Abstract
Critical or sensitive periods in the life of an organism during which certain experiences or conditions may exert disproportionate influence (either for harm or benefit) on long-term developmental outcomes have been the subject of investigation for over a century. This chapter reviews research in the context of the development of social preferences and sensory systems, with a summary of the criteria for defining such a period and the evidence necessary to establish its existence. The notion of nutritional programming, central to the Barker/Developmental Origins hypotheses of health and disease, represents a variant of the critical/sensitive period concept. It is implicit in these hypotheses that the fetal period is a time during which metabolic and physiological systems are malleable and thus susceptible to either insult or enhancement by nutrient intake. Evidence for critical/sensitive periods or nutritional programming requires a systematic manipulation of the age at which nutritional conditions or supplements are implemented. While common in research using animal models, the approach is difficult to establish in epidemiological studies and virtually nonexistent in human clinical trials. Future work seeking to establish definitive evidence for critical/sensitive periods or programming may be advanced by harmonized outcome measures in experimental trials across which the timing, duration, and dose of nutrients is varied.
Keywords: critical period, sensitive period, nutritional programming
Critical and Sensitive Periods in Development
The idea that early nutritional status is critical to lifelong health is pervasive in the scientific literature [1]. Although much of the writing on this topic has been focused on the potential early-life determinants of adult obesity [2–5], much has also been written about the importance of nutrition in the first 1000 days following conception [6] and the potential impact of nutrition and nutritional status on both biological [7] and behavioral [8] systems later in life.
In many of these papers, authors make direct reference to critical periods as a developmental basis for these proposals [9, 10]. While the critical period phenomenon has been a topic of extensive discussion in the biobehavioral and developmental sciences, there have been few detailed expositions of the concept and its implications within the nutrition literature. One objective of this chapter is to provide background on the history of and criteria for critical periods for nutrition researchers. A second objective is to integrate the notion of fetal/neonatal programming - a common concept within the nutrition field - within the framework of critical periods and developmental science. Finally, the chapter seeks to delineate the implications of critical/sensitive periods for the design of future preclinical research and clinical trials.
History of the Concept of Critical Periods
As noted above, the concept of critical periods has a long history in the field of developmental psychology [11–13]. The basic phenomenon was first identified from research in embryology [14], where the effect of exposures to toxic substances on developing embryos was observed to vary systematically with the timing of the exposure. Toxic exposures occurring in the embryonic period produced pervasive and severe effects across multiple biological systems; however, the same exposure or dose later in development resulted in somewhat milder effects, which were constrained more narrowly to particular or specific systems. Indeed, the same exposure applied even later in development might have no demonstrable effects or result in effects evident only upon systemic challenges or stressors. This common sequelae led investigators to the logical conclusion that the biological systems were broadly malleable very early in life, and that as the organism matured and those systems became settled in form and function, they became less vulnerable to environmental insult.
Imprinting and Critical Periods.
The extension of this work to the behavioral sciences came with Lorenz’ exposition of imprinting in birds [15]. In this phenomenon, precocial bird species developed strong social preferences for objects to which they were exposed immediately after hatching; young birds would then attach emotionally and maintain proximity to such objects until fledging. The evolutionary adaptiveness of this phenomenon is obvious, as hatchlings are typically exposed immediately after hatching to their own mother (or at least, a conspecific from the same species), and a neural mechanism that promoted hatchlings’ emotional and physical affiliation with their mother very likely increased the probability of their survival. Indeed, this framework was adapted for use in the early evolutionary-based accounts for explaining human infants’ attachment to their own mothers [16].
Of critical importance to the current discussion, however, two points shaped future thinking about the nature of critical periods in development. First, the nature of the objects to which hatchlings could be imprinted was extremely general; during this period young birds could be manipulated to form social preferences for nearly any object, whether it was Lorenz himself [17] or a moving tennis ball [18]. The other points were derived from Lorenz’ claim that the development of these strong social affiliations could only be formed during a very brief period of time during the hatchlings’ development: once imprinting had occurred, it not be undone [19], and that non-imprinted organisms were not able to imprint beyond the hatchling period [20]. Thus, the effects of exposure during this early period of life were claimed to be both irreversible and unrecoverable, thus bringing about the label of the period as critical. However, much of the literature that emerged immediately after these initial claims demonstrated substantial reversibility and flexibility [21] in imprinting. Thus while the early period of life might represent heightened malleability or plasticity, the period might not be as rigidly bound or essential as it had been originally designated making the term sensitive period might more appropriate. The phenomenon was later generalized to the notion of food imprinting [22–25] in several species, where the food preferences typically exhibited by certain animals could be substantially altered by early exposure to alternate foods.
Critical Periods in the Development of the Visual System.
The 1960s and 1970s produced the most comprehensive descriptions of critical periods in mammalian biology and behavior in Hubel and Wiesel’s program of research on the development of the visual system in the cat [26–28]. Briefly, these investigators used techniques for measuring the activity of single neurons in the cat visual cortex, mapped the responsiveness of these neurons to different visual stimuli, and then sought to map the maturation of this neuronal activity from birth to adulthood. While some neurons in the visual cortex were dedicated from birth to processing specific types of input (e.g., accepting from one or both eyes, or responding to horizontal vs. vertical bars), they also determined through careful experimentation that the fate of many cells in the cortex was determined by both the quantity and quality of postnatal input [29, 30], and that the period during which that input was received was limited to the first 4–7 weeks of life. Similar to imprinting, recovery of normal vision after deprivation of input during that period of life was initially reported to be limited [31], suggesting that this was another clear manifestation of a true “critical” period. These findings from the cat were largely confirmed in primates [32, 33], and observational studies of humans deprived of various visual input were found to be generally consistent with the principles outlined in this work [34–36].
Since the emergence of this seminal line of research in biobehavioral development, numerous refinements have been explored in isolating the specific mechanisms underlying the early plasticity of the system and the processes which bring that plasticity to an end [37]. For example, it is clear that this is a sensitive period, rather than a critical period, as some level of recovery of visual function can be attained after the end of the period [38, 39]. In addition, eye movements play a major role in the neural processing that contributes to the dedication of neurons to visual inputs [30], and t both the onset and the eventual end of the sensitive period is triggered by the initiation of visual input [40]. In keeping with the general principles of early plasticity, early disruptions in the normal course of sensory exposure have been found to alter the order in which sensory systems develop and in which sensory preferences or priorities are expressed in postnatal life [41, 42].
Summary.
The phenomenon of critical/sensitive periods in biobehavioral development has been explored in domains beyond that of imprinting and sensory systems; for example, there is also a substantial literature on a critical/sensitive period for language development [43–45]. Several generalities can be drawn from this brief and admittedly perfunctory review, however. First, the principles regarding early vulnerabilities of organisms to frank environmental insult or compromise appear to be reliable and robust; early damage will yield severe and widespread effects while later damage will tend to be less severe and more specifically localized. Second, in the behavioral realm, wherever a “critical” period has initially been described, including claims of absolute irreversibility or inability to recover from deprivation, subsequent work has generally shown that some degree of recovery is possible under special conditions or with targeted remedial actions. Organisms may be both relatively more vulnerable to environmental deprivation and relatively better able to benefit from environmental enhancement early in life, but it is likely better to characterize these early periods of malleability as sensitive periods rather than truly critical periods [13]. Figure 1 schematically represents the difference between “critical” and “sensitive” periods and their interaction with both positive (beneficial) and negative (harmful) events. That said, given that evidence suggests that early interventions will be relatively (rather than absolutely) more effective than later interventions, there is clear economic value in understanding these developmental principles.
Figure 1.
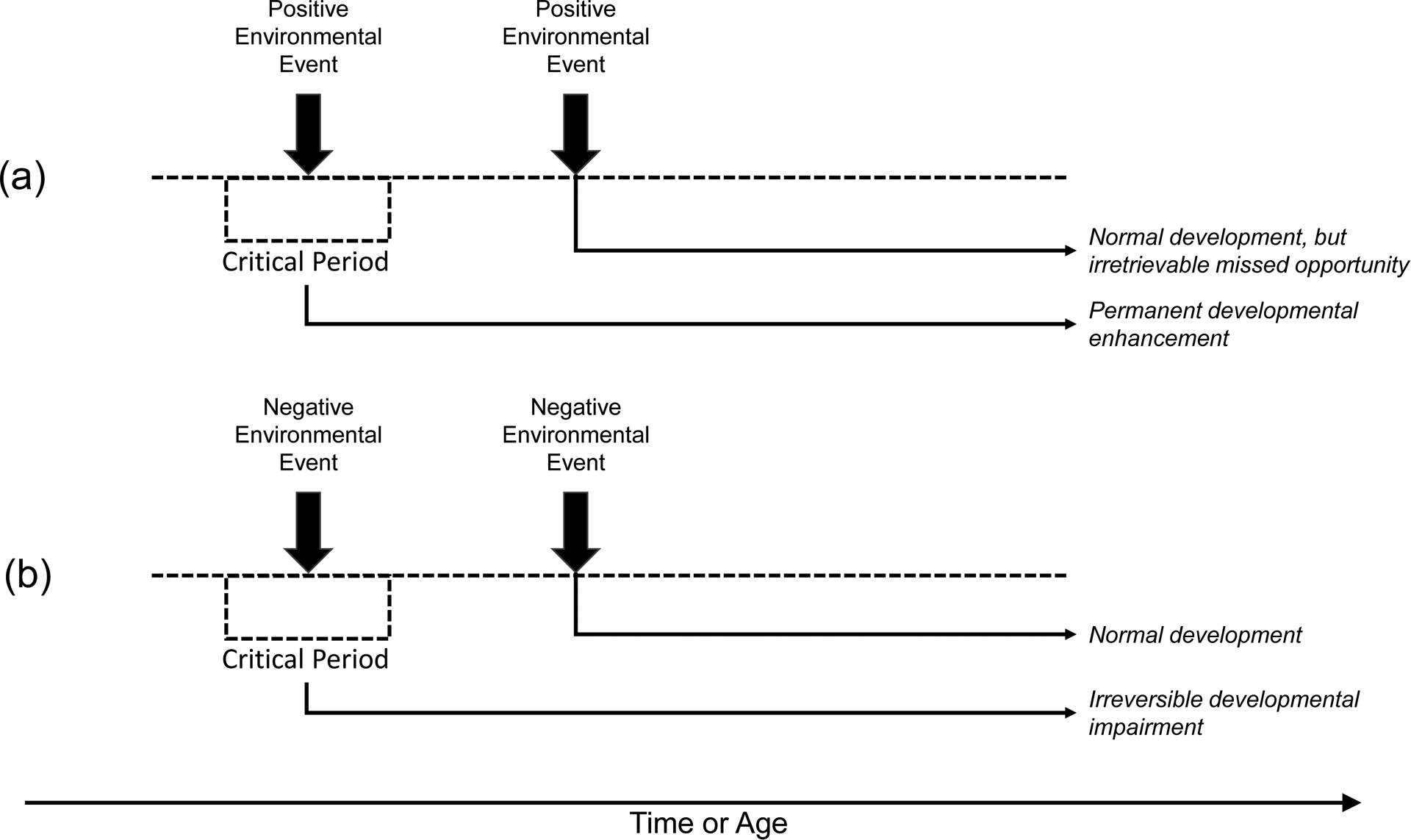
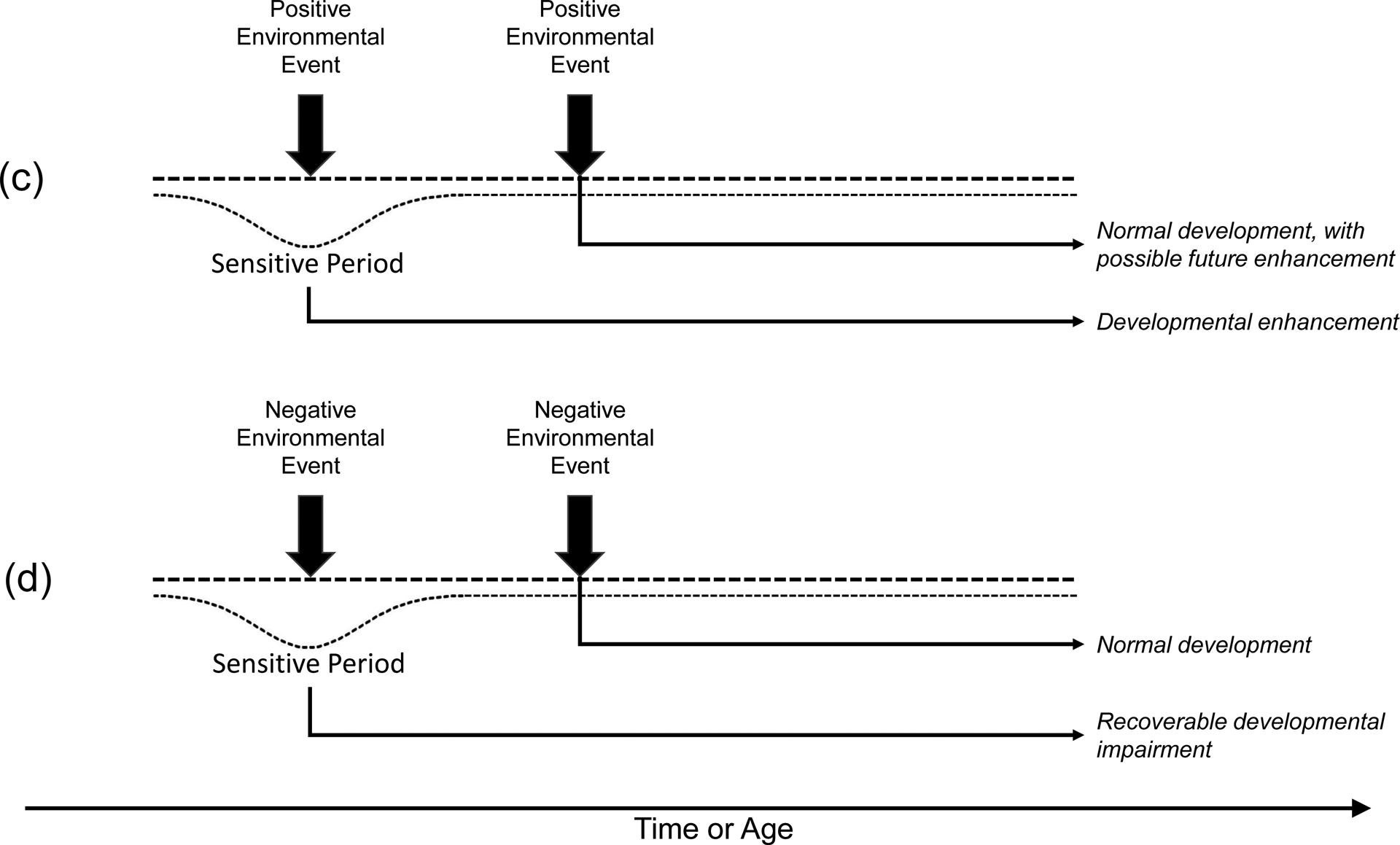
Schematic representation of the difference between a critical period (panels a, b) and a sensitive period (panels c, d). Time/age moves from left to right. Note that, in a critical period, the period of malleability or plasticity is sharply defined as a box, with a clear beginning and end, and no gradient over time. In a sensitive period, the degree of plasticity is relatively higher but plasticity never ends. As a result, the end states from a critical period are irreversible or irretrievable, while in a sensitive period some degree of future enhancement or future recovery from harm is possible in the future.
Scott and colleagues [46] have offered one characterization of these phenomena in development, noting that critical/sensitive periods merely represent periods of rapid development within systems, such that enhancement or deprivation during these periods of emergent and rapid maturation can respectively bring either substantial benefit or wreak substantial havoc on the systems involved. As has been summarized previously [11], if there are qualitatively distinct stages of malleability in development, then one must define them in terms of the specific system involved, as well as by the onset and terminus of the period and the specific inputs that are presumed to enhance or disrupt normal development.
At this point, we turn to discuss programming, a phenomenon similar to the critical/sensitive period as referenced in the nutrition literature.
Early Programming and Critical Periods
The notion of nutritional programming [47] is a popular one among the nutrition science community; a search on the phrase in Google Scholar™ in late 2019 generated over 190,000 entries. This notion emerged from a comprehensive epidemiological study of the Dutch hunger winter [48] in which food shortages precipitated by weather, bad crops, war and a Nazi embargo of food transport to parts of The Netherlands limited pregnant women’s nutritional intake to only 400–800 calories per day. This restricted intake resulted in a remarkable increase in the incidence of coronary heart disease in the offspring whose mothers’ were exposed to restricted food intake early in gestation, markers of reduced renal function among those exposed in mid-gestation, and lifetime growth restriction among those exposed late in gestation (Schutz LC, the Dutch Hunger Winter and the developmental origins of health and disease. PNAS 2010; 107:16757–16758). The Barker hypothesis was derived from observations in the UK that disproportionate fetal growth in middle to late gestation programmed later coronary heart disease in the offspring. The hypothesis regarding the fetal origins of adult disease expanded to the Developmental Origins hypothesis [49–52], the notion that, by influencing epigenetic processes, metabolic set-points, or early inflammatory status, prenatal nutrition in some way “programs” the fetus or maladaptively prepares the fetus for an environment that will induce adiposity/obesity [53, 54] or other metabolic-based diseases [55]. It is a clear implication of the Barker/Developmental Origins hypothesis that the early part of life is in some way special in its malleability or capacity for enacting long-term changes in the organism. Such studies would presume to reveal a critical-period phenomenon in that it is the early stages of the organism’s development that serves as a causal vehicle for the efficacy of the exposure. Furthermore, the notion that the organism is “programmed” comes from the fact that the outcomes associated with fetal conditions reach far into the future and represent health and neurodevelopmental status in adulthood.
A key point about the original Barker study was that, for an observational study, it controlled fairly well for the timing of the deprivation. For example, subsequent secondary analyses noted that the effects varied as a function of the gestational state of the fetus [56]; malnutrition in early pregnancy was associated with a higher risk of coronary heart disease and accelerated cognitive aging [57], mid-gestation exposure had an increased prevalence of bronchial disease, and late/mid-gestation exposure was related to poorer glucose metabolism. It is not a far reach to extrapolate this to the idea that early nutrition extending into the postnatal period may also bring about programming effects; indeed, this case has been made for a number of different functions [58–60], and this argument takes on immediate weight given what is known about the postnatal development of the central nervous system and the potential effects of certain nutrients on brain and behavioral function [61–63].
Age and Timing in Nutritional Studies
Like much of the critical/sensitive-period research, studies lending support to early nutritional programming have largely been conducted with animal models [64]. While it has been argued that the animal data coupled with human clinical trials showing the effects of early nutritional manipulations are compelling [65], in the absence of systematic experimental data in which the age of exposure is manipulated, claims about early nutritional programming remain largely speculative.
In order to definitively establish a true critical/sensitive period or programming effects, one must manipulate the timing of the early intervention [11]. That is, it must be shown that vulnerability to risk or ability to benefit from enhanced conditions at a particular time during development is either absolutely or relatively higher at one time during development over others. Of course, human studies to experimentally vary the timing of adverse interventions to demonstrate the critical/sensitive period-programming effects are unethical, but it is possible and ethical to focus on timing in clinical trials that purport to provide interventions that benefit to their participants; indeed, from an economic point of view, one could argue that such a focus is necessary. Furthermore, going back to the original point in the critical period phenomenon about the dose of exposure interacting with timing [11], one might further argue that designs featuring dose × timing interactions would be ideal.
Even a quick perusal of the literature, however, shows that the extant nutrition clinical trials almost entirely exclude the timing or age at which manipulations are implemented. For the most part, nutritional interventions are implemented as early as possible in infancy and if they show efficacy that persists, as has been established in some cases [66], it is tempting to propose that an early programming effect has taken hold. However, in the absence of exposure to a nutrient for an equivalent duration at a later age, it is by no means clear that this programming effect is endemic to early prenatal or postnatal life. Those who design such trials likely understand the potential importance of timing well, but the conduct of such trials obviously requires tremendous resources to simply establish efficacy; establishing that a nutrient’s efficacy is greater at one age than at another may seem like a luxury. However, until there is evidence that benefit varies with the age at which a nutrient is provided, one can not have evidence for a critical/sensitive period, or for an age-specific programming effect.
In the absence of clinical trials that comprehensively address the issue of age and timing in their designs, one way to examine the relative efficacy across ages is to compare completed trials that have varied the age of their interventions, but where outcome measures were more or less harmonized. This has been done to some degree for examination of differences in outcome as a function of dose [67], although dose still remains an understudied factor in much of the literature on early nutrition. One potential example approximating this approach is represented by two trials conducted in our laboratory over the last two decades. The DIAMOND trial [66, 68] involved postnatal supplementation with four doses of docosahexaenoic acid (DHA) but with a constant level of arachidonic acid (ARA) compared to a placebo. The KUDOS trial [69–71] involved prenatal supplementation with one dose of DHA, again compared to a placebo. While the trials are too different in their manipulation and in their fundamental sample demographics to compare directly here, they do share a fair number of harmonized outcome variables in the domain of postnatal cognitive development to invite a putative inference that postnatal supplementation might produce more pervasive long-term positive effects on infant child neurocognition [72] than prenatal supplementation. On the other hand, the prenatal supplementation produced clear metabolic effects [73] that were not evident from the postnatal trial. While these outcomes and comparisons cannot be considered definitive, they do invite a vision of what might be possible with broadly harmonized outcomes for clinical trials in the future in the field of nutrition.
Summary and Conclusions
Critical and sensitive developmental periods have been key concepts in developmental science for over a century; they have a long history for biobehavioral development and have particularly special importance with respect to the plasticity of the brain. In such developmental periods certain experiences, exposures, or conditions are thought to exert disproportionate influence over the long-term development of the organism due to the fact that the organism is in a particularly malleable state. Examples of putative critical/sensitive periods in biobehavioral development include the establishment of social and food preferences (imprinting), shaping the structure and function of sensory systems, and possibly in the area of language and language acquisition. There is still considerable debate over the nature of critical/sensitive periods, but one hypothesis is that such phases are simply the epiphenomenon of systems that are undergoing rapid maturation or change.
While critical and sensitive period concepts have been used often with respect to studies of early nutrition, they also underlie the concept of nutritional programming, as the implication of programming (particularly within the context of the Fetal/Developmental Origins hypothesis) is that the prenatal period is presumably a time when various metabolic systems are malleable and can be influenced by conditions of maternal physiology and environmental exposures, including nutrient intake.
Critical to the establishment of any critical/sensitive period (and by extension, to any claim for prenatal programming) is the demonstration that an intervention shows improved efficacy when implemented at one age, relative to other ages. For example, in order to establish the existence of a critical period for omega-3 effects on neurodevelopment, one would have to show that supplementation at, say birth to 6 months of age, would have far more influence on outcome measures than supplementation from 6 to 12 months; obviously, from a design standpoint, this would necessitate feeding two age groups for an equivalent duration. While parametric manipulation of the age of nutritional interventions is relatively commonplace in animal models, the results of preclinical studies do not necessarily translate to human trials [74] and so any conclusion about the critical/sensitive periods in nutrition or nutrition programming must be viewed as speculative. It may be that if enough trials have harmonized outcomes, meta-analyses that include age of feeding, duration of feeding, and dose would advance the field as close as possible to answering this question.
Key Messages.
The concept of critical period is often invoked with reference to phenomena in the field of nutrition. The history and evolution of the critical period concept in development is briefly reviewed.
A critical period (or its less restrictive form, a sensitive period) carries with it a number of methodological criteria that are typically not met in the literatures on early nutrition
The phenomenon of programming is placed within this developmental concept.
Implications of these developmental phenomena for the design of preclinical research and clinical trials that seek to demonstrate true programming or critical/sensitive period effects are described
Acknowledgements and Disclosure Statement
This work was supported by NIH grants U54 HD090216, R01 HD086001, R01HD083292, and R01 HD083292. The writing of this article was supported by Nestlé Nutrition Institute. The authors declare no other conflicts of interest
References
- 1.Jang H, Serra C: Nutrition, epigenetics, and diseases. Clinical nutrition research 2014; 3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dietz WH: Critical periods in childhood for the development of obesity. The American journal of clinical nutrition 1994; 59(5):955–959. [DOI] [PubMed] [Google Scholar]
- 3.Power C, Parsons T: Nutritional and other influences in childhood as predictors of adult obesity. Proceedings of the nutrition Society 2000; 59(2):267–272. [DOI] [PubMed] [Google Scholar]
- 4.Giles L, Whitrow M, Rumbold A, Davies C, De Stavola B, Pitcher J, Davies M, Moore V: Growth in early life and the development of obesity by age 9 years: are there critical periods and a role for an early life stressor? International Journal of Obesity 2013; 37(4):513. [DOI] [PubMed] [Google Scholar]
- 5.Cioffi L: General theory of critical periods and development of obesity. Bibliotheca nutritio et dieta 1978;(26):17–28. [DOI] [PubMed] [Google Scholar]
- 6.Cusick SE, Georgieff MK: The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. The Journal of pediatrics 2016; 175:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCance R: Critical periods of growth. Proceedings of the Nutrition Society 1976; 35(3):309–313. [DOI] [PubMed] [Google Scholar]
- 8.Georgieff MK, Brunette KE, Tran PV: Early life nutrition and neural plasticity. Development and psychopathology 2015; 27(2):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilich JZ, Brownbill RA: Nutrition through the life span: needs and health concerns in critical periods In Handbook of stressful transitions across the lifespan. Springer; 2010: 625–641. [Google Scholar]
- 10.Herman DR, Baer MT, Adams E, Cunningham-Sabo L, Duran N, Johnson DB, Yakes E: Life Course Perspective: evidence for the role of nutrition. Maternal and child health journal 2014; 18(2):450–461. [DOI] [PubMed] [Google Scholar]
- 11.Colombo J: The critical period concept: Research, methodology, and theoretical issues. Psychological Bulletin 1982; 91(2):260–275. [PubMed] [Google Scholar]
- 12.Bornstein MH: Sensitive periods in development: Structural characteristics and causal interpretations. Psychological Bulletin 1989; 105(2):179. [DOI] [PubMed] [Google Scholar]
- 13.Bailey DB Jr, Bruer JT, Symons FJ, Lichtman JW: Critical thinking about critical periods: Paul H Brookes Publishing; 2001. [Google Scholar]
- 14.Stockard CR: Developmental rate and structural expression: an experimental study of twins,’double monsters’ and single deformities, and the interaction among embryonic organs during their origin and development. American Journal of Anatomy 1921; 28(2):115–277. [Google Scholar]
- 15.Lorenz K: Imprinting. Auk 1937; 54(1):245–273. [Google Scholar]
- 16.Bowlby J: The nature of the child’s tie to his mother. International journal of psychoanalysis 1958; 39:350–373. [PubMed] [Google Scholar]
- 17.Lorenz KZ: The companion in the bird’s world. The Auk 1937; 54(3):245–273. [Google Scholar]
- 18.Hess EH: Imprinting in birds. Science 1964; 146(3648):1128–1139. [DOI] [PubMed] [Google Scholar]
- 19.Klopfer PH: Stimulus preferences and imprinting. Science 1967; 156(3780):1394–1396. [DOI] [PubMed] [Google Scholar]
- 20.Haywood HC, Zimmerman DW: Effects of early environmental complexity on the following response in chicks. Perceptual and motor skills 1964; 18(2):653–658. [DOI] [PubMed] [Google Scholar]
- 21.Salzen EA, Meyer CC: Imprinting: Reversal of a preference established during the critical period. Nature 1967; 215(5102):785. [DOI] [PubMed] [Google Scholar]
- 22.Burghardt GM, Hess EH: Food imprinting in the snapping turtle, Chelydra serpentina. Science 1966; 151(3706):108–109. [DOI] [PubMed] [Google Scholar]
- 23.Darmaillacq A-S, Chichery R, Dickel L: Food imprinting, new evidence from the cuttlefish Sepia officinalis. Biology letters 2006; 2(3):345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank LH, Meyer ME: Food imprinting in domestic chicks as a function of social contact and number of companions. Psychonomic Science 1970; 19(5):293–295. [Google Scholar]
- 25.Bernays E, Weiss M: Induced food preferences in caterpillars: the need to identify mechanisms. Entomologia Experimentalis et Applicata 1996; 78(1):1–8. [Google Scholar]
- 26.Hubel DH, Wiesel TN: The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of physiology 1970; 206(2):419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiesel TN: Postnatal development of the visual cortex and the influence of environment. Nature 1982; 299(5884):583. [DOI] [PubMed] [Google Scholar]
- 28.Barlow H: Visual experience and cortical development. Nature 1975; 258(5532):199. [DOI] [PubMed] [Google Scholar]
- 29.Wiesel TN, Hubel DH: Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of neurophysiology 1965; 28(6):1029–1040. [DOI] [PubMed] [Google Scholar]
- 30.Freeman R, Bonds A: Cortical plasticity in monocularly deprived immobilized kittens depends on eye movement. Science 1979; 206(4422):1093–1095. [DOI] [PubMed] [Google Scholar]
- 31.Wiesel TN, Hubel DH: Extent of recovery from the effects of visual deprivation in kittens. Journal of neurophysiology 1965; 28(6):1060–1072. [DOI] [PubMed] [Google Scholar]
- 32.Harwerth RS, Smith III EL, Crawford M, von Noorden GK: Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behavioural brain research 1990; 41(3):179–198. [DOI] [PubMed] [Google Scholar]
- 33.Kiorpes L: Visual development in primates: neural mechanisms and critical periods. Developmental neurobiology 2015; 75(10):1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis TL, Maurer D: Effects of early pattern deprivation on visual development. Optometry and Vision Science 2009; 86(6):640–646. [DOI] [PubMed] [Google Scholar]
- 35.Hickey TL: Postnatal development of the human lateral geniculate nucleus: Relationship to a critical period for the visual system. Science 1977; 198(4319):836–838. [DOI] [PubMed] [Google Scholar]
- 36.Lewis TL, Maurer D: Multiple sensitive periods in human visual development: evidence from visually deprived children. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology 2005; 46(3):163–183. [DOI] [PubMed] [Google Scholar]
- 37.Hensch TK: Critical period mechanisms in developing visual cortex. Current topics in developmental biology 2005; 69:215–237. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell DE, Cynader M, Anthony Movshon J: Recovery from the effects of monocular deprivation in kittens. Journal of Comparative Neurology 1977; 176(1):53–63. [DOI] [PubMed] [Google Scholar]
- 39.Movshon JA: Reversal of the behavioural effects of monocular deprivation in the kitten. The Journal of physiology 1976; 261(1):175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cynader M, Berman N, Hein A: Recovery of function in cat visual cortex following prolonged deprivation. Experimental Brain Research 1976; 25(2):139–156. [DOI] [PubMed] [Google Scholar]
- 41.Lickliter R: Prenatal visual experience alters postnatal sensory dominance hierarchy in bobwhite quail chicks. Infant Behavior and Development 1994; 17(2):185–193. [Google Scholar]
- 42.Lickliter R: Prenatal Sensory Ecology and Experience: Implications for Perceptual and Behavioral Development in Precocial Birds In Advances in the Study of Behavior. vol. 35: Academic Press; 2005: 235–274. [Google Scholar]
- 43.Werker JF, Hensch TK: Critical Periods in Speech Perception: New Directions. Annual Review of Psychology 2015; 66(1):173–196. [DOI] [PubMed] [Google Scholar]
- 44.Kral A, Dorman MF, Wilson BS: Neuronal Development of Hearing and Language: Cochlear Implants and Critical Periods. Annual Review of Neuroscience 2019; 42(1):47–65. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JS, Newport EL: Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognitive Psychology 1989; 21(1):60–99. [DOI] [PubMed] [Google Scholar]
- 46.Scott JP, Stewart JM, De Ghett VJ: Critical periods in the organization of systems. Developmental Psychobiology 1974; 7(6):489–513. [DOI] [PubMed] [Google Scholar]
- 47.Langley-Evans SC: Nutrition in early life and the programming of adult disease: a review. Journal of Human Nutrition and Dietetics 2015; 28(s1):1–14. [DOI] [PubMed] [Google Scholar]
- 48.Schulz LC: The Dutch Hunger Winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences 2010; 107(39):16757–16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillman MW: Developmental origins of health and disease. N Engl J Med 2005; 353(17):1848–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gluckman PD, Hanson MA: The Developmental Origins of Health and Disease In Early Life Origins of Health and Disease. Edited by Wintour EM, Owens JA. Boston, MA: Springer US; 2006: 1–7. [Google Scholar]
- 51.Hanson M, Gluckman P: Developmental origins of noncommunicable disease: population and public health implications. The American Journal of Clinical Nutrition 2011; 94(suppl_6):1754S–1758S. [DOI] [PubMed] [Google Scholar]
- 52.Barker DJP: The Developmental Origins of Adult Disease. Journal of the American College of Nutrition 2004; 23(sup6):588S–595S. [DOI] [PubMed] [Google Scholar]
- 53.Symonds ME, Mendez MA, Meltzer HM, Koletzko B, Godfrey K, Forsyth S, van der Beek EM: Early Life Nutritional Programming of Obesity: Mother-Child Cohort Studies. Annals of Nutrition and Metabolism 2013; 62(2):137–145. [DOI] [PubMed] [Google Scholar]
- 54.Budge H, Gnanalingham MG, Gardner DS, Mostyn A, Stephenson T, Symonds ME: Maternal nutritional programming of fetal adipose tissue development: Long-term consequences for later obesity. Birth Defects Research Part C: Embryo Today: Reviews 2005; 75(3):193–199. [DOI] [PubMed] [Google Scholar]
- 55.Tarrade A, Panchenko P, Junien C, Gabory A: Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. The Journal of Experimental Biology 2015; 218(1):50–58. [DOI] [PubMed] [Google Scholar]
- 56.Roseboom TJ, van der Meulen JHP, Ravelli ACJ, Osmond C, Barker DJP, Bleker OP: Effects of Prenatal Exposure to the Dutch Famine on Adult Disease in Later Life: An Overview. Twin Research 2001; 4(5):293–298. [DOI] [PubMed] [Google Scholar]
- 57.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ: Prenatal undernutrition and cognitive function in late adulthood. Proceedings of the National Academy of Sciences 2010; 107(39):16881–16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel MS, Srinivasan M: Metabolic Programming in the Immediate Postnatal Life. Annals of Nutrition and Metabolism 2011; 58(suppl 2)(Suppl. 2):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiedmeier JE, Joss-Moore LA, Lane RH, Neu J: Early postnatal nutrition and programming of the preterm neonate. Nutrition Reviews 2011; 69(2):76–82. [DOI] [PubMed] [Google Scholar]
- 60.Neu J, Hauser N, Douglas-Escobar M: Postnatal nutrition and adult health programming. Seminars in Fetal and Neonatal Medicine 2007; 12(1):78–86. [DOI] [PubMed] [Google Scholar]
- 61.Colombo J: Recent advances in infant cognition: Implications for long-chain polyunsaturated fatty acid supplementation studies. Lipids 2001; 36(9):919–926. [DOI] [PubMed] [Google Scholar]
- 62.Wainwright PE, Colombo J: Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. American Journal of Clinical Nutrition 2006; 84(5):961–970. [DOI] [PubMed] [Google Scholar]
- 63.Carlson SE, Colombo J: Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv Pediatr 2016; 63(1):453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Symonds ME, Gardner DS: Experimental evidence for early nutritional programming of later health in animals. Current Opinion in Clinical Nutrition & Metabolic Care 2006; 9(3):278–283. [DOI] [PubMed] [Google Scholar]
- 65.Lucas A: Role of nutritional programming in determining adult morbidity. Arch Dis Child 1994; 71(4):288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, Gustafson KM, Brez C: Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr 2013; 98(2):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yelland LN, Gajewski BJ, Colombo J, Gibson RA, Makrides M, Carlson SE: Predicting the effect of maternal docosahexaenoic acid (DHA) supplementation to reduce early preterm birth in Australia and the United States using results of within country randomized controlled trials. Prostaglandins, Leukotrienes and Essential Fatty Acids 2016; 112:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T: Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res 2011; 70(4):406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ: DHA supplementation and pregnancy outcomes. Am J Clin Nutr 2013; 97(4):808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colombo J, Gustafson KM, Gajewski BJ, Shaddy DJ, Kerling EH, Thodosoff JM, Doty T, Brez CC, Carlson SE: Prenatal DHA supplementation and infant attention. Pediatr Res 2016; 80(5):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colombo J, Shaddy DJ, Gustafson K, Gajewski BJ, Thodosoff JM, Kerling E, Carlson SE: The Kansas University DHA Outcomes Study (KUDOS) clinical trial: long-term behavioral follow-up of the effects of prenatal DHA supplementation. Am J Clin Nutr 2019; 109(5):1380–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lepping RJ, Honea RA, Martin LE, Liao K, Choi I-Y, Lee P, Papa VB, Brooks WM, Shaddy DJ, Carlson SE et al. : Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Developmental Psychobiology 2019; 61(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerling EH, Hilton JM, Thodosoff JM, Wick J, Colombo J, Carlson SE: Effect of Prenatal Docosahexaenoic Acid Supplementation on Blood Pressure in Children With Overweight Condition or Obesity: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open 2019; 2(2):e190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Symonds ME, Budge H, Stephenson T: Limitations of models used to examine the influence of nutrition during pregnancy and adult disease. Arch Dis Child 2000; 83(3):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]


