Abstract
Background
Depression is associated with an increased risk of cardiovascular disease in human immunodeficiency virus (HIV). We hypothesized that reducing depressive symptoms would improve HIV-related cardiovascular risk.
Methods
We conducted a single-center, randomized (1:1), controlled, parallel-group, assessor-blinded, pilot trial comparing Beating the Blues US (BtB)—an evidence-based, 8-session, internet cognitive-behavioral therapy for depression—with usual care (UC) in HIV-positive participants receiving virologically suppressive antiretroviral therapy and with Patient Health Questionnaire (PHQ)-9 scores ≥10. The primary endpoint was change in brachial artery flow-mediated dilation (FMD) at 12 weeks. Secondary endpoints were FMD change at 24 weeks and inflammation, coagulation, and metabolic biomarker changes at 12 and 24 weeks.
Results
Fifty-four participants were randomized (27 in each arm). Mean reductions in PHQ-9 scores were significantly greater with BtB versus UC at 12 weeks (−5.60 vs −1.52; P = .007) and 24 weeks (−6.00 vs −1.38; P = .008); reductions in the Hopkins Symptom Checklist Depression Scale-20 scores were also significantly greater with BtB versus UC at 24 weeks (−0.72 vs −0.35; P = .029). Changes in FMD between arms were not significantly different at 12 or 24 weeks. Significantly larger reductions in soluble (s)CD14 and sCD163 with BtB versus UC were found at 12 and 24 weeks, respectively.
Conclusions
Compared with UC, internet cognitive-behavioral therapy using BtB resulted in greater improvements in depressive symptoms and monocyte activation markers but did not improve FMD in this pilot trial. These data support performing larger studies to determine the potential salutatory effects of behavioral therapies for depression on HIV-related inflammation.
Keywords: cognitive-behavioral therapy, depression, endothelial function, HIV, inflammation
In this pilot randomized trial, we found that internet cognitive-behavioral therapy, compared with usual care, improved depressive symptoms and monocyte activation, but not endothelial function, in HIV-positive patients.
Depression has been linked to a higher risk of cardiovascular disease (CVD) in the general population [1, 2], and preliminary evidence suggests that successful depression treatment may reduce the risk of first CVD events [3]. Although behavioral factors (smoking, poor medication adherence, sedentary lifestyle) associated with depression may partially account for this increased risk, depression may also contribute to atherosclerosis development through its association with heightened systemic inflammation [4, 5] and endothelial dysfunction [6]. Inflammation, and specifically monocyte activation [7, 8], is greater in people with human immunodeficiency virus (PWH) compared with human immunodeficiency virus (HIV)-negative people, even when PWH are receiving virologically suppressive antiretroviral therapy (ART) [9]. Moreover, greater circulating levels of markers of systemic inflammation (high-sensitivity C-reactive protein [hsCRP], interleukin-6 [IL-6]) [10] and monocyte activation (soluble [s]CD14, sCD163) [11, 12] are associated with incident HIV-CVD. Thus, it stands to reason that depression, possibly through greater levels of systemic inflammation and worse endothelial function, may contribute to the increased risk of CVD observed in PWH [13]. In fact, we recently demonstrated an increased risk of acute myocardial infarction [14] and congestive heart failure [15] in PWH with depression versus those without depression. As such, we hypothesized that treating depressive symptoms would reduce HIV-CVD risk, specifically through improved endothelial function measured as brachial artery flow-mediated dilation (FMD) and through a reduction in systemic inflammation. To test this hypothesis, we performed a pilot, randomized, controlled trial comparing an internet cognitive-behavioral therapy (CBT) program for depression called Beating the Blues US (BtB) with usual care (UC) for depression to improve FMD and reduce markers of systemic inflammation in PWH on ART.
METHODS
Study Design
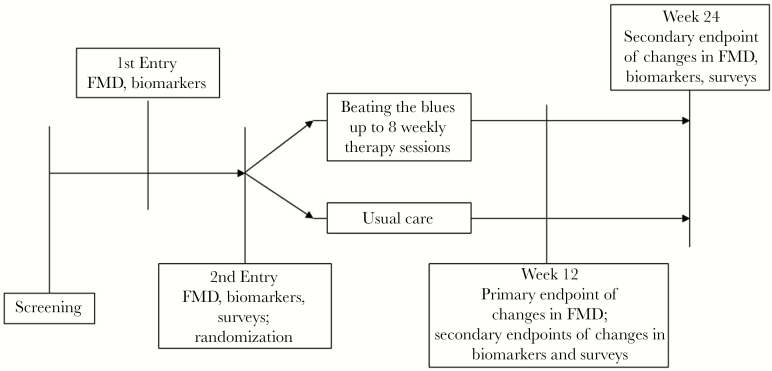
The design of our 24-week pilot trial is shown in Figure 1. The blood specimens obtained during the trial were archived for later batch testing of biomarkers of interest, and FMD of the brachial artery was measured as a marker of physiologic endothelial function. Because we have observed a possible “regression to the mean” effect in FMD in our previous trials with high outlier values at baseline [16, 17], we chose to perform 2 Entry visits, spaced between 1 and 15 days apart, for which biomarker and FMD measurements were averaged to obtain more stable baseline estimates. At the Second Entry Visit, participants were randomized 1:1, stratified by current use of antidepressant medications (yes/no), to either BtB or UC. The randomization sequence was computer-generated with study arm allocation assigned centrally. Participants then underwent similar procedures at Week 12 and Week 24 after the Second Entry Visit. Mental health questionnaires were also completed at the Second Entry, Week 12, and Week 24 Visits. Fasting and smoking cessation for at least 8 hours were required before each study visit. The endpoint assessors were blinded to study arm assignment. Those randomized to BtB underwent up to 8 weekly therapy sessions between the Second Entry Visit and Week 12.
Figure 1.
Design of the 24-week, pilot, randomized, controlled trial showing study visits and associated study measurements. FMD, flow-mediated dilation.
All participants provided written, informed consent. The study was conducted in accordance with the Helsinki Declaration, was approved by the Indiana University (IU) Institutional Review Board, and was registered at ClinicalTrials.gov (NCT02309372).
Study Population
All participants were recruited from the HIV clinics at the IU Medical Center. All were HIV-positive, at least 18 years of age, had received ART (of any kind) for at least 1 year before screening, had an HIV-1 ribonucleic acid (RNA) level <75 copies/mL at screening, and had a Patient Health Questionnaire (PHQ)-9 score ≥10 at screening, which indicates clinically relevant depression symptoms [18]. Exclusion criteria included active suicidality, history of schizophrenia or bipolar disorder, known CVD or other proinflammatory condition (except for hepatitis B or C coinfection), systolic/diastolic blood pressures >160/110 mmHg, estimated glomerular filtration rate <50 mL/min per 1.73 m2, screening glucose ≥140 mg/dL or HgbA1c >8.0%, screening total cholesterol >240 mg/dL, and pregnancy or breastfeeding during the study. Use of any other form of depression treatment was allowed throughout the trial.
Study Intervention
Beating the Blues US (UPMC Health Plan; see www.beatingthebluesus.com for a video tutorial) is an empirically supported, stand-alone, internet cognitive-behavioral treatment program for depression appropriate for adults with little computer experience and at least a 5th–6th grade reading level [19]. Beating the Blues US utilizes an interactive, multimedia format to deliver eight 50-minute, weekly sessions, the structure and content of which mirror face-to-face CBT. Although sessions are tailored to each patient’s problems, general topics include challenging dysfunctional thoughts, activity scheduling, problem solving, graded exposure, task breakdown, sleep management, and relapse prevention. Patients are also assigned tailored home work.
Beating the Blues US sessions occurred at a location selected by the participant where they could access the internet or an investigator’s (J.C.S.) laboratory. Participant preference determined the location. Regardless of location, study personnel provided support to participants working through BtB by scheduling sessions, identifying and collecting homework, assisting with technical issues, and addressing questions. Participants worked alone through the BtB sessions at their own pace.
We chose to use BtB as our intervention, because it is efficacious for depression, acceptable to patients [19], and scalable because of its internet delivery. Beating the Blues US has considerable empirical support, including positive clinical trials of 274 [20, 21] and 704 [22] primary care patients. Beating the Blues US is a potent intervention, with effect sizes comparable to face-to-face CBT [20, 21]. Moreover, we chose a behavioral intervention to avoid drug interactions between antidepressants and ART and to avoid confounding by potentially direct effects of antidepressants (as opposed to effects mediated through depression) on our study endpoints.
Those randomized to UC did not receive any study-provided treatments for depression. The UC participants were informed of their depression diagnosis and were encouraged to follow-up with their primary care or HIV provider. We also sent their primary provider a letter immediately after the Second Entry Visit indicating that their patient had a depressive disorder and was randomized to UC; the letter also included a list of local mental health services. Otherwise, there was no formal interaction with the UC participants between the Second Entry Visit and the Week 12 Visit. No restrictions on receipt of new depression therapies were placed on any study participant at any time; any new or changes in depression treatments (pharmacologic or behavioral) were recorded at each study visit.
Study Endpoints
The primary endpoint was change in FMD at Week 12. Secondary endpoints were change in FMD at Week 24 and changes in the biomarkers at Weeks 12 and 24.
Flow-mediated dilation was measured per consensus guidelines [23] using a GE LOGIQe high-resolution ultrasound with a 15-MHz vascular transducer. A blood pressure cuff was placed around the forearm and inflated to 250 mmHg for 5 minutes. Changes in brachial artery diameter from preinflation to 60 and 90 seconds after cuff deflation were measured with the greater change in diameter recorded for analysis. Nitroglycerin-mediated dilation (NTGMD) was also assessed as an internal control. Measurements were made using AccessPoint 2011 software (version 8.2; Freeland Systems).
Biomarkers of systemic and vascular inflammation (hsCRP, R&D Systems), IL-6 (R&D Systems), soluble vascular cell adhesion molecule-1 ([sVCAM-1] R&D Systems), monocyte immune activation (sCD14 and sCD163, both from R&D Systems), and coagulation (D-dimer, Thermo Scientific; fibrinogen, Abnova) were measured by standard enzyme-linked immunosorbent assay kits per manufacturer instructions at each study visit. Also measured were cholesterol fractions, glucose, and insulin levels. All assays were performed by the Center for Diabetes and Metabolic Diseases Translational Core at the IU School of Medicine.
Changes in depression symptom severity scores were assessed using both the PHQ-9 and the Hopkins Symptom Checklist Depression Scale (SCL)-20. Changes in smoking status, antiretroviral and antidepressant medications, and new diagnoses were ascertained at each study visit.
Statistical Analysis
Continuous variables were summarized by treatment groups using descriptive statistics. Categorical variables were summarized using frequency counts and percentages. Student’s t tests were performed to compare changes for the primary and secondary endpoints between the BtB group (both the full BtB group and the subset of those who completed at least 6 sessions [BtB6-8]) and the UC group from Entry to both Week 12 and Week 24. Because this was a pilot trial, no corrections for multiple testing were performed to identify potential relationships of interest to be verified in future larger trials. Pearson correlations were also calculated between FMD and the depression severity measures. Two-sided P < .05 were considered statistically significant.
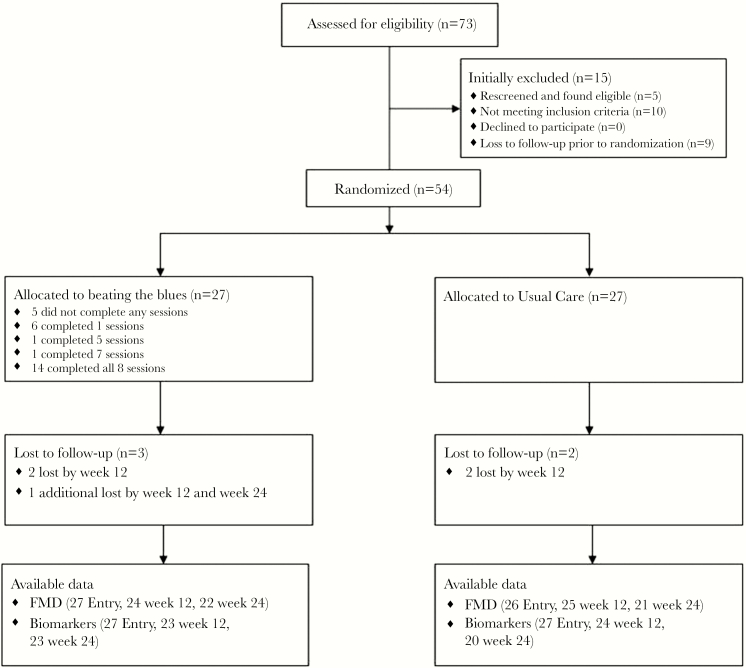
Due to the few missing FMD and SCL-20 data (Figure 2), multiple imputation methods using SAS PROC MI were used to substitute imputed values where FMD, baseline diameter, and SCL-20 were missing at each time point, based on the nonmissing values from other participants and all participants’ age, sex, and race.
Figure 2.
Flow of the participants through the trial. FMD, flow-mediated dilation.
Because we specifically wished to evaluate the effects of covariates on the primary outcomes, we also performed multiple linear regressions adjusted for study group on the changes in FMD. In these models, the indicator variable for study arm was kept regardless of its significance, whereas other potential baseline covariates were then included. All analytic assumptions were verified and all analyses were performed with SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
The characteristics of the study groups at Entry are shown in Table 1. The study group overall consisted primarily of black men. Overall, the participants had high CD4 cell counts with two thirds receiving antidepressant medications. The types of antidepressants received were similar in both groups (Supplementary Table 1). All participants had HIV-1 RNA <75 copies/mL, both at screening and at the Second Entry Visit at the time of randomization. The majority of participants were receiving emtricitabine/tenofovir combined with integrase inhibitors (Supplementary Table 2), with similar numbers in each group receiving efavirenz and abacavir. The BtB and UC groups were similar except for somewhat lower PHQ-9 scores in the UC group (although the SCL-20 scores were similar between groups). Also shown in Table 1 are the characteristics of the BtB6-8 subgroup of 15 participants. These latter participants did not appreciably differ from the total 27 randomized to BtB.
Table 1.
Characteristics of the Study Groups at Entrya
| Characteristic | Usual Care (n = 27) |
Beating the Blues (n = 27) | Beating the Blues6-8 (n = 15) |
Total (n = 54) |
|---|---|---|---|---|
| Age, years | 45.6 (11.9) | 44.6 (9.8) | 47.5 (10.1) | 45.1 (10.8) |
| Female sex | 5 (19) | 4 (15) | 4 (27) | 9 (17) |
| Black race | 15 (56) | 21 (78) | 12 (80) | 36 (67) |
| Current smoker | 12 (44) | 10 (37) | 2 (13.3) | 22 (41) |
| CD4 cell count, µL | 743 (396) | 713 (243) | 760 (245) | 728 (325) |
| Body mass index, kg/m2 | 30.1 (6.7) | 30.0 (7.0) | 30.4 (8.7) | 30.1 (6.7) |
| Systolic blood pressure, mmHg | 123.5 (11.9) | 127.7 (12.9) | 127.3 (13.2) | 125.6 (12.4) |
| Diastolic blood pressure, mmHg | 77.7 (9.7) | 80.0 (9.8) | 78.4 (8.9) | 77.4 (9.7) |
| Hemoglobin A1C, % | 5.4 (0.7) | 5.6 (0.5) | 5.7 (0.6) | 5.5 (0.6) |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 88.5 (23.5) | 98.9 (18.4) | 91.9 (17.2) | 93.7 (21.5) |
| Triglycerides, mmol/L | 1.27 (0.52) | 1.44 (0.97) | 1.48 (1.15) | 1.35 (0.77) |
| Total cholesterol, mmol/L | 5.02 (0.96) | 4.83 (1.02) | 5.02 (1.09) | 4.92 (0.99) |
| High-density lipoprotein cholesterol, mmol/L | 1.26 (0.34) | 1.21 (0.38) | 1.20 (0.43) | 1.23 (0.36) |
| Low-density lipoprotein cholesterol, mmol/L | 3.53 (1.00) | 3.21 (1.05) | 3.47 (1.09) | 3.37 (1.03) |
| Glucose, mmol/L | 5.77 (0.57) | 5.73 (0.71) | 5.79 (0.84) | 5.75 (0.64) |
| Insulin, µU/mL | 13.93 (10.48) | 16.47 (16.25) | 18.28 (19.89) | 15.20 (13.60) |
| HOMA-IR | 3.72 (2.90) | 4.50 (5.00) | 5.12 (6.22) | 4.1 (4.1) |
| PHQ-9 score | 14.04 (4.32) | 17.13 (4.96) | 17.13 (5.08) | 15.58 (4.87) |
| SCL-20 score | 2.09 (0.63) | 2.27 (0.92) | 2.27 (1.06) | 2.18 (0.78) |
| Antidepressant medication use | 19 (70) | 18 (67) | 10 (67) | 37 (69) |
| hsCRP, mg/L | 4.32 (3.84) | 4.65 (4.46) | 5.53 (5.02) | 4.49 (4.12) |
| IL-6, pg/mL | 2.59 (1.65) | 2.80 (2.15) | 3.76 (2.36) | 2.69 (1.90) |
| D-dimer, ng/mL | 2430 (845) | 2536 (878) | 2880 (882) | 2483 (855) |
| Fibrinogen, µg/mL | 2756 (756) | 2707 (667) | 2094 (770) | 2731 (706) |
| sCD14, ng/mL | 2233 (621) | 2325 (849) | 2302 (829) | 2279 (732) |
| sCD163, ng/mL | 43.30 (21.27) | 46.61 (12.03) | 50.77 (10.14) | 44.86 (17.19) |
| sVCAM-1, ng/mL | 378 (108) | 375 (135) | 367 (84) | 376 (121) |
Abbreviations: HOMA-IR, homeostasis model assessment-insulin resistance; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; PHQ-9, Patient Health Questionnaire 9; s, soluble; SCL-20, Hopkins symptom checklist depression scale-20; sVCAM-1, soluble vascular cell adhesion molecule-1.
aData presented as mean (standard deviation) or n (%). The Beating the Blues6-8 subgroup comprised those randomized to Beating the Blues and completed at least 6 of the 8 treatment sessions.
The flow of the participants through the trial is shown in Figure 2. The 54 participants were evenly allocated to each study group. Of the 27 in each study arm, 25 completed the Week 12 visit; 24 and 25 completed the Week 24 study visit in the BtB and UC arms, respectively. There were few losses in FMD (1 at Week 12 and 2 and Week 24 in BtB; 4 at Week 24 in UC) and biomarker data (2 at Week 12 and 1 at Week 24 in BtB; 1 at Week 12 and 5 at Week 24 in UC) due to unreadable imaging and sample unavailability as outlined in Figure 2.
Human immunodeficiency virus-1 RNA levels increased to ≥75 copies/mL at Week 12 in 4 BtB participants (actual values: 154, 225, 664, and 5070 copies/mL) and 3 UC participants (actual values: 111, 121, and 822 copies/mL). At Week 24, HIV-1 RNA levels were ≥75 copies/mL in 4 BtB participants (actual values: 84, 95, 153, and 24 100 copies/mL) and 1 UC participant (actual value: 3950 copies/mL). Changes in ART during the trial were infrequent with 2 switches from efavirenz to bictegravir in the UC arm and 2 switches from efavirenz to bictegravir and elvitegravir-cobicistat in the BtB arm (1 each). No changes in antidepressant medications were observed during the trial.
Depression Symptoms Severity Changes
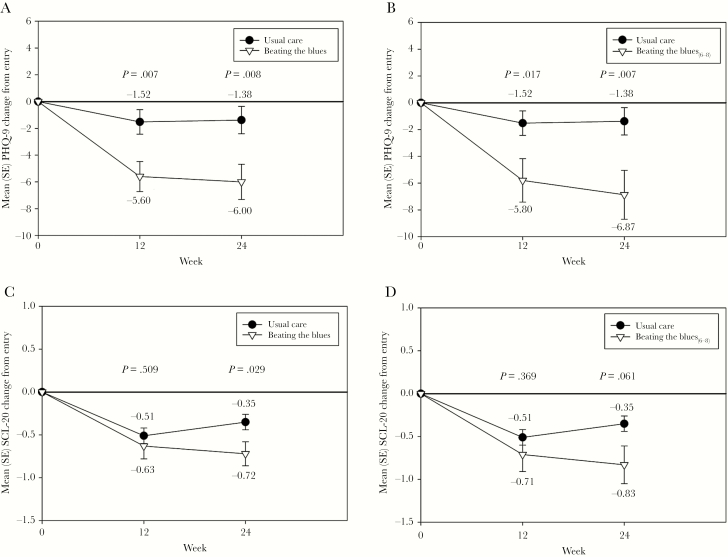
The PHQ-9 and SCL-20 scores decreased (ie, improved) in both study groups as shown in Figure 3. The PHQ-9 scores improved significantly more in the BtB group compared with the UC group at Week 12 (P = .007) and Week 24 (P = .008). The SCL-20 scores also improved more in the BtB group compared with UC at Weeks 12 and 24, but only significantly so at Week 24 (P = .029). Even greater decreases in both scores were observed in the BtB6-8 subgroup compared with UC.
Figure 3.
Changes in Patient Health Questionnaire (PHQ)-9 and Hopkins Symptom Checklist Depression Scale (SCL-20) depression severity symptom scores during the trial between the Beating the Blues US (BtB) and Usual Care (UC) groups (A and C) and the BtB6-8 subgroup and UC group (B and D). SE, standard error.
Vascular Function Changes
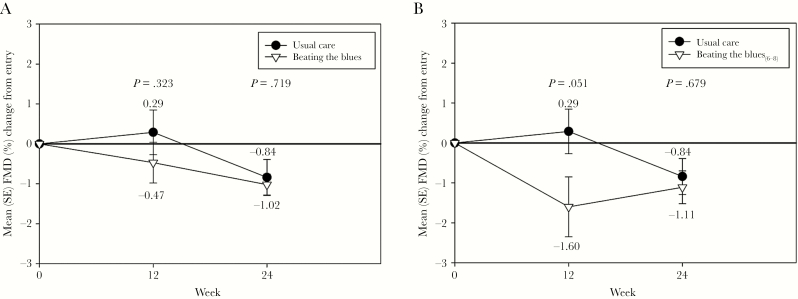
The values of the vascular measurements are shown in Table 2. Brachial artery diameters, NTGMD, and reactive hyperemia velocity time integrals were stable in each group during the trial. Flow-mediated dilation decreased (ie, worsened) in both groups, with no significant differences between arms in unadjusted analyses (Figure 4).
Table 2.
Vascular Function Parameters of the Study Groups During the Triala
| Vascular Parameter | Time Point | Usual Care | Beating the Blues | Beating the Blues6-8 | Total |
|---|---|---|---|---|---|
| Baseline Diameter, cm | |||||
| Entry | 0.42 (0.06) | 0.45 (0.06) | 0.43 (0.06) | 0.44 (0.06) | |
| Week 12 | 0.42 (0.06) | 0.46 (0.05) | 0.44 (0.06) | 0.44 (0.06) | |
| Week 24 | 0.42 (0.06) | 0.46 (0.06) | 0.44 (0.06) | 0.44 (0.06) | |
| FMD, % | |||||
| Entry | 3.39 (1.78) | 2.77 (1.76) | 3.26 (2.00) | 3.08 (1.78) | |
| Week 12 | 3.68 (2.99) | 2.30 (2.19) | 1.66 (2.39) | 2.99 (2.69) | |
| Week 24 | 2.56 (2.51) | 1.75 (1.19) | 2.15 (1.24) | 2.15 (1.99) | |
| NTGMD, % | |||||
| Entry | 15.64 (4.99) | 14.12 (5.55) | 14.76 (6.26) | 14.88 (5.28) | |
| Week 12 | 17.47 (6.87) | 14.70 (5.58) | 15.04 (6.49) | 16.05 (6.32) | |
| Week 24 | 15.60 (6.33) | 12.89 (4.31) | 12.87 (4.49) | 14.25 (5.51) | |
| RHVTI, cm | |||||
| Entry | 0.64 (0.17) | 0.53 (0.13) | 0.50 (0.10) | 0.58 (0.16) | |
| Week 12 | 0.59 (0.19) | 0.51 (0.18) | 0.49 (0.17) | 0.56 (0.19) | |
| Week 24 | 0.61 (0.23) | 0.52 (0.16) | 0.52 (0.17) | 0.56 (0.20) |
Abbreviations: FMD, flow-mediated dilation; NTGMD, nitroglycerin-mediated dilation; RHVTI, reactive hyperemia velocity time integral.
aData presented as mean (standard deviation). The Beating the Blues6-8 subgroup comprised those randomized to Beating the Blues and completed at least 6 of the 8 treatment sessions.
Figure 4.
Changes in flow-mediated dilation (FMD) during the trial between the Beating the Blues (BtB) and Usual Care (UC) groups (A) and the BtB6-8 subgroup and UC groups (B). SE, standard error.
However, in models adjusted for (1) Entry FMD, (2) Entry FMD and persistent HIV-1 RNA level <75 copies/mL during the trial, (3) Entry FMD and Entry ART type (protease inhibitor vs nonnucleoside reverse-transcriptase inhibitor vs integrase strand transfer inhibitor), (4) Entry FMD and Entry SCL-20, and (5) Entry FMD, Entry SCL-20, and Entry use of antidepressant medications, FMD at Week 12 (but not at Week 24) was significantly lower in the BtB6-8 subgroup compared with UC (all P < .05). The only adjusted model in which significantly lower FMD was observed at Week 12 in the overall BtB group versus UC was that which included Entry FMD and ART type; no significant differences were otherwise found between the 2 main study groups in any other model at Week 12 or at Week 24.
Entry FMD and SCL-20 scores were significantly correlated (rho = −0.28; P = .04); Entry FMD and PHQ-9 scores were not correlated (rho = −0.082; P = .56). There were no significant correlations between changes in either SCL-20 or PHQ-9 with changes in FMD at Weeks 12 or 24 in the overall study cohort or in either study group (all rho <|0.18|; all P > .2).
Inflammation, Coagulation, and Metabolic Biomarker Changes
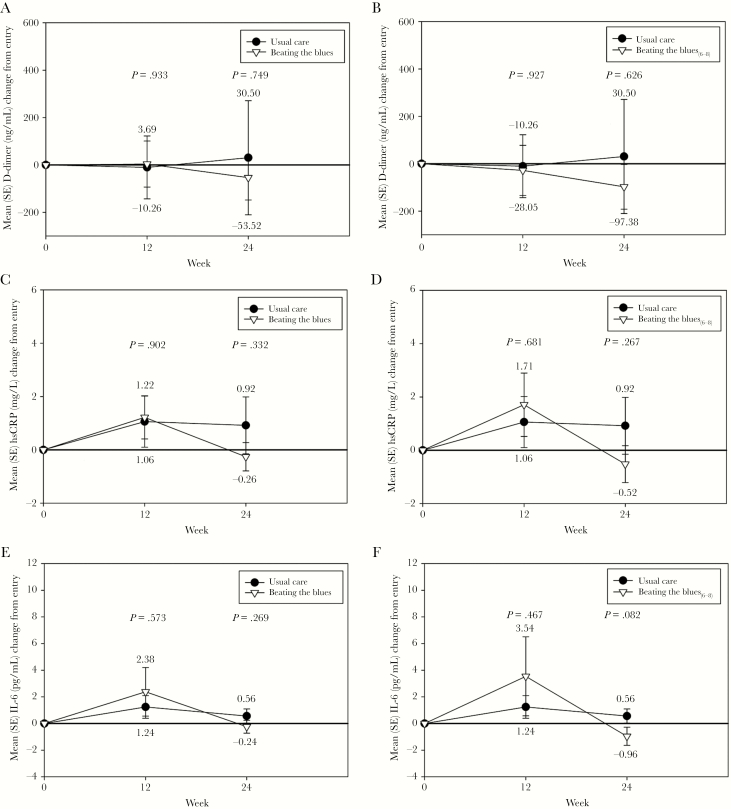
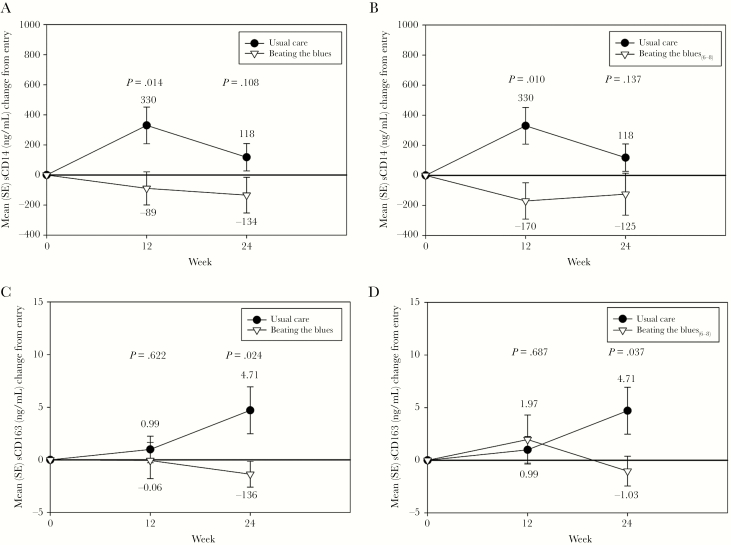
As shown in Figure 5, there were no significant differences in the changes between study groups in hsCRP, IL-6, or D-dimer at either Week 12 or Week 24. In addition, no significant differences were found between the BtB6-8 subgroup and UC for these biomarkers. There were significantly greater reductions observed in sCD14 both in the BtB and BtB6-8 subgroup versus UC at Week 12; these differences persisted to a somewhat smaller degree at Week 24 and thus were no longer significant (Figure 6). There were no significant differences in sCD163 between groups at Week 12, although there were significantly lower levels of this biomarker at Week 24 in the BtB and BtB6-8 subgroup compared with UC (Figure 6). There were no significant differences between study groups during the trial in either fibrinogen or sVCAM-1 levels (data not shown).
Figure 5.
Changes in high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), and D-dimer during the trial between the Beating the Blues (BtB) and Usual Care (UC) groups (A, C, and E) and the BtB6-8 subgroup and UC groups (B, D, and F). SE, standard error.
Figure 6.
Changes in soluble (s)CD14 and sCD163 during the trial between the Beating the Blues (BtB) and Usual Care (UC) groups (A and C) and the BtB6-8 subgroup and UC group (B and D). SE, standard error.
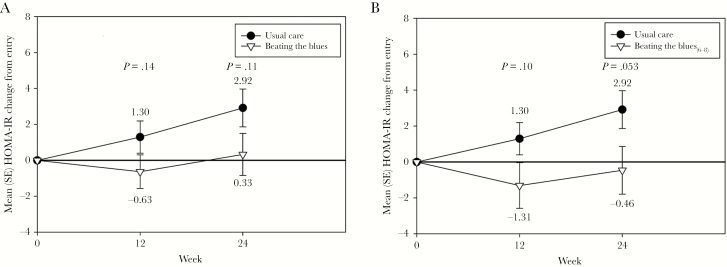
We did not observe significant differences between groups at Week 12 or 24 in cholesterol fractions (data not shown). As shown in Figure 7, there were nonsignificant improvements in Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) values in the BtB versus UC at Weeks 12 and 24 and between the BtB6-8 subgroup versus UC at Weeks 12 and 24. We found significant improvements in systolic blood pressure with BtB compared with UC at Week 12 (−5.5 vs 2.8 mmHg; P = .009), but this did not persist at Week 24 (−3.9 vs 2.8 mmHg; P = .15). We did not find significant differences in diastolic blood pressures between BtB and UC at either Week 12 (−3.1 vs 1.3 mmHg; P = .10) or Week 24 (−4.0 vs 1.1 mmHg; P = .11).
Figure 7.
Changes in Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) during the trial between the Beating the Blues (BtB) and Usual Care (UC) groups (A) and the BtB6-8 subgroup and UC group (B). SE, standard error.
DISCUSSION
To our knowledge, this is the first trial assessing the efficacy of an internet CBT program for depression to improve CVD risk in PWH. We found that Beating the Blues US provided clinically relevant [24] and statistically significant reductions in PHQ-9 scores soon after treatment was completed (Week 12) and in PHQ-9 and SCL-20 scores at least 12 weeks afterwards (Week 24). These improvements are similar to those found with BtB [20, 21] and with antidepressant medications [25] in general population studies. It is interesting to note that these improvements were even more pronounced in the 15 participants who completed at least 6 of the planned 8 sessions (subgroup BtB6-8). Of note, these significant improvements occurred despite the number of participants in the intervention arm who did not take up BtB (11 of 27 completed only 0 or 1 session), which is similar to the nonuse rate found in BtB trials in other populations [20, 21]. Given that this depression treatment modality is highly practical for both patients and healthcare systems (it can be completed remotely, typically in the patient’s home), does not require access to behavioral health professionals, avoids the potential drug interactions and adverse effects of antidepressant medications, and is readily scalable to large numbers of patients, there should be strong consideration towards using internet-based mental health strategies to address the widespread unmet need for depression treatment in PWH.
Several studies have found cross-sectional associations between depression and impaired endothelial function [6], but causal relationships remain unclear. Thus, our primary objective was to determine whether improvement in depressive symptom severity translated to improvement in FMD in PWH. As found by others, we observed an inverse relationship between depressive symptom severity by SCL-20 score and FMD at Entry. However, we did not find differences between study groups in FMD change. In fact, FMD seemed to worsen in both study groups, with an unexpectedly larger reduction in FMD in the BtB6-8 subgroup compared with UC at Week 12, although these differences did not persist at Week 24. It is unclear whether the acute reductions in FMD were due to the observed modest increases in hsCRP and IL-6 at Week 12, were simply due to chance, or whether there was a true relationship between initial improvement in depression severity and transient worsening of endothelial function. Future studies are required to assess these possibilities.
Of note, there were similarly few participants in each study group who lost virologic control during the trial, with only 1 in each group with a marked increase in HIV viral load at Weeks 12 and 24. As such, the within-group levels of FMD and biomarkers and the between-group comparisons should not have been appreciably affected by these viral load elevations.
We also did not observe differences in changes between groups in several markers of systemic inflammation and coagulation (hsCRP, IL-6, sVCAM-1, D-dimer, fibrinogen). However, we did find significant improvements in the BtB group compared with UC in both sCD14 and sCD163, markers of monocyte activation. Depression has been linked to monocyte activation in the non-HIV population [26] and with higher levels of sCD14 in the HIV-positive population [27]. Other studies have suggested that depression, perhaps through vagus nerve neurocommunication, may alter gut microbiomes and enhance bacterial translocation, thereby triggering monocyte activation [28]. Thus, there is support for our findings of a possibly true causal relationship between depression and monocyte activation. Given that heightened monocyte activation has been linked to an increased risk of atherosclerosis [29], insulin resistance [30], cognitive impairment [31], and overall mortality in PWH [12], our results suggest a mechanism by which depression is linked to these outcomes and also suggest an intervention by which to reduce these risks.
We acknowledge several limitations to this study. The sample size was modest and may have precluded finding true differences between study groups in this pilot trial. In a post hoc analysis using the actual sample sizes, the observed variability in changes in FMD from Entry to Week 12 (our primary study endpoint), and an alpha = 0.05 and beta = 0.20, we estimate that the minimally detectable difference in FMD change between study groups was 2.3%. We consider a difference of 1.0% to be clinically relevant because a 1.0% higher baseline FMD predicted a 6% lower risk of CVD events over 5 years in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort study [32, 33]. As such, our study was underpowered to detect such clinically relevant differences in physiologic endothelial function measured using brachial artery FMD. In addition, although we performed repeated measurements at Entry for FMD and the various biomarkers, we only made these measurements once at Weeks 12 and 24 to minimize participant study burden and to reduce overall costs; as such, there may have been increased variability in these measurements during follow-up. The few numbers of women limited our ability to determine whether there were sex-specific effects of BtB versus UC; this will need to be addressed in future trials given that women living with HIV may have greater levels of systemic inflammation and immune activation [34, 35] and, thus, might benefit more than men from successful depression treatment. We cannot extrapolate our results to HIV-positive patients not on suppressive ART. We also performed several comparisons, so there is a risk for false-positive findings; although we found similarly reduced levels of both depression scores and monocyte activation biomarkers, suggesting these effects are real. However, these limitations are offset by the strengths of the study, including its randomized study design, the blinding of the outcome assessors, and the few participants lost to follow-up.
CONCLUSIONS
In conclusion, an internet CBT program for depression significantly improved depressive symptom severity and monocyte activation in PWH on suppressive ART. Larger trials are warranted to verify these preliminary results to improve depression and reduce the risk for both HIV and non-HIV serious events.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We gratefully thank the study participants for their time and effort in the successful completion of this trial. We also thank Danielle Huber, Jule Scott, Paula Johnson, Patricia Anderson, and Dr. Jessica Berntson for study coordination, Paul Sparks and Sheila Ellinger for regulatory assistance, and Dr. Robert Considine and Anthony Acton for performing the laboratory assays.
Financial support. This work was funded by the National Heart, Lung, and Blood Institute (R01HL126557), the National Center for Advancing Translational Sciences (UL1TR002529) at the National Institutes of Health, and the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK097512).
Potential conflicts of interest. S. K. G. has received travel support and advisory fees from Gilead Sciences and advisory fees and investigator-initiated research grant funding from GlaxoSmithKline/ViiV. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Van der Kooy K, van Hout H, Marwijk H, et al. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007; 22:613–26. [DOI] [PubMed] [Google Scholar]
- 2. Gan Y, Gong Y, Tong X, et al. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 2014; 14:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med 2014; 76:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006; 27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71:171–86. [DOI] [PubMed] [Google Scholar]
- 6. Cooper DC, Tomfohr LM, Milic MS, et al. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med 2011; 73:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med 2003; 65:362–8. [DOI] [PubMed] [Google Scholar]
- 8. Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology 2004; 29:1119–28. [DOI] [PubMed] [Google Scholar]
- 9. Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duprez DA, Neuhaus J, Kuller LH, et al. ; INSIGHT SMART Study Group Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandler NG, Wand H, Roque A, et al. ; INSIGHT SMART Study Group Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull 2005; 131:260–300. [DOI] [PubMed] [Google Scholar]
- 14. Khambaty T, Stewart JC, Gupta SK, et al. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus-infected adults: veterans aging cohort study. JAMA Cardiol 2016; 1:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White JR, Chang CC, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation 2015; 132:1630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta SK, Kamendulis LM, Clauss MA, Liu Z. A randomized, placebo-controlled pilot trial of N-acetylcysteine on oxidative stress and endothelial function in HIV-infected older adults receiving antiretroviral treatment. AIDS 2016; 30:2389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta SK, Mi D, Dubé MP, et al. Pentoxifylline, inflammation, and endothelial function in HIV-infected persons: a randomized, placebo-controlled trial. PLoS One 2013; 8:e60852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marks IM, Cavanagh K, Gega L.. Hands-on Help: Computer-aided Psychotherapy. Hove, UK: Psychology Press, 2007. [Google Scholar]
- 20. Proudfoot J, Goldberg D, Mann A, et al. Computerized, interactive, multimedia cognitive-behavioural program for anxiety and depression in general practice. Psychol Med 2003; 33:217–27. [DOI] [PubMed] [Google Scholar]
- 21. Proudfoot J, Ryden C, Everitt B, et al. Clinical efficacy of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. Br J Psychol 2004; 185:46–54. [DOI] [PubMed] [Google Scholar]
- 22. Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial. JAMA Psychiatry 2018; 75:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corretti MC, Anderson TJ, Benjamin EJ, et al. ; International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–65. [DOI] [PubMed] [Google Scholar]
- 24. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals 2002; 32:509–15. [Google Scholar]
- 25. Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA 2001; 286:2947–55. [DOI] [PubMed] [Google Scholar]
- 26. Grosse L, Carvalho LA, Wijkhuijs AJ, et al. Clinical characteristics of inflammation-associated depression: monocyte gene expression is age-related in major depressive disorder. Brain Behav Immun 2015; 44:48–56. [DOI] [PubMed] [Google Scholar]
- 27. Lu H, Surkan PJ, Irwin MR, et al. Inflammation and risk of depression in HIV: prospective findings from the Multicenter AIDS Cohort Study. Am J Epidemiol 2019; 188:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiecolt-Glaser JK, Wilson SJ, Bailey ML, et al. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018; 98:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reid M, Ma Y, Scherzer R, et al. Higher CD163 levels are associated with insulin resistance in hepatitis C virus-infected and HIV-infected adults. AIDS 2017; 31:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DʼAntoni ML, Paul RH, Mitchell BI, et al. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV after dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr 2018; 79:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol 2013; 168:344–51. [DOI] [PubMed] [Google Scholar]
- 33. Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation 2009; 120:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siedner MJ, Zanni M, Tracy RP, et al. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis 2018; 218:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr 2005; 39:44–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.