715. [DOI] [PubMed] [Google Scholar]
- 30.Dhanapal B, Sistla SC, Badhe AS, Ali SM, Ravichandran NT, Galidevara I. Effectiveness of continuous wound infusion of local anesthetics after abdominal surgeries. J Surg Res. 2017;212:94–100. doi: 10.1016/j.jss.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Moslemi F, Rasooli S, Baybordi A, Golzari SE. A comparison of patient controlled epidural analgesia with intravenous patient controlled analgesia for postoperative pain management after major gynecologic oncologic surgeries: A randomized controlled clinical trial. Anesth Pain Med. 2015;5(e29540) doi: 10.5812/aapm.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrientos-Ruiz I, Ortiz-Cruz EJ, Serrano-Montilla J, Bernabeu-Taboada D, Pozo-Kreilinger JJ. Are biopsy tracts a concern for seeding and local recurrence in sarcomas? Clin Orthop Relat Res. 2017;475:511–518. doi: 10.1007/s11999-016-5090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathy S, Rath S, Agrawal S, Rao PB, Panda A, Mishra TS, Nayak S. Opioid-Free anesthesia for breast cancer surgery: An observational study. J Anaesthesiol Clin Pharmacol. 2018;34:35–40. doi: 10.4103/joacp.JOACP_143_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathy S, Mandal I, Rao PB, Panda A, Mishra T, Kar M. Opioid-free anesthesia for breast cancer surgery: A comparison of ultrasound guided paravertebral and pectoral nerve blocks. A randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2019;35:475–480. doi: 10.4103/joacp.JOACP_364_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlaifer A, Sharfman ZT, Martin HD, Amar E, Kazum E, Warschawski Y, Paret M, Brill S, Drexler M, Rath E. Preemptive analgesia in hip arthroscopy: A randomized controlled trial of preemptive periacetabular or intra-articular bupivacaine in addition to postoperative intra-articular bupivacaine. Arthroscopy. 2017;33:118–124. doi: 10.1016/j.arthro.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Manchikanti L, Manchikanti KN, Kaye AD, Kaye AM, Hirsch JA. Challenges and concerns of persistent opioid use in cancer patients. Expert Rev Anticancer Ther. 2018;18:705–718. doi: 10.1080/14737140.2018.1474103. [DOI] [PubMed] [Google Scholar]
- 37.Pinkerton R, Hardy JR. Opioid addiction and misuse in adult and adolescent patients with cancer. Intern Med J. 2017;47:632–636. doi: 10.1111/imj.13449. [DOI] [PubMed] [Google Scholar]
- 38.Paice JA. Cancer pain management and the opioid crisis in America: How to preserve hard-earned gains in improving the quality of cancer pain management. Cancer. 2018;124:2491–2497. doi: 10.1002/cncr.31303. [DOI] [PubMed] [Google Scholar]
- 39.Ok SH, Hong JM, Lee SH, Sohn JT. Lipid emulsion for treating local anesthetic systemic toxicity. Int J Med Sci. 2018;15:713–722. doi: 10.7150/ijms.22643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuwirth MG, Song Y, Sinnamon AJ, Fraker DL, Zager JS, Karakousis GC. Isolated limb perfusion and infusion for extremity soft tissue sarcoma: A contemporary systematic review and meta-analysis. Ann Surg Oncol. 2017;24:3803–3810. doi: 10.1245/s10434-017-6109-7. [DOI] [PubMed] [Google Scholar]
- 41.Bleloch JS, Ballim RD, Kimani S, Parkes J, Panieri E, Willmer T, Prince S. Managing Sarcoma: Where have we come from and where are we going? Ther Adv Med Oncol. 2017;9:637–659. doi: 10.1177/1758834017728927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohberger B, Stuendl N, Leithner A, Rinner B, Sauer S, Kashofer K, Liegl-Atzwanger B. Establishment of a novel cellular model for myxofibrosarcoma heterogeneity. Sci Rep. 2017;7(44700) doi: 10.1038/srep44700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gale AL, Liberman SR, Berry S, Zavlin D, Echo A. Fluorescent imaging evaluation of lidocaine distribution following bier block in the upper extremity. Surg Technol Int. 2017;31:31–34. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.