Abstract
There is evidence that exposures to polycyclic aromatic hydrocarbons (PAH) and fine particles in air pollution are associated with higher childhood body mass index (BMI). Birth cohort analyses of prenatal exposures to PAH and child BMI Z-scores from age 5–14 years were conducted. African-American and Hispanic children born in the Bronx or Northern Manhattan, New York (1998–2006), whose mothers underwent personal air monitoring for airborne PAH exposure during pregnancy, were followed up with measurements of height and weight at approximate ages 5, 7, 9, 10, 11, 12.5 and 13.5 years. Multivariable generalized estimating equation analyses were used to relate prenatal airborne PAH exposures to child BMI Z-scores through time. The analyses adjusted for many known risk factors for childhood obesity and included interactions terms between age and exposure tertiles and age squared and exposure tertiles. In total, 535 children had at least one height and weight measure during follow-up. The prevalence of obesity was 20.6% at age 5 and increased across follow-ups until age 11 when it was 33.0%. At age 5, BMI Z-scores were significantly greater for children in the third tertile of exposure relative to the first tertile (0.35 Z-score units, 95% CI 0.09, 0.61, p=0.007) and were non-significantly higher for the second tertile of exposure compared to the first tertile (0.25 Zscore units, 95% CI −0.02, 0.52, P=0.075). The trajectories of BMI Z-scores by tertiles of exposure converged as the children aged, such that by age 11 years the estimated mean BMI Z-scores associated with each tertile of exposure were not different. Prenatal exposures to airborne PAH were associated with higher childhood BMI Z-scores at a young age, but growth trajectories converged by age 11 years. Accordingly, highly exposed children spend a greater proportion of their childhood with higher BMI Z-scores.
Keywords: Air Pollution, Polycyclic Aromatic Hydrocarbons, Obesity, Pediatric, Body Mass Index
Introduction
Childhood obesity has multiple causes, including individual-level behavioral risk factors (e.g. high fat diets and sedentary behavior) and more distal societal level risk factors that affect diet and physical activity (e.g. suburbanization, car dependence, and agricultural policies) (Sallis et al. 2006; Sallis and Glanz 2006, 2009). Exposures to airborne polycyclic aromatic hydrocarbons (PAH), a family of chemicals which are created during incomplete combustion processes, and are known human carcinogens and have endocrine disrupting effects, may alter the behavior of adipocytes and promote obesity (Davis et al. 1993; Kummer et al. 2008; McConnell et al. 2016; Santodonato 1997). Mouse studies have found that benzo[a]pyrene (B[a]P) exposed mice had decreased lipolytic response to epinephrine and higher weight gain compared with non-exposed control mice (Irigaray et al. 2006). Another study found that starting at week 3 of life, 10 weeks of exposure to a PM2.5 particle mixture representative of the northeastern U.S. and believed to contain PAH, caused weight gain in C57Bl/6J mice due to increases in subcutaneous and visceral fat mass (Sun et al. 2005; Xu et al. 2010). Additional mouse studies have shown that exposure during pregnancy to a mixture of airborne PAH, similar in composition to that measured in air monitoring studies in the Columbia Center for Children’s Environmental Health (CCCEH) cohort study, resulted in significantly higher weight gain trajectories in wildtype pups from postnatal day 21 to day 60 and higher fat accumulation measured at day 60 (Yan et al. 2014).
PAH are found bound to particulates in air pollution and are in the gas phase of air pollution and fine particulate matter (PM2.5) and near roadway air pollution both contain PAH. Levels may also proxy measures for other airborne chemical pollutants(Klein et al. 2006; Murillo-Tovar et al. 2018; Zielinska et al. 2004). A study in Boston found that prenatal exposure to PM2.5 was associated with measures of larger body size at age 4 years, but the effect differed by child sex and timing of exposure during pregnancy(Chiu et al. 2017). Similarly a study at the Boston Medical Center in Massachusetts found that prenatal exposure to PM2.5 was associated with greater risk of childhood overweight/obesity(Mao et al. 2017). Recent work from the Southern California Children’s Health Study has found that proximity to roadway air pollution exposure is associated with higher BMI Z-scores and weight gain trajectories in children (Jerrett et al. 2014; McConnell et al. 2013; McConnell et al. 2015). Similarly, Project VIVA found that children, whose mothers lived close to major roadways at the time of birth, exhibited reduced fetal growth, more rapid postnatal weight gain and higher total fat mass in mid-childhood(Fleisch et al. 2015; Fleisch et al. 2017). Prior analyzes of the CCCEH birth cohort data found that prenatal exposures to PAH in ambient air pollution measured via personal air monitoring during the third trimester were associated with higher body mass index (BMI) Z-scores at ages 5 and 7, higher risk of obesity at ages 5 and 7, and higher fat mass at age 7 (Rundle et al. 2012). Here, with further follow-up of this cohort, we report on analyses of whether these prenatal PAH measures were associated with trajectories of higher BMI Z-scores from age 5 to 14 years.
Materials and Methods
Study participants were from a longitudinal birth cohort of mothers and their children born between 1998 and 2006 and described elsewhere (Perera et al. 2003; Whyatt et al. 2003). Non-smoking pregnant women were recruited from three prenatal clinics at New York Presbyterian Medical Center and Harlem Hospital. The cohort was restricted to women ages 18–35 years who self-identified as either African American or Dominican and had resided in Northern Manhattan or the South Bronx in New York City for at least one year prior to pregnancy. Bilingual interviewers administered a questionnaire during the third trimester of pregnancy, to collect demographics, history of active and passive smoking, education and income level, receipt of public assistance during pregnancy, maternal height and maternal pre-pregnancy weight. Information on infant sex and birth weight were abstracted from medical records following delivery. As described in detail previously, during the third trimester of pregnancy the women wore a small backpack holding a personal ambient air monitor; the backpack was worn during the daytime for two consecutive days and at night the women placed it the near the bed(Perera et al. 2003; Perera et al. 2009). In ambient air, PAH are found in vapors and aerosols and bound to fine particle matter; the particle fraction less than 2.5 micrometers in diameter (PM2.5) is of primary health importance because fine particles of this size can penetrate deeply into the lung (Pope et al. 2002). The personal air sampling pumps operated continuously at 4 liters per minute, collecting PM2.5 on a pre-cleaned quartz microfiber filter and collecting volatile and semi-volatile vapors and aerosols on a polyurethane foam (PUF) cartridge. As described previously (Perera et al. 2003), particle-bound, volatile and semi-volatile PAH were extracted from the filter and PUF via a Soxhlet Extractor and extracts were assayed by gas chromatography-mass spectroscopy for eight carcinogenic PAH: benz[a]anthracene, chrysene, benzo[b]fluroanthene, benzo[k]fluroanthene, B[a]P, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene and benzo[g,h,i]perylene. Total PAH exposure was expressed as the sum of the measured concentrations for these eight PAH and the measurement qualities of this metric have been described previously(Rundle et al. 2012). Personal air monitoring and measurement of PAH of acceptable quality were completed for 687 women who remained enrolled in the study after the child’s birth.
Weight and height data were collected by trained research workers or physicians at multiple times points during follow-up of the cohort including: follow-ups at ages 5, 7, 9 and 11 years; an ancillary project that collected data from a subset of the cohort children between ages 8.5 and 12 years (P01); and projects that collected data from subsets of the cohort children between ages 9.2 and 14.3 (TAPAS I) years and again between ages 11.3 and 14.5 years (TAPAS III). The P01 project included a meeting between the parent and a pediatric endocrinologist to discuss any concerns the parent might have around the child’s height and weight and the physician referred children who were obese at the P01 follow-up to the Columbia University Irving Medical Center’s Department of Pediatrics and their Program for Overweight Education and Reduction Clinic. The Columbia University IRB provided oversight and approval for the cohort enrollment and follow-up and data analyses.
While all anthropometric data were collected using the same standardized data collection protocol (described below), the number of observations collected between age 5 and 14 varied per child (range: 1 – 7 measures). In total, 535 children provided at least one height and weight measure during follow-up and Table 1 documents the available sample size and key demographic characteristics of the children included at each assessment. Weight at age 5 was measured to the nearest 0.1 kilogram using a Detecto Cardinal 750 digital scale while the child was wearing light clothes and no shoes and from age 7 onwards weight was measured using a Tanita scale (Model BC-418). Height to the nearest 0.1 cm was measured using a SECA wall mounted stadiometer. In some instances, children were not followed up at one of the main cohort follow-ups but were included in pilot studies that occurred around the same age as the missed cohort follow-up. In these instances, anthropometric data from the pilot study were used for the missing cohort follow-up data. This occurred in 4 instances for age 5 year data, 1 instance for the age 7 year data, and 3 instances for age 9 year data. Data on child sex, age at anthropometric measurement, weight and height were used with the Centers for Disease Control and Prevention’s SAS macro to calculate child BMI Z-scores at each follow-up.
Table 1.
Follow-up of Columbia Children’s Center for Environmental Health Participants from age 5 to 14 years
| 5 year follow-up, N=477 | 7 year follow-up, N=490 | 9 year follow-up, N=444 | 8.5–12 year follow-up, N=373 | 11 year follow-up, N=354 | 9–14 year follow-up, N=157 | 11–14.5 follow-up, N=94 | |
|---|---|---|---|---|---|---|---|
| Age in months (SD) | 60.61 (2.21) | 84.8 (2.30) | 108.79 (2.53) | 119.64 (14.00) | 132.91 (4.58) | 150.67 (14.09) | 160.68 (9.65) |
| BMI Z-score (SD) | 0.61 (1.31) | 0.82 (1.13) | 0.96 (1.06) | 1.03 (1.05) | 0.98 (1.08) | 0.82 (1.10) | 0.72 (1.20) |
| Obesity Status | |||||||
| Not Obese | 379 (79.45%) | 370 (75.51%) | 309 (69.59%) | 240 (64.34%) | 237 (66.95%) | 120 (76.43%) | 74 (78.72%) |
| Obese | 98 (20.55%) | 120 (24.49%) | 135 (30.41%) | 133 (35.66%) | 117 (33.05%) | 37 (23.57%) | 20 (21.28%) |
| Race/Ethnicity | |||||||
| Dominican | 287 (60.17%) | 296 (60.41%) | 266 (59.91%) | 219 (58.71%) | 218 (61.58%) | 97 (61.78%) | 59 (62.77%) |
| African American |
190 (39.83%) | 194 (39.59%) | 178 (40.09%) | 154 (41.29%) | 136 (38.42%) | 60 (38.22%) | 35 (37.23%) |
| Sex | |||||||
| Female | 254 (53.25%) | 258 (52.65%) | 236 (53.15%) | 195 (52.28%) | 192 (54.24%) | 80 (50.96%) | 54 (57.45%) |
| Male | 223 (46.75%) | 232 (47.35%) | 208 (46.85%) | 178 (47.72%) | 162 (45.76%) | 77 (49.04%) | 40 (42.55%) |
| Prenatal PAH Tertile | |||||||
| 1st (<1.7483 ng/m3) | 167 (35.01%) | 169 (34.49%) | 153 (34.46%) | 116 (31.1%) | 98 (27.68%) | 42 (26.75%) | 23 (24.47%) |
| 2nd (1.7483 – <3.0721 ng/m3) | 156 (32.7%) | 164 (33.47%) | 158 (35.59%) | 132 (35.39%) | 127 (35.88%) | 56 (35.67%) | 35 (37.23%) |
| 3rd (>=3.0721 ng/m3) | 154 (32.29%) | 157 (32.04%) | 133 (29.95%) | 125 (33.51%) | 129 (36.44%) | 59 (37.58%) | 36 (38.3%) |
Data Analyses:
Trajectory analyses:
Multivariable generalized estimating equation (GEE) regression models with an autoregressive 1 correlation structure were used in SPSS v.24 to analyze the repeated measures of BMI Z-scores through time. The children were categorized into low, middle and high prenatal PAH exposure groups using tertiles of the distribution of measured total PAH concentrations across the entire study population (Rundle et al. 2012). The initial GEE model included indicator variables for middle and high exposure categories, age in months at anthropometric measurement, age*exposure category and age2*exposure categories. To test whether the relationship between age and BMI Z-score was non-linear in models that account for interactions of PAH exposure categories and age, we used a test for nested models. The first used age and age*exposure categories as the predictors and the second used age, age2, age*PAH exposure categories and age2*PAH exposure categories as the predictors; both models accounted for repeated measures using GEE and were fit using geepack in R (Halekoh et al. 2006). A test comparing these models found that the model with an age2 and age2*PAH exposure categories was a significantly better fit to the data than a model with just age and age*PAH exposure categories (P<0.001). BMI Z-score data from ages 5 to 14.5 years were analyzed with age offset by −60 months so that regression coefficients for the middle and high exposure categories could be interpreted as the difference in BMI Z-score compared to low exposure at age 5 years. Mean BMI z-scores were estimated using the GEE EMMEANS command at ages 5, 6, 7, 8, 9, 10, and 11 years from a model that used only age and age2 as the predictors and were plotted in Figure 1.
Figure 1.
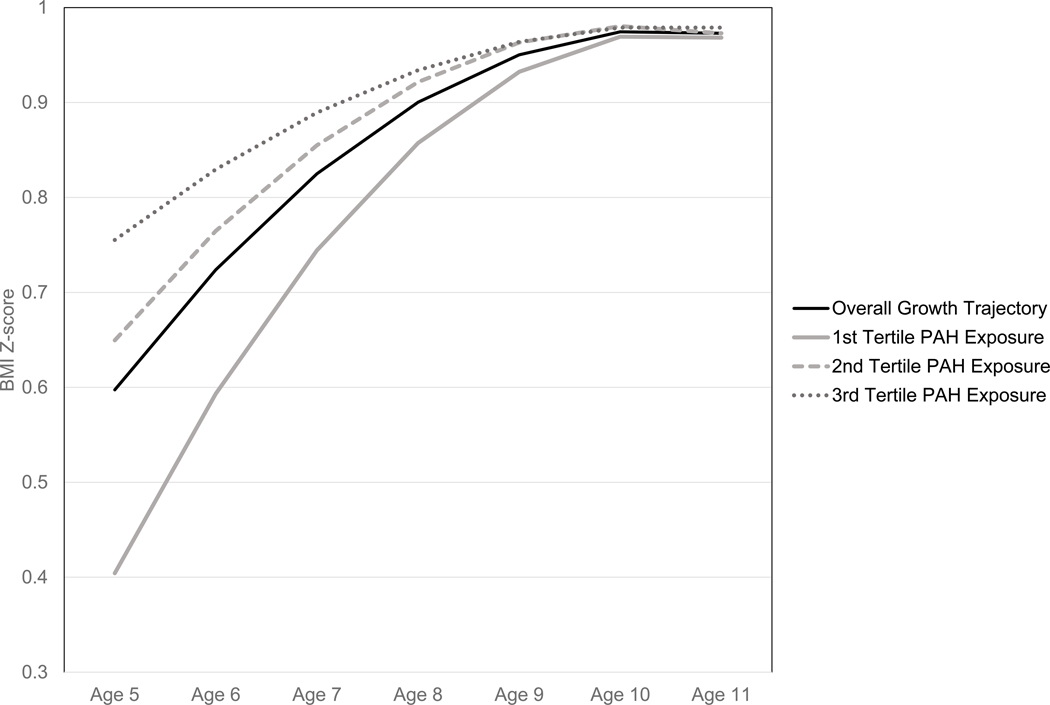
The solid black line represents the estimated mean BMI z-score for the entire study population from a regression model that includes the variables age and age2. The solid grey, dashed grey and dotted grey lines represent the covariate adjusted, estimated BMI Z-scores derived from Model 2 by tertile of prenatal airborne exposures to PAH.
Covariates:
Variables for factors known or hypothesized to predict child BMI Z-scores were added to the initial model, including: material hardship during pregnancy and at child follow-up visits as measured by the Material Hardship Scale(Mayer and Jencks 1989), Institute of Medicine (IOM) gestational weight gain categories, maternal pre-pregnancy obesity, child sex, child race/ethnicity, child birth weight, child age in months at anthropometric measures and child age in months squared at anthropometric measures. Some covariate data were missing and so multiple imputation by chained equations was used to impute missing covariate data for the 535 subjects for whom at least one anthropometric measure was available(Sterne et al. 2009; White et al. 2011). Following the advice of White and colleagues that the number of imputed data sets be at least 100 times the proportion of subjects missing data for a given covariate, 20 data sets were imputed(White et al. 2011). To maximize the amount of available information for imputation of covariates, data from all 687 participants were utilized(Kontopantelis et al. 2017). Imputation used available data regarding the mother during her pregnancy, the child, and the family during follow-up. Maternal variables were: tertiles of prenatal PAH exposure, mother’s height, mother’s weight at beginning of pregnancy, IOM gestational weight gain categories, mother’s marital status, mother’s satisfaction with living conditions during pregnancy, number of material hardships reported by mother during pregnancy, family income, family receipt of public assistance during pregnancy and mother’s educational attainment at pregnancy. The child variables were: sex, birthweight, race/ethnicity, BMI Z-scores during follow-up, child age in months at anthropometric measures, and interaction terms for PAH exposure tertiles and child sex and interaction terms for PAH exposure tertiles and child race/ethnicity. The variables collected about the family during the age 5, 7, 9 and 11 years follow-up were: the family’s receipt of public assistance, mother’s educational attainment, and the number of material hardships reported by mother. Results of the analyses of the imputed datasets were combined using Rubin’s Rules(Rubin 1987). Covariate adjusted estimated marginal mean BMI Z-scores were calculated at ages 5, 6, 7, 8, 9, 10, and 11 years for children in the low, middle and high PAH exposure categories and were plotted in Figure 1.
Analyses also were conducted to assess whether the association between prenatal PAH exposure and BMI Z-scores varied by child sex and by child race/ethnicity. As some of the cell sizes were small, the second and third tertiles of prenatal PAH exposure concentrations were combined into a high exposure category and compared to the first tertile of PAH exposure concentrations. An overall model was fit assessing associations between high PAH exposure and child BMI z-scores and then a model was fit with an interaction term for child sex and likewise a model was fit with an interaction term for child race/ethnicity.
Possible Bias Due to Clinical Referrals:
There was a concern that the clinical referral protocol in the P01 project could have flattened the growth trajectories of obese children, and, that because prenatal PAH exposure was associated with higher BMI Z-scores at age 5 and 7, the referred children might have had higher prenatal exposures. Therefore, sensitivity analyses were conducted in which anthropometric measures collected from children after their P01 follow-up visit(s) were removed from the analyses.
Loss to Follow-Up:
To understand and account for possible bias due to differential loss to follow-up, inverse probability weights for successful follow-up were calculated for each follow-up period(Curtis et al. 2007; Hernan et al. 2004; Rundle et al. 2012). A logistic regression model was fitted predicting successful follow-up at each time point, using data on tertile of prenatal PAH exposure, number of material hardships during pregnancy, maternal education status at pregnancy, family receipt of public assistance during pregnancy, mother’s report of satisfaction with living conditions during pregnancy, maternal marital status during pregnancy, maternal pre-pregnancy obesity, IOM gestational weight gain categories, and the child’s birth weight, sex, and race/ethnicity. Predicted probabilities of successful follow-up were estimated for each child at each follow-up from these models and the predicted probabilities were inverted to create inverse probability weights(Curtis et al. 2007; Hernan et al. 2004; Rundle et al. 2012). Sensitivity analyses were conducted incorporating these weights into the GEE models.
Results
In total, 535 children had at least one BMI Z-score calculated at follow-up. The most common number of BMI Z-score measures per child was 5 (153 children - 29%), 28 children provided 1 measure (5% of the cohort) and 73 children provided 7 measures (14% of the cohort). Table 1 reports the number of participants who provided data at each time point and their anthropometric and demographic characteristics. The prevalence of obesity was 20.5% at age 5, a prevalence that increased across follow-ups until age 11.
Results from GEE analyses of BMI Z-score data for our unadjusted analyses (Model 1) and fully adjusted analyses using multiple imputed data sets (Model 2) are shown in Table 2. Results of sensitivity analyses using complete case analyses (Model 3) and IPW for successful follow-up (Model 4) are also shown in Table 2. The beta coefficients from Model 2 indicate that at age 5, BMI Z-score was significantly greater for participants the third tertile of exposure relative to the first tertile (0.35 Z-score units, 95% CI 0.09, 0.61, p=0.007) and BMI Z-score was non-significantly higher for the second tertile of exposure compared to the first tertile (0.25 Z-score units, 95% CI −0.02, 0.52, P=0.075). The tertile of exposure *age and tertile of exposure*age2 interaction beta coefficients describe how the differences in BMI Z-score across tertiles of exposure change with age. Covariate adjusted, estimated mean BMI Z-score generated from Model 2 for the first, second, and third tertiles of exposure through age 11 show that the trajectories of BMI Z-scores converge as the participants get older (see Figure 1). By age 11 years, the estimated mean BMI Z-scores associated with each tertile of exposure are essentially the same.
Table 2.
Associations between Prenatal Airborne PAH Measures and Child BMI Z-Scores from Age 5 to 14 Years
| Beta Coefficient, (95% CI), P-value | |||||
|---|---|---|---|---|---|
| Predictor Variables | Primary Analyses a | Sensitivity Analyses b | |||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Age in Months | 0.21 (0.15, 0.27) P<0.01 | 0.21 (0.15, 0.27) P<0.01 | 0.20 (0.14, 0.27) P<0.01 | 0.21 (0.14, 0.27) P<0.01 | 0.23 (0.16, 0.30) P<0.01 |
| Age in Months 2 | −0.02 (−0.03, −0.01) P<0.01 | −0.02 (−0.03, −0.01) P<0.01 | −0.02 (−0.03, −0.01) P<0.01 | −0.02 (−0.03, −0.01) P<0.01 | −0.03 (−0.04, −0.01) P<0.001 |
| PAH Exposure 1st Tertile | Ref | Ref | Ref | Ref | Ref |
| PAH Exposure 2nd Tertile | 0.31 (0.03, 0.6) 0.029 | 0.25 (−0.02, 0.52) P=0.075 | 0.23 (−0.05, 0.51) P=0.112 | 0.25 (−0.04, 0.54) P=0.095 | 0.25 (−0.02, 0.52) P=0.07 |
| PAH Exposure 3rd Tertile | 0.38 (0.11, 0.65) P=0.006 | 0.35 (0.09, 0.61) P=0.007 | 0.39 (0.12, 0.65) P=0.005 | 0.33 (0.06, 0.61) P=0.017 | 0.36 (0.11, 0.62) P=0.005 |
| PAH Exposure 1st Tertile * Age in Months | Ref | Ref | Ref | Ref | Ref |
| PAH Exposure 2nd Tertile * Age in Months | −0.08 (−0.17, 0.00) P=0.051 | −0.08 (−0.16, 0.00) P=0.056 | −0.07 (−0.16, 0.02) P=0.124 | −0.08 (−0.17, 0.01) P=0.082 | −0.10 (−0.11, 0.09) P=0.047 |
| PAH Exposure 3rd Tertile * Age in Months | −0.13 (−0.21, −0.05) P=0.002 | −0.13 (−0.2, −0.05) P=0.002 | −0.15 (−0.23, −0.06) P=0.001 | −0.11 (−0.19, −0.03) P=0.01 | −0.16 (−0.25, −0.07) P=0.001 |
| PAH Exposure 1st Tertile * Age in Months 2 | Ref | Ref | Ref | Ref | Ref |
| PAH Exposure 2nd Tertile * Age in Months 2 | 0.01 (0, 0.02) P=0.148 | 0.01 (0, 0.02) P=0.16 | 0.01 (−0.01, 0.02) P=0.326 | 0.01 (−0.01, 0.02) P=0.311 | 0.01 (0.00, 0.03) P=0.11 |
| PAH Exposure 3rd Tertile * Age in Months 2 | 0.01 (0, 0.02) P=0.015 | 0.01 (0, 0.02) P=0.017 | 0.01 (0, 0.02) P=0.013 | 0.01 (0, 0.02) P=0.079 | 0.02 (0.01, 0.04) P=0.008 |
Primary Analyses Model 1: no covariate adjustment. Based on 535 subjects with 2,389 BMI z-score measures. Model 2: covariate adjusted analyses of 20 Multiple Imputed datasets, adjusting for material hardship during pregnancy and at child follow-ups, Institute of Medicine gestational weight gain categories, maternal pregnancy obesity, child sex, child race/ethnicity, and child birth weight. Based on 535 subjects with 2,389 BMI z-score measures.
Secondary Analyses Model 3: covariate adjusted complete case analyses of complete cases adjusting for material hardship during pregnancy and at child follow-ups, Institute of Medicine gestational weight gain categories, maternal pregnancy obesity, child sex, child race/ethnicity, and child birth weight. Based on 477 subjects with 2,108 BMI Z-score measures. Model 4: covariate adjusted analyses of 20 Multiple Imputed datasets with inverse probability weights for complete follow-up, adjusting for material hardship during pregnancy and at child follow-ups, Institute of Medicine gestational weight gain categories, maternal pregnancy obesity, child sex, child race/ethnicity, and child birth weight. Based on 535 subjects with 2,389 BMI z-score measures. Model 5: omitting BMI z-score values collected after a child participated in the P01 follow-up. Covariate adjusted analyses of 20 Multiple Imputed datasets with inverse probability weights for complete follow-up, adjusting for material hardship during pregnancy and at child follow-ups, Institute of Medicine gestational weight gain categories, maternal pregnancy obesity, child sex, child race/ethnicity, and child birth weight. Based on analyses of 535 children and 1,827 BMI Z-score measures.
When the data were re-analyzed using a complete case analysis without imputed covariate data, the results were not meaningfully different from those reported for Model 2. GEE analyses that used inverse probability weights to account for incomplete follow-up at each time point produced results that were not meaningfully different from those reported for Model 2 suggesting that loss to follow-up did not bias the analyses. Results of analyses that omitted BMI Z-score values collected after a child participated in the P01 follow-up were not meaningfully different from those reported for Model 2. The consistency of the two sets of analyses suggests that the clinical referrals based on height and weight data collected at the P01 follow-up did not bias the primary analyses presented from Model 2.
Table 3 presents analyses stratified by sex then by race/ethnicity with p-values for the interaction terms between sex and high PAH exposure. In these models, there is only modest power to observe statistically significant differences in the associations between exposure and BMI Z-score by child sex or by race/ethnicity, as some tests involve a 4-way interaction term (e.g. High exposure * age * age * sex). However, the beta coefficients relating prenatal PAH exposure to BMI Z-score do not differ by sex or by race/ethnicity.
Table 3.
Associationsa between High Prenatal Airborne PAH Exposure and Child BMI Z-Scores from Age 5 to 14 Years by Child Sex and by Child Race/Ethnicity
| Beta Coefficient, (95% CI), P-value | |||||||
|---|---|---|---|---|---|---|---|
| Overall | Stratified by Sex | Stratified by Race/Ethnicity | |||||
| Females | Males | P for Interaction | Dominican | African American | P for interaction | ||
| Age in months | 0.21 (0.15, 0.27) <0.001 | 0.19 (0.11, 0.28) <0.001 | 0.22 (0.13, 0.31) <0.001 | 0.66 | 0.24 (0.16, 0.32) <0.001 | 0.16 (0.08, 0.24) <0.001 | 0.21 |
| Age in months2 | −0.02 (−0.03, − 0.01) <0.001 | −0.02 (−0.03, − 0.01) <0.001 | −0.02 (−0.03, − 0.01) <0.001 | 0.83 | −0.03 (−0.04, − 0.02) <0.001 | −0.01 (−0.02, 0.00) 0.04 | 0.02 |
| High PAH Exposure | 0.30 (0.07, 0.53) 0.01 | 0.22 (−0.10, 0.54) 0.18 | 0.38 (0.06, 0.70) 0.02 | 0.18 | 0.40 (0.10, 0.71) 0.01 | 0.14 (−0.19, 0.47) 0.40 | 0.25 |
| Age*High PAH Exposure | −0.1 (−0.17, − 0.03) <0.001 | −0.11 (−0.21, − 0.01) 0.03 | −0.09 (−0.20, 0.01) 0.09 | 0.78 | −0.14 (−0.24, − 0.05) <0.001 | −0.04 (−0.14, 0.06) 0.38 | 0.17 |
| Age2*Hig h PAH Exposure | 0.01 (0.00, 0.02) 0.03 | 0.01 (0.00, 0.02) 0.03 | 0.01 (−0.01, 0.02) 0.40 | 0.43 | 0.01 (0.00, 0.03) 0.01 | 0.00 (−0.01, 0.01) 0.79 | 0.11 |
Covariate adjusted analyses of 20 Multiple Imputed datasets, adjusting for material hardship during pregnancy and at child follow-ups, Institute of Medicine gestational weight gain categories, maternal pregnancy obesity, child sex, child race/ethnicity, and child birth weight.
Discussion
As reported previously, this cohort of children experienced a high prevalence of obesity by age 5 and a higher BMI Z-score at age 5 was associated with increased prenatal exposures to airborne PAH(Rundle et al. 2012). Now we report that with longitudinal follow-up, the trajectories of BMI Z-score by age across prenatal exposure categories converge such that there is no material difference in BMI Z-score by exposure tertile at age 11. Given that this cohort has a high prevalence of overweight and obesity, the primary effect of exposure appears to be that children with higher prenatal exposures to airborne PAH enter the overweight or obese state at a younger age and spend a larger proportion of their childhood with a higher BMI.
After the first year of life, BMI generally decreases as babies and toddlers grow quickly in length; however, at around age 5 to 6 years there is a period of growth known as the “adiposity rebound” during which BMI starts to increase(Koyama et al. 2014; Whitaker et al. 1998). There is substantial inter-individual variation in the timing of the BMI nadir and subsequent rebound(Koyama et al. 2014; Whitaker et al. 1998). The higher BMI Z-scores associated with higher prenatal exposures observed at age 5 years may represent an earlier BMI nadir and earlier start to the adiposity rebound associated with prenatal exposures to PAH. Early adiposity rebound is associated with future obesity and metabolic consequences of higher triglycerides, atherogenic index, apolipoprotein B, and blood pressure and lower high-density lipoprotein cholesterol at 12 years of age(Koyama et al. 2014). Therefore, a consequence for children who spend a larger proportion of their childhood at a higher BMI Z-score is the likelihood of increased susceptibility to future adverse metabolic effects of obesity. Sen et al. reported a twofold increase of metabolic syndrome prevalence per one-point increase in BMI Z-score in obese children(Sen et al. 2008). Moreover, childhood obesity continues into adulthood (Dietz 1998; Serdula et al. 1993) and is associated with increased susceptibility to hypertension, dyslipidemia, and glucose intolerance. Obesity is also a major contributor to the insulin resistant syndrome which often precedes type 2 diabetes. The higher prevalence of obesity in minority and lower socio-economic populations, such as those who participated in the CCCEH, may contribute to excess disease and mortality rates among these groups.(Hruby and Hu 2015; Psaltopoulou et al. 2017)
Children who were found to be obese at the P01 follow-up, which occurred between the ages of 8.5 and 12 years, were referred to the Department of Pediatrics and their weight loss program. Because high prenatal PAH were associated with higher BMI Z-score at ages 5 and 7, there was concern that the obese children referred to the clinic would also be more highly exposed, which might cause bias in trajectory analyses that included anthropometric data collected after the referral. While data are not readily available on which participants completed their referrals and whether the referrals led to weight loss, our analyses (Model 5) suggest that these referrals did not bias our primary analyses from Model 2. It is possible that few families acted on the referral or that the referrals did not result in weight loss.
PAH also act as a class of pollutants that can have hormone-like activity as measured via in vitro and in vivo test systems(Davis et al. 1993; Kummer et al. 2008; Santodonato 1997). Hydroxy-PAHs, in particular, are structurally similar to estrogen and have been shown to have estrogenic activity in T47D breast adenoma cell lines(Wenger et al. 2009). Likewise, PAH-containing particulate air pollution has been shown to be estrogenic in the T47D cell line, BG1Luc4E2 ovarian carcinoma cell line, and in yeast (Klein et al. 2006; Wang et al. 2003; Wang et al. 2005; Wenger et al. 2009). Data are not available for this cohort on pubertal timing or Tanner staging at the study follow-ups so the analyses cannot be adjusted for or stratified by pubertal stage. However, an effect of PAH exposures on growth that reflects an endocrine disrupting mechanism may suggest that associations between exposure and BMI Z-score trajectories would differ by child sex (Chiu et al. 2017; Hoepner et al. 2016; Maresca et al. 2016; Teitelbaum et al. 2012). However, there were no prominent differences in the relationship between PAH exposure and BMI Z-score by sex in the data.
The major strengths of this study include personal air monitoring for prenatal exposures to PAHs, the availability of repeated measures of height and weight at multiple ages, and the availability of data on many important covariates. We previously documented the measurement characteristics of our personal air monitoring data and showed that neighborhood-level socioeconomic covariates and sources of PAH pollution do not confound the associations between prenatal PAH exposures and BMI Z-scores at age 5 and 7(Rundle et al. 2012). Our data analysis plan addressed two common threats to validity for cohort studies; missing covariate data and loss to follow-up. The sensitivity analyses using complete case-analyses and inverse probability weights for successful follow-up at each round of follow-up, suggest that our results are robust to possible bias due to missing covariate data and missing follow-up data. The sensitivity analyses for loss to follow-up are consistent with our prior analyses of BMI in this cohort (Hoepner et al. 2016; Maresca et al. 2016; Rundle et al. 2012).
In conclusion, in this cohort of African American and Dominican children born in New York City, prenatal exposures to PAH were associated with higher BMI Z-score at age 5. The data suggest that prenatal measures of PAH in ambient air are associated with an earlier adiposity rebound in young children. Although the trajectories of BMI Z-scores as a function of PAH exposure converged by age 11, the fact that children of the more highly exposed mothers had higher BMI Z-scores at a young age and thus spent more of their childhood in a heavier state, is of concern(Koyama et al. 2014).
Highlights.
Obesity was common in this cohort of African American and Hispanic children.
Prenatal exposure to PAH are associated with higher age 5 body mass index z-scores.
Trajectories of BMI Z-scores by PAH exposure converge by age 11 years.
Acknowledgements:
Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA): NIEHS/EPA P01ES09600/ RD82702701, NIEHS/EPA P01ES09600/RD832141, NIEHS/EPA P01ES09600/RD834509, NIEHS/EPA P50ES09600/RD83615401, NIEHS R01ES014393, NIEHS RC2ES018784, NIEHS R01ES13163, and NIEHS R01ES08977, the New York Community Trust, Trustees of the Blanchette Hooker Rockefeller Fund, and the John and Wendy Neu Foundation.
Footnotes
The Authors have no competing financial interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew G. Rundle, Columbia University Mailman School of Public Health, New York, United States
Dympna Gallagher, Columbia University Vagelos College of Physicians & Surgeons, New York, United States.
Julie B. Herbstman, Columbia University Mailman School of Public Health, New York, United States
Jeff Goldsmith, Columbia University Mailman School of Public Health, New York, United States.
Darrell Holmes, Columbia University Mailman School of Public Health, New York, United States.
Abeer Hassoun, Columbia University Vagelos College of Physicians & Surgeons, New York, United States.
Sharon Oberfield, Columbia University Vagelos College of Physicians & Surgeons, New York, United States.
Rachel L. Miller, Columbia University Vagelos College of Physicians & Surgeons, New York, United States
Howard Andrews, University Mailman School of Public Health, New York, United States.
Elizabeth M Widen, University of Texas Austin, Austin, TX, United States.
Lori A. Hoepner, SUNY Downstate Medical Center, School of Public Health, Brooklyn, United States
Frederica Perera, Columbia University Mailman School of Public Health, New York, United States.
References
- Chiu YM, Hsu HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. 2017. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ Res 158:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. 2007. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care 45:S103–107. [DOI] [PubMed] [Google Scholar]
- Davis DL, Bradlow HL, Wolff M, Woodruff T, Hoel DG, Anton-Culver H. 1993. Medical hypothesis: Xenoestrogens as preventable causes of breast cancer. Environ Health Perspect 101:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. 1998. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics 101:518–525. [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. 2015. Prenatal exposure to traffic pollution: Associations with reduced fetal growth and rapid infant weight gain. Epidemiology 26:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, et al. 2017. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes 12:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halekoh U, Hojsgaard S, Yan J. 2006. The r package geepack for generalized estimating equations. Journal of Statistical Software 15:1–11. [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15:615–625. [DOI] [PubMed] [Google Scholar]
- Hoepner LA, Whyatt RM, Widen EM, Hassoun A, Oberfield SE, Mueller NT, et al. 2016. Bisphenol a and adiposity in an inner-city birth cohort. Environ Health Perspect 124:1644–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby A, Hu FB. 2015. The epidemiology of obesity: A big picture. Pharmacoeconomics 33:673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigaray P, Ogier V, Jacquenet S, Notet V, Sibille P, Mejean L, et al. 2006. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice. A novel molecular mechanism of toxicity for a common food pollutant. FEBS J 273:1362–1372. [DOI] [PubMed] [Google Scholar]
- Jerrett M, McConnell R, Wolch J, Chang R, Lam C, Dunton G, et al. 2014. Traffic-related air pollution and obesity formation in children: A longitudinal, multilevel analysis. Environmental health : a global access science source 13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GP, Hodge EM, Diamond ML, Yip A, Dann T, Stern G, et al. 2006. Gas-phase ambient air contaminants exhibit significant dioxin-like and estrogen-like activity in vitro. Environ Health Perspect 114:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopantelis E, White IR, Sperrin M, Buchan I. 2017. Outcome-sensitive multiple imputation: A simulation study. BMC medical research methodology 17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi T, Arisaka O. 2014. Adiposity rebound and the development of metabolic syndrome. Pediatrics 133:e114–119. [DOI] [PubMed] [Google Scholar]
- Kummer V, Maskova J, Zraly Z, Neca J, Simeckova P, Vondracek J, et al. 2008. Estrogenic activity of environmental polycyclic aromatic hydrocarbons in uterus of immature wistar rats. Toxicol Lett 180:212–221. [DOI] [PubMed] [Google Scholar]
- Mao G, Nachman RM, Sun Q, Zhang X, Koehler K, Chen Z, et al. 2017. Individual and joint effects of early-life ambient exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ Health Perspect 125:067005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca MM, Hoepner LA, Hassoun A, Oberfield SE, Mooney SJ, Calafat AM, et al. 2016. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ Health Perspect 124:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S, Jencks C. 1989. Poverty and the distribution of material hardship. The Journal of Human Resources 24:88–114. [Google Scholar]
- McConnell R, Shen E, Gilliland F, Jerrett M, Wolch J, Chang C, et al. Obesogenic effects of exposure to tobacco smoke and air pollution in children. In: Proceedings of the International Society for Environmental Epidemiology, 2013. Basal, Switzerland. [Google Scholar]
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, et al. 2015. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: The southern california children’s health study. Environ Health Perspect 123:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Gilliland FD, Goran M, Allayee H, Hricko A, Mittelman S. 2016. Does near-roadway air pollution contribute to childhood obesity? Pediatr Obes 11:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Tovar M, Barradas-Gimate A, Arias-Montoya M, Saldarriaga-Norena H. 2018. Polycyclic aromatic hydrocarbons (pahs) associated with pm2.5 in guadalajara, mexico: Environmental levels, health risks and possible sources. Environments 5:1–12. [Google Scholar]
- Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, et al. 2003. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect 111:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, et al. 2009. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child iq at age 5 years. Pediatrics 124:e195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. 2002. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou T, Hatzis G, Papageorgiou N, Androulakis E, Briasoulis A, Tousoulis D. 2017. Socioeconomic status and risk factors for cardiovascular disease: Impact of dietary mediators. Hellenic J Cardiol 58:32–42. [DOI] [PubMed] [Google Scholar]
- Rubin D. 1987. Multiple imputtion for nonresponse in surveys. New York:Wiley. [Google Scholar]
- Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. 2012. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. American journal of epidemiology 175:1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, Cervero RB, Ascher W, Henderson KA, Kraft MK, Kerr J. 2006. An ecological approach to creating active living communities. Annu Rev Public Health 27:297–322. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Glanz K. 2006. The role of built environments in physical activity, eating, and obesity in childhood. Future Child 16:89–108. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Glanz K. 2009. Physical activity and food environments: Solutions to the obesity epidemic. Milbank Q 87:123–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodonato J. 1997. Review of the estrogenic and antiestrogenic activity of polycyclic aromatic hydrocarbons: Relationship to carcinogenicity. Chemosphere 34:835–848. [DOI] [PubMed] [Google Scholar]
- Sen Y, Kandemir N, Alikasifoglu A, Gonc N, Ozon A. 2008. Prevalence and risk factors of metabolic syndrome in obese children and adolescents: The role of the severity of obesity. Eur J Pediatr 167:1183–1189. [DOI] [PubMed] [Google Scholar]
- Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. 1993. Do obese children become obese adults? A review of the literature. Preventive medicine 22:167–177. [DOI] [PubMed] [Google Scholar]
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. 2009. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, et al. 2005. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294:3003–3010. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, et al. 2012. Associations between phthalate metabolite urinary concentrations and body size measures in new york city children. Environ Res 112:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu W, Henkelman B, You I, Kettrup A, Schramm K. 2003. Presence of estrogenic activity from emission of fossil fuel combustion as detected by a recombinant yeast bioassay. Atmospheric Environment 37:3225–3235. [Google Scholar]
- Wang J, Xie P, Kettrup A, Schramm KW. 2005. Inhibition of progesterone receptor activity in recombinant yeast by soot from fossil fuel combustion emissions and air particulate materials. Sci Total Environ 349:120–128. [DOI] [PubMed] [Google Scholar]
- Wenger D, Gerecke AC, Heeb NV, Schmid P, Hueglin C, Naegeli H, et al. 2009. In vitro estrogenicity of ambient particulate matter: Contribution of hydroxylated polycyclic aromatic hydrocarbons. J Appl Toxicol 29:223–232. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. 1998. Early adiposity rebound and the risk of adult obesity. Pediatrics 101:E5. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30:377–399. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. 2003. Contemporaryuse pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect 111:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, et al. 2010. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arterioscler Thromb Vasc Biol 30:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zhang H, Maher C, Arteaga-Solis E, Champagne FA, Wu L, et al. 2014. Prenatal polycyclic aromatic hydrocarbon, adiposity, peroxisome proliferator-activated receptor (ppar) gamma methylation in offspring, grand-offspring mice. PloS one 9:e110706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska B, Sagebiel J, Arnott WP, Rogers CF, Kelly KE, Wagner DA, et al. 2004. Phase and size distribution of polycyclic aromatic hydrocarbons in diesel and gasoline vehicle emissions. Environ Sci Technol 38:2557–2567. [DOI] [PubMed] [Google Scholar]


