Abstract
Background
Insomnia seriously affects people’s health and quality of life. Short-term use of Western drugs may also be harmful. Traditional Chinese medicine has been widely used to treat diseases in world. Therefore, this paper aims to study the therapeutic effect of berberine based on the insomnious rat model.
Material/Methods
The insomnia rat model was established by intragastric administration of caffeine and parachlorophenylalanine (PCPA). Berberine and diazepam were used to treat the established insomnia rats. Then, the pathological changes of insomnia rats were detected. In addition, transcriptome sequencing and data analysis were carried out using rat hippocampus. The expression of key genes was verified by quantitative polymerase chain reaction and western blot.
Results
After 7 days of intragastric administration of berberine, the body weight, memory, and sleep quality of insomnia rats were significantly improved. The key roles of Erbb4, Erbb2, Ar, and Grin2a in berberine treatment were identified. Through the analysis of biological functions and signaling pathways, berberine was shown to play a salutary role through nervous system development and ErbB signaling pathway. Gene-set enrichment analysis (GSEA) results showed that berberine treatment affected more metabolic pathways. Compared with diazepam, berberine can play a faster role, and also improve the overall health level of insomnia rats.
Conclusions
These results suggest that berberine can alleviate insomnia in rats through a neuroprotective effect and improved metabolic level. Berberine has great potential in treatment of insomnia and might have better clinical significance.
MeSH Keywords: Berberine; Genes, erbB; Sleep Initiation and Maintenance Disorders
Background
Insomnia is a serious public health problem because of its high prevalence and treatment challenges [1]. Epidemiological studies of the general population show that about one-third of adults complain of insomnia symptoms [2]. The self-report rate of sleep problems in one study was 55.8%, of which 18.0% often had sleep problems [3]. More and more studies have shown that insomnia or short sleep time or lack of sleep have an adverse impact on personal health [4]. Such adverse impacts include increased hypertension, subclinical cardiovascular disease, coronary heart disease and heart failure, cardiovascular disease morbidity and mortality [5–8]. In addition, insomnia has been found to have an association with cognitive impairment, decreased quality of life, reduced work efficiency, mental illness complications, higher medical costs, and higher risk of death [9]. The correlation between insomnia and these conditions is not clear, which is mainly attributed to little knowledge on the causes of insomnia.
The main pathophysiological mechanisms of insomnia are linked to cognitive, self-referential processes, affective, and sleep-wake-promoting changes in related structures or circuits [10–12]. In addition, high frequency cortical dynamics, brain glucose metabolism, impaired systemic metabolic rate and heart rate variability, and increased cortisol and norepinephrine levels were also contributors [13–15]. Recent evidence has indicated that neuregulin-1 (NRG1) and its ErbB receptors played an essential role in neural development and function [16]. The presence of ErbB receptors in the brainstem is involved in motor function [17]. More importantly, many observational studies have found that chronic low-grade inflammation is one of the potential pathways leading to adverse health consequences of insomnia [18,19]. Sleep insufficiency and sleep interruption experiments have shown subsequent elevations in blood pressure and inflammatory cytokines, including C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-alpha) [20–22]. The longer course of extreme sleep was also associated with CRP, IL-6 and TNF-alpha [23]. Studies support that chronic low-grade inflammation might be the last common pathway to adult morbidity [24]. Therefore, it is necessary to identify the molecular mechanisms of insomnia and to diagnose and treat these adverse health outcomes early in life.
Current use of hypnotics was seen in 7.9% of adults in a study of a representative sample of Norwegian adults [25]. In fact, effective treatment for insomnia exists. However, the treatment of insomnia is not standardized [26]. Treatment of insomnia is challenging because both drug and non-drug therapies have limitations [27]. There is no doubt about the effect of cognitive-behavior therapy for insomnia (CBT-I) on insomnia, but the feasibility and cost-effectiveness of CBT-I makes it difficult for various individuals with chronic insomnia to benefit from this approach [28]. Benzodiazepines increase the risk of cognitive impairment and dementia by 50% [29]. Traditional Chinese medicine has been widely studied for its long-term efficacy and safety in the treatment of insomnia [30,31]. The herbs Suanzaoren, Fuling, and Gancao have been found to have potential pharmacological mechanisms for insomnia [32]. Surprisingly, traditional Chinese medicine has been reported to have a good therapeutic effect on elderly insomnia patients with hypertension, which helps to reduce the risk of polypharmacy [33]. Previous studies have shown that Jiao-Tai-Wan, composed of berberine and other herbal formulas, can improve insomnia in rats [34,35]. The specific effect and mechanism of berberine are not clear.
The aim of our study was to identify the molecular mechanisms of insomnia by transcriptome sequencing based on an insomnia rat model. Then the restorative effects and targets of berberine on insomnia rats were evaluated by molecular indicators. The results of this study will further provide a theoretical foundation for the clinical application of berberine in the treatment of insomnia.
Material and Methods
Construction of insomnious rat model and treatment protocol
Healthy male specific-pathogen-free (SPF) grade adult 2-month-old Sprague Dawley (SD) rats weighed 200±20 g and were purchased from the Laboratory Animal Center of Xinjiang Medical University, license number: SCXK (new) 2018-0002. The rats were provided with water freely during feeding. Sixty SD rats were weighed and divided into 6 groups according to a random number table, 10 rats in each group. Insomnia rat model was established as follows: caffeine (Xinjiang Pharmaceutical Factory of China Pharmaceutical Group) with saline solution (60 mg/kg) was injected intraperitoneally for 7 days. Then parachlorophenylalanine (PCPA, Sigma) suspension (300 mg/kg) was prepared with dilute alkaline saline and injected intraperitoneally for 3 days. The normal control group was given intraperitoneal injection of normal saline. Berberine (Shanghai Pharmaceutical Chifeng Mengxin Pharmaceutical) was given in 3 doses: low dose (50 mg/kg) group, moderate dose (100 mg/kg) group, and high dose (200 mg/kg) group; and the diazepam (Beijing Yimin Pharmaceutical) group (0.9 mg/kg) were intragastrically administered for 7 days. Then other experiments were carried out, including body weight measurements in each group.
Moral statement
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and all experimental protocols were approved by the Animals Ethics Committee of the Xinjiang Medical University (No. IACUC20190116-01).
Water maze
The wall of the pool was marked with 4 water entry points: east, south, west, and north. The platform was hidden 2.5 cm below the water surface and placed in the third quadrant. The midpoint of each quadrant arc was selected as the fixed entry point of the quadrant. Rats arrestment on the platform for 10 seconds was regarded as a sign of success in finding the platform. If the platform was discovered within 120 seconds, the next quadrant experiment was carried out after 10 second detention on the platform. On the sixth day, the space exploration experiment was conducted, and the platform was withdrawn. The number of times each rat crossed the original platform within 120 seconds was recorded.
Sodium pentobarbital
The rats in each group were intraperitoneally injected with sodium pentobarbital (Sigma) 35 mg/kg, and the sleep latency and duration of each rat were recorded. Rats were supine on a flat plate and maintained for more than 60 seconds as the righting reflex disappeared. Sleep latency lasted from injection of pentobarbital sodium to the disappearance of righting reflex. Sleep duration ranged from the disappearance of the righting reflex to the recovery of spontaneous movement.
Enzyme-linked immunosorbent assay (ELISA)
Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (Chengdu Kelong Chemical Reagent Factory) at a rate of 3.0 mg/kg. There were no exhibited signs of peritonitis after the administration of 10% chloral hydrate in rats. Blood samples were collected from abdominal aorta immediately after anesthesia and the contents of atrial natriuretic peptide (ANP; Jianglai Biology), B-type natriuretic peptide (BNP; Jianglai Biology) and endothelin-1 (ET-1; Jianglai Biology) were measured. The specific detection method was operated according to the instructions of the enzyme-linked immunosorbent assay (ELISA) kit.
Transcriptome sequencing
The rats were decapitation after anesthetized by intraperitoneal injection of 10% chloral hydrate with 3.0 mg/kg. After sacrificed, hippocampus tissue of rat brain was quickly separated and placed on ice, and quickly frozen in liquid nitrogen. Three rats in each group were randomly selected for extracting total RNA from the hippocampus using RNA simple total RNA Extraction kit (Tiangen Biotech). Next, the library was built through NEBNext <UltraTM RNA Library Prep Kit (Illumina). The library was diluted to 1.5 ng/uL and sequenced on the computer.
Difference analysis and protein–protein interaction (PPI) network
DESeq2 software (1.16.1) was used to analyze the differentially expression genes (DEGs) between the 2 comparison combinations. The Benjamini-Hochberg methods were used to adjust the P value to control the error detection rate. DESeq2 showed that genes with P value <0.05 and |log2foldchange| >1 were assigned to DEGs.
First, we map all differentially expressed genes into protein–protein interaction (PPI) networks. A PPI of changed genes in insomniac rats was constructed by extracting interaction pairs containing only these DEGs. Secondly, we are introducing the differential expression of PPIs and their genes into Cytoscape for display. Finally, the degree of genes was sequenced.
Enrichment analysis and gene-set enrichment analysis
Cluster Profiler (3.4.4) software was used for enrichment analysis using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) database for DEGs. Considering that terms with P value less than 0.05 are significantly enriched by DEGs.
We used gene-set enrichment analysis (GSEA) software to perform enrichment analysis on 4 groups of genes. First, we calculated the enrichment score (ES), which reflects the degree of enrichment of the set S at both ends of the entire ranking list L. In the second step, the significance of the enrichment score was evaluated. The statistical significance of the ES was estimated using a phenotype-based displacement test procedure. The third step was multiple hypothesis testing, which generally considers that the standardized enrichment score absolute value is <1.0, and NOM P-value <0.05 is a meaningful path entry.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was reversely transcribed to cDNA with ImProm-II Reverse Transcription System (Promega). All cDNA sample was run in triplicates. The SYBR Green kit (Promega) was used for real time polymerase chain reaction (RT-PCR). The housekeeping gene, β-actin, was used as an internal control. Primer sequences are described in Table 1.
Table 1.
Primer sequences of key genes.
| Erbb4 | Forward | CACAGCCCTCCTCCTGCCTAC |
| Erbb4 | Reverse | GCCTCTGGTATGGTGCTGGTTG |
| Erbb2 | Forward | GGGCTGGCTCCGATGTGTTTG |
| Erbb2 | Reverse | CCGCTGTAGAGGGCTGAGGTC |
| Ar | Forward | GGCAGCAGTGAAGCAGGTAGC |
| Ar | Reverse | GGACAGAGCGAGCGGAAAGTTG |
| Grin2a | Forward | GTGTGATGCCTGTCTGCGGATG |
| Grin2a | Reverse | CTGGAGGGCGTTGTTCTGTGAC |
Western blot
The extract of hippocampus tissue of rat brains was prepared by using the solution buffer (Thermo Scientific) containing the mixture of halt protease inhibitors. The total protein was loaded on sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE), separated by electrophoresis and transferred to polyvinylidene difluoride (PVDF) membrane. After incubation with 5% skimmed milk, the membrane was incubated with specific primary antibody (Bioswamp) at 4°C overnight. After 3 times of cleaning with phosphate-buffered saline plus Tween (PBS/Tween), it was incubated with secondary antibody combined with horseradish peroxidase (HRP). Finally, we use Fujifilm las-4000 mini (Fujifilm) to take pictures. The chemiluminescent signals recorded in a chemiluminescence imager (Chemidoc Touch, Biorad). β-actin antibody was used as control.
Statistical analysis
SPSS16.0 statistical software package was used to process the data. One-way ANOVA was used for comparison between groups. The difference was statistically significant with P<0.05.
Results
Therapeutic effect of berberine on insomnia rats
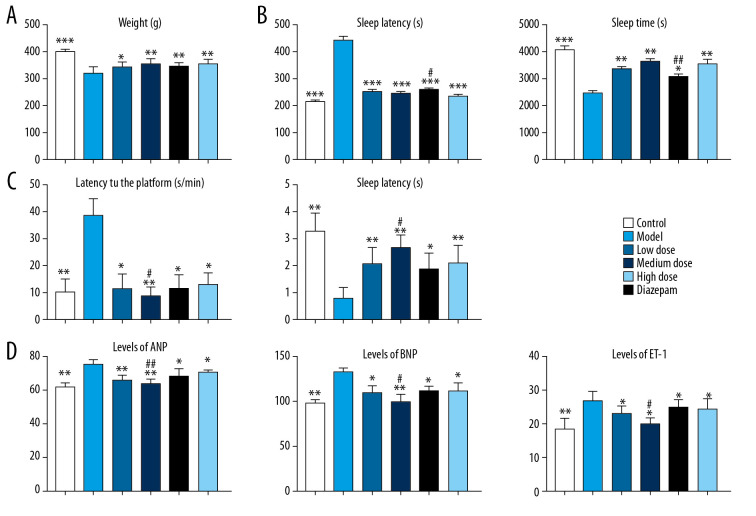
The study flowchart is presented in Figure 1. First, we established an insomnia model in rats. Then, the relieving effect of berberine on insomnia model rats was investigated. Compared with the control group, the weight of insomnia rats decreased significantly. After berberine or diazepam treatment, the body weight of insomniac rats was considerably restored (Figure 2A). Drug treatment significantly improved sleep quality in rats. In the pentobarbital sodium test, berberine and diazepam significantly reduced sleep latency and prolonged the sleep duration in insomniac rats (Figure 2B). In addition, we evaluated the effect of drug treatment on memory ability of insomnia rats by water maze experiment. Insomniac rats treated with berberine and diazepam could find the platform more quickly, and the times of crossing the platform within the prescribed time increased significantly (Figure 2C). In our study, we found that the moderate dose of berberine could alleviate the memory function of insomnia rats better. On the other hand, we also detected the expression of atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), and endothelin-1 (ET-1) in rat plasma. The results showed that berberine and diazepam significantly reduced the pathological index of insomnia rats, especially the moderate dose of berberine (Figure 2D). The results showed that even low doses of berberine sharply increased the body weight of insomniac rats. The salutary effects of moderate and high doses of berberine were similar.
Figure 1.
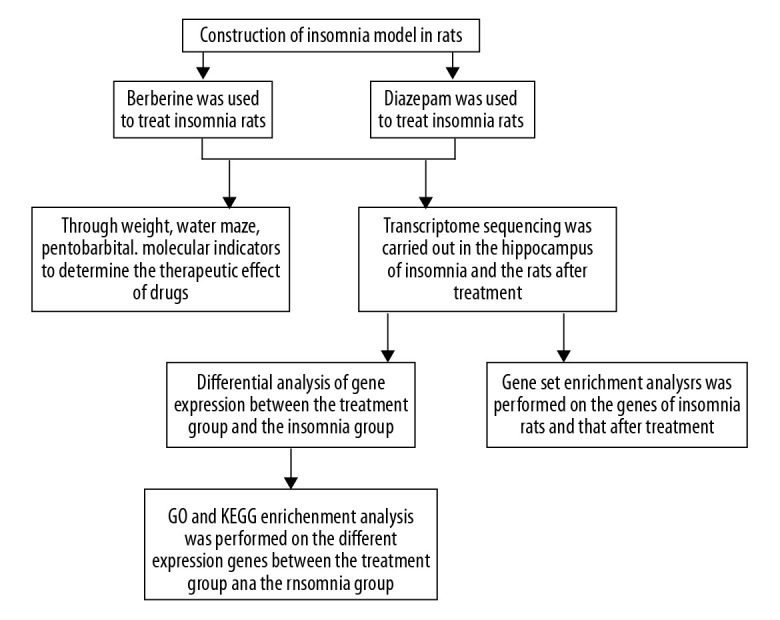
Study flowchart.
Figure 2.
The effect of berberine on the clinical indexes of insomnia in rats. (A) Berberine and diazepam restored the body weight of insomnia rats. (B) Berberine and diazepam reduced the sleep latency of insomnia rats and prolonged the sleep duration in pentobarbital sodium test. (C) In the water maze experiment, the time of finding the platform in insomniac rats treated with berberine and diazepam decreased, and the number of times of crossing the platform increased. (D) The expression of ANP, BNP, and ET-1 in insomnia rats after berberine and diazepam treatment were decreased. * P<0.05, ** P<0.01, *** P<0.001 versus model group; # P<0.05, ## P<0.01 versus diazepam group. ANP – atrial natriuretic peptide; BNP – B-type natriuretic peptide; ET-1 – endothelin-1.
Berberine regulated the expression of disordered genes in insomniac rats
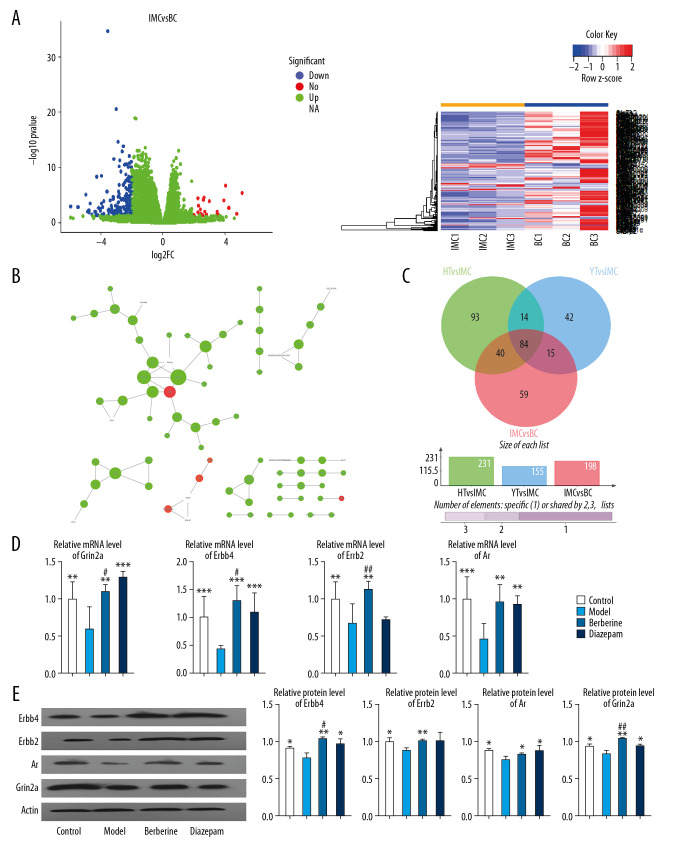
To evaluate the therapeutic effect of berberine, transcriptome sequencing was performed in the hippocampus of rats in 4 groups: blank control (BC), insomnia model (IMC), moderate dose berberine (HT), and diazepam (YT). Compared with the blank control rats, 198 DEGs were identified in insomnia rats. We believe that these genes are potential disorder genes for insomnia in rats (Figure 3A). Then, we constructed a protein–protein interaction (PPI) network of disordered genes, and further identified key genes with high connectivity in the network, which included Erbb4, Erbb2, Ar, and Grin2a (Table 2, Figure 3B). Importantly, we found that 124 of these potential disordered genes were regulated by berberine therapy. Another 99 genes are regulated by diazepam therapy (Figure 3C). Surprisingly, after berberine treatment, the expression of Erbb4, Erbb2, Ar, and Grin2a was restored by comparative analysis. The expression of Erbb4, Ar, and Grin2a also recovered after diazepam treatment. Finally, the expression of key genes in rats was confirmed by qPCR (Figure 3D) and western blot (Figure 3E), which was consistent with the results of transcriptome sequencing. Berberine had a better effect on the expression of Erbb4, Erbb2, and Grin2a. These results suggest that berberine and diazepam can both play therapeutic roles by affecting the expression of insomnia-related genes.
Figure 3.
Berberine regulates gene expression in hippocampus of insomnia rats. (A) Transcription group sequencing identified differentially expressed genes (volcanic and thermal maps) in insomniac rats compared with blank control rats. Red nodes represent upregulated genes and blue nodes represent downregulated genes. (B) The connectivity of disordered genes was screened by PPI network. The larger the node, the higher the connectivity of the gene. Red nodes represent upregulated genes and green nodes represent downregulated genes. (C) Effects of berberine and diazepam on the expression of disordered genes in insomniac rats. (D) qPCR was used to verify the expression of core genes in rats. (E) Western blot was used to verify the expression of core genes in rats. ** P<0.01, *** P<0.001 versus model group, # P<0.05, ## P<0.01 versus diazepam group. PPI – protein–protein interaction; qPCR – quantitative polymerase chain reaction.
Table 2.
Connectivity in protein–protein interaction network of disordered genes.
| Name | Degree | log2 fold change | P-value |
|---|---|---|---|
| Erbb4 | 6 | −2.38692 | 2.39E-08 |
| Erbb2 | 5 | −2.30426 | 0.030524 |
| Ar | 4 | −2.8244 | 1.00E-10 |
| Grin2a | 4 | −2.90201 | 2.06E-05 |
Molecular regulation mechanisms of berberine in relieving insomnia
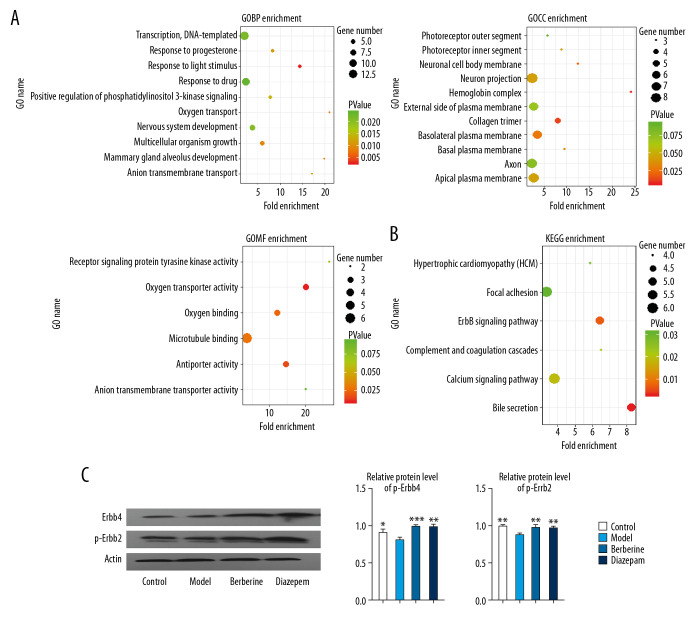
In order to further understand the molecular regulatory mechanism of berberine, we enriched and analyzed the disordered genes. Statistical results showed that the disordered genes were involved in 30 biological processes (BP), 11 cellular components (CC), 6 molecular functions (MF) and 6 KEGG pathways. These include key genes involved in nervous system development, response to drugs and other biological functions (Figure 4A). Complement and coagulation cascades, ErbB signaling pathway, and other signaling pathways (Figure 4B). Importantly, we found that ErbB pathway was activated by berberine and diazepam (Figure 4C). This suggests that berberine and diazepam play a curative role mainly by affecting the nerve function and signaling pathway of rats.
Figure 4.
Enrichment analysis was used to identify the molecular regulatory mechanism of berberine therapy. (A) The key genes involved in GO function in berberine therapy. (B) The key genes involved in KEGG signaling pathway in berberine therapy. (C) Phosphorylation of ErbB2 and ErbB4 proteins. * P<0.05, ** P<0.01, *** P<0.001 versus model group. GO – Gene Ontology; KEGG – Kyoto Encyclopedia of Genes and Genomes.
Comparison of the metabolic mechanisms of berberine and diazepam by GSEA
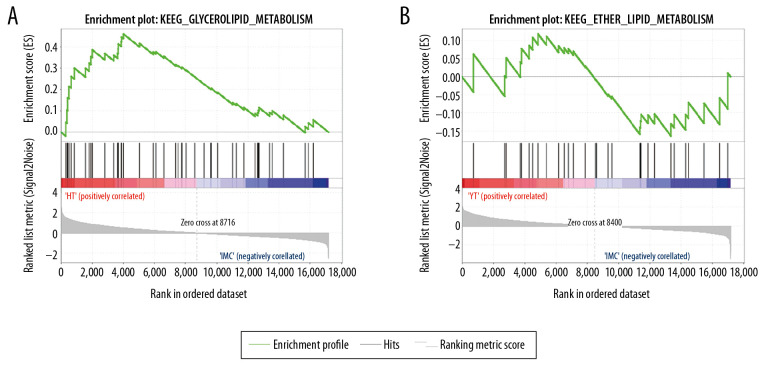
From the molecular mechanism of drug treatment, the targets of the 2 drugs are comparable. In order to compare the time of drug action, we further screened metabolic pathways involved in drug action by GSEA. Seventeen of the 115 signaling pathways in which genes participated in the hippocampus of rats after berberine treatment were metabolically related. Twelve of 108 signaling pathways that changed after diazepam treatment were metabolically related (Table 3, Figure 5). The results showed that berberine might be absorbed faster than diazepam, but the duration of its effect was shorter.
Table 3.
Metabolic signaling pathways involving genes expressed in rat hippocampus after drug treatment.
| Berberine treatment | Diazepam treatment |
|---|---|
| KEGG glycerolipid metabolism | KEGG inositol phosphate KEGG metabolism |
| KEGG inositol phosphate metabolism | KEGG glycerolipid metabolism |
| KEGG glycerophospholipid metabolism | KEGG beta alanine metabolism |
| KEGG ether lipid metabolism | KEGG glycerophospholipid metabolism |
| KEGG propanoate metabolism | KEGG sphingolipid metabolism |
| KEGG sphingolipid metabolism | KEGG purine metabolism |
| KEGG purine metabolism | KEGG cysteine and methionine metabolism |
| KEGG drug metabolism other enzymes | KEGG propanoate metabolism |
| KEGG porphyrin and chlorophyll metabolism | KEGG alanine aspartate and glutamate metabolism |
| KEGG starch and sucrose metabolism | KEGG starch and sucrose metabolism |
| KEGG galactose metabolism | KEGG nicotinate and nicotinamide metabolism |
| KEGG alpha linolenic acid metabolism | KEGG tryptophan metabolism |
| KEGG beta alanine metabolism | |
| KEGG glycine serine and threonine metabolism | |
| KEGG butanoate metabolism | |
| KEGG nicotinate and nicotinamide metabolism | |
| KEGG linoleic acid metabolism |
Figure 5.
GSEA identified metabolic signaling pathways associated with berberine and diazepam treatment. (A) Berberine treatment activates metabolic signaling pathways. (B) Diazepam activate metabolic signaling pathways. Abbreviations: GSEA, gene-set enrichment analysis.
Discussion
Insomnia, as a disease, poses serious risks to the development of cardiovascular and psychiatric diseases, including cognitive deficits [26]. Drug therapy for insomnia has been changing over the past decades. This is mainly attributed to the growing concern about overuse of drugs [36], the change in prescription habits [37], and the increased access to non-drug treatment options [38]. Western drugs for insomnia can easily lead to addiction and other side effects [39]. Therefore, exploring the therapeutic effect and mechanism of traditional Chinese medicine on sleep disorders has become an important research direction. Herein, we evaluated the therapeutic effect of berberine on insomnia rats. Compared with diazepam, berberine had better therapeutic effect on memory recovery. High dosage of berberine did not noticeably increase the body weight of insomniac rats, which might have a bearing on the inhibition of appetite and stomach by high berberine dose. In addition, berberine treatment also alleviated the imbalance of cardiovascular related factors in insomnia rats, and the effect was better than diazepam.
On the other hand, we identified the therapeutic mechanism of berberine by transcriptome sequencing in the hippocampus of 4 groups of rats. We believe that the DEGs between insomnia rats and control rats are disease-related genes of insomnia. Through the PPI network, we identified the core genes in the disordered gene network. After berberine and diazepam treatment, the expression of some genes in insomniac rats changed. We believe that these genes are related to the therapeutic effect of drugs. Surprisingly, the expression of core genes was altered after berberine treatment. Erbb4 is an important NRG-1 receptor involved in many key functions such as neurodevelopment and synaptic plasticity [40,41]. Nerves and synapses were significantly correlated with sleep activity [42]. ErbB 4 plays a key role in regulating the function of cortical-thalamic reticular nucleus (TRN) -thalamic circuit [43]. TRN is critical in the sleep process [44]. Reduced activity of TRN neurons may explain abnormal sleep function [45]. Erbb2 receptor tyrosine kinase plays a major role in early development and regulation of various cell behaviors [46]. It has been found that androgen receptor (Ar) in the main circadian clock of suprachiasmatic nucleus regulates the effect of light on male activity [47]. In addition, changes in GluN2A, a N-methyl-d-aspartate receptor (NMDAR) subunit encoded by GRIN2A, are associated with neurodevelopmental disorders and are critical to sleep-related physiological and pathological processes [48,49]. The aforementioned analysis showed that berberine can achieve therapeutic effect by influencing the expression of key disorder factors.
Our research showed that berberine mainly affects neurological functions and signaling pathways in the treatment process. This is consistent with previous studies that showed that berberine has central nervous system activity [50]. Berberine has also been shown to have neuroprotective effects on learning and memory impairment in rats with severe diffuse axonal injury by inhibiting inflammation, angiogenesis, and apoptosis [51]. The results of one study showed that berberine affected ErbB signaling pathway in insomniac rats, and it could affect the production of several sleep-promoting substances [52]. EGFR, a member of the ErbB signaling pathway, can play a promotive role in Drosophila sleep [52]. In addition, proteins regulated by coagulation cascades will affect the pathophysiology of the central nervous system (CNS) [53]. The activation of complement cascades in peripheral blood significantly affected the circadian rhythm [54]. Importantly, berberine increased the metabolic rate of insomnia rats compared with diazepam. Not only can berberine exert its pharmacodynamics faster, but also improve the overall health status of insomnia rats [55]. In contrast, berberine has more advantages to relieve insomnia in rats.
Conclusions
Our study results confirm that berberine can improve body weight, learning and memory ability, and sleep quality of insomniac rats. It has neuroprotective and health-enhancing effects on insomnia rats. These results suggest that berberine is a potentially effective drug for the treatment of insomnia to consider in the future.
Acknowledgments
The authors would like to thank Xiaoting Li for help in the data analysis.
Footnotes
Ethics approval and consent to participate
Rat tissue samples were collected according to the International Ethical Guidelines for Biomedical Research involving Subjects.
Conflicts of interest
None.
Source of support: This work was supported by National Natural Science Foundation of China (No. 81960837), and Key Disciplines in the 13th five-year Plan of Xinjiang Uygur Autonomous Region
References
- 1.Burman D. Sleep disorders: Insomnia. FP Essent. 2017;460:22–28. [PubMed] [Google Scholar]
- 2.Hou CL, Li Y, Cai MY, et al. Prevalence of insomnia and clinical and quality of life correlates in Chinese patients with schizophrenia treated in primary care. Perspect Psychiatr Care. 2017;53:80–86. doi: 10.1111/ppc.12139. [DOI] [PubMed] [Google Scholar]
- 3.Bjorvatn B, Meland E, Flo E, Mildestvedt T. High prevalence of insomnia and hypnotic use in patients visiting their general practitioner. Fam Pract. 2017;34:20–24. doi: 10.1093/fampra/cmw107. [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: The Penn State Cohort. Hypertension. 2010;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Zhang X, Winkelman JW, et al. Association between insomnia symptoms and mortality: A prospective study of U.S. men. Circulation. 2014;129:737–46. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javaheri S, Blackwell T, Ancoli-Israel S, et al. Sleep-disordered breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2016;193:561–68. doi: 10.1164/rccm.201503-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016;39:1037–45. doi: 10.5665/sleep.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128:268–75.e2. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37:1189–98. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkelman JW, Plante DT, Schoerning L, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36:991–98. doi: 10.5665/sleep.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh S, Kim H, Dang-Vu TT, et al. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39:161–71. doi: 10.5665/sleep.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Kay DB, Buysse DJ. Hyperarousal and beyond: New insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017;7 doi: 10.3390/brainsci7030023. pii: E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang R, Cai H, Zhang L, et al. Dysregulation of neuregulin-1/ErbB signaling in the prefrontal cortex and hippocampus of rats exposed to chronic unpredictable mild stress. Physiol Behav. 2016;154:145–50. doi: 10.1016/j.physbeh.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Joshi M, Krishnakumar A. Hypoglycemia causes dysregulation of neuregulin 1, ErbB receptors, Ki67 in cerebellum and brainstem during diabetes: Implications in motor function. Behav Brain Res. 2019;372:112029. doi: 10.1016/j.bbr.2019.112029. [DOI] [PubMed] [Google Scholar]
- 18.Irwin MR. Why sleep is important for health: A psychoneuroimmunology perspective. Annu Rev Psychol. 2015;6:143–72. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liukkonen T, Rasanen P, Ruokonen A, et al. C-reactive protein levels and sleep disturbances: Observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 20.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 23.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Mendoza J, Baker JH, Vgontzas AN, et al. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017;61:110–16. doi: 10.1016/j.bbi.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omvik S, Pallesen S, Bjorvatn B, et al. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;25:91–100. doi: 10.1097/YIC.0b013e328334e5e6. [DOI] [PubMed] [Google Scholar]
- 26.Riemann D, Nissen C, Palagini L, et al. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14:547–58. doi: 10.1016/S1474-4422(15)00021-6. [DOI] [PubMed] [Google Scholar]
- 27.Kay-Stacey M, Attarian H. Advances in the management of chronic insomnia. BMJ. 2016;354:i2123. doi: 10.1136/bmj.i2123. [DOI] [PubMed] [Google Scholar]
- 28.Perils ML, Smith MT. How can we make CBT-I and other BSM services widely available? J Clin Sleep Med. 2008;4:11–13. [PMC free article] [PubMed] [Google Scholar]
- 29.Islam MM, Iqbal U, Walther B, et al. Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–91. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 30.Ni X, Shergis JL, Guo X, et al. Updated clinical evidence of Chinese herbal medicine for insomnia: A systematic review and meta-analysis of randomized controlled trials. Sleep Med. 2015;16:1462–81. doi: 10.1016/j.sleep.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Ni X, Shergis JL, Zhang AL, et al. Traditional use of Chinese herbal medicine for insomnia and priorities setting of future clinical research. J Altern Complement Med. 2019;25:8–15. doi: 10.1089/acm.2018.0249. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Zhao K. Treatment of insomnia with traditional Chinese herbal medicine. Int Rev Neurobiol. 2017;135:97–115. doi: 10.1016/bs.irn.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Kwon CY, Lee B, Chung SY, et al. Oriental herbal medicine for insomnia in the elderly with hypertension: A systematic review protocol. Medicine (Baltimore) 7:e12200. doi: 10.1097/MD.0000000000012200. 92018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He W, Liu G, Cai H, et al. Integrated pharmacokinetics of five protoberberine-type alkaloids in normal and insomnic rats after single and multiple oral administration of Jiao-Tai-Wan. J Ethnopharmacol. 2014;154:635–44. doi: 10.1016/j.jep.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 35.Chen N, Guo CE, Chen H, et al. Simultaneous determination of six coptis alkaloids in urine and feces by LC-MS/MS and its application to excretion kinetics and the compatibility mechanism of Jiao-Tai-Wan in insomniac rats. Biomed Chromatogr. 2018;32:e4248. doi: 10.1002/bmc.4248. [DOI] [PubMed] [Google Scholar]
- 36.Moloney ME, Konrad TR, Zimmer CR. The medicalization of sleeplessness: A public health concern. Am J Public Health. 2011;101:1429–33. doi: 10.2105/AJPH.2010.300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999–2010. Sleep. 2014;37:343–49. doi: 10.5665/sleep.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 39.Lin YF, Liu ZD, Ma W, Shen WD. Hazards of insomnia and the effects of acupuncture treatment on insomnia. J Integr Med. 2016;14:174–86. doi: 10.1016/S2095-4964(16)60248-0. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher GM, Kalia LV, Ng D, et al. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–78. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu JM, Li KX, Cao SX, et al. Increased NRG1-ErbB4 signaling in human symptomatic epilepsy. Sci Rep. 2017;7:141. doi: 10.1038/s41598-017-00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dashti HS, Jones SE, Wood AR, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10:1100. doi: 10.1038/s41467-019-08917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahrens S, Jaramillo S, Yu K, et al. ErbB4 regulation of a thalamic reticular nucleus circuit for sensory selection. Nat Neurosci. 2015;18:104–11. doi: 10.1038/nn.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latchoumane CV, Ngo HV, Born J, Shin HS. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95:424–35.e6. doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Thankachan S, Katsuki F, McKenna JT, et al. Thalamic reticular nucleus parvalbumin neurons regulate sleep spindles and electrophysiological aspects of schizophrenia in mice. Sci Rep. 2019;9:3607. doi: 10.1038/s41598-019-40398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Syvyk AE, Syvyk TL. Inducible dominant negative ErbB2 rat spermatogonial line for generation of transgenic rat model and dissecting ERBB2 tyrosine kinase mediated pathways. Exp Oncol. 2019;41:95–105. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-2.13026. [DOI] [PubMed] [Google Scholar]
- 47.Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: Influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strehlow V, Heyne HO, Vlaskamp DRM, et al. GRIN2A-related disorders: Genotype and functional consequence predict phenotype. Brain. 2019;142:80–92. doi: 10.1093/brain/awy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salmi M, Del Gallo F, Minlebaev M, et al. Impaired vocal communication, sleep-related discharges, and transient alteration of slow-wave sleep in developing mice lacking the GluN2A subunit of N-methyl-d-aspartate receptors. Epilepsia. 2019;60:1424–37. doi: 10.1111/epi.16060. [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008;589:163–72. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 51.Wang HC, Wang BD, Chen MS, et al. Neuroprotective effect of berberine against learning and memory deficits in diffuse axonal injury. Exp Ther Med. 2018;15:1129–35. doi: 10.3892/etm.2017.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allebrandt KV, Teder-Laving M, Cusumano P, et al. Identifying pathways modulating sleep duration: From genomics to transcriptomics. Sci Rep. 2017;7:4555. doi: 10.1038/s41598-017-04027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Luca C, Virtuoso A, Maggio N, Papa M. Neuro-coagulopathy: Blood coagulation factors in central nervous system diseases. Int J Mol Sci. 2017;18(10) doi: 10.3390/ijms18102128. pii: E2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budkowska M, Ostrycharz E, Wojtowicz A, et al. A Circadian rhythm in both complement cascade (ComC) activation and sphingosine-1-phosphate (S1P) levels in human peripheral blood supports a role for the ComC-S1P axis in circadian changes in the number of stem cells circulating in peripheral blood. Stem Cell Rev Rep. 2018;14:677–85. doi: 10.1007/s12015-018-9836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the crosstalk between the brain and peripheral metabolism. Proc Natl Acad Sci USA. 2011;108(Suppl 3):15609–16. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]