To the Editor:
Tracheal intubation is a high-risk procedure in critically ill adults, and 20–25% of patients experience cardiovascular collapse, defined as hypotension, new vasopressor use, cardiac arrest, or death during or immediately after the procedure (1–3). Despite the high prevalence of this complication, relatively little is known about risk factors for cardiovascular collapse during tracheal intubation, and no reliable prediction model exists for this outcome (2–4). In this secondary analysis of data from three large, pragmatic trials in airway management, we developed and validated a prediction model for cardiovascular collapse for use in analyzing heterogeneity of treatment effect and for prognostic enrichment in trials evaluating interventions to prevent cardiovascular collapse during tracheal intubation of critically ill adults (1, 5–8).
Methods
We performed a secondary analysis of data from the PrePARE (Preventing Cardiovascular Collapse with Administration of Fluid Resuscitation before Endotracheal Intubation), PreVent (Preventing Hypoxemia with Manual Ventilation during Endotracheal Intubation), and Check-UP (Checklists and Upright Positioning in Endotracheal Intubation of Critically Ill Patients) trials, which examined fluid bolus administration, bag-mask ventilation, and ramped positioning, respectively, during tracheal intubation of critically ill adults (1, 5, 6, 8). In each of these trials, any adult undergoing tracheal intubation in a participating intensive care unit (ICU) or emergency department was eligible, unless they were believed to have a specific contraindication to the study intervention or unless the procedure was too emergent to obtain a randomization envelope. The Vanderbilt Institutional Review Board (160158) approved this analysis.
Cardiovascular collapse was defined, as in previous trials, as a new systolic blood pressure (SBP) <65 mm Hg or new or increased vasopressor administration between induction and 2 minutes after intubation or cardiac arrest or death within 1 hour of intubation (1). Patients were excluded from this analysis if the indication for intubation was cardiac arrest or if the SBP at induction was <65 mm Hg. Predictor variables were selected on the basis of prior literature, clinical knowledge, and consistent data collection across trials (2–4, 9, 10). Twenty candidate variables were selected a priori, which are shown in Table 1. An elastic net logistic regression model, which combines ridge and lasso regression coefficient penalties to avoid overfitting, was trained using data from the PrePARE and PreVent trials. Five repeats of 10-fold cross-validation were used to choose the hyperparameters (i.e., ridge and lasso penalty term values) that maximized the area under the receiver operating characteristic curve (AUC) in the training dataset. Restricted cubic splines were used to test for nonlinearity of the continuous variables to determine if this improved accuracy during cross-validation. The model was then externally validated with data from the Check-UP trial, using the AUC for model discrimination and the unreliability index for calibration (11, 12). All analyses were performed using Stata version 16.0 software (StataCorp) and R version 3.6.1 software (R Foundation for Statistical Computing).
Table 1.
Demographics and baseline characteristics of the study population
| Combined Cohort (N = 815) | PrePARE/PreVent (n = 529) | Check-UP (n = 286) | |
|---|---|---|---|
| Age, yr | 59 (47–68) | 60 (48–69) | 57 (47–66) |
| Sex, F | 328 (40.2%) | 225 (42.5%) | 103 (36.0%) |
| Body mass index, kg/m2 | 27.2 (23.2–32.8) | 27.3 (22.8–32.8) | 27.1 (23.9–32.8) |
| Comorbidities | |||
| Cardiac (congestive heart failure or atrial fibrillation) | 196 (24.1%) | 146 (27.6%) | 50 (17.5%) |
| Chronic renal disease* | 129 (15.8%) | 88 (16.6%) | 41 (14.3%) |
| Cirrhosis | 151 (18.5%) | 95 (18.0%) | 56 (19.6%) |
| Oxygen saturation before tracheal intubation | 99% (96–100%) | 99% (96–100%) | 99% (95–100%) |
| Systolic blood pressure before tracheal intubation, mm Hg | 123 (106–142) | 124 (107–144) | 119 (102–142) |
| Receipt of vasopressors in 6 h before tracheal intubation | 152 (18.7%) | 97 (18.4%) | 55 (19.2%) |
| APACHE II score | 21 (16–27) | 21 (16–28) | 22 (18–26) |
| Indication for tracheal intubation† | |||
| Hypoxic respiratory failure | 466 (57.2%) | 306 (57.8%) | 160 (55.9%) |
| Hypercarbic respiratory failure | 157 (19.3%) | 109 (20.6%) | 48 (16.8%) |
| Neurologic (e.g., depressed mental status, airway protection, seizure, agitation) | 312 (38.3%) | 204 (38.6%) | 108 (37.8%) |
| Facilitate procedure | 90 (11.0%) | 54 (10.2%) | 36 (12.6%) |
| Induction agents for tracheal intubation‡ | |||
| Etomidate | 661 (81.1%) | 416 (78.6%) | 245 (85.7%) |
| Propofol | 101 (12.4%) | 79 (14.9%) | 22 (7.7%) |
| Benzodiazepine | 57 (7.0%) | 49 (9.3%) | 8 (2.8%) |
| Ketamine§ | 67 (8.2%) | 40 (7.6%) | 27 (9.4%) |
| Neuromuscular blockade during tracheal intubation | |||
| Succinylcholine | 333 (40.9%) | 198 (37.4%) | 135 (47.2%) |
| Nondepolarizing agent | 450 (55.2%) | 305 (57.7%) | 145 (50.7%) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; Check-UP = Checklists and Upright Positioning in Endotracheal Intubation of Critically Ill Patients; PrePARE = Preventing Cardiovascular Collapse with Administration of Fluid Resuscitation before Endotracheal Intubation; PreVent = Preventing Hypoxemia with Manual Ventilation during Endotracheal Intubation.
Data are presented as median (interquartile range) or count (percent).
Includes chronic kidney disease and end-stage renal disease.
Indications are not mutually exclusive, and subjects may have had more than one indication selected.
Subjects may have received more than one induction agent.
Includes 62 patients who received ketamine, 3 patients who received lidocaine, and 2 with induction agent marked as “other.”
Results
A total of 815 patients were included in the analysis, with 109 events in 529 patients in PreVent and PrePARE and 58 events in 286 patients in Check-UP. Differences in characteristics between the training and validation cohorts are shown in Table 1.
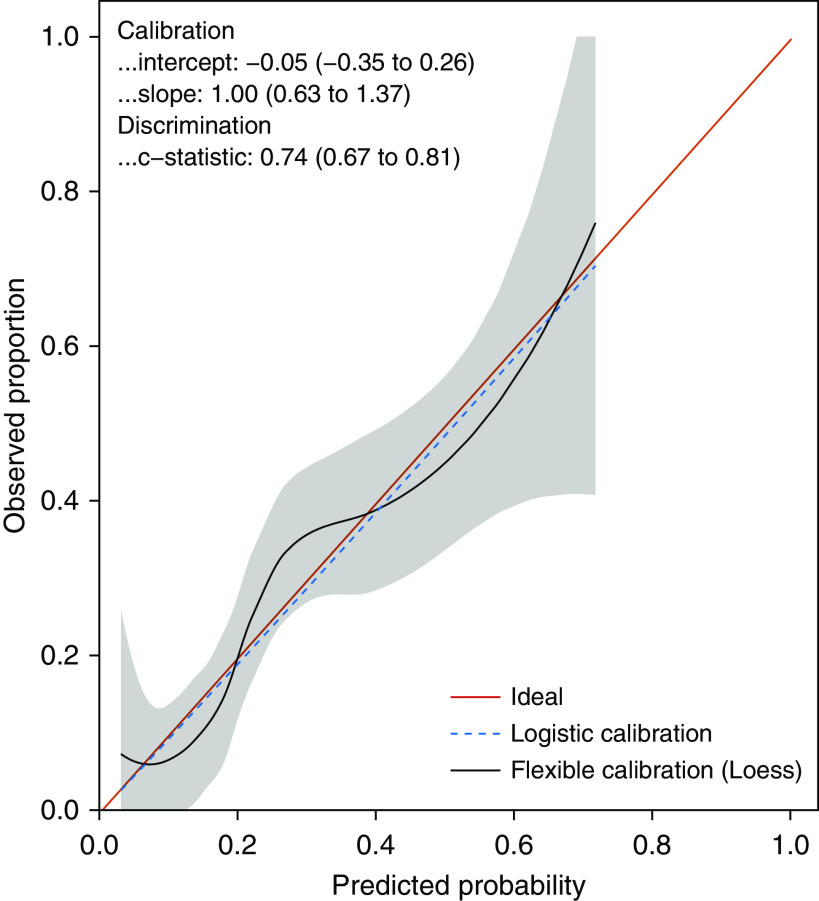
The final elastic net prediction model derived in the PrePARE/PreVent trials excluded 5 of the 20 possible predictor variables and had an AUC of 0.74 (95% confidence interval [CI], 0.67 to 0.81) in the Check-UP trial. Restricted cubic splines did not improve model discrimination in the training dataset and were not included in the final model. Figure 1 shows a variable importance plot for this model, which illustrates which variables contributed the most to model predictions on a 0–100 scale by using the absolute value of the variable coefficients and then scaling to a maximum value of 100. The model was well calibrated with a calibration intercept of −0.05 (95% CI, −0.35 to 0.26), a calibration slope of 0.99 (95% CI, 0.63 to 1.37), and an unreliability index of P = 0.96 (Figure 2).
Figure 1.
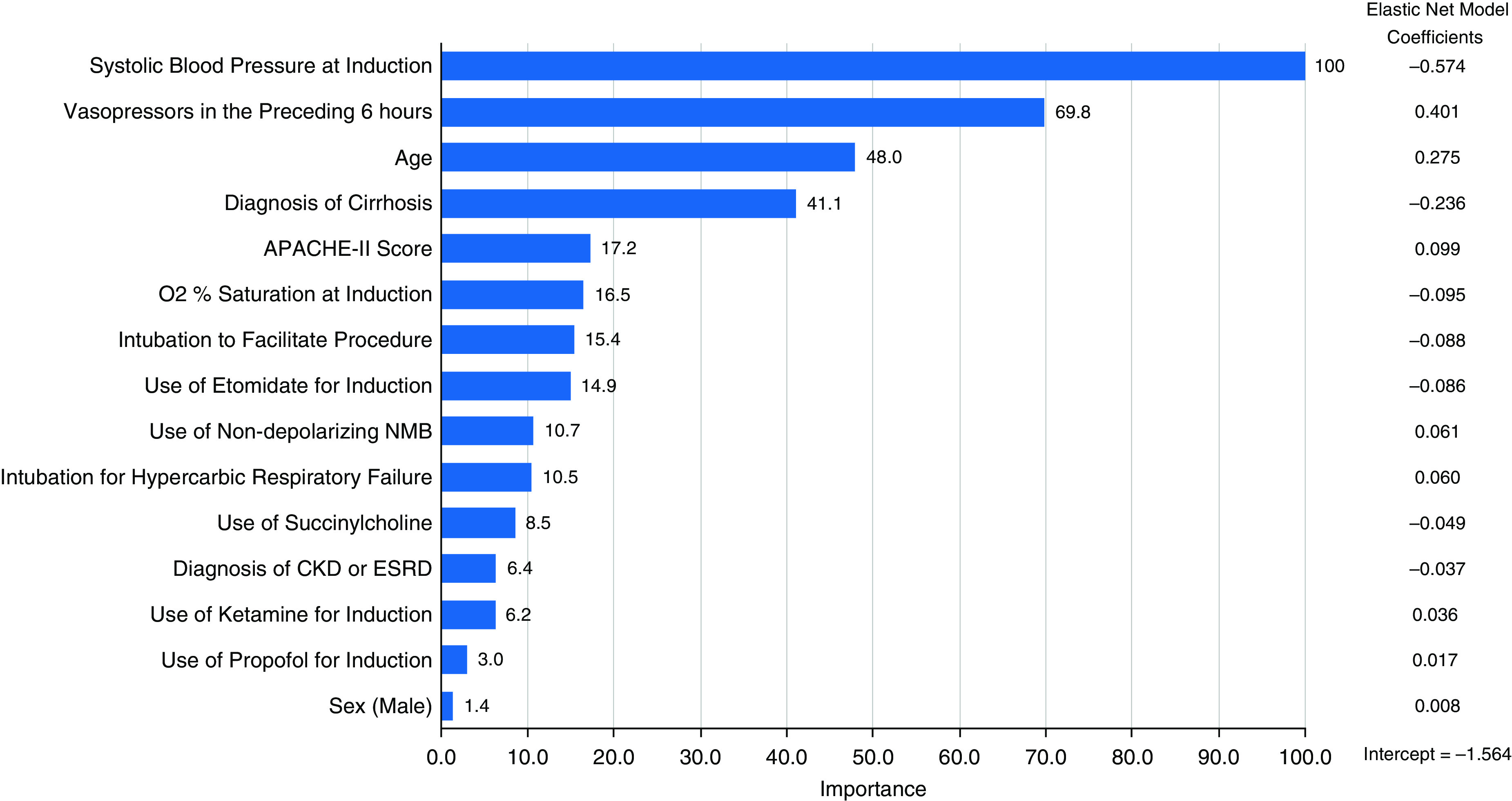
Importance of the predictor variables in the elastic net regression model, scaled to a maximum of 100 by using the absolute value of the variable coefficients and then scaling to a maximum value of 100. Coefficients (logarithmic odds scale) for the variables in the elastic net model are shown on the right. APACHE II = Acute Physiology and Chronic Health Evaluation II; CKD = chronic kidney disease; ESRD = end-stage renal disease; NMB = neuromuscular blockade.
Figure 2.
Model calibration in the Check-UP (Checklists and Upright Positioning in Endotracheal Intubation of Critically Ill Patients) trial (validation cohort), with a calibration intercept of −0.05 (95% confidence interval [CI], −0.35 to 0.26), a calibration slope of 0.99 (95% CI, 0.63 to 1.37), and an unreliability index P = 0.96.
Several variables were significantly different between the training and validation cohorts, including age, sex, prevalence of chronic heart disease, SBP before intubation, and selection of induction and paralytic agents (Table 1). The most important predictors of cardiovascular collapse in the combined dataset, as determined by the elastic net regression model, were SBP at induction, preintubation vasopressors, age, and cirrhosis (Figure 1).
Discussion
In this secondary analysis of data from three large, pragmatic trials, we developed a model with moderate discrimination for predicting cardiovascular collapse during tracheal intubation in critically ill adults. Accurately quantifying patients’ baseline risk of an outcome is fundamental to addressing patient heterogeneity in critical care clinical research (13). Our model predicting cardiovascular collapse may be used to analyze heterogeneity of treatment effect in ongoing trials evaluating interventions to prevent cardiovascular collapse during tracheal intubation of critically ill adults (NCT03787732). In addition, the risk factors identified may be used to selectively enroll patients at high risk of cardiovascular collapse in future trials.
Of the variables tested, SBP at induction, preintubation vasopressors, and age were important independent predictors of cardiovascular collapse. These results are not surprising, but our model helps to quantify the relative degree to which these factors influence risk for cardiovascular collapse. Comorbid cirrhosis, surprisingly, was associated with a reduced risk of cardiovascular collapse. It is uncertain whether this represents differences in the reasons for which patients with cirrhosis undergo intubation, differences in clinician management of vasopressors for this group with chronic vasodilation, or another reason. Choice of induction and paralytic agent, although debated clinically, had little association with risk for cardiovascular collapse in the overall model.
Our study has several strengths: use of high-quality data from clinical trials; validation of the model in an external cohort; and use of a robust, unified statistical approach to perform both variable selection and coefficient estimation. The study also has several weaknesses. Data were derived from a limited number of ICUs in the United States, and the results may not be generalizable to other settings. Cardiovascular collapse is a composite outcome; patients and providers do not perceive low SBP and vasopressor receipt to be equivalent to cardiac arrest and death, and the criteria for vasopressor administration were not predefined in the trials. Because of the pragmatic nature of the trials from which these data were collected, we do not have details of potentially important predictors, such as ejection fraction and induction medication doses. The accuracy of our model when calculated from passively collected electronic health record data or when simplified for use in clinical care is unknown. Therefore, use of our model as a bedside tool for prognostication should be discouraged until further validation studies are performed.
In summary, a model using preintubation risk factors can accurately predict cardiovascular collapse during tracheal intubation of critically ill adults. The model developed and validated in the present study may be used for analysis of heterogeneity of treatment effect or prognostic enrichment of future clinical trials.
Supplementary Material
Footnotes
Supported in part by the Arthur P. and Lisa Wheeler Scholarship Research Grant (J.D.C.). The clinical trials on which this secondary analysis is based used the Research Electronic Data Capture (REDCap) tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from the National Center for Advancing Translational Sciences/National Institutes of Health). J.D.C. was supported in part by the U.S. National Heart, Lung, and Blood Institute (grant K12HL133117). M.W.S. was supported in part by the National Heart, Lung, and Blood Institute (grant K23HL143053). M.M.C. is supported in part by the National Institute of General Medical Sciences (grant R01 GM123193). The funding institutions had no role in 1) conception, design, or conduct of the study; 2) collection, management, analysis, interpretation, or presentation of the data; or 3) preparation, review, or approval of the manuscript.
Author Contributions: S.J.H. designed the study, participated in the analysis, and prepared the manuscript. J.D.C., T.W.R., and M.W.S. designed the study, managed the database, participated in the analysis, and revised the manuscript. D.R.J., D.W.R., J.D., D.J.V., and J.R.W. collected data and revised the manuscript. M.M.C. designed the study, performed the analysis, and prepared the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: for the Pragmatic Critical Care Research Group
References
- 1.Janz DR, Casey JD, Semler MW, Russell DW, Dargin J, Vonderhaar DJ, et al. PrePARE Investigators; Pragmatic Critical Care Research Group. Effect of a fluid bolus on cardiovascular collapse among critically ill adults undergoing tracheal intubation (PrePARE): a randomised controlled trial. Lancet Respir Med. 2019;7:1039–1047. doi: 10.1016/S2213-2600(19)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006;34:2355–2361. doi: 10.1097/01.CCM.0000233879.58720.87. [DOI] [PubMed] [Google Scholar]
- 3.Heffner AC, Swords DS, Nussbaum ML, Kline JA, Jones AE. Predictors of the complication of postintubation hypotension during emergency airway management. J Crit Care. 2012;27:587–593. doi: 10.1016/j.jcrc.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Lin CC, Chen KF, Shih CP, Seak CJ, Hsu KH. The prognostic factors of hypotension after rapid sequence intubation. Am J Emerg Med. 2008;26:845–851. doi: 10.1016/j.ajem.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Janz DR, Semler MW, Joffe AM, Casey JD, Lentz RJ, deBoisblanc BP, et al. Check-UP Investigators; Pragmatic Critical Care Research Group. A multicenter randomized trial of a checklist for endotracheal intubation of critically ill adults. Chest. 2018;153:816–824. doi: 10.1016/j.chest.2017.08.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semler MW, Janz DR, Russell DW, Casey JD, Lentz RJ, Zouk AN, et al. Check-UP Investigators; Pragmatic Critical Care Research Group A multicenter, randomized trial of ramped position vs sniffing position during endotracheal intubation of critically ill adults Chest 2017152712–722.28487139 [Google Scholar]
- 7.McKown AC, Casey JD, Russell DW, Joffe AM, Janz DR, Rice TW, et al. Risk factors for and prediction of hypoxemia during tracheal intubation of critically ill adults. Ann Am Thorac Soc. 2018;15:1320–1327. doi: 10.1513/AnnalsATS.201802-118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey JD, Janz DR, Russell DW, Vonderhaar DJ, Joffe AM, Dischert KM, et al. PreVent Investigators and the Pragmatic Critical Care Research Group. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019;380:811–821. doi: 10.1056/NEJMoa1812405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YF, Wong TW, Lau CC. Midazolam is more likely to cause hypotension than etomidate in emergency department rapid sequence intubation. Emerg Med J. 2004;21:700–702. doi: 10.1136/emj.2002.004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin C, Samuel J, Hu TC. Life-threatening hypotension associated with emergency intubation and the initiation of mechanical ventilation. Am J Emerg Med. 1994;12:425–428. doi: 10.1016/0735-6757(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 12.Miller ME, Langefeld CD, Tierney WM, Hui SL, McDonald CJ. Validation of probabilistic predictions. Med Decis Making. 1993;13:49–58. doi: 10.1177/0272989X9301300107. [DOI] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192:1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.