Abstract
Binge eating is seen across the spectrum of eating disorder diagnoses as well as among individuals who do not meet diagnostic criteria. Analyses of the specific types of foods that are frequently binged upon reveal that sugar-rich items feature prominently in binge-type meals, making the effects of binge consumption of sugar an important focus of study. One avenue to do this involves the use of animal models. Foundational and recent studies of animal models of sugar bingeing, both outlined here, lend insight into the various neurotransmitters and neuropeptides that may participate in or be altered by this behavior. Further, several preclinical studies incorporating sugar bingeing paradigms have explored the utility of pharmacological agents that target such neural systems for reducing sugar bingeing in an effort to enhance clinical treatment. Indeed, the translational implications of findings generated using animal models of sugar bingeing are considered here, along with potential avenues for further study.
Keywords: Animal model, bingeing, pharmacological treatment, sugar, translational research
Introduction
Binge eating is observed across eating disorder diagnoses [ie, binge eating disorder (BED), bulimia nervosa (BN), and the binge-purge subtype of anorexia nervosa]. Further, depending on how it is defined, binge eating is not necessarily limited to those who meet diagnostic criteria for an eating disorder.1 Binge eating is associated with higher body mass index (BMI) and greater prevalence of overweight or obesity, as well as increased risk for several excess weight–related comorbidities, such as hypertension, hypertriglyceridemia, and insulin resistance.2 In addition to physical health risks, binge eating is associated with several markers of psychological distress, including depression, stress, and work-related impairment.3 In light of these concerns, binge eating has been the focus of several research efforts aimed at understanding the factors that motivate, perpetuate, and may help to attenuate this behavior. One such avenue of study includes the use of animal models of binge eating. In addition to reducing the variability that exists in clinical studies, animal models provide an opportunity to study with greater precision both the acute neural effects of bingeing as well as neural adaptations that occur as a result of prolonged periods of binge eating.
Animal models of binge eating also provide a means to isolate the effects of overeating specific diets or macronutrients. During studies of binge meals in humans, women increase their intake of dessert and snack foods,4,5 indicating an important role for palatable foods and sugar-rich items in particular in binge eating behavior. Additionally, both lean and overweight/obese women who binge eat show a preference for sweet foods,6 and craving sweets has been identified as one antecedent for binge eating.7 Thus, studies from our laboratory and others investigating the behavioral and neural effects of binge consumption of sucrose solutions using animal models may lend insight into patterns of binge eating in humans.
Behavioral and Neural Effects of Binge Eating Sugar
Animal models of sugar bingeing
Several approaches have been successful in promoting binge eating in rodents.8 For instance, bingeing behavior has been elicited by offering sugar on a limited, intermittent schedule of 2 h per day on Monday, Wednesday, and Friday9 or by offering palatable food to rats with a history of combined dieting and stress.10 In our laboratory, we have employed an intermittent access paradigm to elicit binge eating that involves 12 h food deprivation and 12 h access to both sugar and chow, which are offered 4 h into the dark (active) period.11 Over time, rats on this schedule tend to increase their consumption of sugar when it is first made available each day (see Figure 1). Thus, this initial meal is considered a “binge.”12
FIGURE 1.
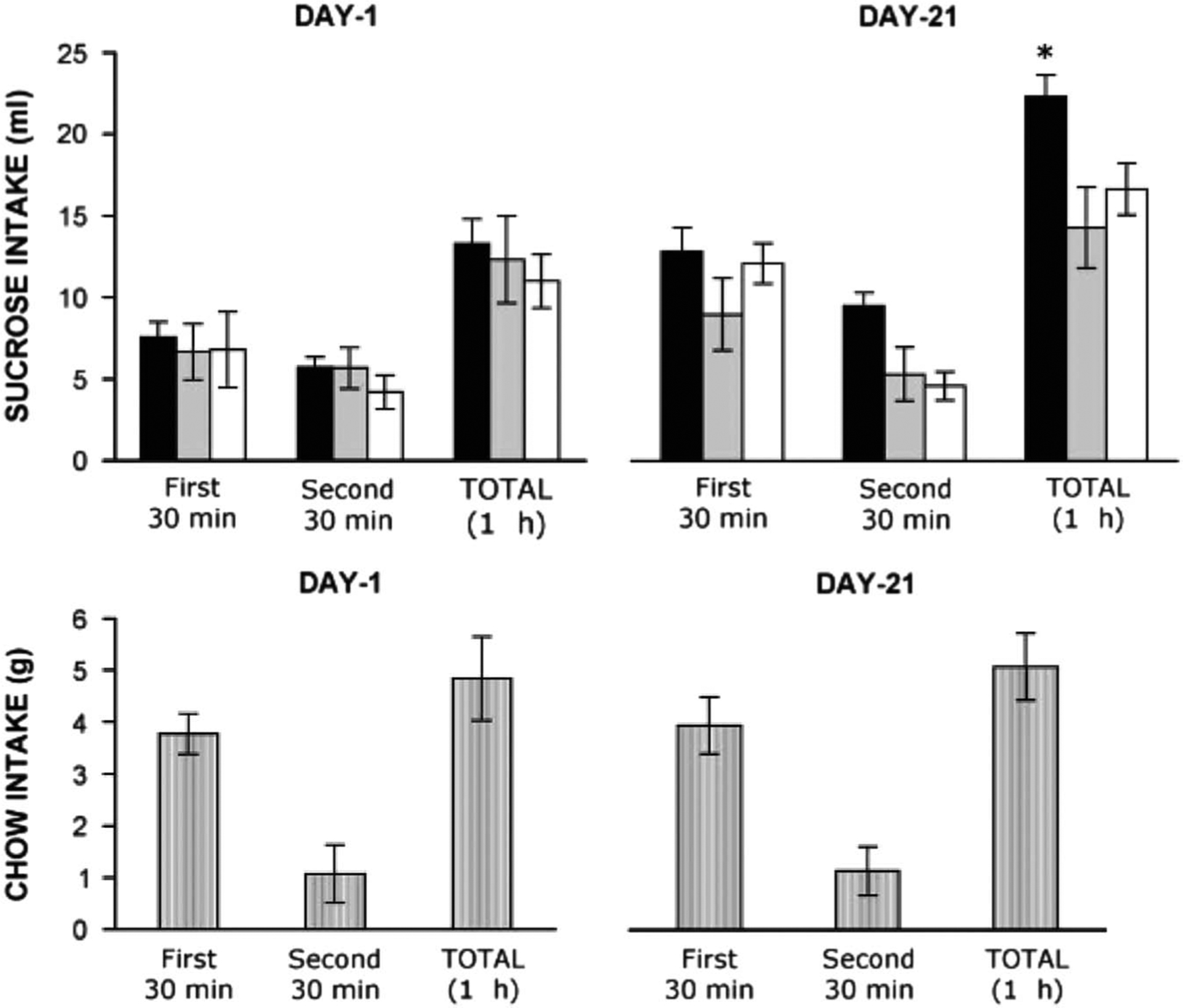
Sugar or chow intake during microdialysis sessions on day 1 and 21. For the sugar drinking groups (top panel), there was no significant difference in sugar intake on day 1. By day 21, only the Daily Intermittent Sugar group showed a difference in intake relative to day 1. Rats in the Daily Intermittent Chow group ate the same amount on day 21 as on day 1 (bottom panel). Top panel: Daily Intermittent Sucrose (black bars); Sucrose Twice (gray bars); Daily Ad Libitum Sucrose (open bars). Bottom panel: Daily Intermittent Chow. *p<0.05. Reprinted with permission from Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744.
Concomitant with this behavior are neurochemical aberrations that may serve to perpetuate sugar bingeing (see Avena and Bocarsly13). For instance, though dopamine (DA) release in the shell of the nucleus accumbens (NAc), a brain region implicated in reward,14 typically wanes with repeated access to a food, even sugar,15 rats with intermittent sugar access show a persistent release of DA in the NAc shell in response to sugar (see Figure 2).16 Notably, this pattern of nonabating DA release is also observed in response to drugs of abuse.17 Although food deprivation has been shown to enhance the release of NAc DA,18 this persistent pattern of DA release is not seen in rats with intermittent food deprivation and access to chow but no sucrose,16 which suggests that the combination of sugar and intermittent food deprivation contributes to this effect. Interestingly, sucrose sham feeding (during which the contents of the stomach are drained via a gastric fistula) on this intermittent schedule also results in increased NAc DA release, an effect that persists with repeated exposure,19 suggesting that the taste of sucrose, and not just its caloric contribution, is important in eliciting this effect. Exposure to this intermittent sugar paradigm is also associated with altered gene expression of DA receptors and opioid peptides in the NAc,20 alterations that resemble in some ways those seen following morphine exposure, such as increased D3 mRNA in the striatal forebrain21 and decreased D2 mRNA in the NAc.22 Intermittent sugar access is also associated with a delayed release of acetylcholine (ACh) in the NAc, which has been implicated in satiety,23,24 though there is some debate surrounding its precise role on the receptor level in satiety signaling.25 Consistent with this, ACh release is significantly attenuated in sucrose sham-fed rats.19 Further, with prolonged food restriction, underweight rats with a history of intermittent sugar access show even greater DA release and lower ACh release in the NAc in response to sugar.26
FIGURE 2.
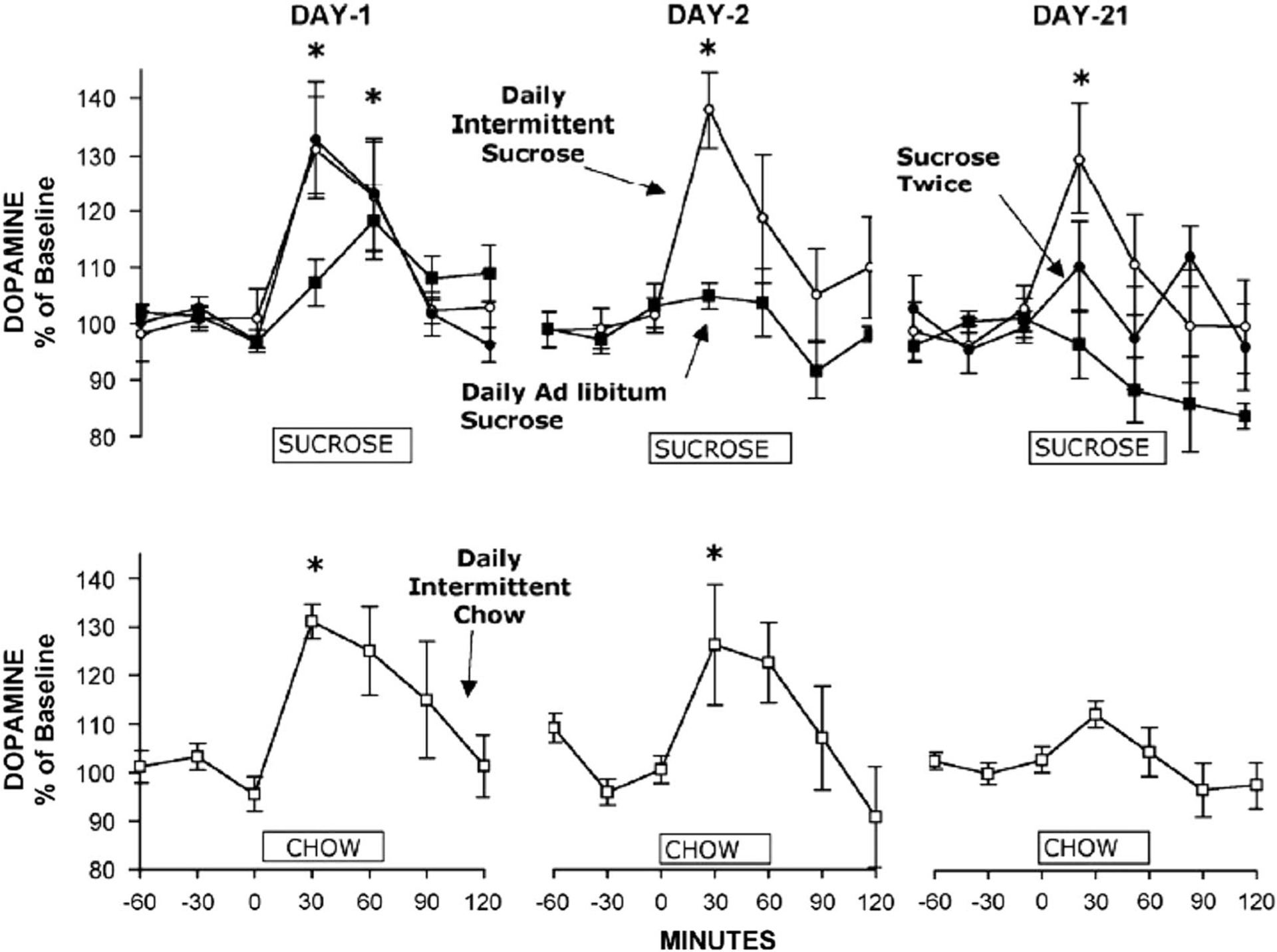
Daily Intermittent Sucrose rats maintain high DA release for 3 weeks. Microdialysis samples were collected on days 1, 2, and 21 of access. DA levels increased for the Daily Intermittent Sucrose rats (open circles) on days 1 and 2 and again on day 21. DA levels also increased significantly for the Sucrose Twice (filled circles), Daily Ad Libitum Sucrose (filled squares), and Daily Intermittent Chow (open squares) control groups on day 1, but there was a blunting of this effect by day 21. The ordinate indicates the hour (0–60 min) of sucrose or chow availability for this test. *p<0.05. Reprinted with permission from Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744.
Food deprivation and administration of the opioid antagonist naloxone both precipitate symptoms of withdrawal, such as anxiety and somatic signs of distress, including teeth chattering, among sugar bingeing rats exposed to this model.27,28 Using a paradigm similar to that used in our laboratory, which involves 12 h food deprivation and 12 h sugar and chow access, Wideman et al29 observed lower body temperature among rats during a 1-week period of abstinence from the sugar solution, which was restored with renewed sugar access. In addition, during abstinence, rats with previous intermittent sugar intake displayed shaking, teeth chattering, and biting. Additionally, rats consumed significantly more sugar in the week following abstinence than previously, suggesting enhanced motivational salience. Compared with control animals, rats with intermittent sugar access also showed increased blood glucose levels. In terms of neural alterations during abstinence, we have observed that 36 hours of withdrawal from both chow and sugar following a period of sugar-bingeing results in reduced DA and increased ACh in the NAc27,28–a pattern similar to what has been seen during withdrawal from drugs of abuse.30
Rats with a history of intermittent sugar access also show increased locomotor activity in response to the DA agonist amphetamine, suggesting sensitization of the dopamine system,31 as well as increased intake of a 9% ethanol solution.32 Rats with intermittent access to chow also show relatively high levels of consumption of this alcohol concentration–though less than those with intermittent sugar access–suggesting a role for food deprivation in producing this effect. This is not surprising given previous reports demonstrating enhanced ethanol consumption in food-deprived rats.33
Sucrose bingeing has also been elicited using a food deprivation paradigm that allows 20 min daily access to sucrose, which is followed shortly thereafter by brief access to chow. Rats on this paradigm show an escalation of sucrose intake, accompanied by increased dopamine active transporter (DAT) binding in the NAc and ventral tegmental area (VTA),34 integral components of the mesolimbic dopamine pathway, and increased DAT mRNA in the VTA. Notably, these effects were limited to rats that were food deprived and predictably received sugar access at the same time each day. This paradigm has also been found to result in lower D2 receptor binding in the NAc than is seen in response to normal food restriction.35
Recent insights
To further elucidate the neural mechanisms that may underlie binge eating, a number of recent studies have assessed appetite regulatory signals in the hypothalamus, a key brain region implicated in the control of feeding behavior, in the context of bingeing behavior. One study did not find differences in appetite regulatory neuropeptides such as neuropeptide Y (NPY), agouti-related peptide (AgRP), pro-opiomelanocortin (POMC), and cocaine-and-amphetamine-regulated transcript (CART) within the hypothalamus of rats and mice after a month of daily 2 h binge access to a sucrose solution (and otherwise ad libitum chow access), nor did the authors find altered gene expression of opioid peptides and D2 and D3 receptors within the NAc.36 Thus, the paradigm used to model sugar bingeing (ie, ad libitum chow access vs. intermittent food deprivation, 2 vs. 12 h sugar access) appears to be important in eliciting certain neural effects. Further, the authors of this study also point out that samples were collected immediately before the normally scheduled binge period. In our previous study that found reduced and increased NAc expression of D2 and D3 receptors in sugar bingeing rats, respectively, samples were selected immediately following binge access.20
Recent research using a drinking in the dark (DID) procedure (in which alcohol or sugar is offered for a period of 2–4 h, 3 h into the dark period) has shown reduced orexin mRNA in the lateral hypothalamus (LH) of mice following 2 h binge consumption of sucrose or saccharin37 and reduced orexin immunoreactivity in the LH of both ethanol- and sucrose-bingeing mice.38 Orexin is known to stimulate feeding; thus, it has been speculated that this reduction may be an appropriate compensatory response to bingeing.37
In addition to the DID procedure, several other paradigms to induce sugar bingeing have recently been tested. For instance, an anticipatory contrast paradigm, which involves giving animals 30 min access to a 4% sucrose solution followed by 30 min access to either an unsweetened solution or a 20% sucrose solution, has recently been shown to elicit high amounts of sucrose licking in rats.39 Additionally, sucrose bingeing has recently been observed in mice given 4 h access to sugar and chow following 20 h of food deprivation.40 In response to these conditions, mice progressively increase their intake of sugar over time and during the first hour of access in particular. Interestingly, in this study, neither systemic administration of glucose nor a chow preload deterred sucrose bingeing; however, repeated access to greater periods of chow access did serve to attenuate sucrose bingeing.
Novel Pharmacological Treatment Approaches
Studying various pharmacological treatments for sugar bingeing imparts additional information regarding the underlying neural mechanisms involved in bingeing on this macronutrient. For instance, antagonism of the orexin type-1 receptor with SB-334867 during the DID paradigm mentioned previously reduces binge-like consumption of sucrose and saccharin.37,38 This suggests that orexin receptors may be a useful target to reduce sugar bingeing behavior. Additionally, during a limited, intermittent schedule of 2 h access, the D2 receptor antagonist raclopride reduced sucrose intake.41 Methylphenidate, which acts on both dopamine and norep-nephrine, also reduces sucrose bingeing but increases chow intake, presumably to compensate for lower caloric intake from sugar. Concomitant with this, methylphenidate treatment leads to increased DAT and D2 receptor binding in the NAc shell.42 GS 455534, an aldehyde dehydrogenase-2 inhibitor that reduces DA synthesis,43 has also been shown to selectively reduce binge consumption of sugar (as opposed to ad libitum sugar access) in the 12 h access paradigm discussed earlier.44 In this same study, GS 455534 attenuated DA release in the NAc in response to sugar by approximately 50%. However, in this experiment, binge sugar conumption was not reduced.44 Together, these data implicate the dopaminergic system and D2 receptors in particular in sugar bingeing behavior.
The opioidergic system also appears to play a role in sugar bingeing. The opioid receptor antagonist naltrexone reduces sucrose intake in rats with limited sugar access41 and in rats consuming sucrose in the anticipatory contrast paradigm mentioned earlier.39 Similar results were seen with administration of the irreversible mu-opioid receptor-specific antagonist, beta-funaltrexamine, into the NAc, indicating that mu-opioid receptor signaling in the NAc is involved in sugar bingeing.39 The combination of drugs that act on different neural systems is also an emerging application of pharmacological agents that may prove beneficial in reducing sugar bingeing. Concomitant administration of naltrexone and baclofen, a GABAB agonist, reduces sugar intake, with the effect approaching statistical significance in the 12 h access paradigm.45 In summary, several different neural circuits, including those involving orexin, dopamine, and opioids, appear to participate in sugar bingeing and represent promising targets for alleviating bingeing behaviors. It is worth noting that pharmacological agents that target the serotonergic system have not yet been studied within this context, despite evidence suggesting their efficacy in clinical populations.46
Translational Implications
Animal models provide unique evidence for a model of the etiology and maintenance of binge eating, in part by drawing upon addiction models. For instance, they have provided insight into the neurobiological changes that can occur with the onset of a binge, which may promote the maintenance of this behavior. In line with reports from individuals who binge with BED or BN and who recall an initial binge episode as providing the onset for their disorder,47,48 the animal literature has shown how truly powerful a 1-trial sugar reinforcement experience can be on the neural reward system in leading to a pattern of compulsive binge eating.
Certain animal studies have provided insights into how the neural changes associated with drug abuse are similar to those associated with binge eating. In human studies, this has spawned further investigation of neural substrates that overlap in addiction and in BED49,50; how compulsive overeating overlaps with disorders of addiction51; and whether individuals can substitute one addiction or reinforcer for another. Examples of the latter include switching food for alcohol after bariatric surgery52 or carbohydrates for nicotine after smoking cessation.53 However, cross-sensitization between sucrose and nicotine may not be as straightforward, with other data suggesting that smokers who quit and use sucrose to cope with acute withdrawal find that although it reduces anxiety, drowsiness, and cravings for carbohydrates and fats, it does not reduce agitation, irritability, or concentration difficulties.54 Hopefully, animal studies will continue to further untangle these human findings and in doing so provide more insights as to how to increase the likelihood of drug cessation without weight gain as well as prevent the escalation of drug use following weight loss surgery.
There are other clinical observations that animal studies will be important in elucidating. For instance, we know that individuals who engage in regular binge eating need to consume more food to feel sated,55 and, despite eating more after a fast, the duration of feeling satiated after a meal is shorter.56 However, we still do not understand the underlying neurobiology of the satiation process of individuals who engage in binge eating, although a variety of peripheral neuropeptides have been ruled out.57,58 The finding that the neurotransmitter orexin, which is associated with satiety, is reduced following an episode of sugar bingeing in animals is an exciting one that may lead to the development of pharmacological treatments for BED and obesity. However, loss of control binge eating is characterized in some by binge eating even when one is not hungry, suggesting that exclusively targeting neural circuitry involved in promoting satiety may not prevent or attenuate all instances of binge eating.
Animal studies are also adding to our understanding of the consequences of binge eating, such as the phenomenon of “withdrawal.” However, we do not understand sugar binge withdrawal in individuals with BED and whether this maintains subsequent binge eating. Behavioral experiments with humans, in order to assess whether a prolonged period of abstinence leads to a “sugar crash” or elicits signs of withdrawal, are unfortunately lacking.
In concert with human studies,59 animal models of binge eating have provided evidence of the importance of dietary restraint as an antecedent to sugar binges. Depriving oneself of food for a period leads to overconsumption or consumption of a “forbidden food” and is the basis of maintenance models for bulimia nervosa and the binge-purge subtype of anorexia nervosa.60 Indeed, the finding that sugar bingeing was reduced with repeated regular access to chow suggests that maintaining consistent periods of healthy food consumption serves to protect against binge eating. The importance of dietary restraint in maintaining binge eating and of eating regularly forms the basis of the first-line treatment for BED–guided self-help cognitive behavior therapy.61 However, as mentioned, we know that binge eating is not always driven by energy deficit and can be precipitated by other factors. For instance, with regard to stress, negative affect appears to be an important antecedent to loss of control binges as seen in ecological momentary assessment studies.62–64 Similarly, some animal models have observed binge eating of highly palatable food in response to stressful stimuli.10
Finally, we hope that animal studies will continue to shed light on our understanding of individual differences in response to factors such as stress, sleep deprivation, and possibly interpersonal factors, and how these may or may not promote binge eating. They may also help us understand why despite some shared neurobiological vulnerabilities, one individual binge eats while another engages in drug abuse. Untangling these factors may be best achieved by the cross-talk and cross-questioning that is occurring between animal and human studies of binge eating.
Conclusion
Knowledge of the physiological mechanisms underlying bingeing behavior is important for both better understanding and treating eating disorders associated with bingeing. Laboratory animal studies continue to reveal many of the neurochemical adaptations associated with this behavior. Specifically, foundational models of sugar bingeing provide evidence of a unique neural and behavioral profile seen in animals that binge on sugar that reflect patterns observed in response to drugs of abuse. This has led to recent findings that elucidate the involvement of additional neural circuitry in binge eating and, as a result, provide a broader picture of the different neural systems involved in or affected by sugar bingeing. Moreover, novel pharmacological agents shown to reduce sugar bingeing in animals may be used to treat individuals who struggle with binge eating sugar-rich foods. Together, animal studies may inform clinical treatment of binge eating and explain salient features of this behavior.
Footnotes
Disclosures
Susan M. Murray has nothing to disclose. Alastair Tulloch has nothing to disclose. Eunice Chen has the following disclosures: Guilford Press, author, annual royalties; Shire Pharmaceuticals, consultant, consulting fees; National Institute of Mental Health, principal investigator. Nicole Avena has the following disclosures: National Institutes of Health, principal investigator, research support; Gilead Sciences, Inc., principal investigator, research support; Bioproject, principal investigator, research support; National Eating Disorders Assoc., principal investigator, research support; Shire Pharmaceuticals, consultant, consulting fees; Rivermend Behavioral Health, LLC, consultant, consulting fees; The Sugar Association, consultant, consulting fees; Columbia University, speaker, honoraria; University of Massachusetts, speaker, honoraria; Washington State University, speaker, honoraria; American Psychological Association, speaker, honoraria; Centers for Medical Weight Loss, speaker, honoraria; Ten Speed Press, author, royalties; Oxford University Press, author, royalties.
REFERENCES:
- 1.Coker EL, von Lojewski A, Luscombe GM, Abraham SF. The difficulty in defining binge eating in obese women: how it affects prevalence levels in presurgical bariatric patients. Eat Behav. 2015; 17: 130–135. [DOI] [PubMed] [Google Scholar]
- 2.Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity. 2014; 22(11): 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Striegel RH, Bedrosian R, Wang C, Schwartz S. Why men should be included in research on binge eating: results from a comparison of psychosocial impairment in men and women. Int J Eat Disord. 2012; 45(2): 233–240. [DOI] [PubMed] [Google Scholar]
- 4.Yanovski SZ, Leet M, Yanovski JA, et al. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr. 1992; 56(6): 975–980. [DOI] [PubMed] [Google Scholar]
- 5.Hadigan CM, Kissileff HR, Walsh BT. Patterns of food selection during meals in women with bulimia. Am J Clin Nutr. 1989; 50(4): 759–766. [DOI] [PubMed] [Google Scholar]
- 6.Dalton M, Blundell J, Finlayson G. Effect of BMI and binge eating on food reward and energy intake: further evidence for a binge eating subtype of obesity. Obes Facts. 2013; 6(4): 348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. J Consult Clin Psychol. 2000; 68(1): 95–102. [PubMed] [Google Scholar]
- 8.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011; 104(1): 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007; 92(4): 566–574. [DOI] [PubMed] [Google Scholar]
- 10.Boggiano MM, Chandler PC. Binge eating in rats produced by combining dieting with stress. Curr Protoc Neurosci. 2006. Chapter 9:Unit9.23A. [DOI] [PubMed] [Google Scholar]
- 11.Colantuoni C, Schwenker J, McCarthy J, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001; 12(16): 3549–3552. [DOI] [PubMed] [Google Scholar]
- 12.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008; 32(1): 20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. 2012; 63(1): 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999; 31(1): 6–41. [DOI] [PubMed] [Google Scholar]
- 15.Bassareo V, Cucca F, Musio P, Lecca D, Frau R, Di Chiara G. Nucleus accumbens shell and core dopamine responsiveness to sucrose in rats: role of response contingency and discriminative/conditioned cues. Eur J Neurosci. 2015; 41(6): 802–809. [DOI] [PubMed] [Google Scholar]
- 16.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005; 134(3): 737–744. [DOI] [PubMed] [Google Scholar]
- 17.Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991; 566(1–2): 348–350. [DOI] [PubMed] [Google Scholar]
- 18.Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999; 11(12): 4389–4397. [DOI] [PubMed] [Google Scholar]
- 19.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006; 139(3): 813–820. [DOI] [PubMed] [Google Scholar]
- 20.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004; 124(2): 134–142. [DOI] [PubMed] [Google Scholar]
- 21.Spangler R, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Elevated D3 dopamine receptor mRNA in dopaminergic and dopaminoceptive regions of the rat brain in response to morphine. Brain Res Mol Brain Res. 2003; 111(1–2): 74–83. [DOI] [PubMed] [Google Scholar]
- 22.Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999; 11(2): 481–490. [DOI] [PubMed] [Google Scholar]
- 23.Avena NM, Rada PV. Cholinergic modulation of food and drug satiety and withdrawal. Physiol Behav. 2012; 106(3): 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mark GP, Rada P, Pothos E, Hoebel BG. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum, and hippocampus of freely behaving rats. J Neurochem. 1992; 58(6): 2269–2274. [DOI] [PubMed] [Google Scholar]
- 25.Pratt WE, Blackstone K. Nucleus accumbens acetylcholine and food intake: decreased muscarinic tone reduces feeding but not food-seeking. Behav Brain Res. 2009; 198(1): 252–257. [DOI] [PubMed] [Google Scholar]
- 26.Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008; 156(4): 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colantuoni C, Rada P, McCarthy J, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002; 10(6): 478–488. [DOI] [PubMed] [Google Scholar]
- 28.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008; 94(3): 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wideman CH, Nadzam GR, Murphy HM. Implications of an animal model of sugar addiction, withdrawal and relapse for human health. Nutr Neurosci. 2005; 8(5–6): 269–276. [DOI] [PubMed] [Google Scholar]
- 30.Rada P, Johnson DF, Lewis MJ, Hoebel BG. In alcohol-treated rats, naloxone decreases extracellular dopamine and increases acetylcholine in the nucleus accumbens: evidence of opioid withdrawal. Pharmacol Biochem Behav. 2004; 79(4): 599–605. [DOI] [PubMed] [Google Scholar]
- 31.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003; 122(1): 17–20. [DOI] [PubMed] [Google Scholar]
- 32.Avena NM, Carrillo CA, Needham L, Leibowitz SF, Hoebel BG. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol. 2004; 34(2–3): 203–209. [DOI] [PubMed] [Google Scholar]
- 33.Meisch RA, Thompson T. Ethanol intake as a function of concentration during food deprivation and satiation. Pharmacol Biochem Behav. 1974; 2(5): 589–596. [DOI] [PubMed] [Google Scholar]
- 34.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003; 284(5): R1260–R1268. [DOI] [PubMed] [Google Scholar]
- 35.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002; 13(12): 1575–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bake T, Duncan JS, Morgan DG, Mercer JG. Arcuate nucleus homeostatic systems are not altered immediately prior to the scheduled consumption of large, binge-type meals of palatable solid or liquid diet in rats and Mice. Journal Neuroendocrinol. 2013; 25(4): 357–371. [DOI] [PubMed] [Google Scholar]
- 37.Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I. Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: pharmacological and molecular evidence of orexin involvement. Behav Brain Res. 2014; 272: 93–99. [DOI] [PubMed] [Google Scholar]
- 38.Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015; 39(1): 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsuura Y, Taha SA. Mu opioid receptor antagonism in the nucleus accumbens shell blocks consumption of a preferred sucrose solution in an anticipatory contrast paradigm. Neuroscience. 2014; 261: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasoshima Y, Shimura T. A mouse model for binge-like sucrose overconsumption: contribution of enhanced motivation for sweetener consumption. Physiol Behav. 2015; 138: 154–164. [DOI] [PubMed] [Google Scholar]
- 41.Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009; 20(5–6): 537–548. [DOI] [PubMed] [Google Scholar]
- 42.Bello NT, Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res Bull. 2006; 70(4–6): 422–429. [DOI] [PubMed] [Google Scholar]
- 43.Yao L, Fan P, Arolfo M, et al. Inhibition of aldehyde dehydrogenase- 2 suppresses cocaine seeking by generating THP, a cocaine use-dependent inhibitor of dopamine synthesis. Nature Med. 2010; 16(9): 1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bocarsly ME, Hoebel BG, Paredes D, et al. GS 455534 selectively suppresses binge eating of palatable food and attenuates dopamine release in the accumbens of sugar-bingeing rats. Behav Pharmacol. 2014; 25(2): 147–157. [DOI] [PubMed] [Google Scholar]
- 45.Avena NM, Bocarsly ME, Murray S, Gold MS. Effects of baclofen and naltrexone, alone and in combination, on the consumption of palatable food in male rats. Exp Clin Psychopharmacol. 2014; 22(5): 460–467. [DOI] [PubMed] [Google Scholar]
- 46.Marazziti D, Corsi M, Baroni S, Consoli G, Catena-Dell’Osso M. Latest advancements in the pharmacological treatment of binge eating disorder. Eur Rev Med Pharmacol Sci. 2012; 16(15): 2102–2107. [PubMed] [Google Scholar]
- 47.Hilbert A, Pike KM, Goldschmidt AB, et al. Risk factors across the eating disorders. Psychiatry Res. 2014; 220(1): 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reas DL, Grilo CM. Timing and sequence of the onset of overweight, dieting, and binge eating in overweight patients with binge eating disorder. Int J Eat Disord. 2007; 40(2): 165–170. [DOI] [PubMed] [Google Scholar]
- 49.Wang G-J, Geliebter A, Volkow ND, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring). 2011; 19(8): 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geliebter A, Ladell T, Logan M, Schweider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006; 46(1): 31–35. [DOI] [PubMed] [Google Scholar]
- 51.Grant JE, Schreiber LR, Odlaug BL. Phenomenology and treatment of behavioural addictions. Can J Psychiatry. 2013; 58(5): 252–259. [DOI] [PubMed] [Google Scholar]
- 52.Ivezaj V, Saules KK, Wiedemann AA. “I didn’t see this coming.”: why are postbariatric patients in substance abuse treatment? Patients’ perceptions of etiology and future recommendations. Obes Surg. 2012; 22(8): 1308–1314. [DOI] [PubMed] [Google Scholar]
- 53.Spring B, Wurtman J, Gleason R, Wurtman R, Kessler K. Weight gain and withdrawal symptoms after smoking cessation: a preventive intervention using d-fenfluramine. Health Psychol. 1991; 10(3): 216–223. [DOI] [PubMed] [Google Scholar]
- 54.Helmers K, Young S. The effect of sucrose on acute tobacco withdrawal in women. Psychopharmacology (Berl). 1998; 139(3): 217–221. [DOI] [PubMed] [Google Scholar]
- 55.Sysko R, Devlin MJ, Walsh BT, Zimmerli E, Kissileff HR. Satiety and test meal intake among women with binge eating disorder. Int J Eat Disord. 2007; 40(6): 554–561. [DOI] [PubMed] [Google Scholar]
- 56.Mirch MC, McDuffie JR, Yanovski SZ, et al. Effects of binge eating on satiation, satiety, and energy intake of overweight children. Am J Clin Nutr. 2006; 84(4): 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geliebter A, Hashim SA, Gluck ME. Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED). Physiol Behav. 2008; 94(5): 696–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munsch S, Biedert E, Meyer AH, Herpertz S, Beglinger C. CCK, ghrelin, and PYY responses in individuals with binge eating disorder before and after a cognitive behavioral treatment (CBT). Physiol Behav. 2009; 97(1): 14–20. [DOI] [PubMed] [Google Scholar]
- 59.Herman CP, Mack D. Restrained and unrestrained eating. J Pers. 1975; 43(4): 647–660. [DOI] [PubMed] [Google Scholar]
- 60.Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav Res Ther. 2003; 41(5): 509–528. [DOI] [PubMed] [Google Scholar]
- 61.Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010; 67(1): 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldschmidt AB, Crosby RD, Cao L, et al. Ecological momentary assessment of eating episodes in obese adults. Psychosom Med. 2014; 76(9): 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schulz S, Laessle R. Stress-induced laboratory eating behavior in obese women with binge eating disorder. Appetite. 2012; 58(2): 457–461. [DOI] [PubMed] [Google Scholar]
- 64.Haedt-Matt AA, Keel PK. Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol Bull. 2011; 137(4): 660–681. [DOI] [PMC free article] [PubMed] [Google Scholar]


