Highlights
-
•
We showed a different presentation of Castleman disease.
-
•
Our service role out parasitic, mycotic, oncologic causes.
-
•
Firstly we treat with antibiotics because the patient did not show any clinical improvement. We opted for a surgical treatment.
-
•
All the decisions were discussed with the patient.
-
•
The surgical treatment for soft tissue disease contributes to returning the patient's social and familial activities.
Keywords: Castleman disease, Skin wound, Wound, Lymphedema
Abstract
Introduction
Castleman disease (CD) is a lymphoproliferative disorder with lymph node hypertrophy. In the unicentric form (UCD), it affects one lymph node or chain of lymph nodes. In the multicentric form (DCM), there is hypertrophy of several lymph node chains with the formation of tumor masses, causing compressive symptoms. This case report showed a case of CD in a different location(inguinal region) associated to a multiple skin lesions.
Presentation of the case
We reported a UCD in a 43-year-old female patient with no previous comorbidities. Since January 2016, this patient developed erysipelas lesions of the left leg (LL) from the thigh root to the foot. Concomitantly, a tumor mass appeared in the inguinal region. In 2019 we performed a biopsy that revealed changes characteristic of CD. Due to extremely poor trophic conditions, the skin area with erysipelas was resected, and the raw surface was grafted.
Discussion
As an inference, the erysipelas may have been responsible for the subsequent lymphangitis, lymphedema and lymph node hypertrophy.
Conclusion
Resection of the diseased skin and lymph node excision constitute the treatment of UCD and result in improvement of the clinical picture. Nevertheless, further study of the inflammatory reaction and of markers such as interleukin-6 and the presence of skin disorders in DC is needed.
1. Introduction
Castleman disease (CD) is a rare clinicopathological disorder. CD is characterized by benign lymphoproliferative changes [1]. This disease can be classified into two major groups: unicentric (UCD) presentation, affecting a single lymph node or a single lymph node chain, or a multicentric (MCD) presentation, in which several lymph node chains are hypertrophied [2].
The etiopathogenesis of CD is still poorly understood; however, some researchers associate it with viral or neoplastic mechanisms and their respective inflammatory or autoimmune responses [2,3]. The lymphoid hyperproliferation is a consequence of an exacerbated immune reaction with a relevant inflammatory response and the overproduction of cytokines (IL-6) [7].
The most common form of the CD is a single lymph node (unicentric Castleman disease), usually intracavitary (chest or abdomen).
Multicentric Castleman disease affects multiple lymph nodes throughout the body and can be associated with human herpesvirus type 8 (HHV-8) and human immunodeficiency virus (HIV).
UCD is typically asymptomatic, but there are cases in which the increased lymph node volume compresses neighboring structures, causing compressive symptoms [4,5]. Laboratory tests are frequently unaltered; therefore, the disease is diagnosed by lymph node biopsy [6,7].
Cutaneous manifestations, including paraneoplastic pemphigus, may occur in 55% of cases [6]. UCD requires surgical treatment for the removal of affected lymph nodes; surgery offers good results and the remission of symptoms [8,4].
MCD requires more complex treatments that combine surgery with radiotherapy, antivirals, polychemotherapy and immunosuppressants, but the prognosis is variable [9,4]. Concomitant paraneoplastic skin lesions are treated with a combination of steroids and siltuximab [10].
This study presents the case of a patient presenting skin lesions associated with CD and comments concerning nosology. This case report showed a case of CD in a different location (inguinal region) associated to a multiple skin lesions.
2. Case
To report this case we followed the SCARE checklist [11]. This case report was submitted to the ethical committee of Hospital das Clínicas – Universidade de São Paulo (CAAE 34949120.0.0000.0068). The patient signed the informed consent
A 43-year-old female patient with no previous comorbidities presented erysipelas lesions in the left leg (LL) from the thigh root to the foot, since January 2016.
There wasn’t any past medical and family history.
At that time, she was treated with ceftriaxone and clindamycin, with partial remission of symptoms. However, the patient developed progressive edema on the left foot, with a diagnosis of lymphedema of unknown ethiology. Since then, this edema has been treated with compression stockings and diosmin.
In February of the same year, the patient developed a neuropathic condition in the LL characterized by severe intermittent pain in the left foot. She received analgesics, pregabalin, chlorpromazine and amitriptyline to treat this neuropathic pain without any remission.
Throughout 2017, the patient presented with progressive lymphedema associated with atrophic skin disorders (skin color and skin fissures). These lesions are slowly enlarging in extensive wounds in the left leg.
At the same time, she presented with sporadic low nocturnal fever, somnolence, and inappetence. The patient denied weight loss at the time. In July 2019, the patient noted a left inguinal mass tumor.
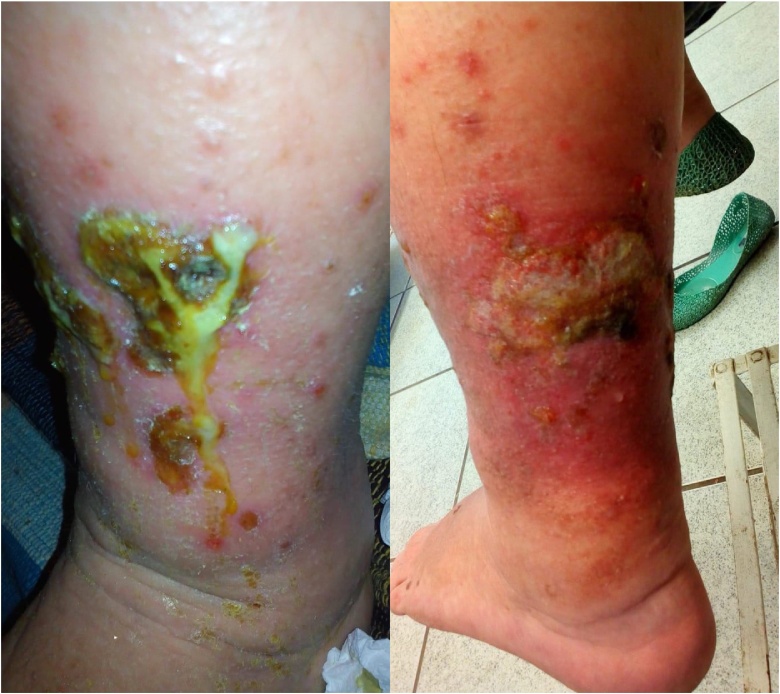
In August 2019, the patient was referred for reassessment. On examination, a non-tender, firm mass was palpated in the left inguinal region associated with a large wound on the LL: a friable, vegetating papillomatous plaque with a fetid odor extending from the pretibial region to the medial aspect of the leg (Fig. 1).
Fig. 1.
Lesion with lymphedema in the left leg. Lateral views.
Laboratory tests showed microcytic and hypochromic anemia, serum albumin within normal values, and a marked increase in C-reactive protein (CRP).
Laboratory tests ruled out mycotic or parasitic disease. We collect the leg exudate to culture, and we treated it with 15 days of ciprofloxacin (500 mg, bid) associated to clindamycin (300 mg, tid) with no wound skin healing
A PET scan showed lymphadenomegaly (5.7 cm, SUVmax 10.3) in the left femoral and inguinal) (Fig. 2) and in the left common-external iliac and paraaortic chain of 3.1 cm (SUVmax 8.3). A magnetic resonance imaging showed subcutaneous cellular tissue edema that did not affect the muscle fascia, known as elephantiasis nostra verrucosa, which presented a cleavage plane of dissection between the injured skin and the intact muscle fascia (Fig. 3).
Fig. 2.
PET SCAN showing: Skin thickening associated with densification of the subcutaneous plane of the left leg; Lymph nodes and lymph node enlargement in the left femoral and inguinal chains.
Fig. 3.
MRI showing the plane of the intact muscle fascia.
In October 2019, an experienced surgeon (more than 10 years of practice) biopsied the lymph node and debridement of the skin lesions. The resected area was treat by a vacuum dressing, and after five days, we performed a skin mesh graft. (Fig. 4, Fig. 5).
Fig. 4.
Left leg after resection of the lesion.
Fig. 5.
Left leg after skin grafting.
The lesion's anatomopathological examination showed a not specific chronic inflammatory process and vascular neoformation in the dermis. The lymph node biopsy showed a cytoarchitectural organization and immunohistochemistry compatible with CD (Fig. 6).
Fig. 6.
Microscopic examination of the lymph node (40× magnification). Lymphoid follicles with atretic germinative centers. Concentric rings of lymphocytes in an “onion skin” pattern.
The skin graft integrated 100%. No locomotion dysfunction or complication was observed.
Until December 2019, the patient is being followed up on an outpatient basis and undergoing physical therapy, with a gradual return to social/occupational activities (Fig. 7).
Fig. 7.
Patient’s follow-up (six months).
3. Discussion
We hypothesized the initial clinical presentation of erysipelas of the leg could be responsible for the onset of lymphangitis and consequent lymphedema [12]. The lymphedema worsened despite the treatment, and the trophic conditions of the skin underwent severe changes that caused lymphedema “nostra verrucose”.
The long-term inflammatory environment, reflected by fever, somnolence, inappetence, and increased CRP aggravated local inflammation.
Increased CRP levels, such as those found in this patient, are in fact indicative of an increase in interleukins tissue level, particularly IL-6. The higher inflammatory activity promoted an increase in this inflammatory protein production. Consequently, at the level of the lymph nodes caused a lymphoproliferation.
As an inference, it can be assumed that the erysipelas triggered the CD; however, there are paraneoplastic lesions that manifest as a result of UCD and present skin thickening, angiomas and telangiectasias [8]. Particular mention should be made of paraneoplastic pemphigus [6], which begins with lesions in the oral mucosa [8]. All these lesions regress with complete resection of the enlarged lymph node, which constitutes effective treatment of the disease [8,14].
Before the lymph node biopsy, the diagnose was lymphedema with an unknown cause. The anatomopathological exam defined the CD.
Regarding to the patient’s neuropathy, the pain may have resulted because the demyelinating peripheral neuropathy observed in CD. However, the neuropathy was more frequent in the multicentric form when compared to unicentric form [13].
In the case under study, we administer antibiotics guided by culture as tempting to solve the dermopathy clinically. However, only with the removal of the hypertrophied lymph nodes associated with the resection of the infected skin area resulted in marked improvement of the patient's clinical condition. This case report showed an unusual CD presentation (different location-inguinal region) associated with multiple skin lesions. Despite the rarity of the CD, it is essential to consider this disease as a possible alternative in the lymphedema differential diagnostic.
Declaration of Competing Interest
None.
Funding
None.
Ethical approval
CAAE 34949120.0.0000.0068/2020.
Consent
We have attached the informed consent.
Author contribution
Modolin MLA – Conceptualization, Project administration, Supervision, Roles/Writing - original draft.
Camargo CP – Project administration, Writing - review & editing.
Milcheski DA – Data curation, Investigation; Methodology.
Cintra Jr. W – Data curation, Investigation; Methodology.
Rocha RI – Data curation, methodology.
Clivatti GM – Data curation, methodology.
Nascimento B – Data curation, methodology.
Gemperli R – Conceptualization, Project administration, Supervision.
Registration of research studies
-
1.
Name of the registry: not applicable- case report
-
2.
Unique identifying number or registration ID: N/A.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): N/A.
Guarantor
Miguel Luiz A Modolin.
Cristina Pires Camargo.
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
M.L.A. Modolin: Conceptualization, Project administration, Supervision, Writing - original draft. C.P. Camargo: Project administration, Writing - review & editing. D.A. Milcheski: Data curation, Methodology. W. Cintra: Data curation, Methodology. R.I. Rocha: Data curation, Methodology. G.M. Clivatti: Data curation, Methodology. B. Nascimento: Data curation, Methodology. R. Gemperli: Conceptualization, Project administration, Supervision.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.ijscr.2020.07.049.
Appendix C. Supplementary data
The following are Supplementary data to this article:
References
- 1.Castleman B., Iverson L., Menendez V.P. Localized mediastinal lymph node hyperplasia resembling thymoma. Cancer. 1956;9(4):822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Fajgenbaum D., Shilling D. Castleman disease pathogenesis. Hematol. Oncol. Clin. N. Am. 2018;32:11–21. doi: 10.1016/j.hoc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Talat N., Belgaumkar A.P., Schulte K.M. Surgery in Castleman’s disease: a systematic review of 404 published cases. Am. Surg. 2012;255(4):677–684. doi: 10.1097/SLA.0b013e318249dcdc. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira C.V.C., Gonçalves C.E.F., Almeida V.F.S., Oliveira A.M.P., Pimenta F.C.F. Doença de Castleman localizada abdominal. Bras. Hemat. Hemoterm. 2005;27(2):133–137. [Google Scholar]
- 5.Sherman D., Ramsay B., Theodorou N.A., Woodrow D., Paradina F.P., Cream J.J., Murray-Lyon I.M. Reversible plane xanthoma, vasculitis and peliosis hepatis in giant lymph node hyperplasia (Castleman’s disease): a case report and review of the cutaneous manifestations of giant lymph node hyperplasia. J. Am. Acad. Dermatol. 1992;26:105–109. doi: 10.1016/0190-9622(92)70016-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.J., Han J.H., Chul H.B., Park K.S., Seok-Goo C., Yoo D.S., Kim K.M., Park H.J., Park Y.M., Lee J.Y., Lee J.H. Cutaneous disorders associated with Castleman’s disease. Na Intern. Non-Prof. J. 2019 doi: 10.2340/00015555-3253. [DOI] [PubMed] [Google Scholar]
- 7.Yabuhara A., Komiyama A. Castleman’s disease and interleukin 6. Leuk. Lymphoma. 1990;2:369–380. doi: 10.3109/10428199009069289. [DOI] [PubMed] [Google Scholar]
- 8.Wong R.M. Unicentric Castleman disease. Hematol. Oncol. Clin. N. Am. 2018;65:73. doi: 10.1016/j.hoc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Swetha I., Muhammada I.B., Halliday Mark. Castleman’s disease: a case report. Int. J. Surg. Case Rep. 2010;1(3):25–26. doi: 10.1016/j.ijscr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teipel R., Ordemam R., Proske U., Dietrich F., Minde M., Ehminger G., Kroschinsky F., Platzbecker U. Am Assoc for Cancer Research; 2015. Siltuximab for Multicentric Castleman Disease – Letter. [DOI] [PubMed] [Google Scholar]
- 11.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Modolin M.L.A., Cintra Jr W., Paggiaro A.O., Faintuch J., Gemperli R., Ferreira M.C. Massive localized lymphedema (MLL) in bariatric candidates. Obs. Surg. 2006;16(16):1126–1130. doi: 10.1381/096089206778392185. [DOI] [PubMed] [Google Scholar]
- 13.Szalat R., Munshi N. Diagnosis of Castleman disease. Hematol. Oncol. Clin. N. Am. 2018;32:53–64. doi: 10.1016/j.hoc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Ramires J., Posada M., Sanches-Urdazpal L., Martin-Perez E., Del Campo L., Garcia I., Martin J.L., Larranaga E. Case report Castleman’s disease: a case report of the unicentric type. Case Rep. Surg. 2012 doi: 10.1155/2012/175272. Article ID 175272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.