Abstract
Introduction
The success of CD19 chimeric antigen receptor (CAR)-T cell therapy for treatment of CD19 positive malignancies has led to the FDA approval of two CD19 CAR-T cell products, tisagenlecleucel and axicabtagene ciloleucel, and ongoing clinical trials of new products. Cytokine release syndrome (CRS) and neurotoxicity are common toxicities associated with CD19 CAR-T cell therapies.
Areas Covered
This review will discuss CRS and neurotoxicity associated with CD19 CAR-T cell therapies, including clinical presentation, risk factors, pathophysiology and therapeutic or prophylactic interventions.
Expert Opinion
In conjunction with improved understanding of the pathophysiology of CRS and neurotoxicity, we expect that the recent development of consensus guidelines for the evaluation of these toxicities will enhance management of patients undergoing CD19 CAR-T cell therapies.
Keywords: CAR-T cell, CD19 malignancies, chimeric antigen receptor, cytokine release syndrome, immunotherapy, leukemia, lymphoma, neurotoxicity
1. Introduction
Treatment of relapsed/refractory CD19-expressing B cell malignancies with CD19-targeted chimeric antigen receptor (CAR)-modified T (CD19 CAR-T) cells has been remarkably successful [1–11]. Response rates have been impressive and durable in a subset of recipients, leading to the approval in 2017 by the U.S. Food and Drug Administration (FDA) of two CD19 CAR-T cell products, tisagenlecleucel and axicabtagene ciloleucel (axi-cel) [12–14]. Typical approaches to generate autologous CAR-T cells involve in vitro modification of patient-derived T cells to express a CAR, which consists of an extracellular tumor antigen recognition domain linked to spacer, transmembrane, and one or more intracellular signaling domains. The tumor antigen recognition domain is often comprised of a single-chain variable fragment derived from a monoclonal antibody, and the intracellular signaling domains usually consist of sequences from CD3ζ and one or more co-stimulatory molecules (e.g. CD28 or 4-1BB). Modified T cells are generated over 1–4 weeks then infused back into the patient, usually after lymphodepletion chemotherapy.
2. Toxicities of CAR-T cell therapy
When CD19 CAR-T cells recognize normal or malignant CD19+ cells in the patient, signaling through the CAR induces CAR-T cell proliferation, cytokine production, and lysis of target cells [15]. CAR-T cell therapy can be associated with significant toxicities that occur as a result of T cell activation and other toxicities that are independent of T cell activation. Toxicities independent of CAR-T cell activation include the effects of lymphodepleting chemotherapy and infusion reactions, as well as rare events such as anaphylaxis, malignant transformation of engineered T cells or generation of replication-competent retrovirus or lentivirus [16–19]. Toxicities directly associated with CAR-T cell activation include cytokine release syndrome (CRS), neurotoxicity, B cell aplasia and hypogammaglobinemia [20,21]. In this review, we will discuss assessment and management of CRS and neurotoxicity, two key toxicities associated with CD19 CAR-T cell therapy.
3. Cytokine Release Syndrome
3.1. Clinical presentation
CRS has been observed in a majority of patients treated with effective CD19 CAR-T cell therapies (Table 1); however, the incidence, presentation and kinetics of onset and resolution may differ between distinct CAR-T cell products. The onset of CRS is defined by the presence of fever. Typically, this occurs in the first 7 days following CAR-T cell infusion and, depending on the management strategy, symptoms and signs resolve within 1–2 weeks. In addition to fever, signs of CRS can include hypotension, capillary leak, coagulopathy, and/or multi-organ dysfunction or failure, with severity varying between patients and CAR-T cell products [11]. Cardiac arrhythmias are relatively common, but fatal CRS is infrequent [2,11]. CRS often occurs when the patient is neutropenic after lymphodepleting chemotherapy, making the distinction between CRS and infection challenging [20]. Some patients with severe CRS have clinical and laboratory findings that are also found in hemophagocytic lymphohistiocytosis (HLH) or macrophage activation syndrome such as extreme hyperferritinemia, high sIL-2Rα, and high CRP [2,11,22,23]. In patients with late onset or persistent CRS, the distinction between CRS and HLH can be difficult to discern. Poor hematopoietic recovery and secondary failure of hematopoiesis have been observed in some patients [11].
Table 1:
Cytokine release syndrome, neurotoxicity and response rates in selected CD19 CAR-T cell trials.
| Cell product | Malignancy | Study | Population | Costim-ulatory Domain | Lymphodepletion | Cell dose | CRS grading system | CRS Total/ severea | NT grading system | NT Total/ severeb | Best ORR/CR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tisagenlecleucel | ALL | Maude, et al [6] | 75 pediatric/ young adult patients | 4-1BB | Flu/Cy in 71 patients and cytarabine/etoposide in 1 patientc | 0.2–5.4 x 106/kg | PENN grading [26] | 77%/47% | CTCAE 4.03[47] | 40%/13% | N/A/81% d |
| Tisagenlecleucel | NHL | Schuster, et al [7] | 111 adult patients | 4-1BB | Flu 25mg/m2/day and Cy 250mg/m2/day x 3 days or Bendamustine 90mg/m2/day x 2 days |
0.1–6 x 108 | PENN grading | 58%/22% | CTCAE 4.03 | 21%/12% | 52%e/40% |
| Axicabtagene ciloleucel | NHL | Neelapu, et al [2] | 101 adult patients | CD28 | Flu 30mg/m2/day and Cy 500mg/m2/day x 3 days | 2 x 106/kg | Lee, et al [25] | 93%/13% | CTCAE 4.03 | 64%/28% | 82%f/54% |
| Lisocabtagene maraleucel | NHL | Abramson, et al [48,61] | 91 adult patients | 4-1BB | Flu 30mg/m2/day and Cy 300mg/m2/day x 3 days | 0.5–1 x 108 (1:1 ratio of CD4:CD8 CAR-T cells) |
Lee, et al | 35%/1% | CTCAE 4.03 | 19%/12% | 74%e/52% |
ALL - acute lymphoblastic leukemia
CRS - cytokine release syndrome
CTCAE - Common Terminology Criteria for Adverse Events
CR - complete response
Cy - cyclophosphamide
Flu - fludarabine
NT - neurotoxicity
ORR - overall response rate
NHL - non-Hodgkin's lymphoma
severe is grade 3 or higher, grading systems differ
CTCAE 4.03 grading criteria, severe is grade 3 or higher [47]
doses and duration not specified
minimal residual disease negative by flow cytometry with and without complete hematologic recovery
Lugano classification [59]
International Working Group Response Criteria for Malignant Lymphoma [60]
3.2. Definition and grading of CRS
Robust systems to enable grading of CRS associated with CAR-T cell therapy were not widely available when early clinical trials of CD19 CAR-T cell immunotherapy were in progress. Grading according to the Common Terminology Criteria for Adverse Events version 3 (CTCAE v3) was not considered optimal for CRS associated with CD19 CAR-T cell therapy, because this system was directed at grading of CRS associated with infusional therapies (e.g. monoclonal antibodies or blood products) [24]. In 2014, revised CRS grading consensus criteria, were developed to provide a more suitable system for grading of CRS associated with CAR-T cell therapies [25]. Three other CRS grading systems were also developed by different centers in the ensuing years; however, key differences between each system rendered comparisons of CRS between different trials complex [9,26,27]. In June 2018, in an effort to standardize CRS grading, the American Society for Transplantation and Cellular Therapy (ASTCT) developed the ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells (Tables 2–4) [28]. CRS was defined by the ASTCT consensus group as “a supraphysiologic response following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells. Symptoms can be progressive, must include fever at the onset, and may include hypotension, capillary leak (hypoxia) and end organ dysfunction” [28]. While fever is required for the initial diagnosis of CRS, its resolution occurs when all signs and symptoms of CRS are no longer present [28]. In the ASTCT consensus criteria, grade 1 CRS is defined by the presence of fever (temperature ≥38°C) not attributable to another cause (Table 2) [28]. The presence of hypotension and/or hypoxia responsive to fluid boluses or low-flow oxygen by nasal cannula at ≤6L/minute, respectively, escalates the severity to grade 2 CRS. Grade 3 CRS includes patients who have fever with hypotension requiring 1 vasoactive medication (with or without vasopressin) or hypoxia requiring high-flow nasal cannula, facemask, nonrebreather or Venturi mask. Grade 4 CRS includes fever and hypotension requiring multiple vasopressors (excluding vasopressin) or hypoxia requiring positive pressure ventilation.
Table 2:
ASTCT grading for CRS (modified from [28])
| Fever | Hypotension | Hypoxia | ||
|---|---|---|---|---|
| Grade 1 | ≥ 38°Ca | None | AND/ORb | None |
| Grade 2 | ≥ 38°C | Responsive to fluids | Low flow nasal cannula (≤ 6L/minute) or blow-by | |
| Grade 3 | ≥ 38°C | Requiring one vasoactive agent (± vasopressin) | High-flow nasal cannula (>6L/minute), facemask, non-rebreather mask or Venturi mask | |
| Grade 4 | ≥ 38°C | Requiring more than one vasoactive agent (excluding vasopressin) | Positive pressure ventilation, such as CPAP, BIPAP, intubation with mechanical ventilation |
CPAP – continuous positive airway pressure
BiPAP – bilevel positive airway pressure
Fever not attributable to another cause
Grade is based on parameter with highest grade
Table 4:
ASTCT grading for ICANS (modified from [28])
| ICE scorea | CAPD scoreb | Depressed level of consciousness | Seizure | Motor findings | Elevated ICP/cerebral edema | |
|---|---|---|---|---|---|---|
| Grade 1 | 7–9 | 1–8 | Awakens spontaneously | None | None | None |
| Grade 2 | 3–6 | 1–8 | Awakens to voice | None | None | None |
| Grade 3 | 0–2 | ≥ 9 | Awakens only to tactile stimuli | Any clinical seizure focal or generalized that resolves rapidly or nonconvulsive seizures on EEG that resolve without intervention | None | Focal/local edema on imaging |
| Grade 4 | 0 | Unable to perform | Unarousable or requires vigorous or repetitive tactile stimuli to arouse; stupor or coma | Life-threatening prolonged seizure (>5min); or repetitive clinical or electrical seizures without return to baseline in between | Deep focal motor weakness (e.g. hemiparesis or paraparesis) | Decerebrate or decorticate posturing, cranial nerve VI palsy, papilledema, Cushing’s triad, or signs of diffuse cerebral edema on imaging |
ICANS grade is based on the grade of the most severe parameter
patients ≥ 12 years old
patients < 12 years old and patients with developmental delay up to 21 years of age
3.3. Pathophysiology of CRS
The clinical manifestations of CRS range from mild pyrexia through severe multi-organ failure with fatal outcome and are initiated by activation of CAR-T cells, resulting in excessive cytokine production by CAR-T cells and other immune cells, particularly those of the monocyte/macrophage lineage [8,11,22,27,29–31]. Numerous inflammatory mediators and cytokines are elevated during CRS, including C-reactive protein (CRP), ferritin, IFN-γ, IL-2Rα, IL-6, IL-8, IL-10, IL-15, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor receptor p55 (TNFR p55), macrophage inflammatory protein-1B and soluble gp130 [2,6,8,11,22]. Cytokines such as IFN-γ, IL-6, IL-8 and MCP-1 can contribute to endothelial activation, resulting in hemodynamic instability, capillary leak, and consumptive coagulopathy. Angiopoietin (ANG)-2 is produced by activated endothelial cells and can exacerbate endothelial activation and permeability [11,32]. Human and animal data suggest that macrophage activation and cytokine production may contribute to an inflammatory cascade during CRS, which can be mitigated by therapies targeting pathways activated by distinct cytokines, such as IL-6 and potentially IL-1 and GM-CSF [2,11,22,23,33–35]. Pre-clinical studies in murine models suggest that catecholamines may also contribute to the development of CRS by activating a self-amplifying feed forward loop of adrenaline and cytokine production in immune cells [36].
3.4. Risk factors contributing to the development of severe CRS
The ability to identify patients who are at risk of developing severe CRS may enable interventions to mitigate the effects of toxicity. Factors that lead to robust CAR-T cell activation are associated with more severe CRS. For example, higher marrow tumor burden and a high infused CAR-T cell dose were associated with greater CAR-T cell proliferation and cytokine secretion, and a consequently higher risk of severe CRS [9–11,22]. It is not clear whether patients with acute lymphoblastic leukemia (ALL) are independently at higher risk than those with non-Hodgkin’s lymphoma (NHL) or chronic lymphocytic leukemia [11]. The selection of lymphodepletion chemotherapy also impacts CAR-T cell proliferation and the risk of CRS [11]. A low pre-treatment platelet count was independently associated with an increased risk of CRS, suggesting that other factors such as low concentrations of the platelet-derived endothelial stabilizer, ANG-1, might also contribute [11]. Distinct CAR-T cell products may impart a higher incidence of CRS, suggesting that the CAR construct, co-stimulatory domain and manufacturing strategies may contribute to the risk (Table 1). However, this has not been confirmed in randomized studies. Following CAR-T cell infusion, early onset of fever and rapid changes in distinct cytokines may be associated with subsequent development of more severe CRS [11].
3.5. Assessment and management of CRS
After CAR-T cell infusion, patients should be monitored for the development of CRS. This may be performed in the hospital or in the outpatient setting, depending on the CAR-T cell product, age of the patient, comorbidities, and the institutional practice. Due to an earlier median onset of CRS in studies of adults receiving axi-cel compared to tisagenlecleucel, we usually infuse the former in the hospital and the latter in the outpatient setting [2,6,7]. Outpatients are initially evaluated 1 day after CAR-T cell infusion then at least weekly for 4 weeks with a clinical assessment that incorporates evaluation of vital signs, physical examination, and assessment for evidence of neurotoxicity. Laboratory analyses, including a complete blood count, differential, complete metabolic panel, lactate dehydrogenase, uric acid, ferritin, CRP and coagulation panel, are obtained twice weekly for the first 2 weeks then weekly for 4 weeks. Visits become less frequent later after CAR-T cell infusion. Patients are instructed to monitor their temperature at home if they feel unwell and to call the immunotherapy service if their temperature is above 38.3°C. They are admitted to the hospital at the first fever or if indicated by other events.
In most cases, CRS resolves without intervention beyond supportive care. When indicated, the goal of treatment is to minimize development of life threatening and/or irreversible complications without compromising anti-tumor efficacy. Although consensus guidelines are being developed to inform therapy, practices vary between different centers. Despite this, there are general approaches that can be considered in the management of CRS (Table 5). Key facets include supportive care and interventions that abrogate potentially deleterious CAR-T cell effector functions or downstream immune effects. For patients with grade 1 CRS according to the ASTCT consensus criteria, hospital admission for observation and supportive management form the mainstays of care. Patients who receive CD19 CAR-T cell therapy are also at risk for infections in part due to immune dysfunction from underlying malignancy, prior therapies, and lymphodepleting chemotherapy. Signs of CRS overlap with infection; therefore, in the case of fever after CAR-T cell therapy it is pertinent to obtain an infectious work-up and empirically treat with antibiotics. One study found that infections were more frequent in CD19 CAR-T cell patients with ALL, more lines of prior therapy, higher CAR-T cell dose and more severe CRS [20]. The onset of hypotension indicates the development of grade ≥2 CRS, necessitating IV fluid with or without vasopressor support. Tocilizumab is a humanized anti-human IL-6 receptor monoclonal antibody that was initially approved for distinct rheumatologic conditions, and later found to be an effective mitigator of fever and hypotension during CRS associated with CAR-T cells [37,38]. Coincident with the approval of tisagenlecleucel for treatment of pediatric ALL in 2017, tocilizumab was approved by the FDA in 2017 for the treatment of CRS in patients aged ≥2 years [37,39]. Although practices vary, tocilizumab (8mg/kg, maximum 800mg/dose) is often considered for patients with CRS and increasing IV fluid requirements in an effort to prevent the need for ICU admission and vasopressors. Because tocilizumab binds the IL-6 receptor and blocks the action of IL-6 produced by monocytes and macrophages downstream of T cell activation, we routinely commence corticosteroids (e.g. dexamethasone 10 mg bid x 2 doses then review) at the same time as tocilizumab to counter the effects of T cell-derived cytokines that might be associated with endothelial activation. Support for the use of corticosteroids concurrently with tocilizumab has been found in two small studies of approaches to decrease the impact of CRS by early intervention [40,41]. Despite this, many centers successfully treat CRS with tocilizumab without corticosteroids. Most centers administer corticosteroids with cytokine-directed therapy for patients with persistent fever and refractory hypotension. In patients with prolonged fever or failure to respond to anti-cytokine and corticosteroid therapies, consideration should be given to increased infection surveillance and broadening of antimicrobial coverage. Up to 3 additional doses of tocilizumab may be given at approximately 8–24 hour intervals to refractory patients, depending on their clinical status [42]. There is little data to determine whether repeated doses of tocilizumab or the addition of or a change to other cytokine-directed therapies is optimal in this scenario. Other strategies have been considered; for example, hemofiltration was reported to successfully treat severe CRS in a 10 year old boy [43].
Table 5:
General guidelines for initial management of CRS and ICANS in adults.
These general guidelines for initial management of CRS and ICANS (ASTCT grading criteria) for adults are based on our clinical practice at the Fred Hutchinson Cancer Research Center. Practices vary between institutions. Other factors such as co-morbidities, tumor burden, the CAR-T cell product, the cell dose, and kinetics of progression of CRS/ICANS can influence management decisions.
| CRS Grade | Cytokine directed therapy | Corticosteroids | |
|---|---|---|---|
| 1 | Not recommended | Not recommended unless otherwise indicated (e.g. ICANS) | |
| 2 | Clinically stable | Not recommended | Not recommended unless otherwise indicated (e.g. ICANS) |
| Unstable (e.g. requiring increasing fluid support or hypoxic) | Consider tocilizumab 8mg/kg (max 800mg) | If tocilizumab given, recommend dexamethasone 10mg BID x 2 doses then review | |
| 3 | Tocilizumab 8mg/kga | Dexamethasone 10mg BID to QID | |
| 4 | Tocilizumab 8mg/kga Consider other therapies if failure to improvea,b | Dexamethasone 10mg QID; consider methylprednisolone 1g daily x 3 doses if no rapid improvement | |
| ICANS Grade | Cytokine directed therapy | Corticosteroids |
|---|---|---|
| 1 | Not recommended unless otherwise indicated (e.g. CRS) | Not recommended unless otherwise indicated (e.g. CRS) |
| 2 | Not recommended unless otherwise indicated (e.g. CRS) | Consider dexamethasone 10mg BID x 2 doses then review |
| 3 | Not recommended unless otherwise indicated (e.g. CRS) | Dexamethasone 10mg BID to QID |
| 4 | Not recommended unless otherwise indicated (e.g. CRS) Consider other therapies if failure to improvea,b | Dexamethasone 10mg QID; consider methylprednisolone 1g daily x 3 doses |
Note the package insert for axicabtagene ciloleucel states that patients with Grade 2 or higher CRS may receive tocilizumab every 8 hours as needed if not responsive to intravenous fluids or increasing supplemental oxygen; no more than 3 doses in a 24 hour period or a total of 4 doses are recommended [42]. At our institution, we consider using other cytokine directed therapies (e.g. anakinra) if CRS is refractory to 2 doses of tocilizumab.
Other approaches for refractory and life-threatening CRS/ICANS have been considered. However, at this point there is limited data to support efficacy or safety of these approaches (e.g. cytokine-directed therapies, hemofiltration, plasma exchange, IVIG).
In an effort to minimize the severity and incidence of CRS, prophylactic and early intervention approaches are being investigated. Tocilizumab has a half-life of up to 13 days so it is possible that early administration could reduce the severity of CRS [37,44]. Although concerns have been voiced about the potential impact of early intervention on anti-tumor efficacy, in the setting of toxicity due to robust CAR-T cell activation it appears that tocilizumab and/or corticosteroids do not prevent anti-tumor responses [1,2,41]. However, it is not known if their use might impair response in patients with marginal CAR-T cell function or if they are administered prior to CAR-T cell infusion. In a pilot study, compared to patients who received tocilizumab for grade 2–3 CRS (Lee, et al), those who received prophylactic tocilizumab on day 2 following axi-cel infusion had a lower incidence and severity of CRS [25,45]. An ongoing clinical trial using CART-19 T cells, which have a 41BB co-stimulatory domain, is evaluating whether administration of tocilizumab at the onset of the second fever in pediatric ALL patients with high tumor burden (>40% marrow blasts) might decrease the severity of CRS (NCT02906371) [46]. Earlier use of corticosteroids after infusion of axi-cel is also being explored with preliminary data reporting a reduced severity of CRS and in patients who received early corticosteroid intervention at grade 1 CRS (Lee, et al) that had not improved after 3 days of supportive care, compared to those treated for established severe CRS [25,40,47]. Another study utilizing a 4-1BB:zeta CD19 CAR T cell showed lower rates of severe CRS in pediatric ALL patients who received tocilizumab and dexamethasone for fever ≥39°C for 10 hours, persistent hypotension after initial fluid bolus, or hypoxia requiring oxygen supplementation, compared to patients who received tocilizumab with or without steroids for more severe toxicities [41].
4. Neurotoxicity
4.1. Clinical Presentation
Neurotoxicity typically presents after the onset of CRS, and often after its resolution. Infrequently, it can occur in the absence of prior CRS. The median time to onset of neurotoxicity ranges from 4–10 days following CAR-T cell infusion, but the incidence (Table 1), kinetics and clinical findings may differ between those receiving different CAR-T cell products. Most neurotoxicity is mild and self-limited; symptoms can include headache, lethargy, impaired attention, dysgraphia, apraxia, aphasia, agitation, tremor, encephalopathy, and seizures [2,7,11,27,28,32,48,49]. Dysfunction of higher centers, such as dysgraphia and dyscalculia are often early findings that are detected by objective evaluation tools [27]. Speech disorders are characteristic and can progress to global aphasia in more severe cases [32]. Electroencephalography (EEG) done in encephalopathic patients typically shows background slowing suggesting diffuse encephalopathy [32,49,50]. Cerebral hemorrhage and diffuse cerebral edema have been reported as manifestations of severe neurotoxicity, but appear to be uncommon [45,49,51,52]. Most patients with neurotoxicity do not have new imaging changes on CT or MRI [8,32,53]. However, when abnormal imaging findings are present, they may be associated with a higher risk of a poor outcome [53]. Multiple patterns of abnormal MRI imaging of the brain have been reported in patients with severe neurotoxicity, including white matter changes, cytotoxic edema, microhemorrhages, and/or diffuse leptomeningeal enhancement (Figure 1) [32,49].
Figure 1:
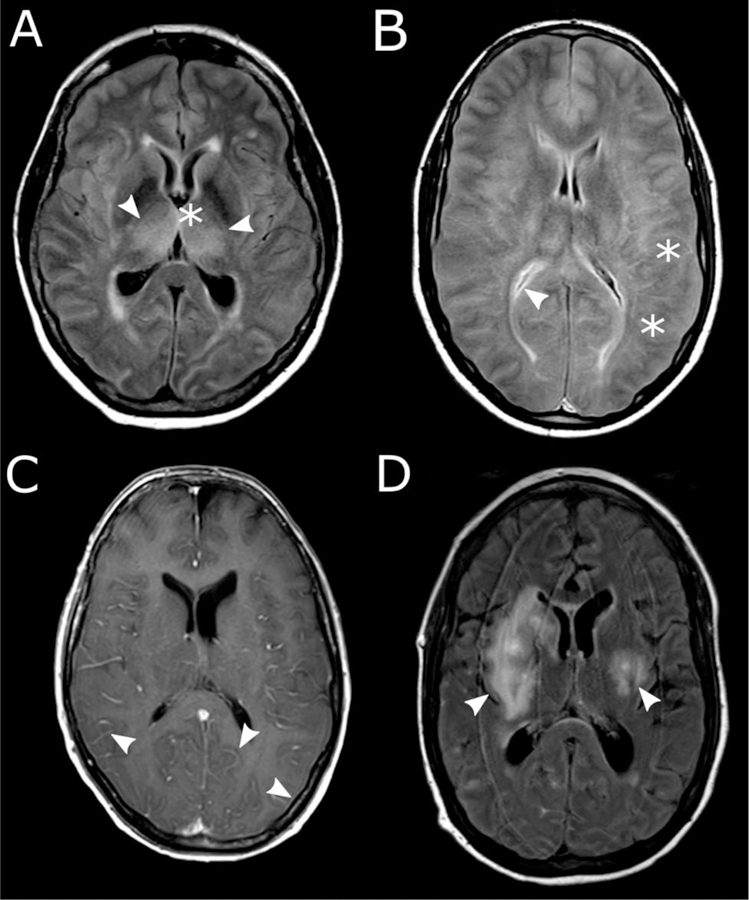
Brain MRI images from patients with severe neurotoxicity following CD19 CAR-T cell therapy. A, Symmetric edema of deep structures (arrowheads). B, Global cerebral edema with blurring of gray-white junction (stars) and slit-like ventricles (arrowhead). C, Diffuse leptomeningeal enhancement (arrowheads). D, focal white matter hyperintensities (arrowheads). (Modified and reprinted from [49])
4.2. Definition and grading of neurotoxicity
Similar to the grading of CRS, robust systems to grade neurotoxicity were not available during early clinical trials; therefore, neurotoxicity was usually graded according to the CTCAE [47]. In the ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells, the terminology Immune effector Cell-Associated Neurotoxicity Syndrome (ICANS) was proposed to encompass neurotoxicity associated with immune effector cell therapies [28]. ICANS is defined as “a disorder characterized by a pathologic process involving the central nervous system following any immune therapy that results in the activation or engagement of endogenous or infused T cells and/or other immune effector cells. Symptoms or signs can be progressive and may include aphasia, altered level of consciousness, impairment of cognitive skills, motor weakness, seizures and cerebral edema [28].” To improve grading of and surveillance for neurotoxicity the CARTOX-10 criteria, a 10-point screening tool, was developed in 2017 [27]. A modified version of CARTOX-10 (the Immune effector Cell-associated Encephalopathy (ICE) score) was incorporated into the ASTCT consensus ICANS grading system to improve the objectivity of neurotoxicity grading. The ICE score comprises assessment of orientation, naming, ability to follow commands, writing, attention, level of consciousness, seizure, motor findings and elevated intracranial pressure (ICP)/cerebral edema [28] (Tables 3 and 4). For children ≤12 years of age the ASTCT consensus group recommends using the Cornell Assessment of Pediatric Delirium (CAPD) in place of the ICE score [28,54]. The CAPD scoring system has been validated for patients up to 21 years of age (with developmental delay) [55].
Table 3:
Immune Effector Cell-Associated Encephalopathy score (modified from [28])
| Parameter | Instruction | Points |
|---|---|---|
| Orientation | Orientation to year, month, city, hospital | 4 |
| Naming | Name 3 objects that are indicated (e.g. point to clock, pen, button) | 3 |
| Following commands | Simple commands (e.g. “Show me 2 fingers”) | 1 |
| Writing | Write a standard sentence (e.g. “Our national bird is the bald eagle”) | 1 |
| Attention | Ability to count backwards from 100 by 10 | 1 |
| Total | 10 |
4.3. Pathophysiology
The severity of neurotoxicity correlates with the severity of CRS, suggesting that the systemic inflammatory response associated with CD19 CAR-T cell activation-induced CRS contributes to neurotoxicity [56]. High serum levels of IL1α, IL2, IL3, IL6, IL10, IP10, IL15, IFNγ, IL2Rα, G-CSF, GM-CSF, MCP-1, granzyme B and ferritin have been associated with neurotoxicity of CTCAE grade 3 or higher [2,32,49]. Studies in adult patients with severe CRS and neurotoxicity demonstrated marked endothelial activation, evidenced by signs of consumptive coagulopathy and higher levels of Von Willebrand factor (VWF) and ANG-2 in patients with CTCAE grade ≥ 4 neurotoxicity compared to patients with grade ≤ 3 neurotoxicity [32,49]. Endothelial activation may lead to disruption of the blood brain barrier allowing infiltration of immune effector cells and inflammatory mediators into the central nervous system (CNS) [1,32,53]. Additionally, incubation of brain pericytes with IFN-γ and TNF-α resulted in increased IL-6 and VEGF secretion, which can in turn activate endothelial cells leading to amplification of the permeability of the blood brain barrier [49]. Autopsies performed on brains of adults who had fatal neurotoxicity showed evidence of endothelial dysfunction and blood brain barrier disruption [49,52]. In contrast to the studies of adults, endothelial activation was not reported in a study of pediatric ALL patients treated with CD19 CAR-T cells [53]. Evaluation of cerebral spinal fluid (CSF) from patients during acute neurotoxicity showed increases in cytokines including IFN-γ, TNF-α, TNFR p55, IL-6, IL-8, IL-10, MCP-1, IP10 and granzyme B [32,49,53]. The concentrations of these cytokines were often higher in serum than in CSF, suggesting that the permeable blood brain barrier did not shield the CSF from high blood cytokine concentrations. However, the finding of higher concentrations of IL-8, IP10 and MCP-1 in CSF compared to serum during acute neurotoxicity suggests that local production within the CNS could also contribute [32]. Studies of animal models of CAR-T cell therapy have not yet reproduced the identical features of neurotoxicity observed in humans, but have provided insights into the pathophysiology. In a non-human primate model using CAR-T cells targeting CD20, neurotoxicity was associated with increases of pro-inflammatory cytokines in the CSF and infiltration of predominantly CD8+ CAR-T and unmodified T cells in the parenchyma and perivascular space [57]. T cell infiltration was also identified in a perivascular distribution in an autopsy of a patient with prior severe neurotoxicity [49]. Studies of immunodeficient mice bearing human hematopoiesis treated with CAR-T cells suggest that monocytes play a key role in the pathogenesis of CRS and neurotoxicity and that and abrogation of monocyte derived IL-1 but not IL-6 might ameliorate neurotoxicity [33,34]. Blockade of GM-CSF with lenzilumab (a GM-CSF neutralizing antibody) decreased manifestations of CRS and neurotoxicity in murine xenograft models without affecting efficacy, suggesting that GM-CSF may be a target in the clinical setting [35].
4.4. Risk factors contributing to the development of severe neurotoxicity
Because the severity of CRS correlates with the severity of neurotoxicity, factors that contribute to severe CRS may place patients at higher risk for development of severe neurotoxicity [6,32,49]. Baseline patient characteristics that may contribute to severe neurotoxicity include higher pre-treatment tumor burden, younger age and pre-existing neurological conditions [32,49]. After CAR-T cell infusion risk factors include earlier and higher fever, higher grade of CRS and higher peak expansion and area under the curve of CAR-T cells [2,32,49]. Different CAR-T cell products and manufacturing may also confer different risks [11,32,51].
4.5. Assessment and management of neurotoxicity
Patients should have baseline neurological assessment prior to infusion of CAR-T cells followed by close monitoring for development of new neurological symptoms and signs after CAR-T cell infusion. Assessment may be performed by history and physical examination as well as by serial monitoring of the ICE score (Table 3) [28]. Because neurotoxicity usually presents following CRS, most patients are already hospitalized before they develop neurotoxicity. The median duration of neurotoxicity signs ranges from 5–14 days [7,32,49].
Strategies to treat neurotoxicity due to CAR-T cells vary between different centers and guidelines have been formulated largely through observational studies; only limited data is available to guide the efficacy and safety of different approaches [25,27,31]. Treatment for neurotoxicity consists of supportive care, with or without corticosteroids and/or cytokine-directed therapies, depending on the presence of active CRS (Table 5) [50,54,56]. An ICE score of 7–9 indicates grade 1 ICANS and patients often have waxing and waning mild confusion, somnolence, headache and intermittent mild speech disturbance [28]. These patients are usually admitted to the hospital for monitoring, including frequent neurologic assessment and laboratory monitoring, especially of sodium levels and platelet counts. Corticosteroids are often not administered for grade 1 ICANS. However, it is not uncommon that the patient may have already received steroids and/or cytokine-directed therapy for antecedent CRS. Seizure prophylaxis is commonly employed, but strategies vary, with different centers starting at the time of infusion, the first fever, following severe CRS or with the onset of new neurological symptoms [27,53,54]. If symptoms deteriorate to grade 2 ICANS (ICE score 3–6, increased disorientation, delayed response in following commands and decreased level of consciousness but still arousable to voice) treatment with corticosteroids is more frequently considered. Dexamethasone has good CNS penetration and is often selected as a first-line corticosteroid therapy for neurotoxicity. It is unclear whether dexamethasone directly improves established neurotoxicity or whether it acts to decrease CAR-T cell effector functions to reduce the severity of subsequent neurotoxicity. Brain imaging, for example by non-contrast head CT or MRI, should be considered to assess for abnormalities, such as edema or hemorrhage. EEG monitoring may be considered to detect seizure activity and/or lumbar puncture may be performed in suitable patients without severe coagulopathy to exclude other causes of the neurological findings. ICU level care is often instituted for grade ≥3 ICANS. Interventions could include continuous EEG monitoring, head imaging, and neuroprotective measures if there is concern for increased ICP. Patients that are not arousable, have repetitive or prolonged seizures lasting more than 5 minutes or signs of increased ICP are classified as having grade 4 ICANS and are usually treated with higher doses of dexamethasone (e.g. 10 mg every 6 hours) or methylprednisolone (e.g. 1 gram every 24 hours) [28].
As neurotoxicity symptoms usually start after CRS, many patients may have previously received tocilizumab for concurrent CRS [2,4,6,7,11,32,49]. However, tocilizumab is not usually recommended for treatment of neurotoxicity in the absence of concurrent CRS, because it does not cross the blood brain barrier and may paradoxically increase the IL-6 concentration in the CNS [32,58]. Whether tocilizumab increases the severity of neurotoxicity may be dependent on the timing of intervention (early versus during established neurotoxicity) and whether it was administered with concurrent corticosteroids. In one study, patients who received prophylactic tocilizumab on day 2 following axi-cel infusion had a higher incidence of Grade ≥ 3 neurotoxicity (CTCAE v4.03) compared to patients who did not receive prophylactic tocilizumab [25,45,47]. In another study with a 4-1BB:zeta CD19 CAR-T cell, when tocilizumab and steroids were administered for fever ≥39°C for 10 hours, persistent hypotension after initial fluid bolus, or hypoxia requiring oxygen supplementation, compared to those with more severe toxicities, there was no difference in the rates of neurotoxicity [41]. One study with axi-cel found a lower incidence of neurotoxicity in patients who received earlier corticosteroids at grade 1 neurotoxicity (CTCAE v4.03) that had not improved after 3 days of supportive care, compared to those who were treated for established severe neurotoxicity [40].
5. Experience in key clinical trials
The FDA has approved two CD19 CAR-T cell products, tisagenlecleucel, which contains a 4-1BB costimulatory domain for relapsed/refractory pediatric ALL and adult NHL and axi-cel, which contains a CD28 costimulatory domain for relapsed/refractory adult NHL [12–14]. Lisocabtagene maraleucel, which also contains a 4-1BB costimulatory domain is in ongoing study and is not currently FDA-approved [48]. CRS and neurotoxicity have been reported in patients treated with each of these products, but the incidences and severities vary (Table 1).
The ELIANA pivotal trial was a phase 2 study of 75 children and young adults treated with 0.2–5.4 x 106 CD19 CAR-T cells/kg for CD19+ relapsed or refractory B-ALL, leading to the first FDA approval of tisagenlecleucel [6,12]. In this study, the complete remission rate (minimal residual disease negative by flow cytometry) was 81%. Seventy-one patients received fludarabine and cyclophosphamide for lymphodepletion and 1 patient received cytarabine and etoposide. CRS occurred in 58 of 75 patients (77%) with a median onset of 3 days and median duration of 8 days [6]. Forty-seven percent of the patients had grade ≥3 CRS according to the PENN criteria [26]. Nineteen (35%) patients required high dose vasopressors. Oxygen supplementation was required in 33 (44%) and mechanical ventilation in 10 (13%) of the patients. There were no deaths related to CRS. Neurotoxicity was graded according to the CTCAE version 4.03 and 40% of the patients developed neurologic events with 13% developing grade ≥3 neurologic events [47]. There were no cases of cerebral edema [6]. The most common neurological events included encephalopathy (11%), confusional state (9%), delirium (9%), tremor (8%), agitation (7%), and somnolence (7%). One patient had a seizure. Tocilizumab was given to patients who demonstrated hemodynamic instability requiring “high-dose” vasopressor support or worsening respiratory distress requiring high-flow oxygen or rapid clinical deterioration [6,26]. Twenty-eight patients (37%) received tocilizumab. The number of patients that received steroids were not reported.
The JULIET pivotal trial was a phase 2 clinical which led to the FDA approval of tisagenlecleucel for adults with relapsed/refractory NHL [7,14]. One hundred and eleven patients received tisagenlecleucel infusions and 93 with relapsed/refractory diffuse large B cell lymphoma were evaluated for efficacy. Best overall response rate (ORR) was 52% with 40% of the patients achieving complete response based on the Lugano classification [59]. Patients received 0.1–6 x 108 cells following lymphodepletion with fludarabine 25mg/m2/day and cyclophosphamide 250mg/m2/day x 3 days or bendamustine 90mg/m2/day x 2 days. The most common adverse event was CRS, which occurred in 58% of the patients. Twenty-two percent had grade ≥3 CRS (PENN grading scale) [26]. The median time to onset of CRS was 3 days with a range of 1–9 days. Median time to CRS resolution was 7 days with a range of 2–30 days. Twenty four percent of patients with CRS required oxygen supplementation, 7% required endotracheal intubation, 6% required high dose vasopressors and 5% required dialysis [7]. There were no deaths attributed to CRS. Neurotoxicity was graded according to the CTAEC 4.03. Twenty-eight percent of the patients had at least one neurological event and 12% had grade ≥3 neurotoxicity. There were no cases of fatal cerebral edema [47]. The median time to onset of neurotoxicity was 6 days (range 1–17) with a median duration of 14 days. Common neurological events included confusional state (9%), encephalopathy (6%), tremor (5%), dysphagia (4%), aphasia (3%), delirium (3%), disturbance in attention (3%) and mental status changes (3%). Two percent of the patients had seizures. Management of CRS and neurotoxicity was similar to management in the ELIANA trial. Fourteen percent received tocilizumab and 10% received tocilizumab and glucocorticoids [6,7]. No patients received more than 2 doses of tocilizumab.
The ZUMA-1 trial was the pivotal phase 2 trial that led to the FDA approval of axi-cel for adults with relapsed or refractory large B cell lymphoma [2,4,13]. The best overall response in 101 treated patients was 82% with a complete response rate of 54% according to the International Working Group Response Criteria for Malignant Lymphoma [60]. Patients received 2 x 106 cells/kg following lymphodepletion with fludarabine 30mg/m2/day and cyclophosphamide 500mg/m2/day x 3 days. Ninety-four of the 101 evaluable patients (93%) had CRS, with 13% having grade ≥3 CRS [2,4]. Grading of CRS was based on criteria developed by Lee, et al [25]. The median time to onset of CRS was 2 days with a range of 1–12 days. The median time to CRS resolution was 8 days. The most common symptoms of grade ≥3 CRS were pyrexia (11%), hypoxia (9%) and hypotension (9%). Vasopressor support was needed in 17% of the patients with grade ≥3 CRS. Deaths of 2 patients were attributed to treatment: one from cardiac arrest and one from HLH. Neurological events were graded according to the CTCAE 4.03 [47]. Sixty-five (64%) patients had one or more neurological events and 28% had grade ≥3 neurotoxicity. The most common events in patients with grade ≥3 neurotoxicity were encephalopathy (21%), confusional state (9%), aphasia (9%) and somnolence (7%) [2]. There were no cases of fatal cerebral edema. Tocilizumab with or without corticosteroids was considered for patients with grade 2–3 CRS and corticosteroids were recommended for grade 4 CRS. Tocilizumab was considered for grade ≥2 neurotoxicity and corticosteroids for grade ≥3 neurotoxicity. Forty-three percent of the patients received tocilizumab and 27% received corticosteroids [2].
TRANSCEND-NHL-001 is a phase 1 clinical trial for lisocabtagene maraleucel, which is not yet FDA approved [48,61]. This trial included 91 adult patients with relapsed or refractory large B-cell NHL. The best ORR according to the Lugano criteria was 74% with a CR of 52%. Lymphodepletion with fludarabine 30mg/m2/day and cyclophosphamide 300mg/m2/day × 3 days was given prior to infusion of 0.5–108 CAR-T cells at a 1:1 CD4:CD8 CAR-T cell ratio. CRS grading was based on the Lee criteria and neurotoxicity grading based on the CTCAE 4.03 [25,47,]. Thirty-five percent of the patients had CRS and only 1% had grade ≥3 CRS. The median time to CRS onset was 5 days and the median time to onset of neurotoxicity was 10 days. Nineteen percent of the patients developed neurotoxicity. Twelve percent developed grade ≥3 neurotoxicity [48]. Tocilizumab was administered to 12% of the patients and steroids to 16% [61].
The different grading criteria used in each study raises considerable challenges in assessing and comparing toxicities between different CAR-T cell products (Table 1). The ASTCT consensus guidelines will be instrumental in providing consistent assessment of toxicities associated with future trials of CAR-T cell therapy [28].
6. Other interventions for CRS and ICANS
As we understand more about the underlying pathophysiology of CRS and ICANS, targeted therapies and preventive measures might be better utilized in severe CRS and/or ICANS. Other drugs targeting cytokines that are elevated in patients with severe CRS and/or neurotoxicity are being explored in pre-clinical and early phase clinical trials. There are case reports of the use of siltuximab, a monoclonal anti-IL-6 antibody that is currently FDA approved to treat multicentric Castleman’s disease, to treat CRS [62,63]. Anakinra is a recombinant IL-1 receptor antagonist that is approved by the FDA for treatment of adults with rheumatoid arthritis and cryopyrin-associated periodic syndromes and has been used to treat macrophage activation syndrome and HLH [64–66]. Lenzilumab is a monoclonal GM-CSF neutralizing antibody that is currently in pre-clinical development for CAR-T cell associated toxicities [35]. Modulating CAR-T cell responsiveness is another attractive strategy to ameliorate toxicities. Dasatinib has been shown to suppress CAR-T cell function in a dose-dependent and reversible manner in a mouse model without additional toxicity [67]. Robust efficacy data to support the routine use of these agents in humans are not available. Ablation of infused CAR-T cells is another strategy that is often considered to treat CAR-T cell dependent toxicities; however, there is no good data to demonstrate that CAR-T cell ablation in humans will mitigate CRS and/or neurotoxicity [68,69].
7. Conclusion
The success of CD19 CAR-T cell therapy in producing durable responses for relapsed/refractory B cell malignancies is exciting. Therapeutic strategies for CRS and neurotoxicity have been based on observational studies without good evidence to guide the optimal approaches, especially for neurotoxicity. The development of the ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells will be critical in evaluating new management algorithms [28].
8. Expert opinion
The advent of CAR-T cell therapy has created a paradigm-shift in the management of patients with refractory CD19-expressing malignancies. Complete and durable remissions have been achieved in a subset of patients with ALL, NHL and CLL. CRS and neurotoxicity are commonly associated with CD19 CAR-T cell therapy. While most cases of CRS and neurotoxicity are self-limiting and only require supportive care, a subset of patients requires additional intervention, up to the level of ICU care. In rare cases CRS and/or neurotoxicity has been fatal. The incidences and severities of CRS and neurotoxicity vary between clinical trials utilizing different CAR-T cell products and between patients with different diseases. The absence of consensus grading systems for CRS and neurotoxicity has hindered comparisons of toxicity between different clinical trials. The development of the ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells will be instrumental moving forward. Supportive care, with or without corticosteroids and/or cytokine-directed therapies currently form the mainstays of care. However, evidence to support distinct strategies in many clinical scenarios is lacking. As more is learned about key risk factors and the underlying pathophysiology of severe CRS and/or neurotoxicity, as well as the determinants of efficacy, more effective measures may be employed to either prevent or treat these toxicities without compromising therapeutic effect.
Article Highlights.
Cytokine release syndrome and neurotoxicity are common toxicities associated with CD19 chimeric antigen receptor-modified T cell therapy.
Cytokine release syndrome and neurotoxicity are often self-limited, but can be life threatening or fatal.
Severe cytokine release syndrome and neurotoxicity are associated with factors that contribute to more robust CAR-T cell activation.
Management of cytokine release syndrome and neurotoxicity involves supportive care with or without corticosteroids and/or cytokine-directed therapies in selected patients.
Standardized management strategies have been difficult to establish, in part due to differences between grading systems employed in clinical trials of different CAR-T cell products.
Acknowledgments
Funding
Funding for this work was provided by the National Institutes of Health 1R01HL132350-03A1 (CJ Turtle), 5R01CA136551-07 (CJ Turtle) and T32CA009351 (CK Chou).
Abbreviations
- ALL
acute lymphoblastic leukemia
- ANG
angiopoietin
- ASTCT
American Society for Transplantation and Cellular Therapy
- Axi-cel
axicabtagene ciloleucel
- BiPAP
bilevel positive airway pressure
- CAPD
Cornell Assessment of Pediatric Delirium
- CAR
chimeric antigen receptor
- CNS
central nervous system
- CPAP
continuous positive airway pressure
- CR
complete response
- CRS
cytokine release syndrome
- CSF
cerebral spinal fluid
- CTCAE
Common Terminology Criteria for Adverse Events
- Cy
cyclophosphamide
- FDA
Food and Drug Administration
- Flu
fludarabine
- HLH
hemophagocytic lymphohistiocytosis
- ICANS
Immune effector Cell-Associated Neurotoxicity Syndrome
- ICE
Immune effector Cell-associated Encephalopathy
- ICP
intracranial pressure
- MCP-1
monocyte chemoattractant protein-1
- NHL
non-Hodgkin’s lymphoma
- NT
neurotoxicity
- ORR
overall response rate
- TNFR p55
tumor necrosis factor receptor p55
- VWF
Von Willebrand factor
Footnotes
Declaration of Interests
CJ Turtle has received research funding from Juno/BMS, Nektar Therapeutics; is on scientific advisory boards for Precision Biosciences, T-CURX, Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, Arsenal Bio; has equity in Precision Biosciences, Eureka Therapeutics, Caribou Bioscience, Myeloid Therapeutics, Arsenal Bio; acted ad hoc on advisory boards (last 12 months) for , Kite/Gilead, , Allogene, PACT Pharma, Nektar Therapeutics, Astra Zeneca; and has patents pending to cover technology related to cellular therapies with Juno/BMS. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017. June 22;129(25):3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017. December 28;377(26):2531–2544.••ZUMA-1 pivotal trial for axicabtagene ciloleucel in adults with relapsed/refractory NHL
- 3.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol Ther 2017. January 4;25(1):285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. The Lancet Oncology 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. The Lancet 2015;385(9967):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018. February 1;378(5):439–448.••ELIANA pivotal trial for tisagenlecleucel in pediatric and young adult patients with relapsed/refractory ALL
- 7.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019. January 3;380(1):45–56.••JULIET pivotal trial for tisagenlecleucel in adults with relapsed/refractory NHL
- 8.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014. February 19;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018. February 1;378(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016. September 7;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017. November 23;130(21):2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary MC, Lu X, Huang Y, et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin Cancer Res 2019. February 15;25(4):1142–1146. [DOI] [PubMed] [Google Scholar]
- 13.Bouchkouj N, Kasamon YL, de Claro RA, et al. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-cell Lymphoma. Clin Cancer Res 2019. March 15;25(6):1702–1708. [DOI] [PubMed] [Google Scholar]
- 14.FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma 2018. [September 27, 2019]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma
- 15.Hay KA, Turtle CJ. Chimeric Antigen Receptor (CAR) T Cells: Lessons Learned from Targeting of CD19 in B-Cell Malignancies. Drugs. 2017. March;77(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcucci KT, Jadlowsky JK, Hwang WT, et al. Retroviral and Lentiviral Safety Analysis of Gene-Modified T Cell Products and Infused HIV and Oncology Patients. Mol Ther 2018. January 3;26(1):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon D, Lapteva N, Gee AP. Absence of Replication-Competent Retrovirus in Vectors, T Cell Products, and Patient Follow-Up Samples. Mol Ther 2018. January 3;26(1):6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornetta K, Duffy L, Turtle CJ, et al. Absence of Replication-Competent Lentivirus in the Clinic: Analysis of Infused T Cell Products. Mol Ther 2018. January 3;26(1):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz CR, Hanley PJ, Liu H, et al. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy 2010. October;12(6):743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018. January 4;131(1):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Romero FA, Taur Y, et al. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin Infect Dis 2018. August 1;67(4):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov 2016. June;6(6):664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashmi H, Bachmeier C, Chavez JC, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol 2019. August 13;187(2)e35–e38. [DOI] [PubMed] [Google Scholar]
- 24. National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006 Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 25.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014. July 10;124(2):188–95.• Overview of CRS and development of consensus criteria for assessment and management of CRS
- 26.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015. September 2;7(303):303ra139.• Development of PENN CRS grading criteria and management algorithm
- 27.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018. January;15(1):47–62.• Review of CAR -T cell toxicities, development of CARTOX guidelines for grading and management of toxicities associated with cellular therapies.
- 28.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2019. April;25(4):625–638.••Reviews prior grading criterias and describes ASTCT consensus grading criteria for CRS and ICANS
- 29.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016. June 30;127(26):3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T cell immunotherapy. Am J Hematol 2019. May;94(S1):S42–S49. [DOI] [PubMed] [Google Scholar]
- 31.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev 2019. March;34:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santomasso BD, Park JH, Salloum D, et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov 2018. August;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018. June;24(6):739–748. [DOI] [PubMed] [Google Scholar]
- 34.Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018. June;24(6):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019. February 14;133(7):697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staedtke V, Bai RY, Kim K, et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018. December;564(7735):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tocilizumab product insert Sept 2016. [September 21, 2019]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125276s107_125472s018lbl.pdf
- 38.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013. April 18;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le RQ, Li L, Yuan W, et al. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018. August;23(8):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topp MS, Meerten Tv, Wermke M, et al. Preliminary results of earlier steroid use with axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory large B-cell lymphoma (R/R LBCL) [Abstract]. Journal of Clinical Oncology 2019;37(15):7558. [Google Scholar]
- 41.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood. 2019. December 12;134(24):2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yescarta product insert 2017. [January 31, 2020]. Available from: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---YESCARTA.pdf
- 43.Liu Y, Chen X, Wang D, et al. Hemofiltration Successfully Eliminates Severe Cytokine Release Syndrome Following CD19 CAR-T-Cell Therapy. J Immunother 2018. Nov-Dec;41(9):406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008. November 15;112(10):3959–64. [DOI] [PubMed] [Google Scholar]
- 45.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory,Aggressive Non-Hodgkin Lymphoma (NHL). Blood. 2017;130:1547. [Google Scholar]
- 46.Oved JH, Barrett DM, Teachey DT. Cellular therapy: Immune-related complications. Immunol Rev 2019. July;290(1):114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. 2010 Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_4.03.xlsx.
- 48.Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol 2018;36(15)7505-7505. [Google Scholar]
- 49.Gust J, Hay KA, Hanafi LA, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017. December;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol 2019. June;37 Suppl 1:48–52. [DOI] [PubMed] [Google Scholar]
- 51.Poh A JCAR015 in ALL: A Root-Cause Investigation. Cancer Discov 2018. January;8(1):4–5. [DOI] [PubMed] [Google Scholar]
- 52.Torre M, Solomon IH, Sutherland CL, et al. Neuropathology of a Case With Fatal CAR T-Cell-Associated Cerebral Edema. J Neuropathol Exp Neurol 2018. July 6. [DOI] [PubMed]
- 53.Gust J, Finney OC, Li D, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol 2019. July;86(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 2019. January;16(1):45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Traube C, Silver G, Kearney J, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med 2014. March;42(3):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity Associated with CD19-Targeted CAR-T Cell Therapies. CNS Drugs. 2018. December;32(12):1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taraseviciute A, Tkachev V, Ponce R, et al. Chimeric Antigen Receptor T Cell-Mediated Neurotoxicity in Nonhuman Primates. Cancer Discov 2018. June;8(6):750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nellan A, McCully CML, Cruz Garcia R, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018. August 9;132(6):662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014. September 20;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007. February 10;25(5):579–86. [DOI] [PubMed] [Google Scholar]
- 61.Locke FL, Haroun F. Advances in Aggressive Lymphoma From the 2017 American Society of Hematology Annual Meeting and Exposition. Clinical Advances in Hematology and Oncology 2018;16(2):20. [PubMed] [Google Scholar]
- 62.Shah B, Huynh V, Sender LS, et al. High Rates of Minimal Residual Disease-Negative (MRD−) Complete Responses (CR) in Adult and Pediatric and Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia (R/R ALL) Treated With KTE-C19 (Anti-CD19 Chimeric Antigen Receptor [CAR] T Cells): Preliminary Results of the ZUMA-3 and ZUMA-4 Trials [Abstract]. Blood. 2016;128:2803. [Google Scholar]
- 63.van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol 2014. August;15(9):966–74. [DOI] [PubMed] [Google Scholar]
- 64.Eloseily EM, Weiser P, Crayne CB, et al. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis. Arthritis Rheumatol 2020. February;72(2):326–334. [DOI] [PubMed] [Google Scholar]
- 65.Anakinra product insert 2012. [September 17, 2019]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/103950-0_Kineret.cfm
- 66.La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019. June 6;133(23):2465–2477. [DOI] [PubMed] [Google Scholar]
- 67.Weber EW, Lynn RC, Sotillo E, et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv 2019. March 12;3(5):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Chang WC, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011. August 4;118(5):1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou X, Dotti G, Krance RA, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015. June 25;125(26):4103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


