Abstract
Aims:
Pulmonary sarcomatoid carcinoma (PSC) is a poorly differentiated non-small-cell lung carcinoma (NSCLC) with aggressive behaviour. This study aimed to evaluate the prognostic clinicopathological and genetic characteristics of PSCs.
Methods and results:
Fifty-three cases of surgically treated PSCs were selected, 23 of which were subjected to mutation and copy number variation analysis using the 50-gene Ion AmpliSeq Cancer Panel. The majority of the patients were male (32 of 53, 60.3%) and smokers (51 of 53, 96.2%). Overall, 25 (47.1%) patients died within 2–105 months (mean = 22.7 months, median = 15 months) after diagnosis, and 28 were alive 3–141 months (mean = 38.7 months, median = 21.5 months) after diagnosis. Five-year overall survival was 12.5%. KRAS codon 12/13 mutation in adenocarcinomas (P = 0.01), age more than 70 years (P = 0.008) and tumour size ≥4.0 cm (P = 0.02) were associated strongly with worse outcome. TP53 (17 of 23, 74.0%) and KRAS codon 12 of 13 mutations (10 of 23, 43.4%) were the most common genetic alterations. Potentially actionable variants were identified including ATM (four of 23, 17.3%), MET, FBXW7 and EGFR (two of 23, 8.7%), AKT1, KIT, PDGFRA, HRAS, JAK3 and SMAD4 (one of 23, 4.3%). MET exon 14 skipping and missense mutations were identified in two (11.1%) cases with adenocarcinoma histology. Copy number analysis showed loss of RB1 (three of 23, 13%) and ATM (two of 23, 8.7%). Copy number gains were seen in EGFR (two of 23, 13.0%) and in one (4.3%) of each PIK3CA, KRAS, MET and STK11.
Conclusions:
Potentially targetable mutations can be identified in a subset of PSC, although most tumours harbour currently untargetable prognostically adverse TP53 and KRAS mutations.
Keywords: copy number analysis, KRAS, lung, next-generation sequencing, sarcomatoid carcinoma
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a highly aggressive type of non-small-cell lung carcinoma (NSCLC), composed of both epithelial and sarcoma-like components. There are five main histological subtypes in this category: pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma and pulmonary blastoma.1 PSCs are rare and account for fewer than 1% of all pulmonary malignancies; however, compared to other stage-matched NSCLC, they are more resistant to conventional therapies and have poorer prognosis.2,3
Although the molecular characteristics of the more common subtypes of NSCLCs, mainly adenocarcinomas, have been studied extensively, the genetic alterations in PSC have only recently become the target of studies.3–7 This is due perhaps to the rarity of the disease and difficulty in diagnosing PSCs, particularly in small biopsies. The 2015 WHO1 recommends molecular testing in PSCs according to known genetic abnormalities associated with the histological components in the tumour. KRAS mutation has been reported in up to 38% of PSCs and EGFR mutations in up to 25%.6,8–10 Furthermore, few recent studies have identified targetable MET exon 14 skipping in a significant fraction of cases,3,7,9 with a few case reports demonstrating a great response to targeted therapy with MET inhibitors.11,12 Despite these advancements, there are still limited options available for treatment of these tumours. In addition, there are no clinicopathological or molecular features that could predict outcome reliably in PSC patients. In this work, using a targeted next-generation sequencing approach, we explored the genetic profile and clinicopathological characteristics of a cohort of surgically treated PSC.
Materials and methods
PATIENTS AND SPECIMENS
Of 53 consecutive, surgically treated PSCs during a 10-year period (from 2004 to 2014), 23 were selected based on tissue availability for additional studies. All cases were reviewed to confirm the diagnosis applying the 2015 WHO criteria1 and were staged according to the American Joint Committee staging manual (8th edn).13 The study was conducted under an exemption approved by the University of Pittsburgh Institutional Review Board (PRO 12070229).
Clinical information including gender, age, tumour stage and smoking status was obtained from patients’ electronic medical records. Follow-up data regarding survival were collected through the institutional Network Cancer Registry.
IMMUNOHISTOCHEMISTRY
Immunohistochemistry (IHC) for Rb1 (Leica, Allendale, NJ, USA; clone 13A10, monoclonal mouse, 1:50) was performed.
FLUORESCENCE IN - SITU HYBRIDISATION ASSAYS
Fluorescence in-situ hybridisation (FISH) assays for amplification of KRAS, EGFR, PIK3CA and MET were performed as described previously.14,15
NEXT – GENERATION SEQUENCING
DNA sequencing was performed using the Ion AmpliSeq Cancer Panel (Ion Torrent; Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA), as reported previously.16 Briefly, 10 ng of DNA was amplified by polymerase chain reaction (PCR) using the AmpliSeq Cancer Panel Primers pool and Ion AmpliSeq Master Mix version 2.0. Multiplexed barcoded libraries were enriched by clonal amplification using emulsion PCR on ion sphere particles (ISPs) (Ion PGM template OT2 200 kit or Ion PI OT2 200 kit version 3) and loaded onto an Ion 318 chip or P1 chip (Thermo Fisher Scientific). Massively parallel sequencing was carried out on a personal genome machine sequencer or ion proton (Thermo Fisher Scientific).
The raw signal data were analysed using Torrent Suite (version 4.0.1; Life Technologies, Carlsbad, CA, USA). The short sequence reads were aligned to the human genome reference sequence (GRCh37 patch 13, GCF_000001405.25). Variant calling was performed using Variant Caller version 4.4.3.3 plugin (integrated with Torrent Suite) that generated a list of identified sequence variations [single nucleotide variants (SNV) and insertions or deletions (indels)] in a variant calling file (VCF version 4.2; https://samtools.github.io/hts-specs/VCFv4.2.pdf). After removing reference calls from the VCF files, variant calls in each VCF files were normalised17 and sorted based on the chromosome and genomic position. Variant calls were annotated using ANNOVAR18 and the HGVS python module.19 Several publically available databases were used for variant annotation: COSMIC version 81 (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/; last accessed 8/30/2017), dbSNP build 137 (http://www.ncbi.nlm.nih.gov/SNP/; last accessed 8/30/2017), 1000 genomes (http://www.1000genomes.org/; last accessed 8/30/2017), Exome Variant Server (http://evs.gs.washington.edu/EVS/; last accessed 8/30/2017), Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/; last accessed 8/30/2017) and in-silico prediction scores (PolyPhen-2 and SIFT).20,21 Sequence variants with at least 5% allelic fraction and at least ×200 depth of coverage were included for analysis. Integrated Genomics Viewer22 (IGV; Broad Institute, Cambridge, MA, USA) was used for manual review of the sequence read pile-ups to assess variant call quality. A joint cohort analysis of all variants across all samples were performed to identify recurrent low-frequency false positive variants. Variants were prioritised using the Association for Molecular Pathology, American Society of Clinical Oncology and College of American Pathologists joint consensus guidelines on variant interpretation in cancer.23 Copy number analysis from next-generation sequencing data was performed using the copy number variation (CNV) kit.24 A pooled normal reference was generated from targeted sequence analysis of 10 normal peripheral blood samples. Copy number variation (gains or losses) that was supported by deviation of all gene-specific amplicons from the baseline was prioritised and evaluated further. Sequence variants and CNVs were confirmed using DNA Sanger sequencing, FISH and IHC. Visualisation plots were created using JavaScript library jsComut (https://github.com/pearcetm/jscomut; last accessed 8/30/2017).
STATISTICAL ANALYSIS
Categorical data were presented as frequency and percentage, whereas continuous variables were described with mean. Overall survival (OS) was defined as the time from date of commencement of treatment (either surgical resection or beginning of radiation or chemotherapy) to the date of the last follow-up or death. Survival differences between groups for an individual risk factor were examined by the log-rank test. Statistical tests were performed using GraphPad Prism software version 7.03 (GraphPad Software, Inc., La Jolla, CA, USA). All tests were two-sided, and differences were considered significant at P-values ≤0.05.
Results
PATIENT CHARACTERISTICS
Clinicopathological characteristics of the 53 PSC cases are summarised in Table 1. Cases include surgically resected 52 pleomorphic carcinomas (98.2%) and one carcinosarcoma (1.8%), the latter composed of squamous cell carcinoma (SCC) and chondrosarcoma. Overall, adenocarcinoma was found in 35 (66.1%), SCC in 11 (20.7%), adenosquamous carcinoma (AdSC) in five (9.4%) and large cell neuroendocrine carcinoma (LCNEC) in two (3.7%) cases. All 52 cases of PSC had >10% of spindle cell carcinoma and giant cell carcinoma components. Tumours ranged in size from 1.0 to 10.0 cm in diameter with a median of 4.1 cm in greatest dimension.
Table 1.
Clinicopathological characteristics of the study cohort (n = 53)
| Characteristics | Number (%) |
|---|---|
| Gender | |
| Male | 32 (60.3) |
| Female | 21 (39.7) |
| Age range, median (years) | 41‒84, 67 |
| Smoking history | |
| Current or former | 51 (96.2) |
| Never smoker | 2 (3.8) |
| Angiolymphatic invasion | |
| Present | 43 (81.1) |
| Absent | 10 (18.9) |
| Visceral pleural invasion | |
| Present | 24 (45.2) |
| Absent | 29 (54.8) |
| Histology | |
| Adenocarcinoma | 35 (66.1) |
| Squamous cell carcinoma | 11 (20.7%) |
| Adenosquamous carcinoma | 5 (9.4%) |
| Large cell neuroendocrine carcinoma | 2 (3.7%) |
| Stage | |
| I | 29 (54.7%) |
| II | 9 (17.0%) |
| III | 7 (13.2%) |
| IV | 8(15.1%) |
MUTATIONS AND CNV
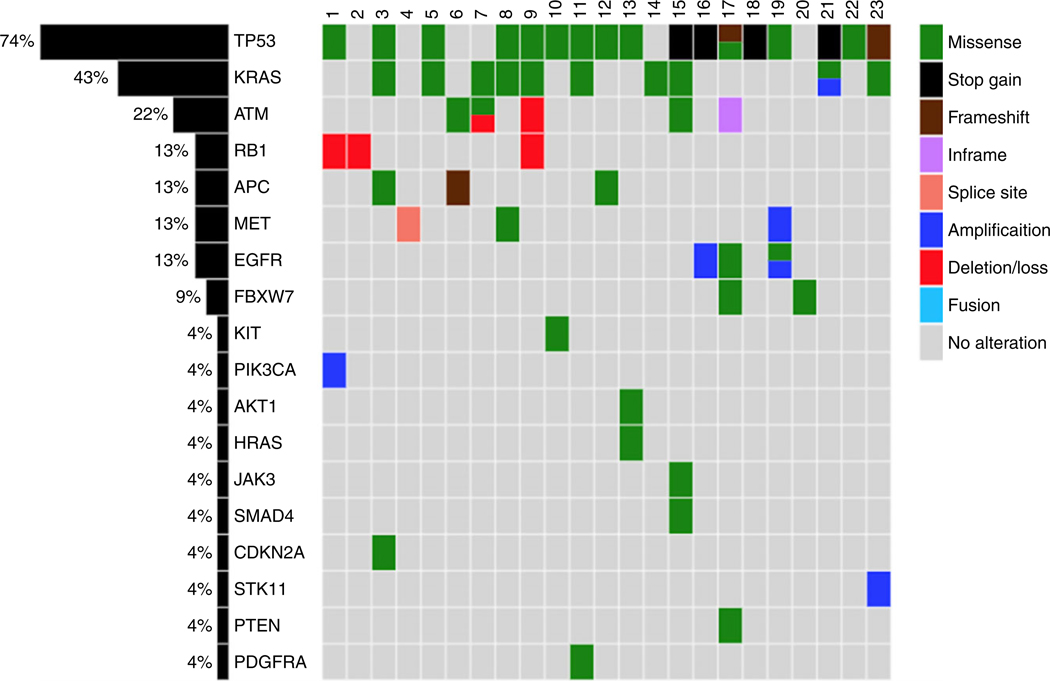
A total of 48 mutations (mean = 2.0; range = 0–6) were identified. The most commonly mutated gene was TP53 (17 of 23, 74.0%) followed by KRAS codon 12 of 13 (10 of 23, 43.4%). KRAS mutations were all found in smokers, distributed among eight (80%) PSCs with adenocarcinoma morphology, one (10%) AdSC and one (10%) SCC (Table 2). Figure 1 and Table 2 summarise the detected actionable and investigational variants by Ion AmpliSeq Cancer Panel. Only one PSC with adenocarcinoma histology had no identifiable mutation. Cases with frequent mutations (≥5) were all adenocarcinomas with a component of giant cell carcinoma.
Table 2.
Actionable and investigational genomic alterations detected by Ion AmpliSeq Cancer Panel* among 23 cases
| Tumour type | Ion AmpliSeq Cancer Panel actionable and investigational genomic alterations | |||||
|---|---|---|---|---|---|---|
| Gene | n (%) | Exon | Protein | cDNA | Mutation type | |
| Adenocarcinoma (n = 18) | KRAS | 8 (44.4%) | 2 | p.G12C (4) p.G12V (3) p.G13D (1) |
c.G34T (4) c.G35T (3) c.G38A (1) |
SNV missense |
| EGFR | 2 (11.1%) | 21 20 |
p.L858R p.G779C |
c.T2573G c.G2335T |
SNV missense | |
| FBXW7 | 2 (11.1%) | 10 7 |
p.L459V p.T267K |
c.T1375G c.C800A |
SNV missense | |
| CDKN2A | 1 (5.5%) | 2 | p.P81S | c.C241T | SNV missense | |
| APC | 3 (16.6%) | 14 | p.E1299Q p.T1538fs |
c.G3895C (2) c.4613_4614insA (1) |
SNV missense (2) Insertion (1) |
|
| ATM | 3 (16.6%) | 17 9 50 |
p.F858L p.V410A p.E2446* |
c.T2572C c.T1229C c.7335_7336TT |
SNV missense (2) Substitution (1) |
|
| MET | 2 (11.1%) | 14 | p.T992I | c.C2975T | Splice (1) Missense (1) |
|
| SMAD4 | 1 (5.5%) | 12 | p.I525V | c.A1573G | SNV missense | |
| JAK3 | 1 (5.5%) | 16 | p.V722I | c.G2164A | SNV missense | |
| PDGFRA | 1 (5.5%) | 15 | p.H687R | c.A2060G | SNV missense | |
| PTEN | 1 (5.5%) | 6 | p.R173L | c.G518T | SNV missense | |
| Squamous cell carcinoma (n = 3) | HRAS | 1 (33.3%) | 3 | p.Q61K | c.181A | SNV missense |
| AKT1 |
1 (33.3%) |
3 |
p.E17K |
c. G49A |
SNV missense |
|
| KRAS | 1 (33.3%) | 2 | p.G12D | c.G35A | SNV missense | |
| Adenosquamous carcinoma (n = 2) | KRAS | 1 (50%) | 2 | p.G12D | c.G35A | SNV missense |
| ATM |
1 (50%) |
9 |
p.V410A |
c.T1229C |
SNV missense |
|
| KIT | 1 (50%) | 18 | p.S850I | c.G2549T | SNV missense | |
Ion Torrent, Life Technologies, Thermo Fisher Scientific, Waltham, Massachusetts.
Figure 1.
coMut plot representation of individual mutations and copy number variants (−c) present in 23 cases of pulmonary sarcomatoid carcinoma. Top: cases 1–23; left: percentages of alterations in each gene.
Among the 23 cases, Ion AmpliSeq Cancer Panel detected a total of 11 CNV (Figure 1 and Table 3). Gains in PIK3CA, EGFR, KRAS and MET were also confirmed by FISH. Additionally, there were copy number losses in RB1, confirmed by immunohistochemistry (Figure 1 and Table 3). There was no co-occurrence of MET amplification and MET exon 14 skipping mutation.
Table 3.
Copy number variants detected by Ion AmpliSeq Cancer Panel* among 23 cases
| Tumour type | Ion AmpliSeq Cancer Panel copy number alterations | |||
|---|---|---|---|---|
| Gene | Gain (%) | Loss (%) | ||
| Adenocarcinoma (n = 18) | R81 | 2 (11.1) | ||
| EGFR | 2 (11.1) | - | ||
| KRAS | 1 (5.5) | - | ||
| ATM | 1 (5.5) | |||
| MET | 1 (5.5) | - | ||
| STK11 | 1 (5.5) | - | ||
| Squamous cell carcinoma (n = 3) | Rs1 | - | 1 (33.3) | |
| P/K3CA | 1 (33.3%) | - | ||
| Adenosquamous carcinoma (n = 2) | ATM | - | 1 (50.0%) | |
ion Torrent, Life Technologies, Thermo Fisher Scientific, Waltham, Massachusetts.
SURVIVAL ANALYSIS
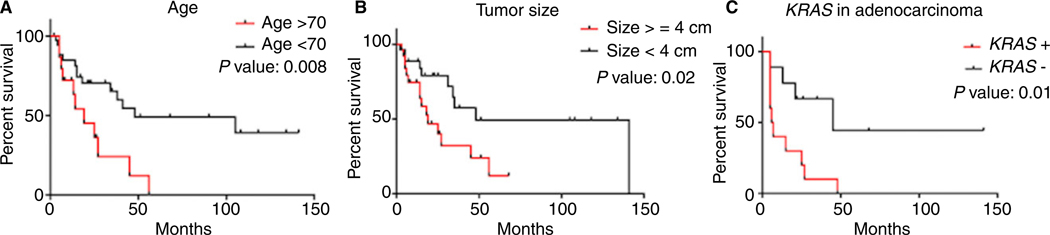
Mean follow-up was 28.8 months (range = 2–141, median = 16 months). Overall, 25 (47.1%) patients died within 2–105 months (mean = 22.7 months, median = 15 months) after diagnosis, and 28 were alive at 3–141 months (mean = 38.7 months, median = 21.5 months) after diagnosis. Five-year overall survival was 12.5% for the whole population. Kaplan–Meier survival analyses showed age greater than 70 years (P = 0.008), tumour size ≥4 cm (P = 0.02) and KRAS mutation (P = 0.01) among adenocarcinomas were associated strongly with worse overall survival (Figure 2A–C). There was no significant association between angiolymphatic invasion, visceral pleural invasion, tumour histology and stage with the outcome.
Figure 2.
Kaplan-Meier survival curves. A, Patient age >70 years; B, tumour ≥4 cm are associated significantly with worse overall survival. C, KRAS mutation in adenocarcinomas was associated significantly with poor survival.
Discussion
Pulmonary sarcomatoid carcinoma is a rare form of NSCLC characterised by high aggressiveness and mortality. The rare occurrence of PSC has restricted the characterisation of its genetic and molecular basis, thus impeding the development of targeted treatment protocols.
In this study, similar to previous reports, most patients were male, in the seventh decade of life and had a history of heavy smoking.2,25 Tumours were found commonly as large masses, with a median diameter of 4.1 cm. We demonstrated that both older age (greater than 70 years) and large tumour size (greater than 4 cm) were associated with significantly worse survival (P = 0.008 and 0.02, respectively). With a mean follow-up period of 28.8 months the overall survival was poor, and only 12.5% of patients were alive at 5 years. Unlike previous studies,2,25 we did not find a significant association between clinical stage and prognosis and this is perhaps because, for diagnostic purposes, we sought to include only surgically treated patients.
In our series, we demonstrated that PSCs harbour a broad spectrum of mutations, the most common being TP53 found in 74.0% of patients. These results are in accordance with those reported by Schrock et al.,3 who also identified TP53 mutations in 74% of their cases.3,26 TP53 mutation often co-occurred with other mutations, with the most common being KRAS. We are uncertain about the significance of co-existing alterations in our study, but they were not of prognostic significance.
KRAS codon 12/13 mutations were the second most common mutation in our series, found in 43.4% of the overall cohort and 46.6% of PSC with adenocarcinoma component. This is slightly higher than the overall frequency of 33% in lung adenocarcinoma according to The Cancer Genome Atlas data;26 however, it is in keeping with previous reports of KRAS mutations in PSC.3,5,6,27 Prognostic significance of KRAS mutations in ‘pure’ lung adenocarcinomas is controversial,28–32 with larger study cohorts indicating no apparent difference in outcome based on KRAS mutation status and subtype. In contrast, PSCs with adenocarcinoma morphology and KRAS codon 12/13 mutations in our study had a significantly worse outcome (P = 0.01) compared to KRAS wild-type. The number of cases is relatively small to make a reliable comparison based on KRAS mutation subtype. Interestingly, KRAS mutation was also identified in a single case of morphologically and immunohistochemically proven squamous cell carcinoma.
Recent studies indicate that inhibition of MET-driven oncogenic pathways has potential as a biomarker-driven targeted approach for PSC therapy.3,7,26,33–35 MET exon 14 mutations have been identified previously in up to 22% of PSC cases,3,7,36 whereas others3,4,9,27 have reported infrequent MET mutations, which may be due to differences in methodologies. In our series, MET amplification was seen in one case (5.5%) with an adenocarcinoma component and MET exon 14 skipping and missense mutations were identified in two (11.1%) cases with adenocarcinoma histology. In contrast to other studies, we did not find co-occurrence of MET amplification and mutation.3,37 However, our findings further argue for the testing for MET mutations in PSC, as they may provide therapeutic options with MET inhibitors such as crizotinib in this setting.
Similar to other studies in the western population, the EGFR-sensitising mutation p.L858R was found in only one PSC (5.5%) with an adenocarcinoma component. Our data confirm previous observations that EGFR mutations are infrequent in PSCs,3,6,27,37 limiting the clinical benefits from EGFR tyrosine kinase inhibitors in patients with PSC. Other targetable alterations, such as mutations in BRAF and HER2 or ALK and ROS1 gene rearrangements, were not identified in our cohort. Although these findings may be explained by a small number of cases, the rarity of these alterations and their associations with lack of smoking history and patient’s relatively younger age may be an alternative explanation. However, our study demonstrates that testing for genes outside the National Comprehensive Cancer Network and the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology guidelines may be potentially beneficial in this aggressive subtype of lung carcinoma.38
Additional actionable and investigational variants were detected in ATM (17.3%), FBXW7 (8.7%), AKT1 (4.3%), PDGFRA (4.3%) and HRAS (4.3%), providing oncologists with options for potential therapeutic targets. In addition to mutations found in known cancer-associated genes, we detected and validated frequent copy number losses in RB1 (three of 23, 13.0%). RB1 deletion in one case was identified as an isolated event, but in the other two cases co-occurred with mutations, particularly p53 and KRAS. While the loss of RB1 has been reported recently in PSC,3,7 its significance is uncertain.
Our study has some limitations. To increase the diagnostic accuracy we restricted our cases to only surgically treated patients, therefore decreasing the total number of cases for the study. Also, tissue blocks were not available for a large subset of cases, limiting molecular and statistical analyses. Further-more, the low number of MET exon 14 alterations in our study may be due to the limitation in coverage provided by the Ion AmpliSeq Cancer Panel. The majority of MET splicing mutations occur at the 3’ end of exon 14 in contrast to the 5’ end.35 In our study and the one by Terra et al.,5 the amplicon in the Ion AmpliSeq Cancer Panel for MET exon 14 covers only the 5’ splice site and some intronic sequence but not the 3’ splice site (Supporting information, Figure S1). Therefore, an alternative sequencing approach may be considered if the initial results are negative for MET exon 14 alterations.
In summary, this study confirms that PSCs frequently harbour mutations in TP53 and KRAS genes among many others, probably contributing to patients’ decreased survival. Furthermore, we identified several actionable and investigational genomic alterations that could potentially increase targeted therapeutic options for these patients.
Supplementary Material
Figure S1. Coverage map for the Ion AmpliSeq Cancer Panel for MET exon 14.
Acknowledgements
This project used the UPMC Hillman Cancer Center Cancer Genomics Facility, supported in part by award P30CA047904 (W.A.LaF.). The authors have no relevant financial interest in the products or companies described in this paper.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon, France: IARC, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Yendamuri S, Caty L, Pine M et al. Outcomes of sarcomatoid carcinoma of the lung: a surveillance, epidemiology, and end results database analysis. Surgery 2012; 152; 397–402. [DOI] [PubMed] [Google Scholar]
- 3.Schrock AB, Li SD, Frampton GM et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J. Thorac. Oncol. 2017; 12; 932–942. [DOI] [PubMed] [Google Scholar]
- 4.Vieira T, Antoine M, Ruppert A-M et al. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014; 85; 276–281. [DOI] [PubMed] [Google Scholar]
- 5.Terra SB, Jang JS, Bi L et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod. Pathol. 2016; 29; 824–831. [DOI] [PubMed] [Google Scholar]
- 6.Italiano A, Cortot AB, Ilie M et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy. Int. J. Cancer 2009; 125; 2479–2482. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Jia Y, Stoopler MB et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. J. Clin. Oncol. 2016; 34; 794–802. [DOI] [PubMed] [Google Scholar]
- 8.Chang Y-L, Wu C-T, Shih J-Y et al. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann. Surg. Oncol. 2011; 18; 2952–2960. [DOI] [PubMed] [Google Scholar]
- 9.Fallet V, Saffroy R, Girard N et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCartaTM Panel: exploring therapeutic targets. Ann. Oncol. 2015; 26; 1748–1753. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher CDM, Dacic STW. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer, 2015. [Google Scholar]
- 11.Zou F, Xie G, Ma J-A et al. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: a case report. Oncol. Lett. 2015; 9; 2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C, Usenko D, Frampton GM et al. MET 14 deletion in sarcomatoid non-small-cell lung cancer detected by next-generation sequencing and successfully treated with a MET inhibitor. J. Thorac. Oncol. 2015; 10; e113–e114. [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Edge SB, Greene FL et al. American Joint Committee on Cancer staging manual. 8th ed New York: Springer, 2017. [Google Scholar]
- 14.Li Z, Dacic S, Pantanowitz L et al. Correlation of cytomorphology and molecular findings in EGFR+, KRAS+, and ALK+ lung carcinomas. Am. J. Clin. Pathol. 2014; 141; 420–428. [DOI] [PubMed] [Google Scholar]
- 15.Schneider F, Derrick V, Davison JM et al. Morphological and molecular approach to synchronous non-small cell lung carcinomas: impact on staging. Mod. Pathol. 2016; 29; 735–742. [DOI] [PubMed] [Google Scholar]
- 16.Dacic S, Villaruz LC, Abberbock S et al. ALK FISH patterns and the detection of ALK fusions by next generation sequencing in lung adenocarcinoma. Oncotarget 2016; 7; 82943–82952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan A, Abecasis GR, Kang HM. Unified representation of genetic variants. Bioinformatics 2015; 31; 2202–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010; 38; e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart RK, Rico R, Hare E et al. A Python package for parsing, validating, mapping and formatting sequence variants using HGVS nomenclature. Bioinformatics 2015; 31; 268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009; 4; 1073–1081. [DOI] [PubMed] [Google Scholar]
- 21.Adzhubei IA, Schmidt S, Peshkin L et al. A method and server for predicting damaging missense mutations. Nat. Methods 2010; 7; 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2013; 14; 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li MM, Datto M, Duncavage EJ et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017; 19; 4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talevich E, Shain AH, Botton T et al. CNVkit: genome-wide copy number detection and visualization from targeted DNA Sequencing. PLoS Comput. Biol. 2016; 12; e1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ung M, Rouquette I, Filleron T et al. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin. Lung Cancer 2016; 17; 391–397. [DOI] [PubMed] [Google Scholar]
- 26.Collisson EA, Campbell JD, Brooks AN et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511; 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lococo F, Gandolfi G, Rossi G et al. Deep sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary sarcomatoid carcinoma. J. Thorac. Oncol. 2016; 11; 1282–1292. [DOI] [PubMed] [Google Scholar]
- 28.Slebos RJ, Kibbelaar RE, Dalesio O et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N. Engl. J. Med. 1990; 323; 561–565. [DOI] [PubMed] [Google Scholar]
- 29.Villaruz LC, Socinski MA, Cunningham DE et al. The prognostic and predictive value of KRAS oncogene substitutions in lung adenocarcinoma. Cancer 2013; 119; 2268–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao M-S, Aviel-Ronen S, Ding K et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J. Clin. Oncol. 2007; 25; 5240–5247. [DOI] [PubMed] [Google Scholar]
- 31.Johnson ML, Sima CS, Chaft J et al. Association of KRAS and EGFR mutations with survival in patients with advanced lung adenocarcinomas. Cancer 2013; 119; 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu HA, Sima CS, Shen R et al. Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J. Thorac. Oncol. 2015; 10; 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paik PK, Drilon A, Fan P-D et al. Response to MET inhibitors in patients with Stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015; 5; 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frampton GM, Ali SM, Rosenzweig M et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015; 5; 850–859. [DOI] [PubMed] [Google Scholar]
- 35.Awad MM, Oxnard GR, Jackman DM et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J. Clin. Oncol. 2016; 34; 721–730. [DOI] [PubMed] [Google Scholar]
- 36.Schrock AB, Frampton GM, Suh J et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J. Thorac. Oncol. 2016; 11; 1493–1502. [DOI] [PubMed] [Google Scholar]
- 37.Ushiki A, Koizumi T, Kobayashi N et al. Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn. J. Clin. Oncol. 2009; 39; 267–270. [DOI] [PubMed] [Google Scholar]
- 38.Lindeman NI, Cagle PT, Beasley MB et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2013; 137; 828–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Coverage map for the Ion AmpliSeq Cancer Panel for MET exon 14.