Abstract
The LIM homeodomain transcription factor Lmx1a shows a dynamic expression in the developing mouse ear that stabilizes in the non-sensory epithelium. Previous work showed that Lmx1a functional null mutants have an additional sensory hair cell patch in the posterior wall of a cochlear duct and have a mix of vestibular and cochlear hair cells in the basal cochlear sensory epithelium. In E13.5 mutants, Sox2-expressing posterior canal crista is continuous with an ectopic “crista sensory epithelium” located in the outer spiral sulcus of the basal cochlear duct. The medial margin of cochlear crista is in contact with the adjacent Sox2-expressing basal cochlear sensory epithelium. By E17.5, this contact has been interrupted by the formation of an intervening non-sensory epithelium, and Atoh1 is expressed in the hair cells of both the cochlear crista and the basal cochlear sensory epithelium. Where cochlear crista was formerly associated with the basal cochlear sensory epithelium, the basal cochlear sensory epithelium lacks an outer hair cell band, and gaps are present in its associated Bmp4 expression. Further apically, where cochlear crista was never present, the cochlear sensory epithelium forms a poorly ordered but complete organ of Corti. We propose that the core prosensory posterior crista is enlarged in the mutant when the absence of Lmx1a expression allows JAG1-NOTCH signaling to propagate into the adjacent epithelium and down the posterior wall of the cochlear duct. We suggest that the cochlear crista propagates in the mutant outer spiral sulcus because it expresses Lmo4 in the absence of Lmx1a.
Keywords: Lmx1a, Crista, Cochlea, Ear, Mouse
Introduction
LMX1A is a LIM-homeodomain transcription factor most closely related to LMX1B and the ISLET factors (Hunter and Rhodes 2005), and is among several such factors expressed in the mammalian ear (Deng et al. 2010; Huang et al. 2008). In chick and mouse ears, Lmx1a is expressed dorsally and posteriorly in the vesicle stage ear (Failli et al. 2002; Giraldez 1998). In the mouse, its initial expression is excluded from the anteroventral neurosensory area and the posterior crista to the non-sensory epithelium, including the developing endolymphatic duct (Nichols et al. 2008). It is also expressed in the constrictions that separate the cochlear part of the ear from the vestibular ear and in the outer spiral sulcus of the cochlear duct (Huang et al. 2008; Koo et al. 2009; Nichols et al. 2008).
LMO4 is a LIM-only transcription cofactor known to participate in diverse transcription complexes that can both activate and suppress transcription (Wadman et al. 1997). It has also been shown to complex with LIM-homeodomain factors, including LMX1A and LMX1B, via the LIM binding domain factor, LDB1 (Agulnick et al. 1996; Matthews et al. 2008). In the mouse ear, Lmo4 expression is required for the formation of a posterior crista and is co-expressed with Lmx1a in the outer spiral sulcus of the cochlear duct (Deng et al. 2014; Deng et al. 2010). In the outer spiral sulcus, its expression is required to prevent the sulcus from forming ectopic cochlear sensory epithelium (Deng et al. 2014).
The DreherJ mouse used here carries a recessive point mutation in the Lmx1a gene (Millonig et al. 2000). DreherJ is one of 14 Lmx1a mutations known to occur in rats and mice, all of which are thought to be functional nulls (Bergstrom et al. 1999; Chizhikov et al. 2006a; Kuwamura et al. 2005; Steffes et al. 2012). Phenotypic manifestations of these mutations include white spotting and ataxia (Bergstrom et al. 1999), skeletal defects, and malformations of the hindbrain (Manzanares et al. 2000), roof plate and cerebellum (Chizhikov et al. 2006b; Glover et al. 2018; Mishima et al. 2009), the midbrain (Deng et al. 2011), and ear (Deol 1964; Huang et al. 2018; Koo et al. 2009; Mann et al. 2017; Nichols et al. 2008; Steffes et al. 2012).
Control of Lmx1a gene expression is likely to be complex. For example, a Lmx1a-cre-containing BAC (bacterial artificial chromosome) with a ROSA26-lacZ reporter to visualize Lmx1a expression in the roof plate and cerebellum of the mouse shows no expression in the ear [e.g., Fig. 3B of (Chizhikov et al. 2006b)], suggesting that the BAC they used lacked an enhancer for ear expression. Furthermore, several human Lmx1a regulatory elements can initiate expression in the zebrafish ear, while others direct expression elsewhere (Burzynski et al. 2013). Thus, while rodents carrying inframe mutations are lethally affected, less traumatic mutations, potentially including those to regions controlling Lmx1a expression, can result in human ear-specific pheno-types (Wesdorp et al. 2018) with variable penetrance, depending on the mutation (Schrauwen et al. 2018). Judging from the various accounts of mouse Lmx1a mutants, it appears that not all descriptions show a similar effect on posterior canal crista and/or interpret the data differently (Huang et al. 2018; Koo et al. 2009; Nichols et al. 2008; Steffes et al. 2012). Specifically, mice mutant for Lmx1a show an enlarged posterior crista and the presence of a novel sensory patch in the lateral wall of the cochlear duct (Nichols et al. 2008). This sensory patch is near the posterior crista and innervated by a branch of the crista’s nerve and is thus termed a papilla neglecta, in keeping with known data on sensory epithelia and their innervation across vertebrates (Fritzsch et al. 2002; Fritzsch and Elliott 2017). We here analyze this proposed relationship in more detail and provide evidence suggesting that this papilla should be named a cochlear crista sensory epithelium.
Fig. 3.
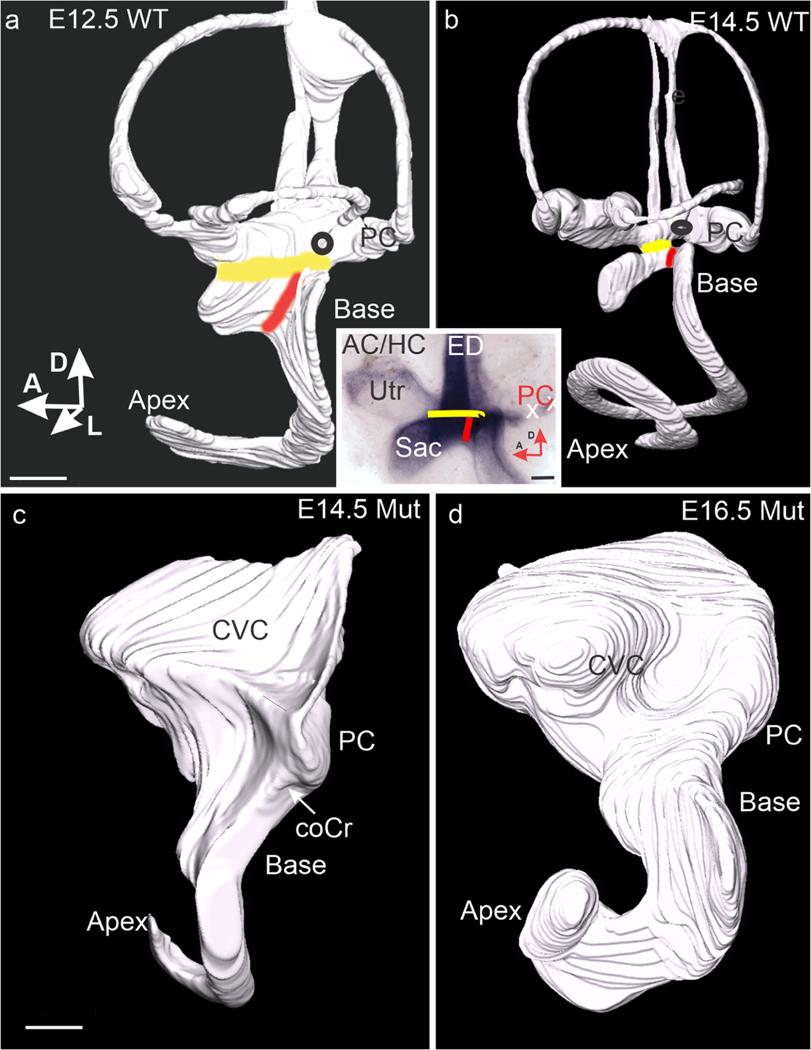
Utriculo-saccular and cochleo-saccular constrictions fail to form in the mutant. (a) Lateral view of the lumen of an E12.5 wild-type ear. Yellow and red bands mark the sites of the utriculo-saccular and cochleo-saccular constrictions respectively. (b) Lateral view of an E14.5 wild-type ear. Note that the constrictions now isolate the base of the cochlear duct from the utricle and saccule (Utr, Sac a/b inset). Black ovals mark the approximate site of a papilla neglecta. Note the segregation of the saccule is fully distinct. (a/b inset) Flat mount of an E14.5 wild-type ear stained for Lmx1a expression. Yellow and red lines mark strong expression in the planes of the utriculo-saccular and cochleo-saccular constrictions, respectively. (c) Lateral view of the lumen of an E14.5 mutant. An arrow marks the cochlear crista, and the site of the transition from basal to apical cochlear sensory epithelia. (d) Lateral view of an E16.5 mutant ear showing an enlarged but unchanged shape. Abbreviations: AC/HC, anterior + horizontal crista; coCr, cochlear crista; CVC, common vestibular cavity; Sac, sacccule; PC, posterior crista; Utr, utricle. All bars are 100 μm
Specifically, we describe that the cochlear crista sensory epithelium forms from what would otherwise be the non-sensory epithelium of the cochlear duct’s outer spiral sulcus. There it forms a prolonged association with sensory epithelia of the basal cochlea when Lmx1a-expressing constrictions fail to form and separate them. Where this association takes place, the basal cochlear sensory epithelium lacks an outer hair cell band, and its inner hair cell band fails to converge. Where the outer hair cell band is lost, the cochlear pattern of Bmp4 expression is reduced to patches separated by sometimes extensive gaps. In contrast, no cochlear crista forms in the mutant’s apical cochlear duct. An imperfectly organized but otherwise intact organ of Corti is present and shows a continuous band of Bmp4 expression (Dvorakova et al. 2016; Dvorakova et al. 2019; Ohyama et al. 2010; Pan et al. 2012; Pan et al. 2011).
Materials
Mice
Atoh1tm2Hzo (Atoh1-lacZ) mice were obtained from Dr. Huda Zoghbi (Bermingham et al. 2001; Fritzsch et al. 2005). B6.C3-Lmx1adr−6J/J (DreherJ) mice were obtained from Jackson Lab. Procedures were performed in accordance with the relevant ethical regulations and had been approved by the local animal use and care authority. Applicable international, national, and institutional guidelines for the care and use of animals were followed. All mice were maintained in an AALAC certified facility under a Creighton University IACUC-approved protocol.
Genotyping for Lmx1a was performed by PCR according to a protocol obtained from the laboratory of Dr. Kathleen Millen using the following primers: Forward ggc aac atc tgt tgc tgt tg and Reverse gaa gca ggc act tac ttc tc. 10 μl of the PCR product was digested with 2 μl of the following mix: 1.2 μl New England Biologicals buffer #4, 0.1 μl HpyCH4V endonuclease (New England Biologicals), 0.7 μl H2O, for 3 h @ 37° C and run on a 3% agarose gel (GenePure HiRes; ISC Bioexpress). The wild type generated bands at 127 and 50 + 40 BP and the mutant at 127 and 90 BP. Genotyping for Atoh1-lacZ mice used primers for the lacZ gene (Echelard et al. 1994; Fritzsch et al. 1995). At least 3, and in many cases eight ears from at least 2 litters, were examined at any given age/genotype/stain. Timed breeding took place overnight, with midnight considered E0.0.
Mice used here were of mixed genetic background. In addition to C57/B6 and SV129, our colony included animals with some outbred CF1 (Charles River) background. Anecdotally, phenotypes changed somewhat as we moved to a purer C57/B6 background, but remained qualitatively similar.
Histology
Atoh1-lacZ was detected using ß-galactosidase/X-gal histo-chemistry (Nichols et al. 1994). E12 and E13 embryos were removed from the uterus to PBS, decapitated, and their mid/ forebrain and facial regions removed. They were then immersion fixed for 20–30 min in 4% paraformaldehyde in PBS (PFA-PBS) at RT. Older embryos and pups were decapitated, and the skin removed. The heads were then hemisected (scissors then scalpel), and the brains removed. They were next rinsed in 0.1 M phosphate buffer, pH 7.4, for 10 min and immersed in multiples of a solution consisting of 3.632 ml phosphate buffer, 40 μl 0.5 M K3Fe(CN)6, 40 μl 0.5 M K4Fe(CN)6, 8 μl 1 M MgCl2, 40 μl 1% Na deoxycholate, 40 μl 2% NP-40, 200 μl 20 mg/ml X-gal (dissolved in dimethylformamide-stock stored at – 20° C). Heads were stained overnight in a light-tight box on a rocker at RT, and staining stopped by refixation in PFA/PBS overnight. Ears were dissected and observed as unflattened (except as noted) whole mounts in 9:1 glycerin/PBS or rapidly embedded in a soft epoxy resin. Embedded ears were sectioned at 5 μm using a histology grade diamond knife (Pella and Dumont). Some sections were counterstained with a 1:50 dilution of 0.5% sodium borate buffered 0.5% toluidine blue stock on an LKB 2208 warming plate. Whole mounts were photographed using an Olympus SZ61 dissecting microscope and DP21 digital camera. Sections were photographed using a Nikon Eclipse 600 light microscope with bright field or phase contrast optics and a QImaging digital camera.
In situ hybridization
Whole mount ISH were carried out according to standard procedures (Pauley et al. 2003) using previously evaluated digoxigenin-labeled riboprobes for Bmp4 (Jones et al. 1991) and Sox2 (Weston et al. 2011). Procedures performed without riboprobes resulted in pale, homogeneous background stains. E12 and E12.5 ears were stained with capsules intact. For E13.5 ears, the otic capsule was partially removed. For older ears, it was necessary to either strip the capsule entirely from the cochlea (wild types) or to cut off the dorsal vestibule and apical cochlea (mutants) to facilitate reagent penetration. Anti-digoxigenin-AP antibody and BM purple (Roche) were used to detect riboprobe binding. Selected ears were embedded in epoxy resin and sectioned as described above.
Three-dimensional reconstruction
This protocol was adapted from (Kopecky et al. 2012) to readily generate three-dimensional renderings of the otic lumen.
Results
A new sensory epithelium forms in the lateral wall of the mutant cochlear duct
On E10.5, Nichols et al. (2008) demonstrate Sox2 expression with discrete margins in the posterior prosensory field of the wild-type ear. In addition to Sox2, hybridization for a second prosensory marker, Bmp4 (Chang et al. 2008; Macova et al. 2019; Morsli et al. 1999; Pan et al. 2011), reveals a similar pattern on E11.0 (Fig. 1a). As previously described, Lmx1a is expressed in and surrounding the crista on E10.5 (Nichols et al. 2008). This expression persists in the non-sensory epithelium surrounding the posterior crista on E13.5, but it has been excluded from the crista itself (Huang et al. 2018; Koo et al. 2009).
Fig. 1.
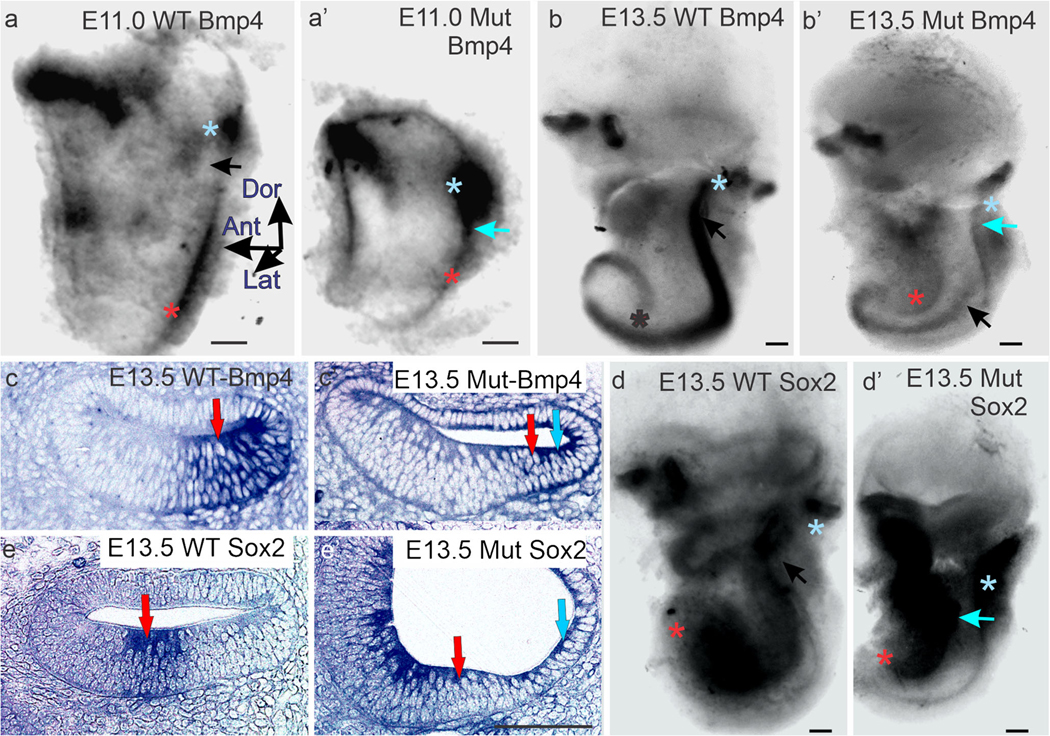
Bmp4 and Sox2 are expressed in the cochlear crista. (a, a’) Whole mounts of E11.0 wild type (a) and mutant Lmx1a null mice (a’) ears stained for Bmp4 expression. Blue asterisks mark posterior crista and red asterisks cochlear Bmp4 expression. Bmp4 expression is absent from the utriculo-saccular constriction of the wild-type ear (black arrow in a), but present in the mutant (blue arrow in a’). (b, b’) Whole mounts of E13.5 wild-type (b) and mutant (b’) ears stained for Bmp4 expression. Black arrow marks the utriculo-saccular constriction in b. Blue arrow mark the cochlear crista, and the black arrow the basal cochlear sensory epithelium in b’. Black (b) and red (b’) asterisks marks the apical tip of the cochlea. (c, c’) Horizontal sections across the cochlear ducts of wild-type (c) and Lmx1a null mutant (c’) ears stained for Bmp4 expression. To the right of the blue arrow crista sensory epithelium (cochlear crista) is stained in the outer spiral sulcus; red arrows mark cochlear sensory epithelium. (d, d’) Whole mounts of E13.5 wild-type (d) and mutant (d’) ears stained for Sox2 expression. Blue asterisks mark the posterior crista, and red asterisks mark the apical tip of the cochlear sensory epithelium. Blue arrow in d’ lies over the cochlear crista. (e, e’) Horizontal sections across the cochlear ducts of E13.5 wild-type (e) and mutant (e’) ears stained for Sox2 expression. Blue arrow marks crista sensory epithelium (cochlear crista) in the outer spiral sulcus, red arrows mark cochlear sensory epithelium. All bars are 100 μm
In the E10.5–11.0 mutant, both Sox2 (Dvorakova et al. 2019; Nichols et al. 2008) and Bmp4 (Fig. 1a’) expressions are enlarged in the posterior prosensory field, and while these expressions are strong at their cores, their edges appear blurred. An extension of Bmp4 expression in the mutant crista forms a bridge to the posterior wall of the cochlear duct (blue arrow). By E13.5, Sox2 (Fig. 1d’) and Bmp4 (Fig.1b’) expressions extend across the site that would form the utriculosaccular constriction in the wild type and down the posterior wall of the basal cochlear duct. Their expressions are broader and stronger proximal to the crista, then become narrower, weaken, and terminate before the duct begins its apical spiral. This cochlear crista subsequently elongates further (Nichols et al. 2008), but only enough to keep pace with the similarly elongating basal cochlear duct. Mann et al. (Mann et al. 2017) similarly describe the expression of Jag1, an additional prosensory marker, extending from the vicinity of the posterior crista down the posterior wall of the cochlear duct in an Lmx1a mutant mouse. Koo et al. (Koo et al. 2009) describe a likely related ventral spread of vestibular gene expressions.
On E13.5, serial sections of mutant ears demonstrate that a Bmp4 and Sox2-expressing cochlear crista is located in the basal duct’s outer spiral sulcus, immediately adjacent to the cochlear sensory epithelium (Fig. 1c, c’ e, e’). In the wild type, the outer spiral sulcus is known to express Lmx1a (Huang et al. 2018; Nichols et al. 2008).
Atoh1-expressing hair cells differentiate in the cochlear crista
By E17.5, an Atoh1-expressing sensory epithelium has formed at the site occupied by the Sox2-expressing cochlear crista sensory epithelium the previous day (Fig. 2a, a’). On E16.5 Sox2 expression appears uniform proximal to the posterior crista but becomes weaker distally (Fig. 2a inset). Subsequent Atoh1 expression frequently forms larger patches proximally and progressively smaller patches distally. This suggests that the cochlear crista’s Sox2 expression field is not uniform but includes sites of stronger and weaker expression. We speculate that stronger sites would go on to form Atoh1-expressing patches, while weaker sites would form intervening non-sensory epithelium (Nichols et al. 2008). At the crista’s distal end, Atoh1 expression can either terminate more compactly (Fig. 2a) or taper into an elongated series of patches and scattered cells (Fig. 2b). The size of the Atoh1 cochlear crista varied widely in our mixed strain animals. Rarely, ears lacked an Atoh1-expressing cochlear crista altogether (not shown). However, a more typical example (Fig. 2a) was 225 μm long and consists of approximately 50 hair cells. Exceptionally, the largest cochlear crista, we encountered was 575 μm long and consisted of approximately 216 hair cells (Fig. 2a’). Approximations were made by multiplying hair cells/unit length by total length and then by an average of hair cells per width taken at five evenly spaced sites along the length of Fig. 2a and ten sites along the length of Fig. 2a’.
Fig. 2.
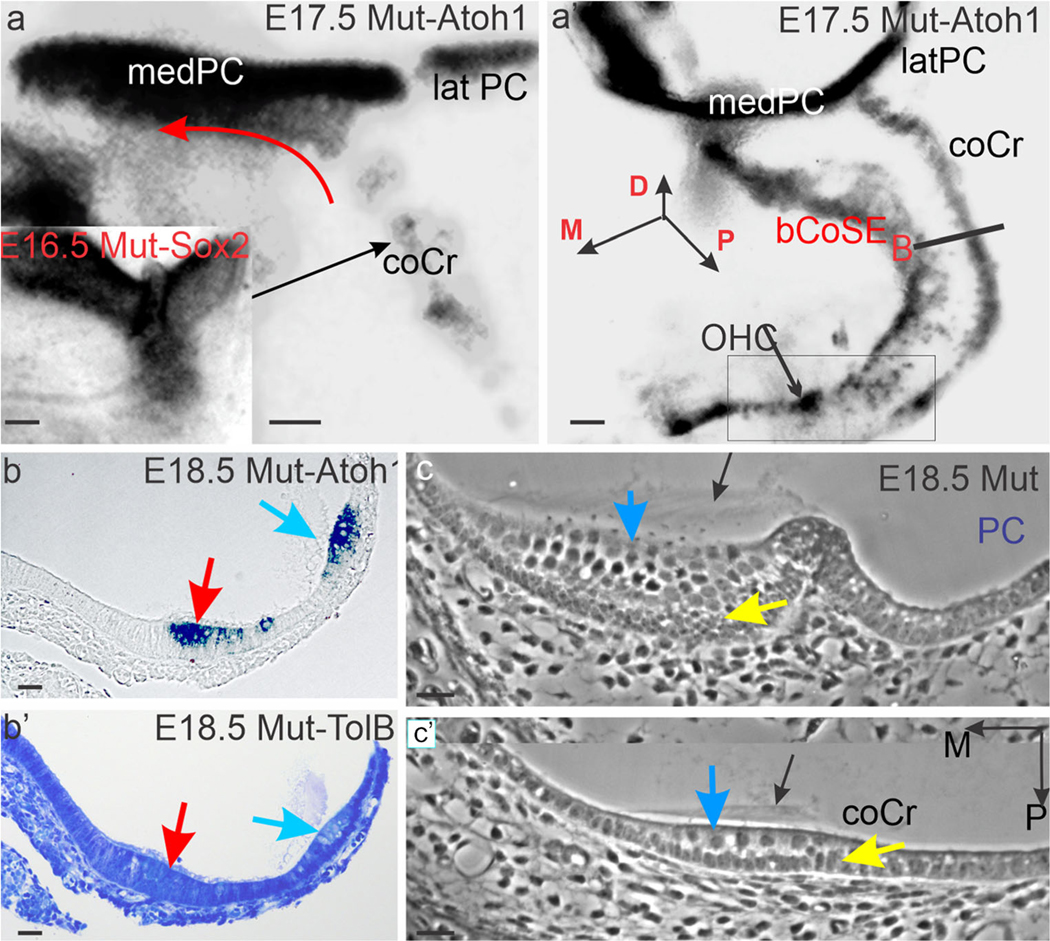
The cochlear crista differentiates Atoh1-expressing hair cells. (a, a’) Whole mounts of E17.5 ears stained for Atoh1 expression in Lmx1a null mice. Note that the enlarged medial arm of the posterior crista (medPC) includes an Atoh1-expressing extension of the cochlear crista (red curved arrow) along its ventral margin. This extension is not present on the ventral margin of the smaller lateral arm (latPC). Boxed area in a’ marks a region in which the cochlear crista ends in scattered cells and the previously associated basal CoSE retains patches of outer hair cells (OHC). (b, b’) Adjacent phase contrast (b) and toluidine blue stained (b’) sections in the plane marked B in a’. Red arrows mark basal cochlear sensory epithelium and blue arrows, cochlear crista. Note cupula-like matrix beneath the blue arrows.(c, c’) Selected phase contrast serial sections across the length of the posterior crista (c) and the width of the cochlear crista (c’). Black arrows mark cupular matrix, blue arrows, hair cells, and yellow arrows mark a supporting cell layer. Abbreviations: bCoSE, basal cochlear sensory epithelium; coCr, cochlear crista; latPC, lateral arm of the posterior crista; medPC, medial arm of the posterior crista; OHC, outer hair cell patch. Bars are 100 μm (a, a’), and 10 μm (b, b’, c, c’)
On E18.5, in horizontal sections across the cochlear duct proximal to the posterior crista, the cochlear crista is bilayered, with Atoh1-expressing hair cells in its luminal layer (Fig. 2b). This figure also clearly demonstrates the separation of the cochlear crista from the cochlear sensory epithelium at this age. A toluidine blue-stained matrix, distinct from tectorial membrane, is present in the lumen above the cochlear crista (Fig. 2b’). In serial horizontal sections, cut from an E18.5 mutant, the posterior ampulla demonstrates the expected cupula matrix capping the crista sensory epithelium (Fig. 2c). In the basal cochlear duct proximal to the crista, a similar matrix caps the cochlear crista (Fig. 2c’).
In mutant ears, the medial arm of the posterior crista is both wider and variably longer than the lateral arm. Its increased width appears to result from incorporating an extension of the cochlear crista along its ventral margin (Fig. 2a, a’).
Utriculo-saccular and cochleo-saccular constrictions fail to form in the mutant
On E11.5, the wild-type cochlear duct opens broadly onto a common vestibular cavity that includes the posterior ampulla (Morsli et al. 1999). However, by E12.5, the posterior aspect of the utriculo-saccular constriction is separating the duct from the posterior ampulla and crista (Fig. 3a). This constriction is completed and the cochleo-saccular constriction (ductus reuniens) added prior to E14.5 (Fig. 3b). Lmx1a is strongly expressed in both constrictions (Fig. 3a/b, inset).
In the E14.5 mutant, the utriculo-saccular and cochleo-saccular constrictions are vestigial (Fig. 3c) and remain so at E16.5 (Fig. 3d). As a result, the posterior ampulla and its crista remain open to the basal cochlear duct and its cochlear sensory epithelium in a persistent common vestibular cavity (CVC). As described below, this continuity, together with the over-growth of the posterior crista to form the cochlear crista, will bring the two sensory epithelia into contact.
The crista makes contact with the basal cochlear sensory epithelium
In the E13.5 wild type, horizontal sections across the cochlear duct identify a Sox2-expressing cochlear sensory epithelium (Fig. 1e), the basal end of which is separated from the saccular macula by the cochleo-saccular constriction by E14.5 (red line, Fig. 3b). In the mutant, however, no cochleo-saccular constriction forms and the cochlear sensory epithelium remains joined to the macula. To maintain this junction, the basal end of the cochlear sensory epithelium turns anteriorly out of the basal cochlear duct and onto the medial wall of a common vestibular cavity. There it joins the saccular macula. The morphology of this junction is best visualized in the perinatal mutant ear (Fig. 4a, b). See also summary Figure 8.
Fig. 4.
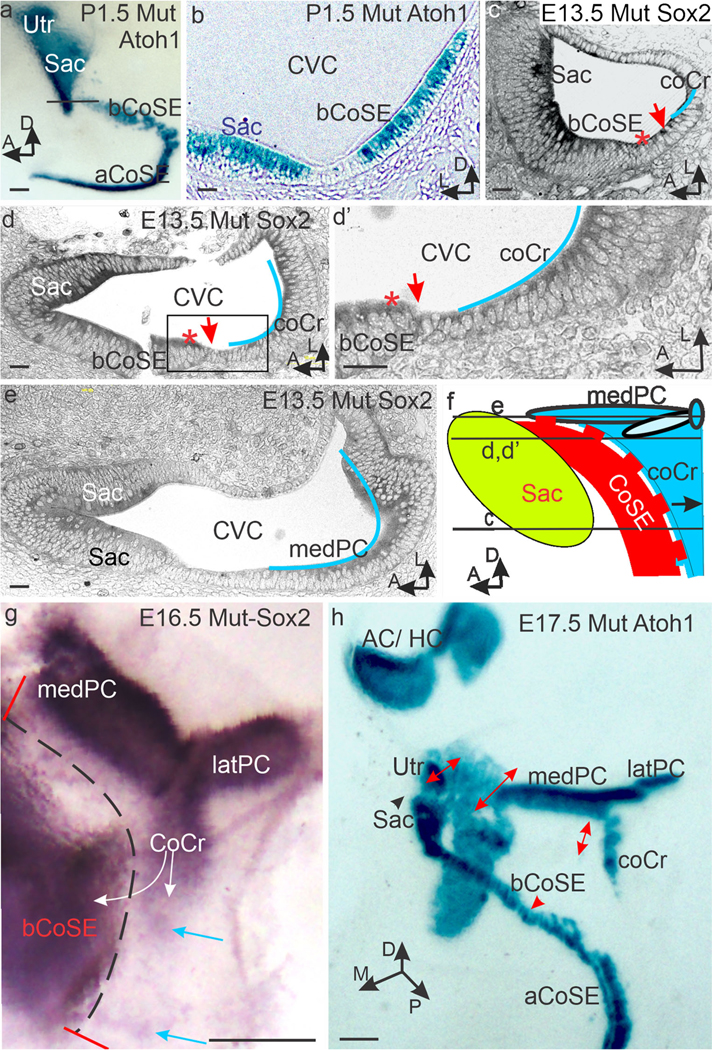
Mutant cochlear and crista sensory epithelia make contact. (a, b) P1.5 Whole mount (a) and section (b) stained for Atoh1 expression in hair cells. Note the crista is parted from the basal cochlear sensory epithelium (bCoSE) at this age and is not included. Black line in (a) marks the plane of section in b where the basal CoSE approaches the saccular macula (Sac) and joins it in an adjacent section (not shown). (c, d, d’,e) Selected horizontal serial sections cut from an E13.5 ear stained for Sox2 expression in planes indicated in f. Blue lines approximate crista sensory epithelium. Red asterisks mark the abneural edge of the basal cochlear sensory epithelium. Red arrows mark the region of contact between crista and cochlear sensory epithelia. The cochlear sensory epithelium is artifactually broken in (d). (d’) shows the boxed area in (d). In (e), the plane of section is dorsal to the cochlear sensory epithelium. (f) Diagram illustrating the association of the crista sensory epithelium with the basal cochlear sensory epithelium along the dashed red line. Here the cochlear sensory epithelium will lack an outer hair cell band and cochlear Bmp4 expression will be patchy. (g) Whole mount of an E16.5 posterior and cochlear crista stained for Sox2 expression. White arrows mark the proposed directions of crista propagation by lateral induction. Blue arrows mark distal patches of Sox2 expression. Note the broad and elongated medial arm of the posterior crista (medPC). Dotted black line marks the now separating zone of contact with the basal cochlear epithelium. (h) By E17.5, the cochlear crista and medial posterior cristae are separated from the basal cochlear sensory epithelium, but the former contact zone can still be recognized (double headed red arrows). Abbreviations: aCoSE, apical cochlear sensory epithelium; AC/HC, anterior and horizontal cristae; bCoSE, basal cochlear sensory epithelium; coCr, cochlear crista; CVC, common vestibular cavity; latPC, lateral arm of the posterior crista; medPC, medial arm of the posterior crista; Sac, saccular macula; Utr, utricle. Bars in a, g, and h are 100 μm. All others are 10 μm
Fig. 8.
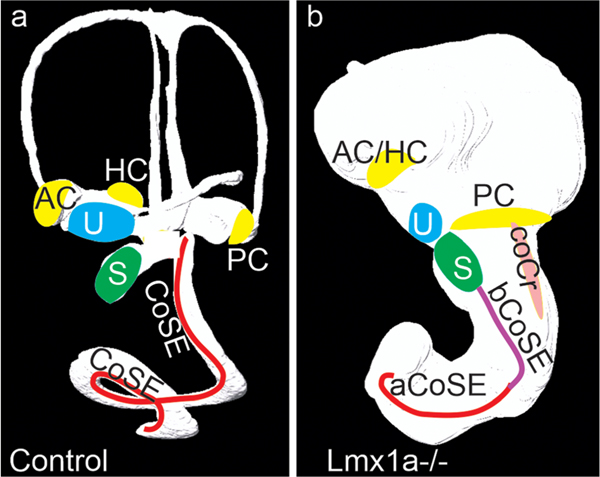
Summary Figure. (a) In the E14.5 wild type, cochlear sensory epithelium forms in an cochlear duct separated from the posterior crista sensory epithelium and saccular macula. (b) In the E16.5 mutant the absence of constrictions has permitted an enlarged medial arm of the posterior crista and an ectopic cochlear crista to interact with the basal cochlear sensory epithelium, eliminating its outer hair cell band and altering its inner hair cell band. At this age these have now separated, but remain adjacent. Note this is a view of the lateral side of the otic lumen, but the elongated arm of the posterior crista and the basal cochlear sensory epithelium are actually facing its medial side. Abbreviations: AC, anterior crista; aCoSE, apical cochlear sensory epithelium; bCoSE, basal cochlear sensory epithelium; CoSE, cochlear sensory epithelium; coCr, cochlear crista; HC, horizontal crista; PC, posterior crista; S, saccule; U utricle
As noted above, the utriculo-saccular constriction is also absent in the mutant. In the absence of both constrictions, the basal cochlear sensory epithelium, on its path to join the saccular macula, associates laterally with the cochlear crista and its extension on the ventral margin on the medial posterior crista. On E13.5, this association is so close that Sox2-expressing cells in the cochlear crista and medial arm of the posterior crista make direct contact with Sox2-expressing cells on the abneural margin of the basal cochlear sensory epithelium (Fig. 4c–d’). In the wild type, the future outer hair cell band would be located on this margin. These relationships are diagrammed in Fig. 4f and Summary Figure 8. While contact between the two Sox2 expressing sensory epithelia is broken by E16.5 and their Atoh1 expressions are separated on E17.5, the former extent of the association can still be visualized in E16.5 and E17.5 whole mounts (Fig. 4g, h).
A Noggin-expressing non-sensory epithelium separates the crista from the basal cochlear sensory epithelium
In the wild-type cochlea, the BMP antagonist, Noggin (Nog), is expressed abneural to the cochlear sensory epithelium on E13.5 (Fig. 5a). By E17.5, this expression extends from the abneural (lateral) margin of the organ of Corti to the nearest margin of the stria vascularis (Fig. 5b).
Fig. 5.
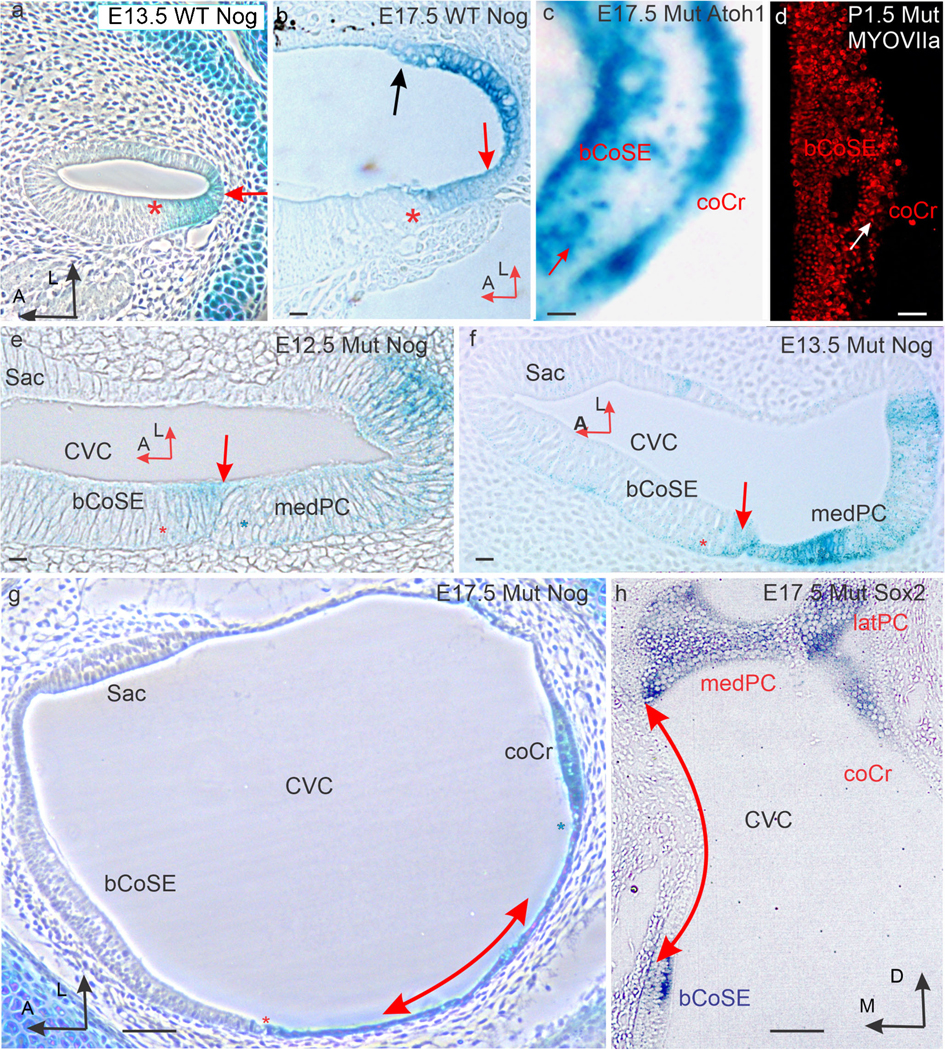
A Nog-expressing epithelium separates basal cochlear sensory epithelium from crista sensory epithelium. (a) Section across the cochlear duct of an E13.5 wild-type ear stained for Nog expression (red arrow). Red asterisk marks the site of the cochlear sensory epithelium. (b) Similar section across an E17.5 wild-type duct stained for Nog expression in the outer spiral sulcus. Red asterisk marks the site of the cochlear sensory epithelium. A black arrow marks the edge of the stria vascularis identified by black melanocytes in its forming intermediate layer. A red arrow marks the outer spiral sulcus. (c) Atoh1 stained cochlear duct from the E17.5 mutant ear in Fig. 2a’. Note several hair cells (e.g., red arrow) are located in the epithelium separating the cochlear sensory epithelium from the crista sensory epithelium. (d) Flat mount of the basal CoSE of a P1.5 mutant immunostained for MYOSIN VIIa to label maturing hair cells. White arrow marks the abneural margin of the basal cochlear sensory epithelium. Note scattered hair cells to the right of the margin. (e) Horizontal section across the posterior crista and basal cochlear sensory epithelium of an E12.5 mutant ear. The medial arm of the crista (blue asterisk) and cochlear sensory epithelium (red asterisk) are in direct planar contact (red arrow). Nog expression stains the interface between the two. (f) similar section from an E13.5 ear. The contacting edge of the crista is thinning. (g) Horizontal section across the common vestibular cavity (CVC) of an E17.5 mutant ear stained for Nog expression. A red asterisk marks the abneural (lateral) margin of the basal cochlear sensory epithelium, and a blue asterisk marks the facing margin of the cochlear crista. The double headed arrow parallels the Nog stained epithelium separating the cochlear sensory epithelium from the crista sensory epithelium. (h) Vertical section across the common vestibular cavity of an E17.5 mutant ear stained for Sox2 expression. The double-headed red arrow parallels the unstained non-sensory epithelium separating the cochlear sensory epithelium from the crista. Abbreviations: bCoSE, basal cochlear sensory epithelium; coCr, cochlear crista; CVC, common vestibular cavity; latPC, lateral arm of the posterior crista; medPC, medial arm of the posterior crista; Sac, saccular macula. Bars in c, d are 100 μm. All others are 10 μm
In the mutant, Nog is expressed at the interface between the crista sensory epithelium and the basal cochlear sensory epithelium beginning on E12.5 (Fig. 5e). At E13.5 this interface temporarily expresses both Sox2 (Fig. 4c) and Nog (Fig. 5f). By E17.5, the cells in the interface have expanded into a flattened monolayer (Fig. 5g) that no longer expresses detectable Sox2 (Fig. 5h). We thus identify this as a non-sensory epithelium. Nevertheless, scattered Atoh1-expressing hair cells are occasionally present (Fig. 5c, d).
The basal cochlear sensory epithelium of the mutant is altered where formerly associated with crista sensory epithelium
Though separated by E16.5, the cochlear crista and medial arm of the E17.5 posterior crista still parallel the basal cochlear sensory epithelium they previously contacted (Fig. 4h). This basal region of the cochlear sensory epithelium is not in the cochlear duct, but on the medial wall of the common vestibular cavity.
In the entire wild-type cochlea, as well as in the apical cochlea of the mutant, a narrow band of inner hair cells, one-hair cell wide, is separated by the tunnel of Corti from a broader band of outer hair cells, three-hair cell wide. In contrast, the basal cochlear sensory epithelium of the mutant completely lacks an outer hair cell band, and the inner band is variably expanded to several hair cells wide (Fig. 6a–d). Both (Koo et al. 2009; Nichols et al. 2008) note that among the inner hair cells of this mutant basal cochlear sensory epithelium are hair cells with stereocilia bundles resembling those of vestibular (crista?) sensory epithelia.
Fig. 6.
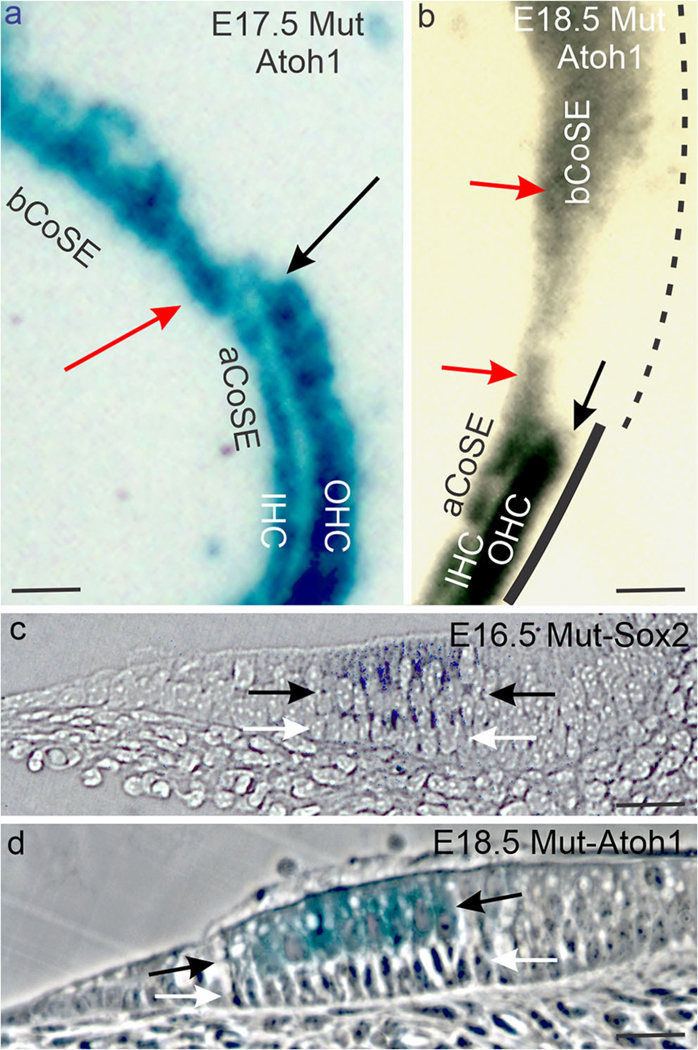
The mutant basal cochlear sensory epithelium is altered. (a, b) Whole mounts of E17.5 (a) and E18.5 (b) basal-apical cochlear sensory epithelial junctions. Red arrows mark the inner hair cell bands, and black arrows the blunt end terminations of the outer hair cell bands at the junctions. The black line in b suggests unbroken cochlear Bmp4 expression in the apical cochlear sensory epithelium (solid line) and patchy expression in the basal cochlear sensory epithelium (dashed line). (c, d) Sections across the basal cochlear sensory epithelium of an E16.5 mutant ear stained for Sox2 expression (c, bright field) and an E18.5 mutant ear stained for Atoh1 (d, phase contrast) expression. Black arrows bracket hair cell layers and white arrows, supporting cell layers. In d note that the inner hair cell row of the basal CoSE is eight hair cells wide and the tunnel of Corti is missing. Abbreviations: aCoSE, apical cochlear sensory epithelium; bCoSE, basal cochlear sensory epithelium; IHC, inner hair cells; OHC, outer hair cells. All bars are 25 μm
An abluminal layer of Sox2-expressing supporting cells is present (Fig. 6c), though differentiated pillar and Deiters’ cells are absent (Nichols et al. 2008). In the absence of pillar cells, a tunnel of Corti is also missing. Where the cochlear crista is more compact, the transition from altered basal cochlear sensory epithelium to intact apical organ of Corti is abrupt (Fig. 6a, b). However, where the crista’s apical termination is elongated into patches and scattered cells, patches of an outer hair cell band can be present in the vicinity of an indistinct junction (Fig. 2a’).
These results suggest that a contact-mediated interaction between crista and basal cochlear sensory epithelium is responsible for altering the basal cochlear sensory epithelium. However, at the cochlear-saccular epithelial basal tip, it retains a junction with the saccular macula through perinatal ages. Thus, this association too might be responsible for altering the peri-junctional cochlear epithelia.
Region of patchy basal cochlear Bmp4 expression approximates that of its former association with the cochlear crista
In whole mounts of E13.5 wild-type ears, an unbroken band of Bmp4-expressing cells is present immediately abneural to the cochlear sensory epithelium (Fig. 1b). In mutants, an unbroken band is present apically, but it weakens basally (Fig. 1b’). In dissected E13.5 mutant ears, the weakened basal band observed in whole mounts resolves into patches that taper to extinction as the cochlear sensory epithelium approaches its junction with the saccular macula (Fig. 7a).
Fig. 7.
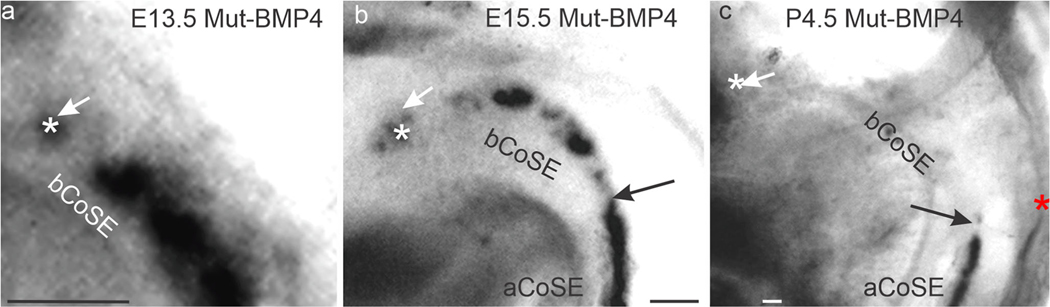
Basal cochlear Bmp4 expression is patchy in the mutant. (a) Flat mount of the posterior and medial walls of the common vestibular cavity in an E13.5 mutant ear stained for Bmp4 expression. The junction of the basal CoSE with the saccular macula is out of view in the direction of the white arrow here and in b, c. Asterisks mark patches of Bmp4 expression here and in b, c. (b) Similar view of an E15.5 ear. Black arrows here and in c mark the transition from unbroken expression apically to patchy expression basally. (c) Similar view of a P4.5 ear. Abbreviation: aCoSE, apical cochlear sensory epithelium; bCoSE, basal cochlear sensory epithelium. All bars are 50 μm
At E15.5, the patchy region has elongated, with longer gaps between patches (Fig. 7b). The region of patchy expression approximates that of the basal cochlear sensory epithelium’s former association with crista sensory epithelium, as suggested in Fig. 6b. Postnatally, gaps are variable in length and can include much of the basal cochlear sensory epithelium (Fig. 7c). Nevertheless, an unbroken band of Bmp4 expression is always present apically.
Discussion
We here demonstrate that Lmx1a expression participates in mechanisms that maintain the separation between posterior crista sensory epithelium and basal cochlear sensory epithelium. In the Lmx1a mutant, this separation is breached, and a cochlear crista forms in the posterior wall of the basal cochlear duct. There we propose it alters the basal cochlear sensory epithelium.
Lateral induction forms an enlarged posterior crista in the mutant
Previous work (Huang et al. 2018; Mann et al. 2017) demonstrates that ectopic expression of Lmx1b (the equivalent of mammalian Lmx1a) suppresses Jag1 expression in the chick ear. Mann et al. (2017) then describe the spread of Jag1 expression beyond core sensory epithelia in an Lmx1a mutant mouse and suggest this results when JAG1-NOTCH signaling propagates itself into the surrounding epithelium by lateral induction (Hartman et al. 2010). Similarly, we find that the expressions of Sox2 and Bmp4 expand beyond the wild-type core of the posterior crista into the surrounding epithelium. We thus propose that this expansion results when LMX1A is unavailable to prevent lateral induction from propagating Jag1 expression beyond the core’s margins (Daudet and Lewis 2005; Neves et al. 2011). JAG1-NOTCH signaling would then sustain Sox2 expression and thus assert a sensory fate (Daudet et al. 2007; Hartman et al. 2010).
Additional factors must be responsible for shaping the cochlear crista. Lmx1a and the LIM transcription cofactor, Lmo4, are normally co-expressed in the posterior wall of the wild-type cochlear duct. In addition to Lmo4’s expression there, it is also required for the formation of the posterior crista (Deng et al. 2010). Thus, one possibility is that the absence of Lmx1a expression in the mutant plus the continued presence of Lmo4 expression makes the wall specifically susceptible to crista sensory epithelium propagation and thus defines the path of its expansion.
However, the crista sensory epithelium fails to propagate beyond the basal cochlear duct, into the outer spiral sulcus of the apical duct. This possibly suggests that its propagation is time constrained. Consistent with this, Sox2 expression in the cochlear crista becomes patchy at its distal end. The contrast between the cochlear crista’s indistinct margins and the more well-defined margins of the posterior crista’s other two arms (Fig. 4g) further suggests that the cochlear crista is less spatially constrained as well. This might allow cochlear crista to propagate into the basal cochlear sensory epithelium, possibly directing some of its hair cells to a vestibular (crista?) fate. Nevertheless, while propagation by lateral induction is suggested, it should be noted that we have not ruled out prosensory cell migration from the core posterior crista as a source of the cochlear crista.
Gene expressions for the formation of crista sensory epithelium are suppressed by LMX1A
Lmx1a expression has been implicated in two mechanisms that oppose the formation of posterior crista sensory epithelium. In the first, Lmx1a expression suppresses the formation of posterior crista sensory epithelium (Huang et al. 2018) by suppressing that of genes essential for crista formation (Deng et al. 2010). These include Bmp4 (Chang et al. 2008) and Fgf10 (Pauley et al. 2003). The expression of Islet1 is also suppressed, but its role sensory epitheliogenesis is not clear. Nevertheless, a role is suggested by its ubiquitous expression in otic prosensory epithelia (Radde-Gallwitz et al., 2004; Li et al., 2004; Huang et al. 2008), its role in reducing noise-related damage to adult hair cells (Huang et al. 2018), and in enhancing Atoh1-mediated conversion of adult supporting cells to hair cells (Yamashita et al. 2018). In the wild-type crista, the suppression of these gene expressions is prevented by the opposing expression of the LIM-only factor, Lmo4 (Huang et al. 2018).
In the second mechanism, Lmx1a expression suppresses Jag1 expression that would otherwise initiate NOTCH signaling and subsequent sensory epitheliogenesis. In the wild-type crista, this suppression is prevented when Jag1 expression instead suppresses that of Lmx1a (Mann et al. 2017). Whether these two suppressions (Lmo4, Jag1-Notch signaling) interact is unknown. However, in Drosophila, a LIM-homeodomain factor, Apterous, is responsible for initiating the expression of the Jag1 homolog, Serrate, in the dorsal compartment of the imaginal wing disc. Furthermore, Apterous expression is subsequently suppressed by the Drosophila LIM-only factor, dLMO (Milán and Cohen 2000). Thus, a precedent exists for an interaction between a LIM-homeodomain factor and both a LIM-only factor and Jag1/Serrate.
Our results combined with those of Mann et al. (2017) suggest wild-type Lmx1a expression can successfully suppress Jag1-mediated sensory epitheliogenesis in the non-sensory epithelium surrounding the posterior crista (Fig. 8). Both suppressive mechanisms might apply in the wild-type outer spiral sulcus, where Lmx1a and Lmo4 are co-expressed. When suppression is successful, not only is a sensory epithelium absent but also the posterior wall of the cochlear duct is freed to pursue other fates (e.g., stria vascularis).
The basal cochlear sensory epithelium is altered in the Lmx1a mutant
The work of others (Mann et al. 2017) suggests that Lmx1a expression creates a barrier that prevents the epithelium surrounding the posterior crista from assuming a sensory fate. Lmx1a is also expressed in constrictions that separate the wild-type posterior crista sensory epithelium from the basal cochlear sensory epithelium. These physically separate the two sensory epithelia into mutually unavailable compartments (Mann et al. 2017). In the Lmx1a mutant, both sensory fate resistance and physical separation fail. As a result, lateral induction propagates ectopic crista sensory epithelium into the posterior wall of the basal cochlear duct and the cochlear crista is transiently located in contact with the basal cochlear sensory epithelium.
Our results further demonstrate that where transient contact has occurred, the resulting organ of Corti is characteristically altered. The outer hair cell band is missing, and while a supporting cell layer is present, it lacks differentiated pillar and Deiters’ cells (Nichols et al. 2008). The absence of Deiters’ cells at least is consistent with the loss of the outer hair cell band, of which they are a part. The remaining hair cell band mingles vestibular-like hair cells with the expected inner hair cells (Koo et al. 2009; Nichols et al. 2008). This suggests that contact has allowed the cochlear crista to propagate a crista hair cell phenotype among uncommitted sensory pre-cursors in the cochlear sensory epithelium. The broad band of inner/vestibular hair cells is consistent with the possibility that it has failed to converge and extend. This failure could reflect its partially vestibular phenotype or possibly its ectopic location on the medial wall of the common vestibular cavity rather than the elongating cochlear duct. Consistent with the presence of both inner, and vestibular-like hair cells, Fgf8 expression is expanded in this band (Nichols et al. 2008).
In addition to defects in the basal cochlear sensory epithelium, cochlear-pattern Bmp4 expression is reduced to patches. We suggest this too results from the cochlear sensory epithelium’s exposure to the propagating cochlear crista. However, the proposed role of cochlear BMP4 signaling in organizing and differentiating the organ of Corti (Deng et al. 2014; Ohyama et al. 2010) suggests that reduced cochlear-pattern BMP4 signaling itself might play a role in suppressing the outer hair cell band (Fig. 8a,b).
The cochlear crista suggests a papilla neglecta
In many vertebrates, including man, a patch of sensory epithelium, the papilla neglecta, is located near the posterior crista (Brichta and Goldberg 1998). In wild-type mice, this patch consists of no more than 5–8 hair cells in the utricular canal, near the lip of the utriculo-saccular constriction (Nichols et al. 2008). Given the presence of this constriction, the papilla’s position is equivalent to the cochlear crista in the Lmx1a mutant. In addition, both are innervated by the nerve to the posterior crista, and a cupula-like matrix has been described capping both (Fritzsch and Wake 1988). However, the papilla is present in an ear capable of expressing Lmx1a, which our results suggest might be expected to suppress it (Fritzsch and Wake 1988; Wu and Oh 1996).
Despite this, we suggest that the wild-type papilla results from the same mechanism we propose responsible for the mutant’s cochlear crista. Thus a Jag1-expressing papilla pre-cursor would originate as a propagation from the posterior crista. It would then be isolated from the crista when additional factors are combined with Lmx1a expression to suppress JAG1-NOTCH signaling expression in the intervening epithelium. Secondarily suppressed NOTCH signaling in a cell population resembling that of a cochlear crista/papilla neglecta was noted by Mann et al. (2017). The existence of secondary factors influencing the extent of the cochlear crista is suggested by the crista’s variable size in our mixed strain mice (Fig. 2a vs. 2b).
Our results suggest that while the papilla neglecta of the wild-type mouse is small and possibly vestigial, its posterior sensory field retains the capacity to generate a larger papilla. Exploiting this potential could permit the evolution of alternate sensory configurations (Elliott et al. 2018; Fritzsch and Elliott 2017; Fritzsch et al. 2013; Fritzsch and Wake 1988).
Mutant crista and cochlear sensory epithelia are eventually separated by a Noggin-expressing non-sensory epithelium.
In the wild-type ear, the posterior crista and basal cochlear sensory epithelium form from spatially separated sites and remain separated from one another (Morsli et al. 1999). Integral to maintaining this separation are Lmx1a dependent mechanisms that confine the crista and form constrictions separating it from the cochlear duct. In the mutant, these mechanisms play no role. Nevertheless, by E16.5 the crista sensory epithelia and the basal cochlear sensory epithelium are separated by a Noggin expressing (for the most part) non-sensory epithelium. Scattered Atoh1-expressing hair cells are, however, occasionally present in this epithelium but their formation is delayed compared to control animals (Nichols et al. 2008). Their presence suggests they have formed from an initially bipotential, sensory/non-sensory epithelium generated at the interface between the crista and cochlear sensory epithelia (Mann et al. 2017). The expression of Noggin in this epithelium suggests that the suppression of BMP signaling might play a role in its formation (Krause et al. 2011).
Acknowledgments
We thank Dr. Garret Soukup, Jason Pecka, and Marsha Pierce for help designing and preparing probes and primers, Dr. Doris Wu for the Bmp4 probe plasmid, and Dr. Huda Zoghbi for mice. We also thank Dr. Kathleen Millen for a helpful discussion regarding her Lmx1a-cre line.
Funding information The study was supported by the grants of NCRR/NIGMS/COBRE (NCRR P20 RR 018788; NIGMS P20GM103471; DHN), NIH (RO1 DC 005590; R01 AG060504 BF), and National Center for Research Resources (G20RR024001). This work was carried out under research programs of Creighton University (D H. Nichols, J E. Bouma, K W. Beisel, D Z. Z. He, H Liu) and University of Iowa (B J. Kopecky, I Jahan, B Fritzsch).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict(s) of interest.
Informed consent All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies involving human participants performed by any of the authors.
Committee ethics All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All were mice were maintained in an AALAC certified facility under a Creighton University IACUC-approved protocol.
References
- Agulnick AD, Taira M, Breen JJ, Tanaka T, Dawid IB, Westphal H, (1996) Interactions of the LIM-domain-binding factor Ldbl with LIM homeodomain proteins. Nature 384 (6606):270–272 [DOI] [PubMed] [Google Scholar]
- Bergstrom DE, Gagnon LH, Eicher EM (1999) Genetic and physical mapping of the dreher locus on mouse chromosome 1. Genomics 59:291–299 [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY (2001) Proprioceptor pathway development is dependent on Math1. Neuron 30:411–422 [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM (1998) The papilla neglecta of turtles: a detector of head rotations with unique sensory coding properties. J Neurosci 18:4314–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynski GM, Reed X, Maragh S, Matsui T, McCallion AS (2013) Integration of genomic and functional approaches reveals enhancers at LMX1A and LMX1B. Mol Gen Genomics 288:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, Wu DK (2008) Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet 4:e1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov V, Steshina E, Roberts R, Ilkin Y, Washburn L, Millen KJ (2006a) Molecular definition of an allelic series of mutations disrupting the mouse Lmx1a (dreher) gene. Mamm Genome 17: 1025. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Currle DS, Rose MF, Monuki ES, Millen KJ (2006b) The roof plate regulates cerebellar cell-type specification and proliferation. Development 133:2793–2804 [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J (2005) Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 132: 541–551 [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J (2007) Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development 134:2369–2378 [DOI] [PubMed] [Google Scholar]
- Deng M, Pan L, Xie X, Gan L (2010) Requirement for Lmo4 in the vestibular morphogenesis of mouse inner ear. Dev Biol 338:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Andersson E, Hedlund E, Alekseenko Z, Coppola E, Panman L, Millonig JH, Brunet J-F, Ericson J, Perlmann T (2011) Specific and integrated roles of Lmx1a, Lmx1b and Phox2a in ventral midbrain development. Development 138:3399–3408 [DOI] [PubMed] [Google Scholar]
- Deng M, Luo X-j, Pan L, Yang H, Xie X, Liang G, Huang L, Hu F, Kiernan AE, Gan L (2014) LMO4 functions as a negative regulator of sensory organ formation in the mammalian cochlea. J Neurosci 34:10072–10077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol M (1964) The origin of the abnormalities of the inner ear in dreher mice. Development 12:727–733 [PubMed] [Google Scholar]
- Dvorakova M, Jahan I, Macova I, Chumak T, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G (2016) Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance. Sci Rep 6:38253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova M, Macova I, Bohuslavova R, Anderova M, Fritzsch B, Pavlinkova G (2020) Early ear neuronal development, but not olfactory or lens development, can proceed without SOX2. Dev Biol 457: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP (1994) Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development 120:2213–2224 [DOI] [PubMed] [Google Scholar]
- Elliott KL, Fritzsch B, Duncan JS (2018) Evolutionary and developmental biology provide insights into the regeneration of organ of Corti hair cells. Front Cell Neurosci 12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Failli V, Bachy I, Rétaux S (2002) Expression of the LIM-homeodomain gene Lmx1a (dreher) during development of the mouse nervous system. Mech Dev 118:225–228 [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL (2017) Gene, cell, and organ multiplication drives inner ear evolution. Dev Biol 431:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Wake M (1988) The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system (Amphibia, Gymnophiona). Zoomorphology 108:201–217 [Google Scholar]
- Fritzsch B, Nichols D, Echelard Y, McMahon A (1995) Development of midbrain and anterior hindbrain ocular motoneurons in normal and Wnt-1 knockout mice. Develop Neurobiol 27:457–469 [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel K, Jones K, Farinas I, Maklad A, Lee J, Reichardt L (2002) Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol 53:143–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei V, Nichols D, Bermingham N, Jones K, Beisel K, Wang V (2005) Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn 233:570–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T (2013) Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evol Dev 15:63–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez F (1998) Regionalized organizing activity of the neural tube revealed by the regulation of lmx1 in the otic vesicle. Dev Biol 203:189–200 [DOI] [PubMed] [Google Scholar]
- Glover JC, Elliott KL, Erives A, Chizhikov VV, Fritzsch B (2018) Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev Biol 444:S14–S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O (2010) Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci 107:15792–15797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Sage C, Li H, Xiang M, Heller S, Chen ZY (2008) Diverse expression patterns of LIM-homeodomain transcription factors (LIM-HDs) in mammalian inner ear development. Dev Dyn 237: 3305–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Hill J, Yatteau A, Wong L, Jiang T, Petrovic J, Gan L, Dong L, Wu DK (2018) Reciprocal negative regulation between Lmx1a and Lmo4 is required for inner ear formation. J Neurosci 38:5429–5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CS, Rhodes SJ (2005) LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep 32:67–77 [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Hogan BL (1991) Involvement of bone morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111:531–542 [DOI] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK (2009) Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol 333:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky BJ, Duncan JS, Elliott KL, Fritzsch B (2012) Three-dimensional reconstructions from optical sections of thick mouse inner ears using confocal microscopy. J Microsc 248:292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C, Guzman A, Knaus P (2011) Noggin. Int J Biochem Cell Biol 43:478–481 [DOI] [PubMed] [Google Scholar]
- Kuwamura M, Muraguchi T, Matsui T, Ueno M, Takenaka S, Yamate J, Kotani T, Kuramoto T, Guenet JL, Kitada K, Serikawa T (2005) Mutation at the Lmx1a locus provokes aberrant brain development in the rat. Brain Res Dev Brain Res 155:99–106 [DOI] [PubMed] [Google Scholar]
- Macova I, Pysanenko K, Chumak T, Dvorakova M, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G (2019) Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. J Neurosci 39:984–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann ZF, Galvez H, Pedreno D, Chen Z, Chrysostomou E, Żak M, Kang M, Canden E, Daudet N (2017) Shaping of inner ear sensory organs through antagonistic interactions between Notch signalling and Lmx1a. Elife 6:e33323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares M, Trainor PA, Ariza-McNaughton L, Nonchev S, Krumlauf R (2000) Dorsal patterning defects in the hindbrain, roof plate and skeleton in the dreher (dr(J)) mouse mutant. Mech Dev 94:147–156 [DOI] [PubMed] [Google Scholar]
- Matthews JM, Bhati M, Craig VJ, Deane JE, Jeffries C, Lee C, Nancarrow AL, Ryan DP, Sunde M (2008) Competition between LIM-binding domains. Biochem Soc Trans 36:1393–1397 [DOI] [PubMed] [Google Scholar]
- Milán M, Cohen SM (2000) Temporal regulation of apterous activity during development of the Drosophila wing. Development 127: 3069–3078 [DOI] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME (2000) The mouse dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature 403:764–769 [DOI] [PubMed] [Google Scholar]
- Mishima Y, Lindgren AG, Chizhikov VV, Johnson RL, Millen KJ (2009) Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J Neurosci 29:11377–11384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK (1999) Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development 126:2335–2343 [DOI] [PubMed] [Google Scholar]
- Neves J, Parada C, Chamizo M, Giráldez F (2011) Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development 138:735–744 [DOI] [PubMed] [Google Scholar]
- Nichols D, Echelard Y, McMahon A, Fritzsch B (1994) Combining bio-tinylated dextran amine neuronal labeling and lac-Z/β-galactosidase reporter gene labeling to study the relationship between identified neuronal populations and gene expression in the embryonic mouse nervous system. Neurosci Protocol 30:1–11 [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B (2008) Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res 334:339–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK (2010) BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci 30:15044–15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B (2011) Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res 275:66–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B (2012) A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One 7:e30358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B (2003) Expression and function of FGF10 in mammalian inner ear development. Dev Dyn 227:203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen I, Chakchouk I, Liaqat K, Jan A, Nasir A, Hussain S, Nickerson DA, Bamshad MJ, Ullah A, Ahmad W (2018) A variant in LMX1A causes autosomal recessive severe-to-profound hearing impairment. Hum Genet 137:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffes G, Lorente-Cánovas B, Pearson S, Brooker RH, Spiden S, Kiernan AE, Guénet J-L, Steel KP (2012) Mutanlallemand (mtl) and belly spot and deafness (bsd) are two new mutations of Lmx1a causing severe cochlear and vestibular defects. PLoS One 7:e51065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH (1997) The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J 16:3145–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesdorp M, de Koning Gans PAM, Schraders M, Oostrik J, Huynen MA, Venselaar H, Beynon AJ, van Gaalen J, Piai V, Voermans N, van Rossum MM, Hartel BP, Lelieveld SH, Wiel L, Verbist B, Rotteveel LJ, van Dooren MF, Lichtner P, Kunst HPM, Feenstra I, Admiraal RJC, Yntema HG, Hoefsloot LH, Pennings RJE, Kremer H (2018) Heterozygous missense variants of LMX1A lead to nonsyndromic hearing impairment and vestibular dysfunction. Hum Genet 137: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, Pierce ML, Jensen-Smith HC, Fritzsch B, Rocha-Sanchez S, Beisel KW, Soukup GA (2011) MicroRNA-183 family expression in hair cell development and requirement of microRNAs for hair cell maintenance and survival. Dev Dyn 240:808–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DK, Oh S-H (1996) Sensory organ generation in the chick inner ear. J Neurosci 16:6454–6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Zheng F, Finkelstein D, Kellard Z, Robert C, Rosencrance CD, Sugino K, Easton J, Gawad C, Zuo J (2018) High-resolution transcriptional dissection of in vivo Atoh1-mediated hair cell conversion in mature cochleae identifies Isl1 as a co-reprogramming factor. PLoS Genet 14:e1007552 [DOI] [PMC free article] [PubMed] [Google Scholar]