Abstract
Background
To identify the association between somatic ataxia-telangiectasia mutated (ATM) mutations and improved radio-sensitivity, we retrospectively reviewed next-generation sequencing data from patients diagnosed with isocitrate dehydrogenase (IDH)-wildtype high-grade glioma.
Methods
We included 39 individuals with (IDH)-wildtype high-grade glioma (diffuse astrocytoma n = 2, anaplastic astrocytoma n = 10, and glioblastoma n = 27) not subjected to gross tumor resection and undergoing radiation therapy with a median total dose of 60 Gy in 30 fractions. The mutational status of the ATM gene was obtained through next-generation sequencing using a TruSight Tumor 170 cancer panel. Disease progression was defined according to the Response Assessment in Neuro-Oncology (RANO) criteria as well as neurologic and clinical findings.
Results
Among the 39 samples, ATM mutations (ATM mut(+)) were detected in 26% of cases (n = 10). No significant differences were observed in the characteristics of the patients or tumors. Among the 10 patients in the ATM mut(+) group, there were 6 patients with glioblastoma and 4 patients with anaplastic astrocytoma. Most mutations were missense mutations (n = 8, 80%). With a median follow-up of 16.5 mo (interquartile range, 11.4–19.8), ATM mut(+) exhibited 1-year in-field control of 100% compared with 44.1% in the ATM mut(−) group (p = 0.002). There was no difference in the out-field control rate or overall survival between the two groups (p = 0.861 and p = 0.247, respectively).
Conclusions
Our results demonstrated that ATM mutations might be involved in the increased radio-sensitivity with excellent in-field control despite the aggressive nature of IDH-wildtype high-grade glioma. Further studies are necessary to uncover the potential role of ATM as a biomarker and candidate therapeutic target in high-grade gliomas.
Keywords: ATM, IDH-wild type high-grade glioma, Radiosensitivity, Next-generation sequencing, Radiation therapy
Background
Isocitrate dehydrogenase (IDH)-wildtype high-grade glioma (diffuse astrocytoma, anaplastic astrocytoma, and glioblastoma multiforme [GBM]), being an aggressive brain tumor, has been correlated with poor prognosis despite the best trimodality treatment approaches (surgery, chemotherapy, and radiation therapy (RT)). This poor prognosis has been attributed to the intrinsic radio- and chemo-resistance of the tumor [1]. Although there are several clinical and molecular factors known to be prognostic factors for high-grade glioma, including methylation of O-6-methylguanine-DNA methyltransferase (MGMT) promoter, age, involvement of the subventricular zone (SVZ), extent of resection, and sex [2], most patients are treated with a uniform adjuvant treatment approach (i.e., one-size-fits-all).
The ataxia-telangiectasia mutated (ATM) gene encodes a serine/threonine protein kinase known to be activated by autophosphorylation upon DNA double-strand breaks arising from ionizing radiation. The prevalence of ATM mutations (ATM mut(+)) in high-grade glioma is known to be less than 5% [3]; hence, owing to this rarity, there have been no clinical reports on such patients. Similar to the approach employing a PARP inhibitor targeting BRCA1 mutations in breast and ovarian cancers [4], several preclinical studies on the inhibition of ATM have been performed to enhance radio-sensitivity in other solid tumors [5, 6] and high-grade gliomas [7–10]. Rainey et al. discovered that transient inhibition of ATM by chemicals (CP466722) sensitizes HeLa cells and cells expressing BCR-Abl to ionizing radiation [6]. Recently, Golding et al. demonstrated that dynamic ATM inhibition with sub-micromolar concentrations of KU-60019 slightly increased RT-induced cell killing in human glioblastoma cells [8]. These studies intrigued many physicians to identify the clinical significance of ATM mut(+) in real practice.
With the emergence of state-of-the-art sequencing, next-generation sequencing (NGS) [11], physicians have easy access to the ATM mutational status of every patient. In this context, we undertook a retrospective analysis of NGS data to address the radio-sensitivity of ATM mut(+) and its impact on clinical outcomes in patients with IDH-wildtype high-grade glioma.
Methods
Patient selection
As NGS data for gliomas have been utilized in our institution from 2017, patients with newly diagnosed WHO grade II with IDH-wildtype, WHO grade III and IV type gliomas, according to the new 2016 WHO classification, were screened between June 2017 and December 2018. Accordingly, we screened for patients treated with RT following surgery and patients with available NGS data (n = 144). Patients were excluded from the study if one of the following criteria was met: (1) they had undergone gross total removal of the tumor, and thus it was not possible to investigate the response of the residual tumor to RT (n = 66); (2) they had been previously diagnosed with a primary CNS tumor (n = 15); (3) they had tumors with IDH mutations (n = 9); (4) they had undergone hypofractionated RT (n = 2); (5) peritumoral edema was not included in RT fields (n = 5); (6) they could not provide follow-up images (n = 5); and (7) they could not complete RT (n = 3). Finally, 39 patients, including 10 patients with ATM mutations (ATM mut(+) group) and 29 patients without ATM mutations (ATM mut(−) group), were included in our cohort. The study protocol was approved by the institutional review board (No.4–2019-0009), and the requirement for the provision of informed consent was waived because of the retrospective nature of this study.
Multi-modal treatments
Treatments and follow-up for every patient were performed by a multidisciplinary neuro-oncology board, including neurosurgeons, radiation oncologists, neuro-radiologists, neuropathologists, and medical oncologists [2]. All patients were evaluated based on perioperative and follow-up magnetic resonance images (MRI) and clinical symptoms. The involvement of the SVZ was assessed via preoperative MRI according to a standardized spatial classification [12]. Navigation-guided surgery following the maximal safe resection protocol was performed for all patients, except for those who underwent stereotactic biopsy. The extent of surgery was evaluated using immediate postoperative gadolinium-enhanced T1-weighted MRI obtained within 48 h after surgery and then categorized as total (absence of visible contrast-enhanced portion), subtotal (at least 90% of the tumor removed), partial (less than 90% of the tumor removed), or biopsy (in case of stereotactic biopsy) [13].
Conventional fractionated RT with a median total dose of 60 Gy (interquartile range, IQR: 60.0–60.0) was applied in 30 fractions to the gross tumor volume. With postoperative MRI, both the resection cavity and residual tumor were included in the gross tumor volume. The clinical target volume was delineated to include the peritumoral edema with a 1- or 1.5-cm margin on T2-weighted fluid-attenuated inversion recovery postoperative MRI, and a median total dose of 49.5 Gy (IQR: 48.0–51.0) in 30 fractions was then applied to the clinical target volume [14, 15]. Following the treatment strategy followed at our institution, there was no difference in the dose prescription of the protocol according to pathology: all patients were treated with intensity-modulated RT using Tomotherapy (Hi-Art TomoTherapy; Accuray, Sunnyvale, CA, USA).
In the case of patients with GBM, during RT, all patients concomitantly underwent a daily administration of temozolomide (75 mg/m2 of body surface area per day, 7 days per week, from the first to the last day of RT), followed by the administration of adjuvant temozolomide (150–200 mg/m2 for 5 days during each 28-day cycle). Regarding patients with anaplastic astrocytoma and WHO grade II with IDH-wildtype, 6 cycles of adjuvant procarbazine, lomustine, and vincristine (oral lomustine; 110 mg/m2 on day 1; oral procarbazine: 60 mg/m2 per d from day 8 to day 21; and intravenous vincristine: 1.4 mg/m2 on day 8 and 29) were administered every 6 weeks for 9 months.
Molecular analysis
Representative formalin-fixed, paraffin-embedded tissues were analyzed by targeted NGS using the commercially available TruSight Tumor 170 panel (Illumina, Inc., San Diego, CA, USA). Detailed methods for the procedure of sequencing and data analysis have been previously described [16, 17]. In the obtained NGS data, along with ATM mutations, we identified other frequent mutations found in gliomas, such as mutations in the Breast Cancer susceptibility gene1/2 (BRCA1/2), phosphatase and tensin homolog (PTEN), telomerase reverse transcriptase (TERT), and tumor protein p53 (TP53) genes. The DNA methylation status of the MGMT promoter was also examined [18]. Additionally, we stratified patients based on the consensus guidelines from the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) for the identification of gliomas appearing histologically as WHO grade II or III with molecular features of GBM using the following criteria: (1) amplification of epidermal growth factor receptor (EGFR) (n = 1), (2) combined whole chromosome 7 gain and whole chromosome 10 loss, or (3) mutation of the TERT promoter (n = 5) [19].
Follow-up
Follow-up of all patients was performed until death or time of analysis. Most patients underwent MRI 1 month after the planned RT as well as every 3 months for the first 2 years, and every 6 to 12 months thereafter according to institutional policy. Disease progression was defined using the Response Assessment in Neuro-Oncology (RANO) criteria [20] as well as neurologic and clinical findings. All patients suspected of having progressive disease were determined as exhibiting progressive disease after discussion and joint decision made by the multidisciplinary team. Either in-field or out-field failure was defined based on the relationship between the RT field (within the region for 95% of the prescribed dose) and the volume of the recurrence tumor assessed by MRI .
Statistical analysis
Parsons’ Chi-square or Fisher’s exact test were used to analyze categorical variables, whereas the Mann-Whitney U test (non-normally distributed data) was used to compare continuous variables when comparing differences in the characteristics of patients and treatments between the two groups. All events (including in-field failure, out-field failure, and death) were measured from the day of diagnosis (day of surgery) to the time of the event. The Kaplan-Meier method was performed to estimate the in-field, out-field, and overall survival (OS) rates with the log-rank test used to assess prognostic significance. Univariable analyses of in-field and out-field control as well as OS were performed using Cox regression analysis. Further multivariable analysis was not performed because there was no statistically significant factor identified in the univariable analysis. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 25.0.0; IBM Corp., Armonk, NY, USA) and R (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/) software.
Results
The median age at diagnosis was 59 years (IQR: 48–63), and most patients presented with a Karnofsky performance scale (KPS) score ≤ 80. Perioperative MRI revealed that 87.2% of tumors were located in the SVZ, and 48.7% of patients underwent subtotal removal. Methylation of the MGMT promoter was observed in 61.5% of patients. There was no significant difference in the characteristics of the patients and treatments between the ATM mut(+) and ATM mut(−) groups (Table 1). In addition, there was no difference in the frequency of mutations in other genes (Additional file 1). Detailed information on each patient with an ATM mutation is listed in Table 2. Missense mutations (8 patients, 80%) were the most common mutations in the ATM mut(+) group. There were six patients diagnosed with GBM and four patients with IDH-wildtype anaplastic astrocytoma. Based on preoperative MRI, the involvement of the SVZ (9 patients, 90.0%) and the presence of gliomatosis (6 patients, 60.0%) were frequently observed in the ATM mut(+) group.
Table 1.
Patient and treatment characteristics
| Total | ATM mut(−) | ATM mut(+) | p-value | |
|---|---|---|---|---|
| N = 39 | N = 29 | N = 10 | ||
| Median age [IQR], years | 59.0 [47.5;63.0] | 60.0 [52.0;64.0] | 49.0 [37.0;59.0] | 0.071 |
| < 60 years, n (%) | 22 (56.4) | 14 (48.3) | 8 (80.0) | 0.169 |
| ≥ 60 years, n (%) | 17 (43.6) | 15 (51.7) | 2 (20.0) | |
| Sex, n (%) | 0.727 | |||
| Male | 20 (51.3) | 14 (48.3) | 6 (60.0) | |
| Female | 19 (48.7) | 15 (51.7) | 4 (40.0) | |
| Preoperative KPS, n (%) | 0.580 | |||
| ≤ 80 | 28 (71.8) | 22 (75.9) | 6 (60.0) | |
| 90–100 | 11 (28.2) | 7 (24.1) | 4 (40.0) | |
| Subventricular zone, n (%) | 1.000 | |||
| Free | 5 (12.8) | 4 (13.8) | 1 (10.0) | |
| Involvement | 34 (87.2) | 25 (86.2) | 9 (90.0) | |
| Gliomatosis, n (%) | 0.515 | |||
| No | 21 (53.8) | 17 (58.6) | 4 (40.0) | |
| Yes | 18 (46.2) | 12 (41.4) | 6 (60.0) | |
| Pathology*, n (%) | 0.380 | |||
| Diffuse astrocytoma | 2 (5.1) | 2 (6.9) | 0 (0.0) | |
| Anaplastic astrocytoma | 10 (25.7) | 6 (20.7) | 4 (40.0) | |
| Glioblastoma | 27 (69.2) | 21 (72.4) | 6 (60.0) | |
| MGMT promoter, n (%) | 1.000 | |||
| Unmethylated | 15 (38.5) | 11 (37.9) | 4 (40.0) | |
| Methylated | 24 (61.5) | 18 (62.1) | 6 (60.0) | |
| Median Ki67 index [IQR], % | 15.0 [6.5;26.2] | 15.0 [6.5;27.5] | 17.5 [7.5;30.0] | 0.617 |
| < 15%, n (%) | 16 (41.0) | 12 (41.4) | 4 (40.0) | 1.000 |
| ≥ 15%, n (%) | 23 (59.0) | 17 (58.6) | 6 (60.0) | |
| Extent of resection, n (%) | 0.562 | |||
| Biopsy | 2 (5.1) | 2 (6.9) | 0 (0.0) | |
| Partial removal | 18 (46.2) | 14 (48.3) | 4 (40.0) | |
| Subtotal removal | 19 (48.7) | 13 (44.8) | 6 (60.0) | |
| Median total RT dose [IQR], Gy | 60.0 [60.0;60.0] | 60.0 [60.0;60.0] | 60.0 [60.0;60.0] | 0.600 |
| Median total RT fractions [IQR], fx | 30.0 [30.0;30.0] | 30.0 [30.0;30.0] | 30.0 [30.0;30.0] | 0.631 |
Abbreviations: IQR interquartile range, ATM ataxia-telangiectasia mutated gene, mut mutation, KPS Karnofsky performance status, MGMT O[6]-methylguanine-DNA methyltransferase, RT radiation therapy, Gy gray, fx fractions
* Pathology refers to the WHO grade
Table 2.
Detailed information on patients harboring the ATM mutation
| Patient number | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mutation | Missense mutation | Missense mutation | Missense mutation | Missense mutation | Missense mutation | Frameshift deletion | Missense mutation | Missense mutation | Frameshift insertion | Missense mutation |
| VAF (%) | 43.01 | 53.22 | 46.3 | 79.92 | 11.8 | 25.88 | 92.49 | 6.45 | 5.08 | 0.369 |
| Amino acid change | p.R2832H | p.R924O | p.K92T | p.P2974L | p.L822S | p.L2946Nfs*9 | p.K92T | p.L413I | p.S28212Vfs*3 | p.P260T |
| Sequence change | c.8495G > A | c.2771G > A | c.275A > C | c.8921C > T | c.2465 T > C | c.8835_8836delGT | c.275A > C | c.1237C > A | c.8432dupA | c.778C > A |
| Age, years | 63 | 51 | 47 | 35 | 32 | 43 | 37 | 58 | 63 | 59 |
| Sex | Male | Female | Female | Female | Male | Male | Female | Male | Male | Male |
| KPS | 70 | 90 | 80 | 90 | 80 | 80 | 90 | 80 | 100 | 60 |
| Pathology | GBM | GBM | GBM | GBM | GBM | GBM | AA, IDH-WT | AA, IDH-WT | AA, IDH-WT | AA, IDH-WT |
| MGMT promoter methylation | (−) | (+) | (+) | (−) | (+) | (+) | (−) | (−) | (+) | (+) |
| SVZ involvement | Yes | None | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Gliomatosis | No | No | Yes | Yes | No | Yes | No | Yes | Yes | Yes |
| Extent of resection | Subtotal | Subtotal | Partial | Subtotal | Subtotal | Subtotal | Partial | Subtotal | Partial | Subtotal |
| Total RT dose, Gy | 60 | 60 | 60.2 | 60 | 60 | 60 | 60 | 60.2 | 60 | 60 |
| PD | Yes | Yes | No | Yes | No | No | No | Yes | No | No |
| PD interval, months | 11.6 | 18.9 | 18.0 | 14.4 | 15.4 | |||||
| Progression site* | Out-field | Out-field | Out-field | Out-field | Out-field | |||||
| Salvage treatment | CTx | Re-RT | BSC | BSC | Re-RT | |||||
| Follow-up, months | 22.7 | 19.8 | 20.8 | 15.0 | 8.1 | 11.4 | 18.7 | 17.3 | 19.2 | 11.1 |
| Survival | Dead | Alive | Dead | Dead | Alive | Alive | Alive | Alive | Alive | Alive |
Abbreviations: VAF Variant Allele Frequency, KPS Karnofsky performance status, GBM glioblastoma, AA anaplastic astrocytoma, IDH isocitrate dehydrogenase, WT wild-type, MGMT O[6]-methylguanine-DNA methyltransferase, SVZ subventricular zone, RT radiation therapy, PD progressive disease, CTx chemotherapy, Re-RT re-irradiation, BSC best supportive care
* Progression site was defined based on relationship between radiation field and recurrence site
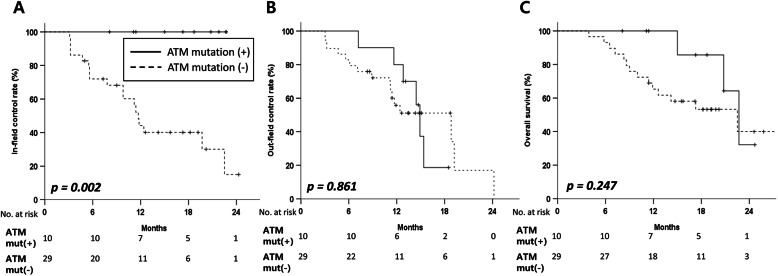
The median follow-up for all patients was 16.5 mo (IQR: 11.4–19.8), and there was no difference in the follow-up period between the two groups (ATM mut(+): 18.0 mo (IQR: 11.4–20.8); ATM mut(−): 15.5 mo (IQR: 10.0–19.2), p = 0.645). Patients with ATM mut(+) showed no in-field failure compared with the ATM mut(−) group (1-y in-field control rate: 100.0% vs. 44.1%, p = 0.002, Fig. 1a). Conversely, there was no difference in the out-field failure between the two groups (1-y out-field control rate: 71.4% vs. 51.9%, p = 0.861, Fig. 1b). Of 23 out-field failures, 6 failures (60.0% of ATM mut(+)) occurred in the ATM mut(+) group and 17 (58.6% of ATM mut(−)) in the ATM mut(−) group. In addition, out-field failures in the ATM mut(+) group were observed to have developed at a median of 15.4 mo (IQR: 11.6–18.9) after diagnosis. In addition, 3 patients died of progressive disease in the ATM mut(+) group with a 2-y OS rate of 32.1%, which was comparable to that of the ATM mut(−) group (39.9%, p = 0.247, Fig. 1c). Using Cox regression univariable analysis, we noted that only the BRCA mutation status was associated with worse out-field control (Hazard Ratio, 2.44) and poor OS (Hazard Ratio, 2.78, Table 3).
Fig. 1.
In-field a and out-field b control rates, and overall survival c of patients according to the mutational status of ATM
Table 3.
Prognostic factors for in-field, out-field control, and overall survival determined using univariable Cox regression analysis
| In-field control | HR | 95% CI | P-value |
| ATM (mut(−) vs. mut(+)) | 0.16 | 0.00–0.48 | 0.036 |
| Age at diagnosis (< 60 vs. ≥60) | 1.02 | 0.39–2.69 | 0.966 |
| Sex (male vs. female) | 1.09 | 0.45–2.78 | 0.857 |
| KPS (90–100 vs. ≤80) | 1.44 | 0.51–4.07 | 0.496 |
| SVZ (free vs. involvement) | 0.97 | 0.28–3.39 | 0.974 |
| Gliomatosis (No vs. Yes) | 1.34 | 0.53–3.39 | 0.534 |
| Extent of resection (subtotal vs. partial/biopsy) | 1.15 | 0.45–2.94 | 0.767 |
| Pathology (WHO Grade II-III vs. WHO grade IV) | 1.47 | 0.34–6.43 | 0.606 |
| MGMT promoter (unmethylated vs methylated) | 0.59 | 0.23–1.48 | 0.260 |
| Ki67 index (< 15% vs. ≥15%) | 1.57 | 0.57–4.32 | 0.380 |
| BRCA status (wild-type vs mutant) | 1.46 | 0.51–4.19 | 0.485 |
| PTEN status (wild-type vs mutant) | 1.32 | 0.47–3.72 | 0.600 |
| TERT status (wild-type vs mutant) | 0.93 | 0.37–2.35 | 0.873 |
| EGFR amplification (No vs. Yes) | 1.98 | 0.74–5.34 | 0.176 |
| TP53 status (wild-type vs mutant) | 1.39 | 0.48–4.01 | 0.546 |
| Out-field control | HR | 95% CI | P-value |
| ATM (mut(−) vs. mut(+)) | 0.92 | 0.35–2.40 | 0.862 |
| Age at diagnosis (< 60 vs. ≥60) | 1.58 | 0.68–3.71 | 0.290 |
| Sex (male vs. female) | 0.95 | 0.40–2.26 | 0.909 |
| KPS (90–100 vs. ≤80) | 1.36 | 0.55–3.39 | 0.506 |
| SVZ (free vs. involvement) | 1.53 | 0.45–5.22 | 0.496 |
| Gliomatosis (No vs. Yes) | 2.32 | 0.96–5.61 | 0.062 |
| Extent of resection (subtotal vs. partial/biopsy) | 0.74 | 0.32–1.74 | 0.497 |
| Pathology (WHO Grade II-III vs. WHO grade IV) | 2.62 | 0.93–7.38 | 0.069 |
| MGMT promoter (unmethylated vs methylated) | 0.49 | 0.21–1.14 | 0.098 |
| Ki67 index (< 15% vs. ≥15%) | 1.35 | 0.55–3.33 | 0.511 |
| BRCA status (wild-type vs mutant) | 2.44 | 1.18–6.07 | 0.035 |
| PTEN status (wild-type vs mutant) | 0.96 | 0.35–2.62 | 0.939 |
| TERT status (wild-type vs mutant) | 0.48 | 0.20–1.14 | 0.096 |
| EGFR amplification (No vs. Yes) | 3.09 | 1.09–8.77 | 0.035 |
| TP53 status (wild-type vs mutant) | 1.80 | 0.67–4.83 | 0.246 |
| Overall survival | HR | 95% CI | P-value |
| ATM (mut(−) vs. mut(+)) | 0.77 | 0.27–2.15 | 0.615 |
| Age at diagnosis (< 60 vs. ≥60) | 2.27 | 0.89–5.84 | 0.088 |
| Sex (male vs. female) | 1.01 | 0.40–2.54 | 0.986 |
| KPS (90–100 vs. ≤80) | 1.96 | 0.69–5.57 | 0.205 |
| SVZ (free vs. involvement) | 1.55 | 0.36–6.79 | 0.559 |
| Gliomatosis (No vs. Yes) | 1.74 | 0.68–4.46 | 0.246 |
| Extent of resection (subtotal vs. partial/biopsy) | 0.74 | 0.30–1.84 | 0.518 |
| Pathology (WHO Grade II-III vs. WHO grade IV) | 0.47 | 0.16–1.43 | 0.184 |
| MGMT promoter (unmethylated vs methylated) | 1.04 | 0.40–2.72 | 0.931 |
| Ki67 index (< 15% vs. ≥15%) | 1.22 | 0.48–3.10 | 0.678 |
| BRCA status (wild-type vs mutant) | 2.78 | 1.05–7.36 | 0.039 |
| PTEN status (wild-type vs mutant) | 1.00 | 0.33–3.04 | 0.992 |
| TERT status (wild-type vs mutant) | 0.88 | 0.36–2.18 | 0.785 |
| EGFR amplification (No vs. Yes) | 1.78 | 0.67–4.72 | 0.249 |
| TP53 status (wild-type vs mutant) | 0.42 | 0.10–1.80 | 0.241 |
*The foreparts of the parentheses were set as the reference group
Abbreviations: HR hazards ratio, CI confidence interval, KPS Karnofsky performance status, SVZ subventricular zone, MGMT O[6]-methylguanine-DNA methyltransferase
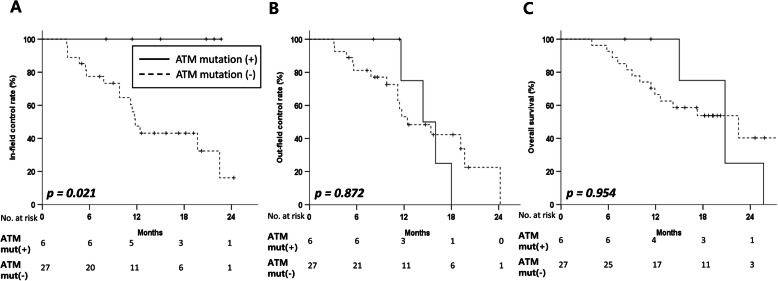
In subsequent analysis employing the recommended diagnostic criteria for the molecular features of glioblastoma according to cIMPACT-NOW (n = 33), ATM mut(+) (n = 6) was shown to be associated with better in-field control than ATM mut(−) (n = 27) (1-y in-field control rate: 100.0% vs. 47.5%, p = 0.021; Fig. 2a, Additional File 2). The out-field control rate and OS values were comparable between the ATM mut(+) and ATM mut(−) groups in tumors with the molecular features of glioblastoma (Fig. 2b-c, Additional File2). Even in tumors in contact with SVZ, the ATM mut(+) group was observed to be significantly associated with better in-field control than ATM mut(−) (1-y in-field control rate: 100.0% vs. 48.1%, p = 0.003, Additional File3). In addition, there were no differences in the out-field control rate and OS values in tumors involving the SVZ (p = 0.711, p = 0.433, respectively, Additional File3).
Fig. 2.
In-field a and out-field b control rates and overall survival c of patients diagnosed with the molecular features of glioblastoma based on cIMPACT-NOW
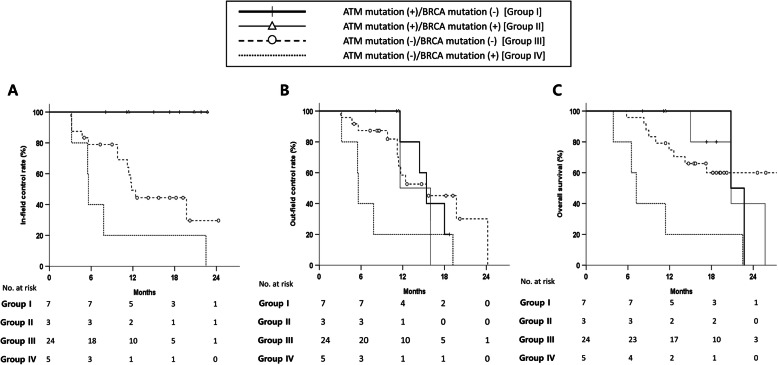
As the status of BRCA mutations was revealed to function as a prognostic factor for out-field control and OS, we performed further subgroup analysis stratified by the mutational status of ATM and BRCA. Accordingly, patients with ATM mut(−) and BRCA mut(+) (n = 5) showed the worst clinical outcomes, with a 1-y in-field control rate of 20.0%, out-field control rate of 20.0%, and OS of 20.0% (Fig. 3a-c). In contrast, patients with ATM mut(+) and BRCA mut(−) (n = 7) were observed to exhibit statistically better clinical outcomes, with a 1-y in-field control rate of 100.0% and 2-y OS rate of 40.0% (p = 0.002, and p = 0.031, respectively). Patients with ATM mut(+) and BRCA mut(+) (n = 3) showed comparable results to the ATM mut(+) and BRCA mut(−) group (1-y in-field control rate and 1-y OS rate of 100.0%; p = 0.809 for OS).
Fig. 3.
In-field a and out-field b control rates and overall survival c of patients stratified by the mutational status of ATM and BRCA
Discussion
We investigated the clinical impact of ATM mutations in patients with intracranial IDH-wildtype high-grade glioma undergoing RT following incomplete tumor resection. Interestingly, although well-known poor prognostic factors, such as either the presence of gliomatosis or the involvement of the SVZ, were predominant in the ATM mut(+) group, we observed excellent in-field control without in-field failure. However, it was shown that the ATM mut(+) group had no impact on the out-field area, demonstrating comparable out-field failures to the ATM mut(−) group. Hence, these clinical results illustrated the increased radio-sensitivity of tumors harboring somatic ATM mutations.
There has been considerable accumulated preclinical evidence that ATM might play a key role in the response to ionizing radiation. Preclinical studies on cell lines from patients with ataxia-telangiectasia syndromes have demonstrated that these cells exhibit increased sensitivity to radiation [21, 22]. Similarly, experimental inhibition of ATM was reported to influence cellular hypersensitivity to radiation [5, 6]. This salutary effect on the radio-sensitivity of cells following the inhibition of ATM was recently observed in GBM cell lines [7, 8], xenograft models of GBM [9], and even patient-derived xenograft models [10]. However, there have been few clinical reports agreeing that ATM mut(+) could be a prognostic biomarker for a favorable response to RT. Jennifer Ma et al. [23] reported that 8 patients with extracranial primary disease (nonglial tumors) harboring ATM mut(+) demonstrated excellent and durable RT responses with a median local control of 4.62 years; moreover, only 2 patients exhibited local recurrence. Especially, 2 patients with breast and non-small cell lung cancers receiving whole brain RT were observed to relapse at 41.0 and 43.2 months after RT. Additionally, Su et al. [24] reported the significant benefit of adjuvant RT in 43 carriers of germline ATM mutations diagnosed with breast cancer; they reported that 13 patients treated with RT (not cobalt therapy) remained locally disease-free, sustaining tolerable toxicities. Recently, we reported that somatic ATM mutations in solid tumors (except brain tumors) were related to markedly improved responses after RT compared with tumors not harboring ATM mutations: an overall response rate of 61.0% (vs. 24.0% in ATM mut(−) tumors) with a durable response for a median period of 11 mo (vs. 3 mo in ATM mut(−)) [25]. In the current study, the ATM mut(+) group was shown to yield excellent in-field control, indicating the radio-sensitive nature of ATM mutations even in patients with intracranial high-grade glioma.
Following the generation of radiation-induced double-strand breaks in the DNA, the ATM gene is known to control the key signaling pathway in the repair process of the DNA double-strand break damage [3, 26]. After it has been recruited to the site of the double-strand break by the MRE11-RAD50-NBS1 complex, ATM is activated by autophosphorylation at Ser1981 [27]. Catalytic activation of ATM is known to subsequently lead to the phosphorylation of many downstream effectors involved in the activation of the G1/S cell-cycle checkpoint arrest (CHk1 and Chk2) [28], homologous recombination or non-homologous end-joining repair of DNA [29, 30], and apoptosis (p53) [31]. Consequently, mutations in ATM might prevent the repair or restoration of radiation-induced damaged DNA, resulting in the radio-sensitivity of cells. Therefore, germline ATM mut(+) is highly involved in radiation-induced risks from its radio-sensitivity. Recently, there is an effort to predict individual response to radiation based on ATM nucleoshuttling rate [32]. Future investigations to increase the understanding of ATM is needed for refinement and development models of personalized ionizing radiation response.
From a clinical point of view, targeting ATM has intrigued many researchers and encouraged them to attempt approaches to enhance the radio-sensitivity of cells. Since then, several ATM inhibitors have been developed and their in vitro or in vivo radio-sensitivity have been reported [17]. However, to date, no ATM inhibitor has yet been clinically used because of the lack of bioavailability, concerns of potential side-effects, and the lack of clinical trials. There is an ongoing prospective phase I trial on the combination of palliative RT and an ATM inhibitor against solid tumors (NCT03225105). In addition to the administration of ATM inhibitors as radio-sensitizers, somatic ATM mutations could also be applied as intrinsic radio-sensitizers. The advent of NGS in routine clinical practice might allow ATM to be used as the sole biomarker for patients treated with RT. Recently, researchers developed a genome-adjusted radiation dose model based on an individualized radio-sensitivity index with genomic features [33]. Beyond personalized radiation dose planning, we could cautiously assume that whole brain or large field RT rather than focal RT might be an effective personalized treatment option with excellent in-field control and prolonged survival in patients with IDH-wildtype ATM mut(+) high-grade glioma.
In addition, the current study showed that BRCA mutations could be associated with poor outcomes of not only intracranial control but also OS. Our results appeared to differ to some extent from those demonstrated on different primary tumors, many of which have reported the survival benefit of BRCA mutations. To date, there has not been any clinical evidence supporting the notion that a somatic BRCA mutation might govern the prognosis of IDH-wildtype high-grade glioma. Recent studies of germline BRCA1/2 mutations in patients with breast cancer have suggested that these mutations have a similar prognosis, though they exhibit different effects on the efficacy of chemotherapy [34, 35]. Additionally, there have been several reports analyzing the survival benefit of BRCA1/2 mutations in patients with ovarian cancer [36]. These data have collectively supported the relatively better short-term prognosis in patients carrying BRCA1/2 mutations. In the current study, a subgroup analysis stratified by the mutational status of both the BRCA and ATM genes revealed that patients without any mutation showed comparable outcomes to the historical data, whereas the ATM mut(−)/BRCA mut(+) group showed the worst outcomes. The ATM mut(+) group with or without BRCA mutations showed comparable results of rates of out-field control and OS, with the same excellent in-field control. These results reflected the fact that BRCA might be a target in the downstream signaling of ATM. Further investigation regarding the prognostic impact of BRCA on high-grade glioma is needed.
Our study had several limitations. First, this was a retrospective analysis; thus, the results should be cautiously reviewed. Second, owing to the limited number of patients, there are issues regarding the statistical insignificance of the results and the unavailability of multivariable analysis to minimize potential confounders. However, to our knowledge, this study has the largest clinical data, to date, on tumors with somatic ATM mutations analyzed by the latest NGS technology. In addition, a relatively frequent ATM mut(+) in the current cohort compared to previous report could overestimate the potential predictive value of ATM mut(+); however, the widespread utilization of NGS is expected to clarify the real-world prevalence of ATM mut(+). An additional limitation was that this was a single-center study; however, its consistent treatment strategy of surgery and RT provides another strength to this study. Lastly, the in-field control rates of the ATM mut(+) group could have been overestimated owing to the short-term follow-up of this study. As most patients with IDH-wildtype high-grade glioma were observed to experience in-field or out-field failures within 1 year after diagnosis, the current follow-up period was considered acceptably reasonable for the analysis of failure patterns. However, long-term follow-up data based on a larger number of patients is still needed to efficiently determine certain effects of ATM mutations.
Conclusions
We demonstrated that ATM mutations in IDH-wildtype high-grade glioma could yield an excellent RT response, irrespective of the adverse features of gliomatosis or the involvement of the SVZ. Owing to the limited number of ATM mutations in high-grade glioma, a further validation in a larger cohort, even including patients under gross total removal status, is needed to identify the prognostic value of ATM mutations. Therefore, we have initiated a multicenter cohort study with Korean Radiation Oncology Group (Protocol No. KROG 19–11). Further preclinical and prospective clinical studies are warranted to elucidate the role of large field RT in patients with ATM mut(+) and the effect of the combination of RT and ATM inhibitors during the course of RT in IDH-wildtype high-grade glioma.
Supplementary information
Additional file 1. Mutations in other genes according to the mutational status of ATM.)
Additional file 2. Clinical outcomes according to the mutational status of ATM in tumors with the molecular features of glioblastoma based on cIMPACT-NOW.
Additional file 3. In-field (a) and out-field (b) control rates and overall survival (c) of patients presenting SVZ involvement.
Acknowledgements
The results were presented at the 61st annual meeting of American Society for Radiation Oncology, October 2019, Chicago, IL, USA and 2019 Annual meeting of Society of Neuro-Oncology, November 2019, Phoenix, AZ, USA.
Abbreviations
- IDH
Isocitrate dehydrogenase
- RT
Radiation therapy
- MGMT
O-6-methylguanine-DNA methyltransferase
- SVZ
Subventricular zone
- ATM
Ataxia-telangiectasia mutated
- NGS
Next-generation sequencing
- IQR
Interquartile range
- MRI
Magnetic resonance image
- cIMPACT-NOW
Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy
- BRCA1/2
Breast Cancer susceptibility gene1/2
- OS
Overall survival
- KPS
Karnofsky performance scale
- GBM
Glioblastoma multiforme
Authors’ contributions
Conceptualization: Hong In Yoon, Jong Hee Chang; Methodology: Nalee Kim, Se Hoon Kim, Hong In Yoon; Formal analysis and investigation: Nalee Kim, Se Hoon Kim, Seok Gu Kang, Ju Hyung Moon, Jaeho Cho, Chang-Ok Suh; Writing-original draft preparation: Nalee Kim, Hong In Yoon, Jong Hee Chang; Writing – review and editing: Nalee Kim, Chang-Ok Suh, Hong In Yoon, Jong Hee Chang; Funding acquisition: Hong In Yoon; Resources: Se Hoon Kim, Seok Gu Kang, Ju Hyung Moon, Jaeho Cho, Hong In Yoon; Supervision: Hong In Yoon, Jong Hee Chang. The author(s) read and approved the final manuscript.
Funding
This work was supported by Radiation Technology R&D program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2017R1C1B2010379).
Availability of data and materials
It is limited due to institutional data protection law and confidentiality of patient data.
Ethics approval and consent to participate
This study was approved by the Health Institutional Review Boards of Yonsei University Hospital (No.4–2019-0009). The requirement for informed consent was waived because of the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong In Yoon and Jong Hee Chang contributed equally to this work.
Contributor Information
Hong In Yoon, Email: yhi0225@yuhs.ac.
Jong Hee Chang, Email: changjh@yuhs.ac.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13014-020-01619-y.
References
- 1.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 2.Kim N, Chang JS, Wee CW, et al. Validation and optimization of a web-based nomogram for predicting survival of patients with newly diagnosed glioblastoma. Strahlenther Onkol. 2019;196(1):58–69. doi: 10.1007/s00066-019-01512-y. [DOI] [PubMed] [Google Scholar]
- 3.Choi M, Kipps T, Kurzrock R. ATM mutations in Cancer: therapeutic implications. Mol Cancer Ther. 2016;15(8):1781–1791. doi: 10.1158/1535-7163.MCT-15-0945. [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Ledermann JA, Kohn EC. PARP inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25(1):32–40. doi: 10.1093/annonc/mdt384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickson I, Zhao Y, Richardson CJ, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64(24):9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 6.Rainey MD, Charlton ME, Stanton RV, et al. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008;68(18):7466–7474. doi: 10.1158/0008-5472.CAN-08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy K, Wang L, Makrigiorgos GM, et al. Methylation of the ATM promoter in glioma cells alters ionizing radiation sensitivity. Biochem Biophys Res Commun. 2006;344(3):821–826. doi: 10.1016/j.bbrc.2006.03.222. [DOI] [PubMed] [Google Scholar]
- 8.Golding SE, Rosenberg E, Adams BR, et al. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle. 2012;11(6):1167–1173. doi: 10.4161/cc.11.6.19576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biddlestone-Thorpe L, Sajjad M, Rosenberg E, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res. 2013;19(12):3189–3200. doi: 10.1158/1078-0432.CCR-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durant ST, Zheng L, Wang Y, et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci Adv. 2018;4(6):eaat1719. doi: 10.1126/sciadv.aat1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11(10):685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 12.Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-Oncology. 2007;9(4):424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roh TH, Park HH, Kang SG, et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: a single-center analysis. Medicine (Baltimore) 2017;96(27):e7422. doi: 10.1097/MD.0000000000007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SH, Kim JW, Chang JS, et al. Impact of including Peritumoral edema in radiotherapy target volume on patterns of failure in Glioblastoma following Temozolomide-based Chemoradiotherapy. Sci Rep. 2017;7:42148. doi: 10.1038/srep42148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im JH, Hong JB, Kim SH, et al. Recurrence patterns after maximal surgical resection and postoperative radiotherapy in anaplastic gliomas according to the new 2016 WHO classification. Sci Rep. 2018;8(1):777. doi: 10.1038/s41598-017-19014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Na K, Kim HS, Shim HS, et al. Targeted next-generation sequencing panel (TruSight tumor 170) in diffuse glioma: a single institutional experience of 135 cases. J Neuro-Oncol. 2019;142(3):445–454. doi: 10.1007/s11060-019-03114-1. [DOI] [PubMed] [Google Scholar]
- 17.Weber AM, Ryan AJ. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Kim SH, Cho J, et al. MGMT gene promoter methylation as a potent prognostic factor in glioblastoma treated with temozolomide-based chemoradiotherapy: a single-institution study. Int J Radiat Oncol Biol Phys. 2012;84(3):661–667. doi: 10.1016/j.ijrobp.2011.12.086. [DOI] [PubMed] [Google Scholar]
- 19.Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. doi: 10.1007/s00401-018-1913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019;8(1):CNS28-CNS. [DOI] [PMC free article] [PubMed]
- 21.Taylor AM, Harnden DG, Arlett CF, et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- 22.Chen PC, Lavin MF, Kidson C, et al. Identification of ataxia telangiectasia heterozygotes, a cancer prone population. Nature. 1978;274(5670):484–486. doi: 10.1038/274484a0. [DOI] [PubMed] [Google Scholar]
- 23.Ma J, Setton J, Morris L, et al. Genomic analysis of exceptional responders to radiotherapy reveals somatic mutations in ATM. Oncotarget. 2017;8(6):10312–10323. doi: 10.18632/oncotarget.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su Y, Swift M. Outcomes of adjuvant radiation therapy for breast cancer in women with ataxia-telangiectasia mutations. JAMA. 2001;286(18):2233–2234. doi: 10.1001/jama.286.18.2233. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJB, Yang AJ, Chang JS, et al. Genomic analysis reveals somatic mutations of ATM gene in DNA repair confer exceptional target lesion response to radiation therapy. J Glob Oncol. 2019;5(suppl):130. doi: 10.1200/JGO.2019.5.suppl.130. [DOI] [Google Scholar]
- 26.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14(4):197–210. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- 27.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282(5395):1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 29.Durant ST, Nickoloff JA. Good timing in the cell cycle for precise DNA repair by BRCA1. Cell Cycle. 2005;4(9):1216–1222. doi: 10.4161/cc.4.9.2027. [DOI] [PubMed] [Google Scholar]
- 30.Durant ST, Paffett KS, Shrivastav M, et al. UV radiation induces delayed hyperrecombination associated with hypermutation in human cells. Mol Cell Biol. 2006;26(16):6047–6055. doi: 10.1128/MCB.00444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blasius M, Bartek J. ATM targets hnRNPK to control p53. Cell Cycle. 2013;12(8):1162–1163. doi: 10.4161/cc.24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berthel E, Foray N, Ferlazzo ML. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers (Basel). 2019;11(7). [DOI] [PMC free article] [PubMed]
- 33.Scott JG, Berglund A, Schell MJ, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18(2):202–211. doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast Cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncol. 2017;3(10):1378–1385. doi: 10.1001/jamaoncol.2017.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin JR, Rosen B, Moody J, et al. Long-term ovarian cancer survival associated with mutation in BRCA1 or BRCA2. J Natl Cancer Inst. 2013;105(2):141–148. doi: 10.1093/jnci/djs494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Mutations in other genes according to the mutational status of ATM.)
Additional file 2. Clinical outcomes according to the mutational status of ATM in tumors with the molecular features of glioblastoma based on cIMPACT-NOW.
Additional file 3. In-field (a) and out-field (b) control rates and overall survival (c) of patients presenting SVZ involvement.
Data Availability Statement
It is limited due to institutional data protection law and confidentiality of patient data.