Abstract
Background
Neurological complications in coronavirus disease 2019 (COVID-19) have been described, but the understanding of their pathophysiology and neuroanatomical correlates remains limited.
Purpose
To report on the frequency and type of neuroradiological findings in COVID-19.
Materials and Methods
In this retrospective study, all consecutive adult hospitalized patients with PCR-positivity for SARS-CoV-2, undergoing neuroimaging at Karolinska University Hospital between March 2 and May 24, 2020, were included. All examinations were systematically re-evaluated by 12 readers. Summary descriptive statistics were calculated.
Results
185 patients with COVID-19 (62±14 years, 138 men) underwent neuroimaging. In total, 222 brain CT, 47 brain MRI and 7 spinal MRI scans were performed. Intra-axial susceptibility abnormalities were the most common finding (29 of 39 [74%, 95%-CI 58–87%]) in patients with brain MRI, often with an ovoid shape suggestive of microvascular pathology, and with a predilection to corpus callosum (23 of 39, [59%, 95%-CI 42–74%]) and juxtacortical areas (14 of 39, [36%, 95% CI 21-53%]). Ischemic and macrohemorrhagic manifestations were also seen, but vascular imaging did not reveal overt abnormalities. Dynamic susceptibility contrast perfusion MRI in 19 patients did not reveal consistent asymmetries between hemispheres or regions. Many patients (18 of 41 [44%, 95%-CI 28–60%]) had leukoencephalopathy and one patient had a cytotoxic lesion of the corpus callosum. Other findings included olfactory bulb signal abnormalities (7 of 37, 19%), prominent optic nerve subarachnoid spaces (20 of 36, 56%), and enhancement of the parenchyma (3 of 20, 15%), leptomeninges (3 of 20, 15%), cranial nerves (2 of 20, 10%), and spinal nerves (2 of 4, 50%). At MRI follow-up, regression of leukoencephalopathy and progressive leptomeningeal enhancement was observed in one patient respectively, suggestive of dynamic processes.
Conclusion
Patients with COVID-19 had a wide spectrum of vascular and inflammatory involvement of both the central and peripheral nervous system.
Summary
In hospitalized adults with COVID-19 undergoing neuroimaging, vascular and inflammatory changes are common, with frequent non-spherical susceptibility abnormalities on MRI suggestive of cerebral microvascular involvement.
Key Results
■ In patients with COVID-19 who required neuroimaging, intra-axial susceptibility abnormalities were the most common finding (29/39, 74%, of patients with brain MRI), often with an ovoid shape suggestive of microvascular pathology, and with a predilection to corpus callosum (23/39, 59%).
■ A predilection of susceptibility abnormalities for juxtacortical white matter was seen in 14/39 (36%) patients with COVID-19, similar to patients with other critical illnesses.
Introduction
Neurological symptoms in coronavirus disease 2019 (COVID-19) are increasingly reported. It remains debated to what extent these symptoms are manifestations of potential neurovirulence, secondary effects, general critical illness, or iatrogenic from complex symptom management (1,2). However, the incomplete understanding of their pathophysiological basis impedes the development of effective treatment strategies and portends long-standing effects on public health.
Wuhan had early reports of acute cerebrovascular lesions and impaired consciousness (3). These findings were later confirmed in other studies reporting macrovascular events (4), even in young patients (5), despite reductions in stroke imaging during the pandemic (6). Other early neuroimaging case reports in COVID-19 showed acute hemorrhagic necrotizing encephalopathy (7), and hippocampal signal abnormalities (8). A later detailed case report demonstrated leukoencephalopathy and susceptibility-weighted imaging (SWI) abnormalities in white matter and posterior hyperperfusion (9).
To date, large neuroimaging studies on COVID-19 are lacking, with most studies focusing on a smaller number of patients in the intensive care unit. A letter on neurological features in intensive care patients reported brain MRI findings in 13 patients, including 3 ischemic strokes, leptomeningeal enhancement in 8 patients, and frontotemporal hypoperfusion in all 11 patients who underwent perfusion imaging. (10). Some of these imaging findings have since been debated (11). In another retrospective intensive care study, 12 of 27 (44%) patients with neurological symptoms had brain MRI findings (12). Also, in hospitalized patients, 51 of 108 (47%) examined patients had neuroimaging abnormalities, most frequently infarcts and hemorrhages but also less commonly cerebral venous thrombosis, encephalopathy, posterior reversible encephalopathy syndrome, Guillain-Barré syndrome, and Miller-Fisher syndrome (13).
In a recent retrospective multicenter study, 37 patients with severe COVID-19 were consecutively sampled and underwent brain MRI with pathology. The results showed frequent signal changes in the mesial temporal lobe, variable contrast-enhancement of multifocal white matter hyperintensities, and SWI-abnormalities (14).
The purpose of this study was to report the frequency and type of neuroradiological findings in patients with COVID-19 based on all consecutive adult hospitalized patients imaged at our institution.
Materials and Methods
Ethical considerations
This retrospective study was approved by the Swedish Ethical Review Authority. Informed consent was waived due to the retrospective nature of the study and to avoid bias in participant inclusion, especially since many patients had impaired consciousness/cognition, were critically ill or deceased.
Study participants
Included in this study were all consecutive adult (≥18 years of age) hospitalized patients with positive reverse transcription-polymerase chain reaction (PCR) for SARS-CoV-2 that underwent neuroimaging at Karolinska University Hospital in Stockholm, Sweden at two geographical sites between March 2 (first patient admitted) and May 24, 2020 (study conclusion). No exclusion criteria were applied in order to avoid biases in the reporting.
Imaging
All imaging was performed according to clinical routine. Scanner allocation was based on the clinical and logistical needs. Iodine-based and gadolinium-based contrast agents were used cautiously as per standard of care due to the high frequency of renal failure and the need for dialysis in the patient group.
CT
Brain CT was performed on the following scanner models: Canon Aquilion ONE Genesis (Canon Medical Systems, Otawara, Japan), GE Discovery CT750 HD and Revolution CT (GE Healthcare, Milwaukee, WI), Philips IQon Spectral CT (Philips Healthcare, Best, Netherlands) and Siemens SOMATOM Definition Flash (Siemens Healthineers, Erlangen, Germany). Scanning was performed with/without contrast agent in standard doses. Sub-millimeter spatial resolution images were acquired and reconstructed in three planes with 5-millimeter slice thickness.
MRI
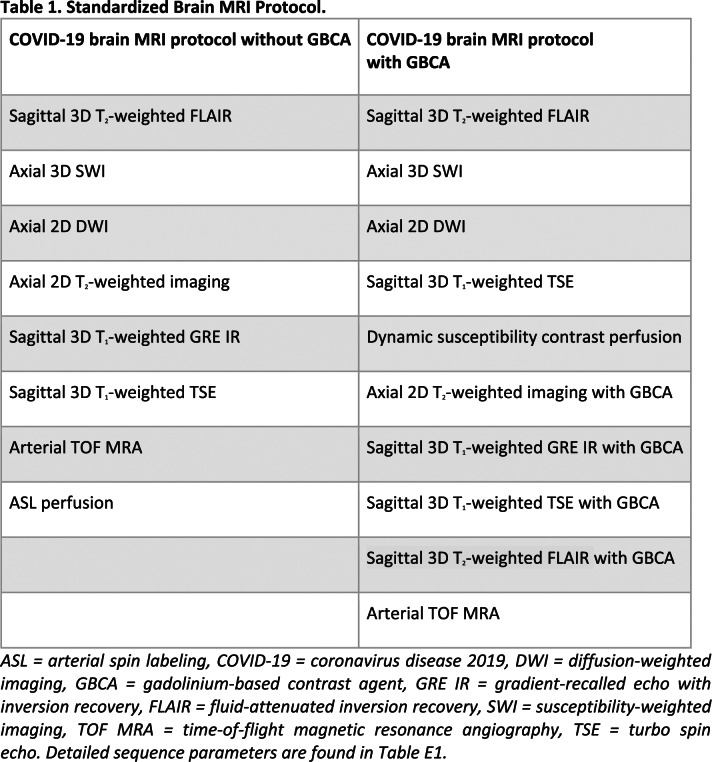
All MRI was performed on the following scanner models using standard clinical coils: GE Optima MR 450w GEM 1.5 T (GE Healthcare, Milwaukee, WI), Philips Ingenia 1.5 T (Philips Healthcare, Best, Netherlands), Siemens MAGNETOM Aera 1.5 T, Prismafit 3.0 T, Skyra 3.0 T, Vida 3.0 T (Siemens Healthineers, Erlangen, Germany). Based on preliminary brain MRI findings on neurologic features in severe SARS-CoV-2 infection (10), we decided to implement a detailed standardized protocol for brain MRI in clinical practice, as detailed in Table 1. The technical MRI parameters are detailed in Table E1. When available, previous brain MRIs were also assessed.
Table 1.
Standardized Brain MRI Protocol.
Visual assessments
All imaging was simultaneously and independently re-evaluated in a structured manner by 12 readers (author initials, clinical title, years of clinical experience in radiology): AT, neuroradiologist, 14; SK, neurologist, fellow in neuroradiology, 4; AK, neuroradiologist, 15; CÖ, neuroradiologist, 9; EK, fellow in neuroradiology, 14; HA, neuroradiologist, 22; ÅA, neuroradiologist, 9; HM, neuroradiologist, 20; JF, neuroradiologist, 10; JL, resident in radiology, 5; AFD, neuroradiologist, 10; TG, fellow in neuroradiology, 6. Some readers (AT, SK, CÖ, EK, JL, TG) were, in parallel, asked to systematically report on specific findings across all scans, to ensure homogenous evaluations across the sample. Meanwhile, some readers were asked to review a broad spectrum of pathology in a subset of CTs (HA, HM, JF), the complete sample of brain MRIs (AK, AFD) or spinal MRIs (SK, ÅA) to ensure that the whole perspective of findings was considered per patient. The initial ratings were performed blinded to the clinical data. All imaging aspects were reviewed by at least one board-certified neuroradiologist and disagreements in findings were resolved by consensus immediately after the all individual ratings had been performed.
We systematically evaluated findings previously reported in COVID-19 (see detailed references in the Introduction) or known from other human coronavirus infections (1) and findings encountered in other diseases and critical illness. When encountering findings in the sample not previously reported in the literature, we reevaluated all scans based on the new aspect. Due to the lack of validated rating scales for COVID-19, we applied the Microbleed Anatomical Rating Scale (MARS) to quantify the burden of susceptibility abnormalities (15). As described by Chung et al, the 3D T2-FLAIR signal of the olfactory bulb was visually assessed (SK, AK, AFD); higher signal intensity than adjacent normal-appearing grey matter was considered abnormal (16). The presence of contrast-enhancement was assessed on 3D T1-weighted MRI.
Perfusion analysis
Brain perfusion MRI was performed using dynamic susceptibility contrast (DSC) according to clinical routine (gadoteric acid; GE Healthcare, Milwaukee, WI; 0.1 mmol/kg). MRI perfusion images were post-processed in Philips IntelliSpace Portal version 10.1 (Philips Healthcare, Best, Netherlands) using the MRI Neuro Perfusion package and manual arterial input function (AFD). Perfusion MRI was, first, independently assessed by two neuroradiologists (AK, AFD) blinded to the clinical data and, immediately afterwards, disagreements were resolved by consensus. To objectively assess the findings, regions of interest were placed in grey and white matter supratentorial regions (AFD), exemplified in Figure E1. Relative cerebral blood flow (rCBF) was normalized against cerebellar blood flow to yield an rCBF ratio. Arterial spin labeling results are not presented due to the uncertainty in the interpretation of the findings when dynamic susceptibility contrast perfusion results were not overtly pathological.
Statistical analysis
Descriptive statistics were obtained using R version 4.0.1 for Linux (R Core Team, Vienna, Austria).
Results
Patient demographics
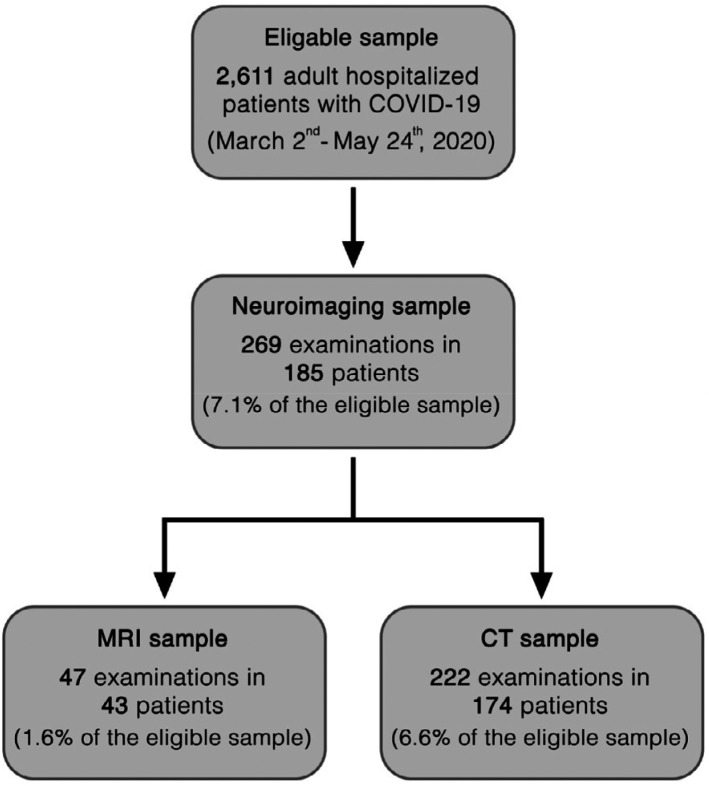
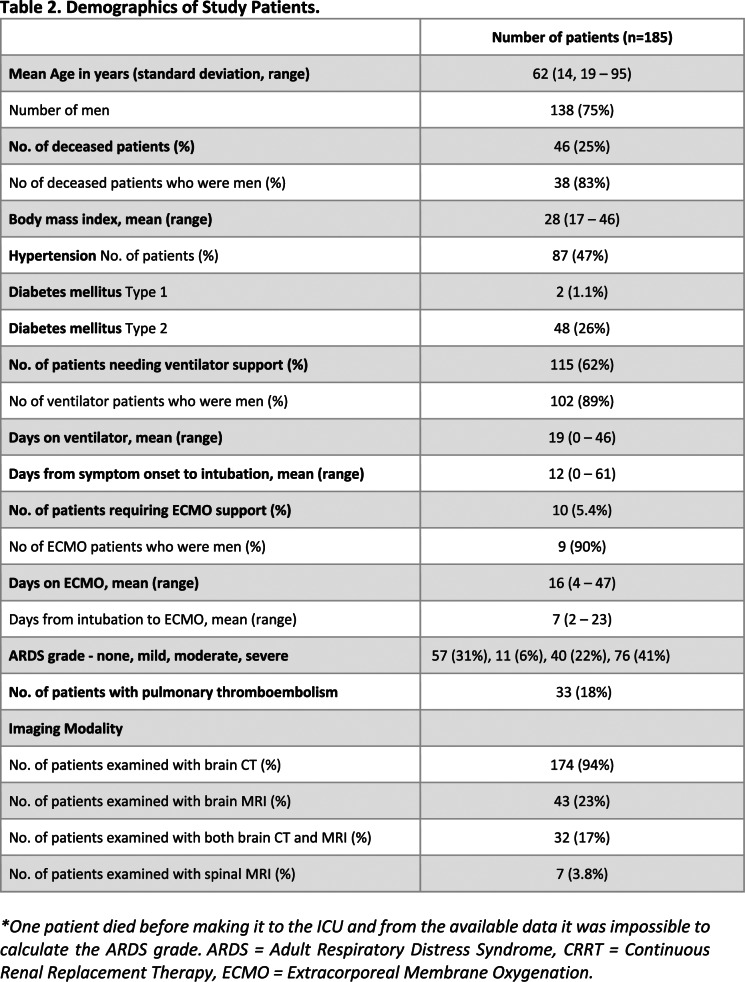
Among 2611 patients with COVID-19, 185 (185/2611, 7.1%, 95% confidence interval [95%-CI] 6.1–8.1%; 62±14 years, 138 men) underwent neuroimaging based on clinical indication. A flow chart of the study is presented in Figure 1. The demographics of the study participants are summarized in Table 2.
Figure 1.
Flow chart of the study. Inclusion criteria were: Positive polymerase-chain-reaction test result for severe acute respiratory syndrome coronavirus 2; age of ≥18 years; neuroimaging performed during the study period. No exclusion criteria were applied to ensure a complete consecutive study sample.
Table 2.
Demographics of Study Patients.
Frequency of imaging and findings
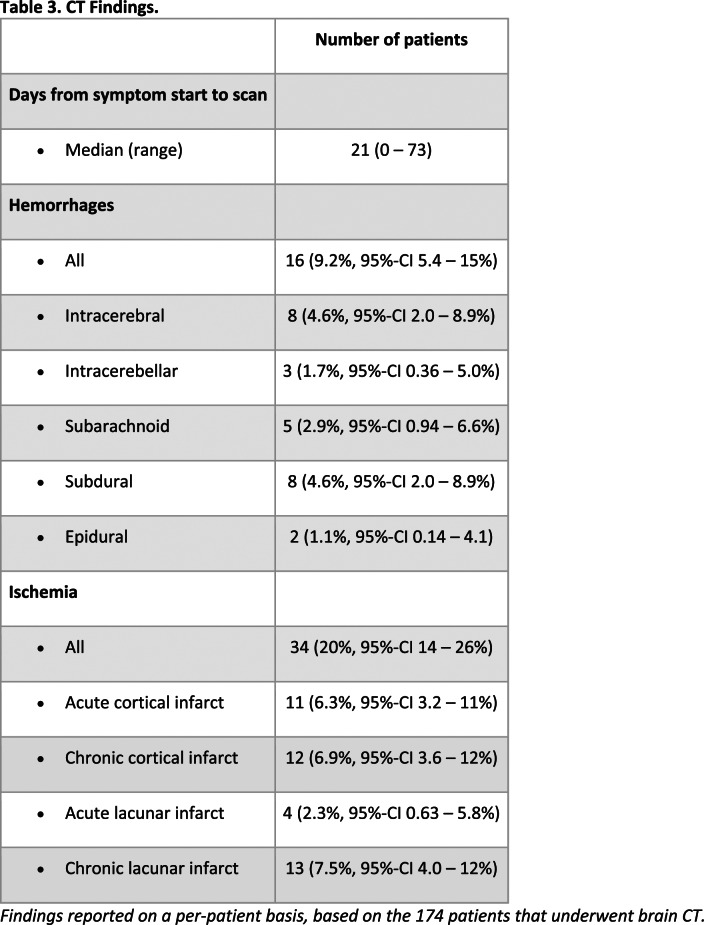
A total of 174 patients (174/2611, 6.6%, 95%-CI 5.7–7.7%) had 222 brain CT scans (median 1, range 1–4 scans). Brain MRI was performed in 43 patients (43/2611, 1.6%, 95%-CI 1.2–2.2%) with 47 brain MRI scans, of which 22 were contrast-enhanced; 4 patients had a follow-up MRI (median follow-up time 12, range 6–24 days). Seven patients underwent a spinal MRI (2 whole-spine, 2 cervical, 1 thoracic-lumbar, 2 lumbar) of which 4 were contrast-enhanced. The median time from symptom onset to scan was 21 days for CT and 34 days for MRI. The frequency of findings is summarized in Tables 3 and 4. All reported percentages refer to the number of patients with appropriate imaging to assess the pathology in question.
Table 3.
CT Findings.
Table 4.
MRI Findings.
In asymptomatic/mildly symptomatic patients with incidental PCR-positivity, 43 brain CT scans (43/185 CT, 23%, 95%-CI 17–30%) and 2 brain MRI scans (2/47, 4.3%, 95%-CI 0.5–15%) were performed on indications unrelated to COVID-19. Head trauma was a common indication for CT. The most common indication for MRI was unexplained, prolonged consciousness impairment after extubation.
Macrovascular pathology
Fifteen patients (15/174, 8.6%, 95%-CI 4.9–14%) had acute ischemic infarcts on CT, two of which showed hemorrhagic transformation (Figure 2 A-D). Eleven patients (11/174, 6.3%, 95%-CI 3.2–11%) had non-traumatic intraparenchymal hematomas on CT. One patient had an acute subdural hematoma related to a posterior cerebral hemorrhagic infarct (Figure 2 C-D). Two patients had intraparenchymal hematomas (1 frontal, 1 cerebellar) on MRI. Six patients had thin circumferential contrast enhancement (distal ICA, distal basilar, P1, M1-2 and A1 segments) on vessel wall (“black blood”) imaging.
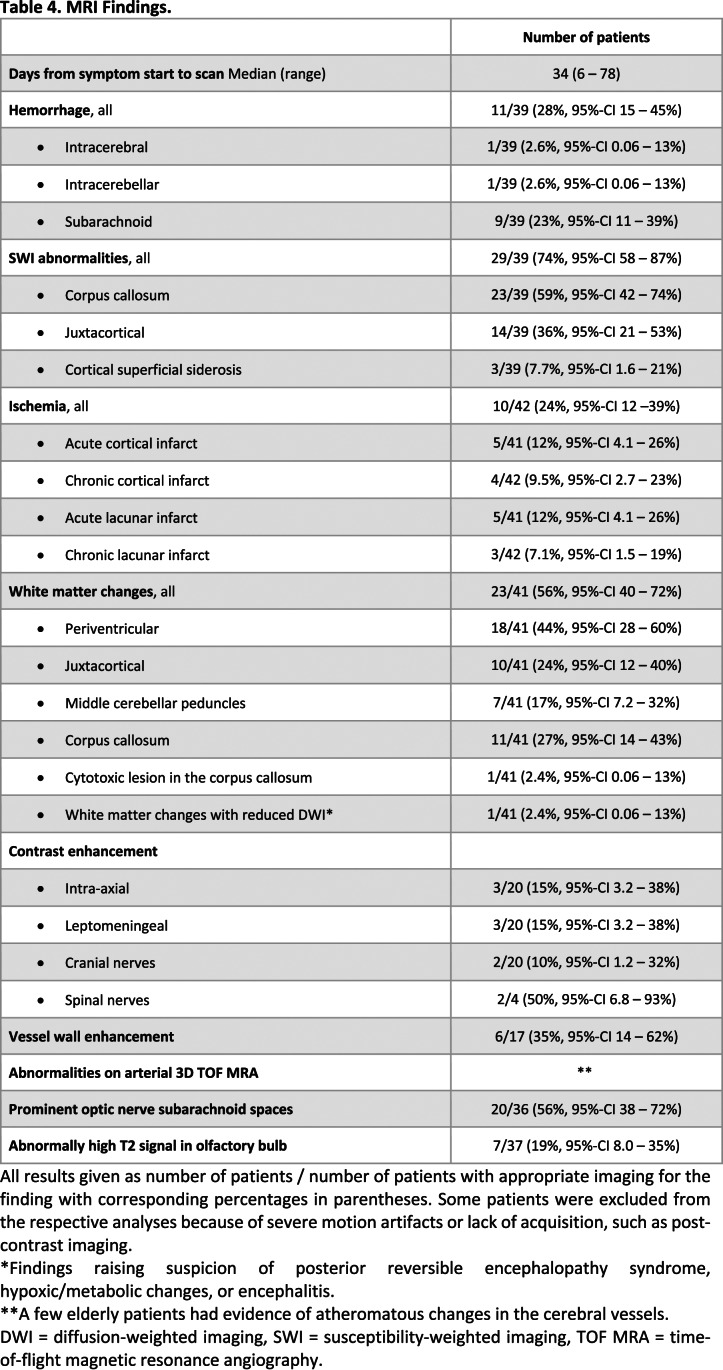
Figure 2.
Macrovascular and diffusion imaging findings.
First row (A, B): Man in his late 60s with COVID-19, left side motor symptoms and left-sided ophthalmoplegia upon a routine wake up test from anesthesia. A, Non-enhanced brain CT in the axial view demonstrating an infarct of the right middle cerebral artery territory. B, Axial non-enhanced brain CT in the axial view a week later revealed hemorrhagic transformation.
First row (C, D): Man in his mid-50s with COVID-19 a month after symptom onset and several weeks in the ICU. Non-enhanced brain CT in the, C, axial and, D, coronal views. Intracerebral hematoma dissecting into the ventricles and along the right tentorium. Low attenuation in the territories of right middle and posterior cerebral arteries were also found.
Second row: Man in his mid-50s with COVID-19 after a month in the ICU and impaired consciousness (GCS 3) after termination of anesthesia. Brain MRI with, E, axial T2-weighted imaging, F, b1000 DWI,and, G, ADC map showing multiple, bilateral acute lacunar infarcts in the semioval centers. H, TOF MRA with normal findings.
Third row: Woman in her late 40s with COVID-19, impaired consciousness and paretic extremities 2 weeks after admission to the ICU. Brain MRI with, I, axial b1000 DWI, J, ADC map, K, T2-weighted imaging, and, L, sagittal T2-weighted FLAIR showed a cytotoxic lesion of the corpus callosum in the splenium.
ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, FLAIR = fluid-attenuated inversion recovery, GCS = Glasgow coma scale, ICU = intensive care unit, SWI = susceptibility-weighted imaging, TOF MRA = time-of-flight magnetic resonance angiography.
Diffusion abnormalities
Five patients presented with acute cortical infarcts (5/41, 12%, 95%-CI 4.1–26%) and five with acute lacunar small (<1.5 cm) infarcts (5/41, 12%, 95%-CI 4.1–26%) on brain MRI, (Figure 2 E-H). One patient presented with a cytotoxic lesion of the corpus callosum (Figure 2 J-L). Reduced diffusion with mild enhancement in the globus pallidus bilaterally was seen in 1 patient.
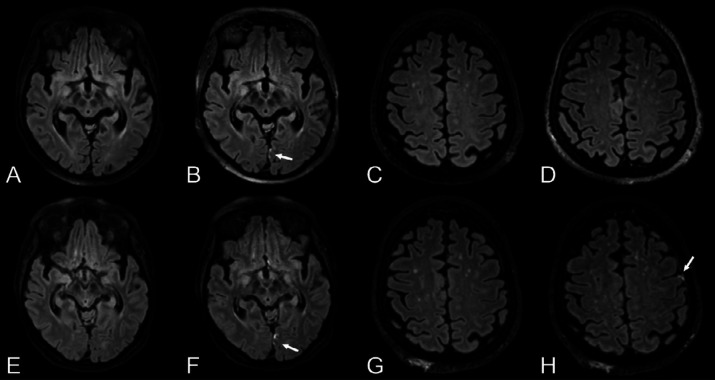
Susceptibility-weighted imaging abnormalities
The most frequent MRI finding, seen in 29/39 (74%, 95%-CI 58–87%) patients, was SWI abnormalities of variable number and extent (Figure 3), often with ovoid or tubular appearance (Figure E2). There was a predilection to the splenium, the juxtacortical U-fibers and the main white matter tracts. SWI abnormalities were detected in the corpus callosum in 23/39 (59%, 95%-CI 42–74%) patients. Some patients with a high number of SWI abnormalities had focal reduced diffusion and/or confluent T2/FLAIR hyperintensities in the most affected areas and other brain areas. No difference in the intraparenchymal SWI abnormalities was seen across time in the patients who had follow-up MRI with SWI (N=3). Another common finding on SWI was the presence of small subarachnoid foci (9/39, 23%, 95%-CI 11–39%) including intraventricular blood (6/39, 15%, 95%-CI 5.9–31%) and cortical superficial siderosis (3/39, 7.7%, 95%-CI 1.6–21%). The median microbleed anatomical rating scale (MARS) score was 6.5 (range 0–26).
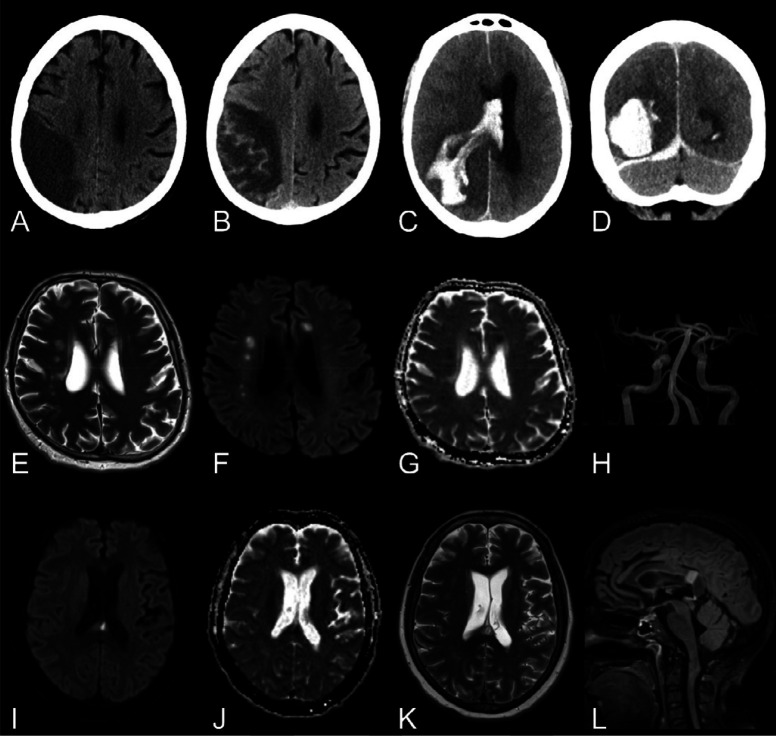
Figure 3.
SWI abnormalities.
First row: Man in his mid 60s with COVID-19, tetraplegia and prolonged confusion (GCS14) after extubation. Brain MRI with, A, axial SWI reveals numerous cerebral susceptibility abnormalities bilaterally in the white matter, most prominently in the corpus callosum and especially in the splenium, many of which have an ovoid shape (as further exemplified in Figure E3). B, Axial T2-weighted FLAIR, C, b1000 DWI, and, D, T2-weighted imaging without any remarkable findings.
Second row: Man in his early 60s with COVID-19 and impaired consciousness (GCS 2+T+1) a week after termination of anesthesia. E, Non-enhanced brain CT in the axial view initially reported as normal. Brain MRI the following day with T2-weighted FLAIR images in the, F, axial and, G, coronal views showing subcortical extension of leukoencephalopathy without associated reduced diffusion (images not shown). H, Axial SWI reveals extensive susceptibility abnormalities with a predilection for subcortical U-fibers, corticospinal tracts, and the corpus callosum.
Third row: Four other patients with COVID-19. Brain MRI with SWI in the axial view showing different degrees and types of susceptibility changes. A few susceptibility abnormalities in the splenium and genu of corpus callosum (I, man in his mid 60s), numerous subcortical cerebral susceptibility artifacts (J, man in his mid 60s), subarachnoid hemorrhage and cortical superficial siderosis (K, man in his late 50s) and dependent intraventricular blood in the posterior horns of the lateral ventricles (L, woman in her mid 40s).
White matter changes
White matter changes were common (23/41, 56%, 95%-CI 40–72%). Eighteen patients (18/41, 44%, 95%-CI 28–60%) had confluent, symmetric, periventricular lesions. Eleven patients (11/41, 27%, 95%-CI 14–43%) had involvement of the corpus callosum. One patient with diffuse symmetric leukoencephalopathy showed partial resolution with clinical improvement at follow-up (Figure 4). Ten patients (10/41, 24%, 95%-CI 12–40%) exhibited juxtacortical white matter changes and seven patients (7/41 17%, 95%-CI 7.2–32%) had white matter changes in the middle cerebellar peduncles.
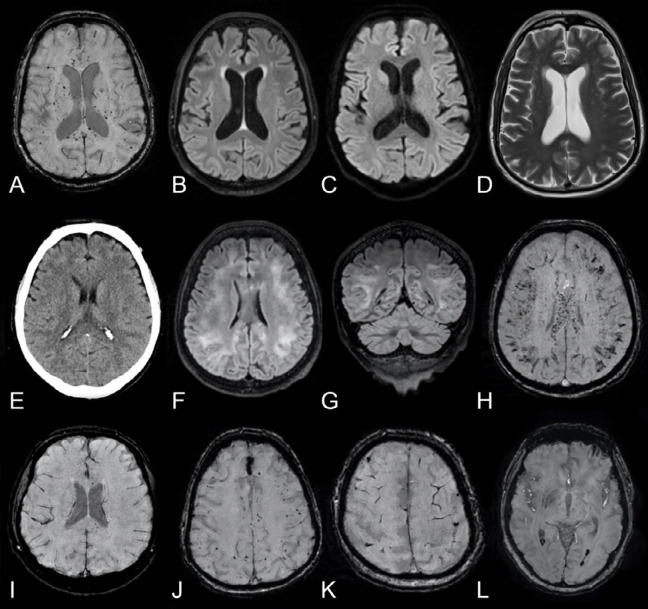
Figure 4.
Longitudinal changes in white matter signal abnormalities.
Top row: Man in his mid 40s with COVID-19 and confusion a month after symptom onset and 2 weeks in the ICU. A-C, Baseline brain MRI with non-enhanced T2-weighted FLAIR images in the axial, coronal, and sagittal views exhibit symmetric confluent white matter changes bilaterally. D, Axial SWI also revealed a few susceptibility abnormalities in the splenium of corpus callosum. There was no reduced diffusion (images not shown). EEG performed 3 days prior to the MRI, found signs of encephalopathy.
Bottom row: E-G, Follow-up brain MRI a week later with non-enhanced T2-weighted FLAIR images in the same views demonstrating partial resolution of the leukoencephalopathy, correlating with an improved mental state. H, The extent of susceptibility abnormalities in the brain parenchyma was unchanged.
FLAIR = fluid-attenuated inversion recovery, ICU = intensive care unit, SWI = susceptibility-weighted imaging.
One patient had bilateral symmetric white matter changes with reduced diffusion in occipital and frontal areas. These findings could represent diffuse leukoencephalopathy possibly related to hypoxemia as previously suggested in (17), or posterior reversible encephalopathy syndrome as previously reported in COVID-19 (13). Toxic/metabolic encephalopathy or encephalitis were considered as alternative diagnoses. Subtle regression of the white matter changes was seen on a follow-up MRI scan in one of the patients (Figure E3), who later succumbed to cardiorespiratory complications.
Contrast enhancement
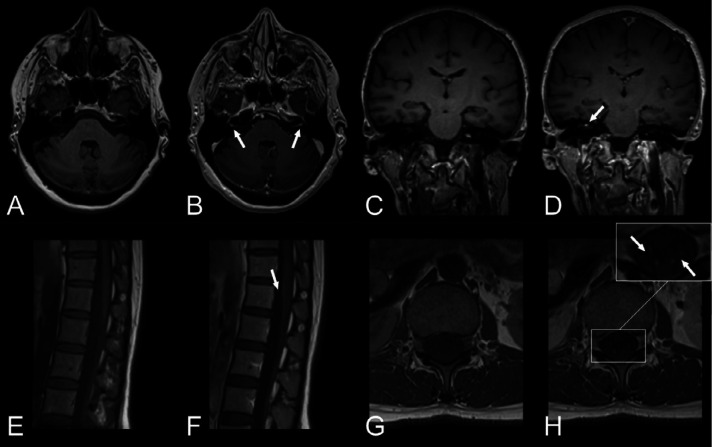
Intraparenchymal enhancement was detected in 3 patients (3/20, 15%, 95%-CI 3.2–38%) with subacute cortical infarction, adjacent to a subacute intracerebral hematoma and in one patient bilaterally in the globus pallidus. Three patients (3/20, 15%, 95%-CI 3.2–38%) had subtle leptomeningeal enhancement, most visible on post-contrast T2-FLAIR; One patient had a follow-up MRI where progression of leptomeningeal enhancement was demonstrated (Figure 5), although the patient improved clinically. Two patients exhibited cranial nerve enhancement; one bilaterally in the facial nerves (Figure 6 A-B), presenting with facial diplegia; the other in the right vestibular nerve (Figure 6 C-D), presenting with vestibular neuronitis. Two patients exhibited enhancement along the cauda equina nerve roots, both presenting with acute demyelinating inflammatory polyneuropathy (Figure 6 E-F).
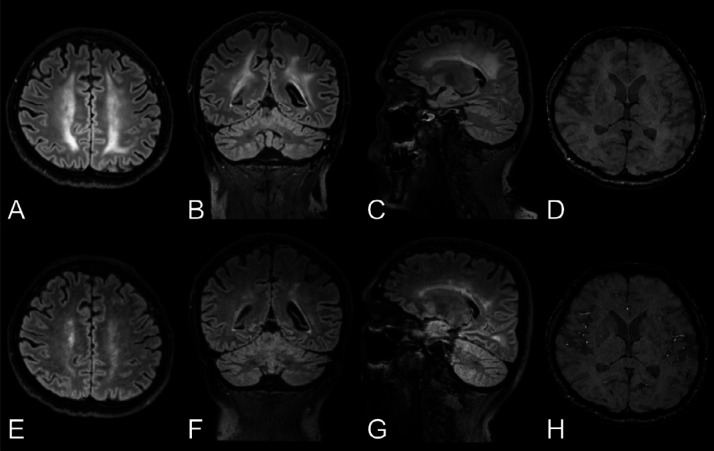
Figure 5.
Progressive leptomeningeal enhancement.
Top row: Woman in her late 60s with COVID-19 with impaired consciousness after termination of anesthesia. Baseline brain MRI with, A, pre- and, B, post-contrast T2-weighted FLAIR images in the axial view revealing a solitary focus of leptomeningeal enhancement (arrow) along the medial aspect of the left occipital lobe.
Bottom row: Follow-up 3.5 weeks later. The original leptomeningeal enhancement focus had increased slightly (E, F with arrow). A new focus of leptomeningeal enhancement had formed along the left inferior frontal gyrus (G, H with arrow) not present on the baseline scan (C, D).
FLAIR = fluid-attenuated inversion recovery, ICU = intensive care unit.
Figure 6.
Cranial nerve and spinal nerve root enhancement.
First row (A, B): Woman in her early 60s with COVID-19, bilateral leg weakness and paresthesias. Brain MRI with T1-weighted images, A, pre- and, B, post-contrast in the axial view showing focal contrast enhancement of the canalicular segment of facial nerve bilaterally. Lumbar puncture showed elevated albumin with no cells and nerve conduction studies were consistent with acute demyelinating polyneuropathy. She received intravenous immunoglobulin treatment on suspicion of Guillain-Barré syndrome and improved clinically.
First row (C, D): Woman in her mid 40s with COVID-19 and typical findings of right-sided vestibular neuronitis. Brain MRI with T1-weighted images, C, pre- and, D, post-contrast in the coronal view depicting focal enhancement of the right vestibular nerve.
Second row: Woman in her mid 30s with COVID-19, pain in the lumbar region and paresthesias in the lower extremities. Lumbar spinal MRI with T1-weighted images, E, pre- and, F, post-contrast in the sagittal view revealed faint contrast enhancement along the cauda equina. T1-weighted images, G, pre- and, H, post-contrast in the axial view verified contrast enhancement of the nerve roots.
Olfactory bulbs and tracts
Increased signal on T2-weighted FLAIR sequences in the olfactory bulbs/tracts was seen in 7/37 (19%, 95%-CI 8.0–35%) patients (Figure E4); in two, there was also subtle contrast enhancement.
Optic nerves
Prominent subarachnoid spaces around the optic nerves were noted in 20 patients (20/36, 56%, 95%-CI 38–72%) on T2-weighted MRI sequences.
Perfusion findings
Perfusion was assessed in 19 patients. rCBF ratios were mostly symmetric between white and grey matter regions respectively (Figure E5). rCBF ratios were similar across patients, with a white matter rCBF ratio of 0.63±0.25 and a grey matter rCBF ratio of 1.05±0.36 (mean±standard deviation).
Previous brain MRI findings
Previous brain MRI was available in 9/43 (21%, 95%-CI 10–36%) patients (time difference: 7.5±7.9, range: 1.2–25 years). Pre-existing conditions included macrovascular events or degenerative/microvascular changes and multiple sclerosis.
Discussion
We report on neuroradiological observations in 2611 consecutive hospitalized adults hospitalized with COVID-19 where 7.1% (95%-CI 6.1–8.1%) underwent neuroimaging. Intraaxial susceptibility abnormalities (29/39, 74%, 95%-CI 58–87%) and leukoencephalopathy (18/41, 44%) was common. We observed ischemic/macrohemorrhagic manifestations but vascular and perfusion imaging did not reveal overt pathology. We also observed contrastenhancement of the parenchyma (3/20, 15%), leptomeninges (3/20, 15%), cranial nerves (2/20, 10%), and spinal nerves 2/4 (50%). At MRI follow-up, regression of leukoencephalopathy and progressive leptomeningeal enhancement were observed in one patient respectively, suggestive of dynamic processes.
The SWI abnormalities and white matter lesions (sometimes with reduced diffusion) both had a striking predilection to the splenium, juxtacortical white matter and long white matter tracts, in line with other recent studies (9,14,18). Their topographical overlap suggests a common cause. Plausible pathophysiological mechanisms may include hypoxia, ischemia, stasis (with deoxyhemoglobin-rich blood), thrombosis, hemorrhages, inflammation, endothelial injury, or a combination of these (19–21). Interestingly, the corpus callosum is also a predilection site for Susac's syndrome, characterized by autoimmune-mediated occlusions of precapillary arterioles due to endothelial autoantigens (22). The distribution of SWI abnormalities is also reminiscent of previous reports in thrombotic thrombocytopenic purpura (23), critical illness (24), H1N1-influenza with extracorporeal membrane oxygenation (ECMO)-treatment (25), high-altitude sickness (26), cerebral malaria (27), and hepatic encephalopathy (28). In our sample, only 1/10 ECMO-treated patients underwent brain MRI; that person had SWI abnormalities but with a low microbleed anatomical rating scale (MARS) score of 2.
While some SWI abnormalities may be punctate (17), many had a non-spherical shape, suggestive of pathology along vessels or microstructure, as also pointed out by others (18). Endotheliopathy, microthrombosis, and microinfarction have been demonstrated in peripheral organs in COVID-19 (20,29,30). Recent neuropathology in COVID-19 has shown hypoxic injuries and inflammatory changes (19) but also frequent microthrombosis (21). Meanwhile, an ex vivo in situ MRI study in COVID-19 showed SWI abnormalities but did not provide histopathological correlates (31). While SWI abnormalities are often referred to as “microbleeds” in radiological reporting, the non-spherical shape of the SWI abnormalities along with evidence for microthrombosis in COVID-19 in other organs and the brain (21,32), suggests that “microbleeds” may be a misnomer in COVID-19. This is supported by the relatively low rate of macroscopic hemorrhages in our study and previous studies (2,12,13), despite routine use of anticoagulation/antiplatelet treatment in COVID-19. Interestingly, hemorrhagic components are not always histopathologically evident in critically ill patients with SWI abnormalities either (33). The terminology may affect the clinical cost-benefit analysis of anticoagulation therapy and since microthrombi is a valid differential diagnosis, this semantic issue is of clinical importance.
The timing of our MRI scans, often in a late disease stage, may contribute to the relatively low frequency of diffusion and perfusion abnormalities. This is supported by the lack of dynamics in intra-axial SWI abnormalities at follow-up, indicating that the microvascular pathologic processes had already waned. The leukoencephalopathic changes in patients with severe COVID-19 seem to have a dynamic nature; the partial resolution of the white matter changes in one case correlated with clinical improvement. Progression of leptomeningeal contrastenhancement did not correlate with clinical deterioration in another. Similar leptomeningeal enhancement has previously been reported in multiple sclerosis (34), and oligoclonal bands (though matched in serum) have been reported in COVID-19 with leptomeningeal enhancement (10,14). While nonspecific, these findings in combination with neuroinflammatory disorders being linked to previous coronaviruses (1,2), hint that neuroinflammatory complications of COVID-19 may occur.
Anosmia is common in COVID-19 (2), and olfactory bulb MRI signal abnormalities have indeed been described (35). Such findings were relatively rare in our study, but their visual assessment can often be equivocal. The presence of prominent subarachnoid spaces around the optic nerves was common (20/43, 47%) and raises potential questions about intracranial pressure in COVID-19. In 4 patients (3 intubated, 1 tracheostomy), this finding could possibly be confounded by positive pressure ventilation. Lumbar punctures were rarely performed, due to contraindications.
Our study has some limitations. First, as with all retrospective studies, it is subject to systematic confounders. Our patients had varying degrees of disease severity, from completely asymptomatic with incidental PCR-positivity to critically ill patients. There naturally exists an indication bias, whereby MRI was performed almost exclusively in severely ill patients. Second, the lack of a control group limits the possibility to draw conclusions on the specificity of our findings to COVID-19. Third, the lack of histopathological data is also a limitation. Fourth, the retrospective nature of this study and the commonly impaired consciousness of the patients limited the ability to perform clinico-radiological correlations, such as olfactory/taste symptomatology. Fifth, the standardized protocol did not include coronal T2-weighted or short tau inversion recovery imaging, which would have been a good complement for assessing the olfactory bulbs. Sixth, the limited number of follow-up MRIs and short observation period does not allow any meaningful conclusions about the long-term clinical outcomes.
In conclusion, brain MRI is essential in order to depict the oftentimes subtle vascular and inflammatory aspects of nervous system involvement in patients hospitalized with COVID-19. The ovoid shape of the susceptibility abnormalities bears similarities to other conditions characterized by endotheliopathy and microthrombosis, both observed in other organs in severe COVID-19. While longitudinal data is limited, the regression of leukoencephalopathy and concurrent clinical improvement in one of our patients is encouraging. The common presence of prominent subarachnoid spaces around the optic nerves raises potential questions about intracranial pressure in COVID-19 and should be evaluated in future studies.
Acknowledgments
Acknowledgments
We would like to thank our colleagues at the Department of Neuroradiology for making this study possible and for their valuable input. We would especially like to acknowledge the contributions of Jessica Bystam, Per Grane, Jens Kolloch, Ola Norbeck, Harriet Nyström, Fredrik Piehl Anna Sjöström and Adrian Szum. We thank Ioannis Koupidis for his help with figure panels. We also want to thank all of our clinical colleagues that have worked so tirelessly and bravely during this pandemic. Finally, we would like to thank all the patients and their families.
S.K. and A.T. contributed equally to this work.
RO was supported by MultipleMS (EU Horizon 2020 grant 733161) and COMBAT-MS (PatientCentered Outcomes Research Institute grant MS-1511–33196). JL was supported by grants from the Stockholm Region Clinical Postdoc program and a private donation by Tedde Jeansson Sr. AFD was supported by grants provided by the Stockholm Region (ALF project). TG was supported by grants from the Stockholm Region Clinical Postdoc program, Stockholm Region ALF and StratNeuro.
Abbreviations:
- 95%-CI
- 95% confidence interval
- COVID-19
- coronavirus disease 2019
- FLAIR
- fluid-attenuated inversion recovery
- PCR
- polymerase-chain-reaction
- rCBF
- relative cerebral blood flow
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- SWI
- susceptibility-weighted imaging
References
- 1.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Román GC, Spencer PS, Reis J, et al. The neurology of COVID-19 revisited: A proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. 2020;414:116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. New England Journal of Medicine. 2020;0(0):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral Effect of Covid-19 on Stroke Evaluation in the United States. New England Journal of Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology. 2020;201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs JR, Gibbs KW, Swor DE, et al. COVID-19-Associated Leukoencephalopathy. Radiology. Radiological Society of North America; 2020;201753. [Google Scholar]
- 10.Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. New England Journal of Medicine. 2020;382(23):2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.More on Neurologic Features in Severe SARS-CoV-2 Infection. New England Journal of Medicine. 2020;382(26):e110. [DOI] [PubMed] [Google Scholar]
- 12.Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology. 2020;201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahammedi A, Saba L, Vagal A, et al. Imaging in Neurological Disease of Hospitalized COVID-19 Patients: An Italian Multicenter Retrospective Observational Study. Radiology. 2020;201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer S, Lersy F, de Sèze J, et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology. 2020;202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregoire SM, Chaudhary UJ, Brown MM, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73(21):1759– 1766. [DOI] [PubMed] [Google Scholar]
- 16.Chung MS, Choi WR, Jeong H-Y, Lee JH, Kim JH. MR Imaging-Based Evaluations of Olfactory Bulb Atrophy in Patients with Olfactory Dysfunction. Am J Neuroradiol. 2018;39(3):532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radmanesh A, Derman A, Lui YW, et al. COVID-19 –associated Diffuse Leukoencephalopathy and Microhemorrhages. Radiology. 2020;202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitsiori A, Pugin D, Thieffry C, Lalive P, Vargas MI. Unusual Microbleeds in Brain MRI of Covid-19 Patients. Journal of Neuroimaging. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological Features of Covid-19. New England Journal of Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020;2020.05.18.20099960. [Google Scholar]
- 22.Dörr J, Krautwald S, Wildemann B, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9(6):307–316. [DOI] [PubMed] [Google Scholar]
- 23.Noorbakhsh-Sabet N, Zand R. Thrombotic Thrombocytopenic Purpura with Concomitant Progressive Cerebral Microbleeds. Journal of Stroke and Cerebrovascular Diseases. 2016;25(11):e214–e215. [DOI] [PubMed] [Google Scholar]
- 24.Fanou EM, Coutinho JM, Shannon P, et al. Critical Illness-Associated Cerebral Microbleeds. Stroke. 2017;48(4):1085–1087. [DOI] [PubMed] [Google Scholar]
- 25.Chow FC, Edlow BL, Frosch MP, Copen WA, Greer DM. Outcome in Patients with H1N1 Influenza and Cerebrovascular Injury Treated with Extracorporeal Membrane Oxygenation. Neurocrit Care. 2011;15(1):156–160. [DOI] [PubMed] [Google Scholar]
- 26.Hackett PH, Yarnell PR, Weiland DA, Reynard KB. Acute and Evolving MRI of High-Altitude Cerebral Edema: Microbleeds, Edema, and Pathophysiology. Am J Neuroradiol. 2019;40(3):464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickerson JP, Tong KA, Raghavan R. Imaging cerebral malaria with a susceptibility-weighted MR sequence. Am J Neuroradiol. 2009;30(6):e85-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achiriloaie AF, Kido D, Wycliffe D, Jacobson JP. White Matter Microsusceptibility Changes in Patients With Hepatic Encephalopathy. J Radiol Case Rep. 2011;5(8):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Annals of Internal Medicine. American College of Physicians; 2020. [DOI] [PubMed] [Google Scholar]
- 30.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020. [DOI] [PubMed] [Google Scholar]
- 32.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. New England Journal of Medicine. Massachusetts Medical Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang J, Arani K, Chew S, et al. Susceptibility Etching on MRI in Patients with Microangiopathy. J Neuroimaging. 2017;27(1):43–49. [DOI] [PubMed] [Google Scholar]
- 34.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Politi LS, Salsano E, Grimaldi M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020. [DOI] [PubMed] [Google Scholar]