Abstract
Carbapenems and tigecycline are two important classes of antimicrobial agents to treat the infections caused by Enterobacterales. Here, we reported a plasmid carrying both blaIMP–26 and tet(A) variant in clinical Klebsiella pneumoniae KP-1572. MIC results showed that K. pneumonia KP-1572 was resistant to a wide range of antimicrobials. The blaIMP–26 and tet(A) variant were located on an identical plasmid, which was indicated by S1-PFGE and southern blotting hybridization and can be successfully transferred by electroporation. Whole-plasmid sequencing and analysis revealed that a 142,993-bp-sized plasmid, designated pIMP1572, contains an IncFIIk backbone and a variable region harboring blaIMP–26 and tet(A) variant. The plasmid pIMP1572 was apparently originated from a tet(A)-carrying IncFIIk plasmid but with a deletion length of 6,216-bp and a multiple drug resistance region (MDRR) insertion of 25,259 bp. The plasmid pIMP1572 in the present study represents the first report of the IncFIIk plasmid co-carrying blaIMP and tet(A) variant, which should be monitored.
Keywords: Klebsiella pneumoniae, carbapenems, tigecycline, resistance, blaIMP–26, tet(A) variant, IncFIIk plasmid
Introduction
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is an increasing problem worldwide (Nordmann et al., 2011; Munoz-Price et al., 2013). Horizontal transfer of plasmid-mediated carbapenemase-encoding genes, especially the predominant blaKPC, is contributing to the dissemination of carbapenem resistance among CRKP (Zhang et al., 2017). Unlike the blaKPC gene, the IMP-type metallo-β-lactamase (MBL) genes, which have been reported carried by IncL/M, IncA/C, IncHI2, and IncN plasmids in Enterobacterales from Australia and China (Villa et al., 2010; Dolejska et al., 2016; Wang et al., 2017), were not frequently detected in CRKP and associated with IncFIIK plasmids (Wang et al., 2018). IMP-26, which differs from IMP-4 by a single amino acid substitution (Phe49Val), was firstly reported from the Pseudomonas aeruginosa isolate in Singapore in 2010 (Koh et al., 2010; Tada et al., 2016) and was demonstrated to possess increased carbapenem-hydrolyzing activity to meropenem than IMP-1 (Tada et al., 2016).
Tigecycline was considered to be the last-resort drug to treat infections caused by CRKP (Tasina et al., 2011). However, the previously described tet(A) variant (Yao et al., 2018) and recently identified tet(X) variants, such as tet(X3), tet(X4), tet(X5), and tet(X6) (He et al., 2019; Sun et al., 2019; Wang L. et al., 2019a; Liu et al., 2020), have been reported to mediate the low-level and high-level tigecycline resistance, respectively. Both tet(A) variant and tet(X) variants are mobilized, indicating that they are posing a higher threat to public health. The association between the tigecycline resistance genes and carbapenem-hydrolyzing enzymes genes in CRKP has not been well explored.
Herein, we characterized an IncFIIk plasmid co-carrying blaIMP–26 and tigecycline-resistant gene tet(A) variant in a clinical K. pneumoniae isolate which displayed resistance to carbapenems and tigecycline.
Materials and Methods
Bacterial Isolation, Antimicrobial Susceptibility Testing, and PCR Detection
Klebsiella pneumonia KP-1572 was obtained from a sputum culture of a 1-day newborn boy hospitalized due to intracranial hemorrhage associated with neonatal infections at a teaching hospital of the Zhengzhou University.
The MICs to imipenem, meropenem, aztreonam, ceftazidime, gentamicin, amikacin, tetracycline, tigecycline, colistin, and fosfomycin were determined using the broth microdilution method and the agar dilution method (for fosfomycin) according to the Clinical and Laboratory Standards Institute guidelines (CLSI) (CLSI, 2020) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST)1. Escherichia coli ATCC 25922 served as the quality-control strain.
PCR was used to determine the presence of the carbapenem-resistance and tigecycline-resistance genes blaKPC, blaNDM, blaIMP, blaVIM, blaOXA, tet(A), and tet(X4) with primers described previously (Poirel et al., 2011; Yao et al., 2018; He et al., 2019).
Multilocus Sequence Typing
Multilocus sequence typing (MLST) of K. pneumoniae KP-1572 were performed as described previously (Diancourt et al., 2005). PCR amplification and sequencing for seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) were carried out. Then, the sequences of these seven housekeeping genes were submitted to a database2 to obtain the ST type.
S1-PFGE and Southern Blotting
S1-PFGE and Southern blotting were performed to detect the location of the resistance genes. The whole-cell DNA of the K. pneumonia KP-1572 isolate in agarose gel plug was treated with S1 nuclease (TaKaRa, Dalian, China) and then separated by PFGE under the conditions reported previously (Qin et al., 2014). The location of the blaIMP–26 and tet(A) variant was indicated by Southern hybridization using a digoxigenin-labeled blaIMP and tet(A) probe, respectively, according to the manufacturer’s instructions for the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics, Basel, Switzerland).
Conjugation Assay and Electrotransformation Experiments
Conjugation assays were performed according to the method described previously with minor modification (Borgia et al., 2012). Briefly, the rifampicin-resistant E. coli isolate EC600 was used as the recipient, and donor and recipient strains were mixed at a ratio of 1:4 on LB agar and cultured for 12 h. The mixtures were collected and then plated on an LB agar containing rifampicin (64 μg/mL) and meropenem (1 μg/mL) or tigecycline (0.5 μg/mL). Electrotransformation was performed as described previously (Yan et al., 2020). Briefly, the plasmid co-harboring the blaIMP–26 and tet(A) variant was extracted from K. pneumoniae KP-1572 and then transferred into the recipient Electro-Cells E. coli DH5α (TaKaRa, Dalian, China) by electroporation (Bio-Rad MicroPulser, 1.8 kV, 5 ms). The electrotransformants were screened by LB agar containing meropenem (1 μg/mL).
Plasmid Sequencing and Analysis
The plasmid was sequenced by the PacBio RS and Illumina MiSeq platforms (Shanghai Personal Biotechnology Co., Ltd., China). The PacBio sequence reads were assembled with HGAP4 and CANU (Version 1.6) and corrected by Illumina MiSeq with Pilon (Version 1.22). The prediction and annotation of ORFs were performed using Glimmer 3.0.
Results and Discussion
Klebsiella pneumoniae KP-1572 exhibited a multiple drug resistance (MDR) profile for a wide range of antimicrobial agents, including imipenem, meropenem, aztreonam, ceftazidime, gentamicin, tetracycline, tigecycline, and colistin, while it was susceptible to amikacin and fosfomycin (Table 1). Resistance gene screening and sequencing revealed that K. pneumonia KP-1572 co-carried the carbapenem-resistance gene blaIMP–26 variant and tigecycline-resistance gene tet(A) variant. The tet(A) variant showed a mutation profile of I5R, V55M, I75V, T84A, S201A, F202S, and V203F compared with tet(A) (X00006) (Waters et al., 1983) and exhibited 100% identity with that in our previous study (Yao et al., 2018). Multilocus sequence typing (MLST) showed that K. pneumonia KP-1572 belonged to uncommon sequence type ST1083, which was reported in carbapenem-resistant K. pneumonia isolated from clinical bovine mastitis in Tunisia (Saidani et al., 2018).
TABLE 1.
Antibiotic susceptibility of KP-1572 isolate and its electrotransformant.
| Isolate | Antibiotic susceptibility (μg/ml) to |
|||||||||
| IPMa | MEM | ATM | CAZ | GN | AK | TET | TIG | CL | FOS | |
| KP-1572 | 64 | >64 | 64 | >64 | 64 | 8 | >64 | 2 | 8 | 8 |
| DKP1572 | 16 | 16 | 8 | 64 | 32 | 1 | >64 | 2 | 0.5 | <1 |
| DH5α | <0.25 | <0.25 | <0.25 | 0.5 | 0.25 | 0.5 | <0.25 | <0.25 | <0.25 | <1 |
aIPM, imipenem; MEM, meropenem; ATM, aztreonam; CAZ, ceftazidime; GN, gentamicin; AK, amikacin; TET, tetracycline; TIG, tigecycline; CL, colistin; FOS, fosfomycin.
S1 nuclease PFGE and Southern blotting confirmed that the gene blaIMP–26 and tet(A) variant were located on an identical plasmid of KP-1572 (Supplementary Figure S1). The conjugation experiments failed after three attempts; however, transformants were successfully obtained by electroporation, which was confirmed by PCR and S1-PFGE (Supplementary Figure S1). The susceptibility testing results indicated that the electrotransformant (designed DKP1572) showed > 64-fold increased resistance to meropenem and imipenem compared to the recipient DH5α. DKP1572 also exhibited an increased resistance level (2 μg/mL, eightfold increase) to tigecycline than that of DH5α (Table 1).
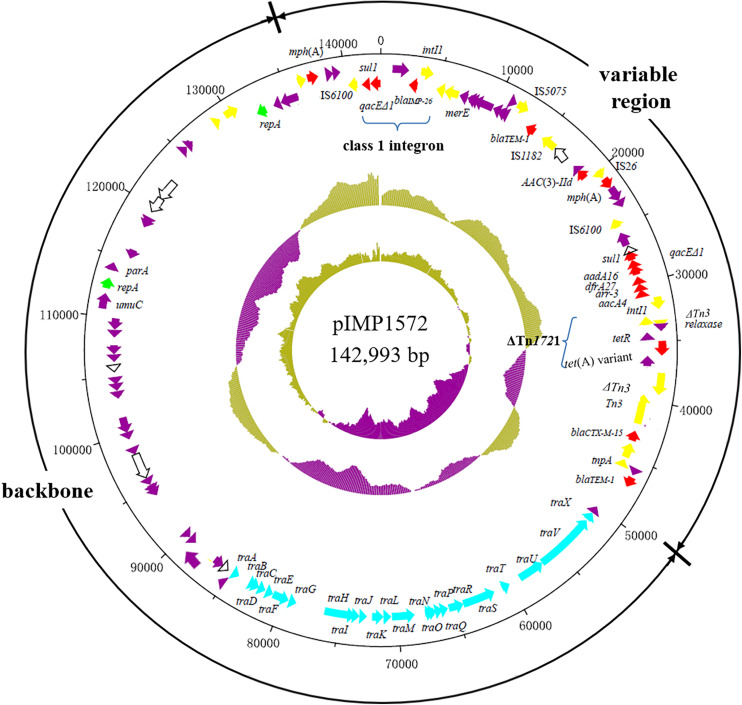
Whole-plasmid sequencing of plasmid in DKP1572 (named pIMP1572) showed that it is an IncFIIk-type plasmid with a length of 142,993 bp and an average GC content of 53.5%, which encodes 117 predicted open reading frames. The plasmid pIMP1572 consisted of an 89,521-bp IncFIIK typical backbone encoding genes responsible for plasmid replication, transfer, and stability functions, and a 53,472-bp variable region (Figure 1). The oriTs, relaxases, T4SS gene clusters, and T4CPs are closely associated with conjugation of plasmids (Li et al., 2018); however, mutations were present in relaxases, TraB, TraD, and T4CP encoding genes in pIMP1572, which might explain the failure of its conjugation. pIMP1572 is a multiple-drug-resistance plasmid that included the aminoglycoside resistance genes aac(3)-IId, aadA16, and aac(6′)-Ib-cr; β-lactam resistance genes blaTEM–1B, blaCTX–M–15, and blaTEM–1C; macrolide resistance gene mph(A); rifampicin resistance gene arr-3, sulfonamide resistance gene sul1; and trimethoprim resistance gene dfrA27 in addition to the blaIMP–26 and tet(A) variant (Figure 1). Multiple transfer elements, such as IS26, were also present in this plasmid (Figure 1), which may promote the dissemination of resistance genes among K. pneumoniae and other Enterobacterales.
FIGURE 1.
The structure of the plasmid pIMP1572 from K. pneumonia KP-1572. The size scale in bp; genes are color-coded, depending on functional annotations: red, antimicrobial resistance; blue, plasmid transfer; green, plasmid replication; yellow, transposition; purple, other functions; and white, hypothetical proteins.
Analysis of the flanking regions of blaIMP–26 revealed that this gene was located in a class 1 integron cassette, IntI1-blaIMP–26-ORF1-qacE△1-sul1, which contained a complete 5′ conserved sequence (5′-CS, integrase intl1) and 3′-CS (qacE△1-sul1). The blaIMP–26-carrying class 1 integron cassette in this study showed 100% identity and 97% query coverage with the corresponding region of a plasmid pIMP26 in Enterobacter cloacae isolated from the bloodstream in China (Wang S. et al., 2019b) but was different from that reported in P. aeruginosa in Vietnam (Tada et al., 2016). Tn1721 was a member of Tn3-family unit transposons, with the complete genetic structure of mcp-res-tnpR-tnpA-tetR-tet(A)-pecM-ΔtnpA (Allmeier et al., 1992). In this study, the tet(A) variant was found in a truncated Tn1721-like transposon with arrangement of the ΔtnpA-relaxase-tetR-tet(A) variant (Figure 1). Recently, the tet(A) variant was found located on a blaKPC–2-carrying plasmid in K. pneumonia and was confirmed to mediate tigecycline resistance (Yao et al., 2018; Zeng et al., 2020). To our knowledge, the current study is the first time to report the presence of a blaIMP–26 and tet(A) variant-co-carrying plasmid, which can render K. pneumonia to be reduced susceptibility significantly to both carbapenems and tigecycline, posing a threat to treatments of CRKP infection in clinic.
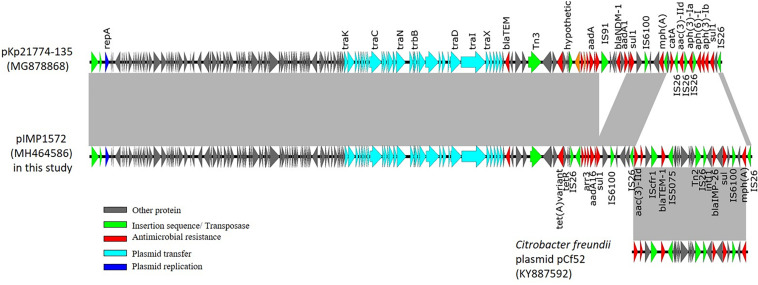
The sequence data revealed that pIMP1572 shares 99.99% identity and 89% query coverage3 with an IncFIIk type pKp21774-135 in K. pneumoniae (accession number in GenBank, MG878868) (Figure 2). Multiple drug resistance regions (MDRR) with a length of 25,259 bp insertion and 6,216 bp deletion were found in pIMP1572 plasmids in this study, when compared with pKp21774-135 (Figure 2). The insertion MDRR that contained multiple resistance genes was bracketed by IS26, including qacE△1-sul1, blaTEM–1, aac(3)-IId, and mph(A) in addition to blaIMP–26. The sequence of the MDRR region showed 99% identity and query coverage to the corresponding region of an IncA/C2 plasmid pCf52 (KY887592) from Citrobacter freundii (Figure 2), indicating that this MDRR may be acquired from C. freundii other than K. pneumonia.
FIGURE 2.
The structure comparison of the plasmid pIMP1572 identified in this study with others published previously. The gray-shaded areas represent genomic regions that share 99% nucleotide sequence identities.
IncFIIk plasmid, a member of the divergent IncFII replicon plasmids, played a significant role in restoring and transferring the blaKPC gene in K. pneumoniae (Feng et al., 2017; Wang et al., 2017; Bi et al., 2018; Fu et al., 2019), which was also reported sporadically to carry MBL-encoding genes, such as blaNDM (Sugawara et al., 2019). The plasmid pIMP1572 identified in this study is different from previously reported blaIMP-harboring plasmids that belonged to incompatibility groups IncL/M, A/C, HI2, and IncN (Carattoli, 2009; Dolejska et al., 2016; Wang et al., 2017) and represents the first report of IncFIIk plasmid carrying blaIMP. Association of blaIMP-like genes with an epidemic IncFIIk plasmid may facilitate their further dissemination among K. pneumonia. Thus, enhanced efforts should be made to monitor the potentially rapid dissemination of blaIMP and tet(A) variant-encoding IncFIIk-type plasmid.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, MH464586.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Life Sciences of Zhengzhou University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SQ and X-DD designed the research and supervised the study. HY, JC, RY, AL, and WZ performed the experiments and analyzed the data. HY, JC, and X-DD wrote the manuscript. All authors revised the manuscript and approved the final version for submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This work was supported by grants from the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (No. 18IRTSTHN020) (X-DD) and the guiding plan for key scientific research projects in universities of Henan Province (No. 19B230014) (HY).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01610/full#supplementary-material
Detection of blaIMP26– and tet(A) variant- co-carrying plasmid by S1-PFGE and Southern hybridization.
References
- Allmeier H., Cresnar B., Greck M., Schmitt R. (1992). Complete nucleotide sequence of Tn1721: gene organization and a novel gene product with features of a chemotaxis protein. Gene 111 11–20. 10.1016/0378-1119(92)90597-i [DOI] [PubMed] [Google Scholar]
- Bi D., Zheng J., Li J. J., Sheng Z. K., Zhu X., Ou H. Y., et al. (2018). In silico typing and comparative genomic analysis of IncFIIk plasmids and insights into the evolution of replicons, plasmid backbones, and resistance determinant profiles. Antimicrob. Agents Chemother. 62:e00764-18. 10.1128/aac.00764-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgia S., Lastovetska O., Richardson D., Eshaghi A., Xiong J., Chung C., et al. (2012). Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM–1, Ontario, Canada. Clin. Infect. Dis. 55 e109–e117. 10.1093/cid/cis737 [DOI] [PubMed] [Google Scholar]
- Carattoli A. (2009). Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53 2227–2238. 10.1128/aac.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement M100, 30th Edn Wayne, PA: CLSI. [Google Scholar]
- Diancourt L., Passet V., Verhoef J., Grimont P. A., Brisse S. (2005). Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43 4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolejska M., Masarikova M., Dobiasova H., Jamborova I., Karpiskova R., Havlicek M., et al. (2016). High prevalence of Salmonella and IMP-4-producing Enterobacteriaceae in the silver gull on five Islands, Australia. J. Antimicrob. Chemother. 71 63–70. 10.1093/jac/dkv306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Yin Z., Zhao Q., Zhao Y., Zhang D., Jiang X., et al. (2017). Genomic characterization of novel IncFII-type multidrug resistant plasmids p0716-KPC and p12181-KPC from Klebsiella pneumoniae. Sci. Rep. 7:5830. 10.1038/s41598-017-06283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P., Tang Y., Li G., Yu L., Wang Y., Jiang X. (2019). Pandemic spread of blaKPC–2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int. J. Antimicrob. Agents 54 117–124. 10.1016/j.ijantimicag.2019.03.014 [DOI] [PubMed] [Google Scholar]
- He T., Wang R., Liu D., Walsh T. R., Zhang R., Lv Y., et al. (2019). Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 4 1450–1456. 10.1038/s41564-019-0445-2 [DOI] [PubMed] [Google Scholar]
- Koh T. H., Khoo C. T., Tan T. T., Arshad M. A., Ang L. P., Lau L. J., et al. (2010). Multilocus sequence types of carbapenem-resistant Pseudomonas aeruginosa in Singapore carrying metallo-beta-lactamase genes, including the novel bla(IMP-26) gene. J. Clin. Microbiol. 48 2563–2564. 10.1128/jcm.01905-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xie Y., Liu M., Tai C., Sun J., Deng Z., et al. (2018). oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 46 W229–W234. 10.1093/nar/gky352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Zhai W., Song H., Fu Y., Schwarz S., He T., et al. (2020). Identification of the novel tigecycline resistance gene tet(X6) and its variants in Myroides, Acinetobacter and Proteus of food animal origin. J. Antimicrob. Chemother. 75 1428–1431. 10.1093/jac/dkaa037 [DOI] [PubMed] [Google Scholar]
- Munoz-Price L. S., Poirel L., Bonomo R. A., Schwaber M. J., Daikos G. L., Cormican M., et al. (2013). Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13 785–796. 10.1016/s1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Naas T., Poirel L. (2011). Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17 1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Walsh T. R., Cuvillier V., Nordmann P. (2011). Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70 119–123. 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Qin S., Fu Y., Zhang Q., Qi H., Wen J. G., Xu H., et al. (2014). High incidence and endemic spread of NDM-1-positive Enterobacteriaceae in Henan Province, China. Antimicrob. Agents Chemother. 58 4275–4282. 10.1128/aac.02813-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidani M., Messadi L., Soudani A., Daaloul-Jedidi M., Chatre P., Ben Chehida F., et al. (2018). Epidemiology, antimicrobial resistance, and extended-spectrum beta-lactamase-producing Enterobacteriaceae in clinical bovine mastitis in Tunisia. Microb. Drug. Resist. 24 1242–1248. 10.1089/mdr.2018.0049 [DOI] [PubMed] [Google Scholar]
- Sugawara Y., Akeda Y., Hagiya H., Sakamoto N., Takeuchi D., Shanmugakani P. K., et al. (2019). Spreading patterns of NDM-producing Enterobacteriaceae in clinical and environmental settings in Yangon, Myanmar. Antimicrob. Agents Chemother. 63:e01924-18. 10.1128/AAC.01924-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Chen C., Cui C. Y., Zhang Y., Liu X., Cui Z. H., et al. (2019). Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 4 1457–1464. 10.1038/s41564-019-0496-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Nhung P. H., Miyoshi-Akiyama T., Shimada K., Tsuchiya M., Phuong D. M., et al. (2016). Multidrug-resistant sequence type 235 Pseudomonas aeruginosa clinical isolates producing IMP-26 with increased carbapenem-hydrolyzing activities in Vietnam. Antimicrob. Agents Chemother. 60 6853–6858. 10.1128/aac.01177-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasina E., Haidich A. B., Kokkali S., Arvanitidou M. (2011). Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 11 834–844. 10.1016/s1473-3099(11)70177-3 [DOI] [PubMed] [Google Scholar]
- Villa L., Garcia-Fernandez A., Fortini D., Carattoli A. (2010). Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65 2518–2529. 10.1093/jac/dkq347 [DOI] [PubMed] [Google Scholar]
- Wang L., Liu D., Lv Y., Cui L., Li Y., Li T., et al. (2019a). Novel plasmid-mediated tet(X5) gene conferring resistance to tigecycline, eravacycline, and omadacycline in a clinical Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 64:e01326-19. 10.1128/aac.01326-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang X., Wang J., Ouyang P., Jin C., Wang R., et al. (2018). Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin. Infect. Dis. 67 S196–S205. 10.1093/cid/ciy660 [DOI] [PubMed] [Google Scholar]
- Wang S., Zhou K., Xiao S., Xie L., Gu F., Li X., et al. (2019b). A multidrug resistance plasmid pIMP26, carrying blaIMP–26, fosA5, blaDHA–1, and qnrB4 in Enterobacter cloacae. Sci. Rep. 9:10212. 10.1038/s41598-019-46777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lo W., Lai R. W., Tse C. W., Lee R. A., Luk W. K., et al. (2017). IncN ST7 epidemic plasmid carrying blaIMP–4 in Enterobacteriaceae isolates with epidemiological links to multiple geographical areas in China. J. Antimicrob. Chemother. 72 99–103. 10.1093/jac/dkw353 [DOI] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. (1983). The tetracycline resistance determinants of RP1 and Tnl721: nucleotide sequence analysis. Nucleic Acids Res. 11 6089–6105. 10.1093/nar/11.17.6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Yu R., Li D., Shi L., Schwarz S., Yao H., et al. (2020). A novel multiresistance gene cluster located on a plasmid-borne transposon in Listeria monocytogenes. J. Antimicrob. Chemother. 75 868–872. 10.1093/jac/dkz545 [DOI] [PubMed] [Google Scholar]
- Yao H., Qin S., Chen S., Shen J., Du X. D. (2018). Emergence of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Lancet. Infect. Dis. 18:25 10.1016/s1473-3099(17)30628-x [DOI] [PubMed] [Google Scholar]
- Zeng Y., Dong N., Zhang R., Liu C., Sun Q., Lu J., et al. (2020). Emergence of an Empedobacter falsenii strain harbouring a tet(X)-variant-bearing novel plasmid conferring resistance to tigecycline. J. Antimicrob. Chemother. 75 531–536. 10.1093/jac/dkz489 [DOI] [PubMed] [Google Scholar]
- Zhang R., Liu L., Zhou H., Chan E. W., Li J., Fang Y., et al. (2017). Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMed 19 98–106. 10.1016/j.ebiom.2017.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of blaIMP26– and tet(A) variant- co-carrying plasmid by S1-PFGE and Southern hybridization.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, MH464586.