Abstract
Chronic obstructive pulmonary disease (COPD) affects one-tenth of the world’s population and has been identified as a major global unmet health need by the World Health Organisation, which predicts that within 10 years, COPD will become the third leading cause of death. Despite active research, there have been no recent major strides in terms of disease modifying treatment for COPD; smoking cessation remains the only intervention known to alter disease progression and improve mortality. As established COPD is a key driver of disease burden, earlier diagnosis coupled with disease-modifying intervention carries promise as a route to address this global health priority. The concept of early COPD is emerging as an area of focus for research and consideration of new treatment modalities, as it has been hypothesised that intervention at this stage may potentially halt or reverse the disease process. However, at present, a globally accepted criteria for defining early COPD does not exist. Several studies propose small airways disease as the earliest stage in the development of COPD, and this has been demonstrated to be a precursor to development of emphysema and to correlate with subsequent development of airflow obstruction. However, treatment strategies for early disease, which pre-date the development of airflow obstruction, remain uncertain. This review addresses the rationale and current evidence base for the diagnosis and treatment of early COPD and highlights the challenges of implementing trials and clinical pathways to address COPD earlier in the life course, particularly in the absence of a universally accepted definition of COPD.
The reviews of this paper are available via the supplemental material section.
Keywords: chronic obstructive pulmonary disease, COPD diagnosis, COPD management, early COPD
Take home message
Emerging data suggest small airways disease as the beginning of early COPD. However, there are no studies exploring pharmacological therapies in this group. A consensus on definition of early COPD is essential to facilitate further research in this area.
Introduction
Chronic obstructive pulmonary disease (COPD) remains a major cause of morbidity and mortality in developed countries, and is emerging as a major disease in developing economies.1 It is a heterogeneous condition arising from various contributing pathophysiological processes, including poor lung development, reduced lung growth in early life, lung damage related to exposure to cigarette smoke, air pollution, infections, and airway remodelling.2
The British Thoracic Society (BTS) defines COPD as ‘a slowly progressive disorder characterised by airflow obstruction that does not vary markedly over several months’ observation.’ It is a highly prevalent disease, affecting 10% of the global population,3 and greatly reduces quality of life and life expectancy in those afflicted.4 Analysis by the WHO (World Health Organisation) predicts that COPD will become the third leading cause of death by 2030. The burden of COPD is projected to increase in the coming decades due to ongoing exposure to risk factors such as tobacco smoke, and an ageing population.5,6
There are several variations in the reported prevalence of COPD by Global Initiative for Obstructive Lung Disease (GOLD) stage. Haughney et al.7 gathered data from 80 general practices (GPs) in the UK and found that 17.1% of patients had stage I, and 52.2% had stage II disease. Regional studies show that in spirometry-confirmed cases of COPD, 42% have mild and 44% moderate disease in England; this compares with 25% and 59% in Norway, 56% and 38% in Spain, 31% and 51% in Poland, and 56% and 38% in Japan.8
COPD is a heterogeneous disease combining different pathological entities to varying degrees in different patients.9 The natural history of emphysema, small airways disease and bronchitis vary in their onset, and the nature of their inter-relation requires further research. As one disease entity may lead to the development of another, early diagnosis and intervention may allow a targeted approach to the instigating disease process, and hence prevent progression to more complex disease states.
At present, most patients are diagnosed with COPD once the condition is established and chronic symptoms have developed as a consequence of demonstrable airflow obstruction. The majority of evidence for interventions is therefore established in patients who have had the disease for several years, with established airflow obstruction. However, as demonstrated in the SPIROMICS study,10 patients develop symptoms prior to development of airflow obstruction, and the conventionally described forced expiratory volume in 1 s (FEV1)/ forced vital capacity (FVC) < 0.7 could be insensitive to early airway disease. This group demonstrated that respiratory symptoms were reported by 50% of current and former smokers with preserved pulmonary function. In addition, symptomatic patients, even with preserved spirometry, had reduced exercise capacity and more frequent exacerbations compared with asymptomatic patients, and even patients with GOLD-defined mild COPD appear to have significant symptoms requiring pharmacological therapy.11,12 The development of airflow obstruction therefore indicates an already established disease process, and this has implications for potential for treatment. So far, no treatment has been shown to halt or reverse the progression of COPD, and it is widely accepted that there is an urgent need for COPD to be identified early enough where lung damage is potentially reversible. A number of initiatives have recently sought to identify undiagnosed COPD and establish diagnosis earlier in the natural history of the condition, and the value of these will ultimately be determined by the impact of interventions in early disease.13
As such, there has been recent renewed interest in the concept of early COPD. It is important to acknowledge that early COPD is not synonymous with mild COPD, as it is possible to have had the disease for several years, albeit at a mild stage. Early COPD relates to a time-point in the natural history of disease,14 and it is unfortunate that several therapeutic trials have used ‘early’ and ‘mild’ COPD interchangeably in their data analyses. At present, little is known about when the earliest changes of COPD begin in susceptible individuals. Several authors suggest that these changes may begin as early as in utero,15,16 progressing during childhood, for example with recurrent infections, exposure to passive smoke, etc., and go on into adolescence, with further active and passive exposure to cigarette smoke, resulting in a reduction in the peak attained lung function, which subsequently increases risk of being diagnosed with COPD in later life. A globally agreed definition of early COPD is currently lacking and poses challenges to studies that attempt to address this important concept.
There are several reasons for a current lack of global consensus on the definition of early COPD. This is due, as mentioned previously, partly to complexities relating to the definition of COPD itself in terms of disease onset, as well as to the heterogeneity of the condition. Spirometry in isolation, especially with a fixed cut-off value, is in accurate in marking the beginning of COPD. Both the FEV1 and FVC reduce with age, but FEV1 declines faster, and therefore the fixed diagnostic cut-off ratio of 0.7, which defines current diagnosis, leads to under-diagnosis of COPD in younger individuals and over-diagnosis in older individuals. In addition, the marked heterogeneity of COPD means that various phenotypes of COPD might have varying natural histories, further complicating a definition describing the onset of disease.17–20
Martinez et al. suggest that early COPD should be defined by detecting the initial events responsible for ultimate development of pathology (Table 1).21 Their group propose defining early COPD as subjects aged under 50 years with a smoking history of at least 10 pack years, with any of FEV1/FVC ratio less than the lower limit of normal, compatible abnormalities on computed tomography (CT), or accelerated FEV1 decline of at least 60 ml/year. A key issue is that this criterion imposes an age limit and therefore has the potential to exclude older patients with early disease.
Table 1.
| Martinez et al.15 | Siafakas et al.16 |
| Age >50 years | FEV1/FVC > 0.7 |
| Smoking history of at least 10 pack years | FEV1 > 80% predicted |
| Any one of: - FEV1/FVC less than LLN - Compatible CT abnormalities - Accelerated FEV1 decline of at least 60 ml/year |
Presence of compatible respiratory symptoms |
COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LLN, lower limit of normal.
Siafakas et al. proposed a different approach, and suggested that the previously described GOLD stage 0 should be used to define early COPD.22 They suggest that this is the pre-clinical stage and therefore is consistent with early disease. However, one should note that GOLD 0 is diagnosed on the basis of symptoms in absence of obstructive spirometry, and therefore this stage is not truly pre-clinical.
Several groups have proposed small airways disease as the earliest stage of COPD, even prior to development of emphysema.23–25 Galban et al., using parametric response mapping (PRM) techniques, demonstrated that functional small airways disease (fSAD) precedes the development of emphysema.26 Hence novel approaches to explore origins of disease are emerging, but methods to diagnose small airways disease are not at present standardised or currently available in routine clinical practice.
Modifiable risk factors
COPD is the result of complex interplay of long-term exposure to inhaled noxious gases or particles, combined with a variety of host factors including genetics, early lung development, and maximal lung growth attainment.
The commonest risk factor for COPD worldwide is tobacco smoking. Analysis of subjects involved in the National Survey of Health and Development (NHSD) showed that cigarette smoking during adolescence and early adulthood modifies how early-life exposures such as childhood respiratory illnesses, social class, overcrowding and pollution levels impact on midlife FEV1.27
Results of the Lung Health Study demonstrated that in men with FEV1/FVC less than 0.7 and FEV1 55–90% predicted, each year of self-reported occupational fume exposure was associated with a 0.25% reduction in FEV1 as a percentage of predicted value.28 This compared with 1.2–1.9% reduction with continued smoking.
At present, risk factor modification often occurs in the context of an established diagnosis, which relies on the presence of airflow obstruction. Identification of and intervention at early COPD, prior to development of established disease, would allow for potential reversal of these early changes, through patient-centred risk reduction strategies.
Diagnostic criteria of COPD
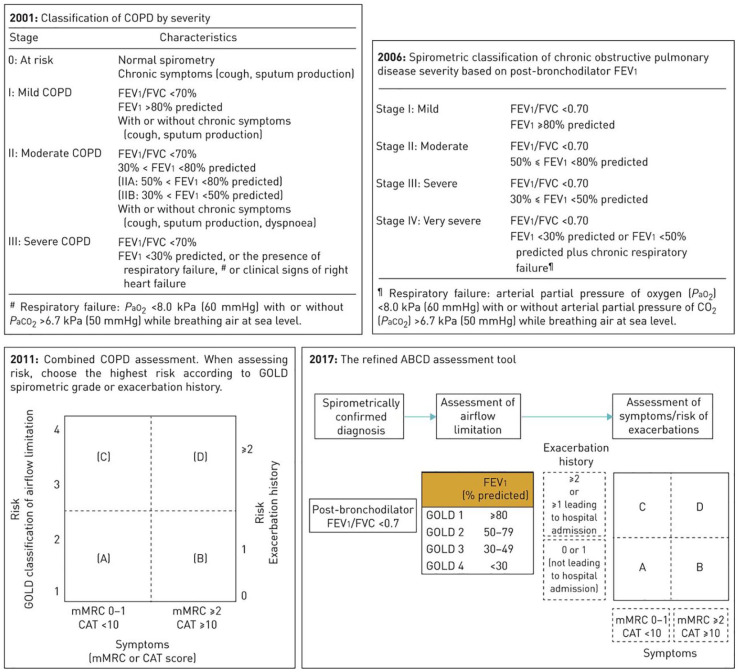
The GOLD was established in 1998 to improve the diagnosis, management and prevention of COPD, and was most recently revised in 2019. The diagnosis of COPD requires a post-bronchodilator FEV1 to FVC ratio of less than 0.7 and is further classified into stages of severity according to FEV1 percentage predicted (Figure 1).29
Figure 1.
Revised GOLD classification of COPD based on spirometry, symptom score, and exacerbation frequency. Reproduced with permission from Vogelmeier et al.29
CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease; mMRC, modified Medical Research Council.
Figure 2.
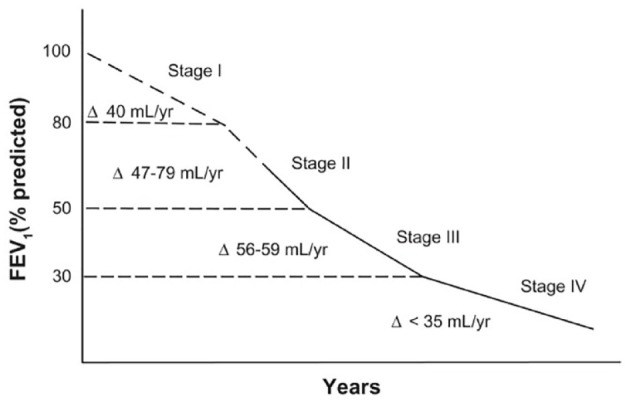
FEV1 decline according to COPD stage. Reproduced with permission from Tantucci et al.30
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.
GOLD classification of COPD on post-bronchodilator FEV1 (% predicted):
I Mild ⩾80%
II Moderate 50–79%
III Severe 30–49%
IV Very severe <30%
The revised GOLD scheme also incorporates assessment of dyspnoea using the modified Medical Research Council scale or symptoms using the COPD Assessment Test, as well as frequency and severity of exacerbations.
Disease trajectory and prognosis in COPD
Tantucci et al. demonstrated that the rate of FEV1 decline is more rapid in earlier stages of COPD than in late stages, in particular, stage II rather than stages III and IV, although the authors highlight a lack of information on stage I COPD.30
A potential explanation for this is that higher lung function provides greater potential for functional decline, whereas in more advanced COPD, lung function is already compromised and potential for further loss is reduced. This means that the potential for halting or reversing progression of disease is best when diagnosis is made at the earliest stage of disease onset.
Several studies report that mortality is affected even in the earlier and milder forms of COPD. Bridevaux et al. studied a cohort of patients with COPD according to GOLD-defined categories and monitored their FEV1 decline for 11 years.31 A total of 610 participants met the modified GOLD criteria for COPD; of these 519 (85.1%) had GOLD stage I COPD. The investigators reported that symptomatic patients with GOLD stage I COPD had a faster rate of FEV1 decline than asymptomatic patients with normal lung function.
The Atherosclerosis Risk in Communities (ARIC) Study assessed mortality in COPD patients according to GOLD classification.32 Subjects aged 43–66 years at baseline were followed for up to 11 years; 2244 subjects were in GOLD stage 0, 1679 in stage 1, and 1484 in stage 2. The death rate according to GOLD category in per 1000 person years was 10.1 for stage 0, which is early COPD as defined by Siafakas et al.,22 9.1 for stage I, 18.1 for stage II, and 42.9 for stage III or IV. This is in comparison with asymptomatic individuals with normal lung function, where the death rate was 5.4 per 1000 person years.
The previously mentioned studies lend credence to evidence that even early stages of the disease affect mortality, stressing the need for earlier detection and intervention, ideally prior to development of clinically evident disease.
Potential diagnostic modalities for early disease
Pulmonary function tests
Spirometry has traditionally been the most often utilised diagnostic test for COPD, through assessment of impairment of FEV1 and airflow obstruction. However, it has clear limitations in the diagnosis of early disease, as obstruction on spirometry indicates an already established disease process. In the COPD Gene cohort,33 smokers with normal spirometry had a significantly worse 6-min walk distance and 42% of these patients had emphysema or airway thickening on CT thorax, lending credence to the fact that use of spirometry alone is not sufficient for detection of early COPD (Table 2).
Table 2.
Investigative modalities with potential for diagnosis of early COPD.
| Spirometry | Useful in conjunction with other investigations but has potential to miss early disease |
| FEF 25–75% | Identifies small airway disease, which has been explored as site of initial development of pathology in COPD |
| DLCO | Identifies airway disease and emphysema prior to identification of disease on spirometry |
| Oscillometry | Has potential to identify small airway disease; however universally accepted standardisation of cut-off values required |
| HRCT | Identifies characteristic changes on lung parenchyma such as emphysema, air trapping and bronchial wall thickening in the absence of spirometry-defined airflow obstruction |
| PRM | Has potential to identify fSAD, which appears to precede development of emphysema |
COPD, chronic obstructive pulmonary disease; DLCO, diffusion capacity for carbon monoxide; FEF, forced expiratory flow at 25–75% of forced vital capacity; fSAD, functional small airway disease; HRCT, high resolution computed tomography; PRM, parametric response mapping.
Other parameters such as forced expiratory flow at 25–75% of FVC (FEF 25–75%), total lung volume, diffusion capacity for carbon monoxide (DLCO) and imaging have important roles in identifying potentially early disease. Harvey et al. monitored a cohort of smokers with normal spirometry for 4 years, and found that among those with a low DLCO (<80% predicted), 10 out of 46 patients (22%) developed obstruction on spirometry (FEV1/FVC < 0.7), whereas among those with normal DLCO, only 2 out of 59 (3%) developed obstruction, suggesting that DLCO has the ability to diagnose COPD prior to development of airflow obstruction.34
Forced oscillometry techniques such as impulse oscillometry (IOS) have recently gained attention as a potential modality for recognising early disease. As mentioned previously, small airways have been proposed as the site of earliest changes in COPD, and oscillometry techniques allow assessment of these changes. Resistance at 20 Hz (R20) represents proximal resistance, whereas the resistance at 5 Hz (R5) represents total airway resistance. R5-20 can therefore be employed as a useful measure of small airways resistance.
Frantz et al. studied subjects with and without symptoms of COPD against GOLD criteria and reported abnormal IOS findings in subjects with symptoms but normal spirometry.35 The authors suggest that using impulse oscillometry is a potentially useful tool to diagnose early COPD prior to development of spirometric impairment.
Oscillometry techniques are not currently available in routine clinical practice, and globally standard references values for diagnosis of COPD have not been defined. Lipworth et al. suggest IOS cut-offs for R5 > 0.5kPa/L/s and R5-20 > 0.10 kPa/L/s as being pathologically abnormal.36 Standardisation of reference values for IOS will bring us a step closer to utisiling this technique in identifying early disease.
Imaging in early COPD
CT, in particular high-resolution CT, is the current imaging modality of choice in the diagnosis of COPD.37–39 Three features, namely, emphysema, air trapping and bronchial wall thickening, can be assessed in the identification of COPD by cross-sectional imaging.40 Emphysema is the most commonly identified abnormality on imaging patients with COPD, and it has been demonstrated that it is possible to have emphysema in the absence of spirometry-defined airflow obstruction.41,42 However, as with spirometry-defined airflow obstruction, emphysema usually indicates an already established disease process, and not necessarily the beginning of pathology in COPD.
Comparison of inspiratory and expiratory scans can identify air trapping, and this has been utilised as a surrogate marker of small airways disease present in early disease through the technique of PRM. Through their work on this technique, Galban and colleagues analysed data from 194 subjects and studied the relationship between fSAD and emphysema.26 The investigators found that many of the subjects had less than 10% emphysema, yet some had fSAD 10–30% of their lung parenchyma, suggesting that fSAD precedes the development of emphysema.
PRM was subsequently tested in the COPDGene cohort,43 which included subjects with normal spirometry but features of chronic bronchitis. The results showed that in this group of subjects, every additional 5% of lung affected by fSAD was associated with an additional FEV1 decline of 2.2 ml/year. This suggests that PRM has potential in early identification of subjects with risk of developing spirometry-defined airflow obstruction.
Potential interventions in early COPD
Smoking cessation
Smoking cessation remains the only intervention definitively known to halt the progression of COPD, and several studies have demonstrated that early smoking cessation has the potential to slow down or even reverse accelerated decline in lung function, highlighting the importance of intervention in early disease.
The Lung Health Study randomized current smokers, on a 2:1 basis, to either an intensive smoking cessation programme (special intervention/SI), or to usual care (UC).44 The SI group was further randomised on a 1:1 basis to receive either inhaled ipratropium bromide (SIA) or placebo (SIP).
Study participants in the SI and UC groups who stopped smoking in year 1 had an average increase in FEV1 of 47 ml. In contrast, continued smokers showed a decline in FEV1 by 49 ml in year 1. Over a 5-year period, continued smokers had a faster rate of FEV1 decline compared with sustained quitters. From year 1 to year 5, sustained quitters had a rate of decline of FEV1 of 31 +/– 48ml/year. In contrast, in continued smokers, FEV1 declined by 62 +/– 55ml/year from year 1 to year 5.
These results also translate into longer-term findings. The Lung Health Study participants were followed up 11 years after initial enrolment.45 This extension study showed that men who stopped smoking at the beginning of the LHS had an FEV1 decline of 30.2 ml/year, whereas women who stopped smoking at the beginning had a rate of decline of 21.5 ml/year. Men who continued to smoke declined at 66.1 ml/year, whereas women who continued to smoke declined at 54.2 ml/year, demonstrating the potential value of early cessation.
Inhaled bronchodilator and steroid therapy
Most literature on treatment for COPD has focused on established disease, and there is a clear deficiency in literature on studies involving early COPD. As mentioned previously, almost all studies reporting intervention on ‘early COPD’ included subjects with mild to moderate COPD in their analyses, without clear distinction between early and mild/moderate COPD (Table 3).
Table 3.
Summary of studies exploring inhaled bronchodilator therapy in early COPD.
| Research trial | Investigational product | Participant characteristics | Study outcome |
|---|---|---|---|
| Lung Health Study | Ipratropium bromide | Mean age 48.4 FEV1 75.1% predicted |
FEV1 increase of 38.8 ml in first year, maintained for 4 years but no further increase in time. Reversal of benefit with cessation of treatment |
| UPLIFT (subgroup) | Tiotropium | Age <50 years At least 10 pack years FEV1/FVC < 0.7 |
FEV1 decline reduced by 20 ml/year (in contrast to primary outcome) |
| Pauwels et al.48 | Budesonide | Age 30–65 Mean FEV1 77% predicted Current smoker |
Small one-time improvement in FEV1 (17 ml/year) with no effect on long-term decline |
| DIMCA | Fluticasone propionate | Normal spirometry Accelerated FEV1 decline of at least 80 ml/year |
No significant effect on FEV1 decline |
COPD, chronic obstructive pulmonary disease; DIMCA, detection, intervention, and monitoring program of COPD and asthma; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; UPLIFT, understanding potential long-term impact on function with tiotropium.
The aforementioned Lung Health Study results showed a decline in FEV1 during the first year of follow up of 34.3 ml in the UC group, an increase of 11.2 ml in the SIP group, and an increase of 38.8 ml in the SIA group.46 This increase in FEV1 in the SIA group at the end of the first year was maintained for the subsequent 4 years but did not increase further with time. Furthermore, when bronchodilator was discontinued towards the end of the study, the improvement in lung function associated with bronchodilator use was reversed, suggesting no long-term benefit of therapy in terms of preventing disease progression. Entry criteria for this study included age range 35–60 years, with mean age in the SIA group of 48.4 years. The mean FEV1 in the treatment group was 75.5% predicted with a mean FEV1/FVC ratio of 62.9% predicted. It is therefore important to note that while this group would have included patients with early COPD as defined by Martinez et al., this would not have been exclusive.21
The Understanding Potential Long-Term Impact on Function with Tiotropium (UPLIFT) Trial47 reported on a pre-specified post hoc analysis of the effect of Tiotropium in patients less than 50 years of age with COPD. This group would also fit the definition of early COPD as proposed by Martinez et al.21 The subgroup consisted of 356 participants under 50 years old, with at least 10 pack-years smoking history, and FEV1/FVC < 0.7. The mean decline in post-bronchodilator FEV1 was 38 ml/year in the treatment group and 58 ml/year in the placebo group (p = 0.01). This is in contrast to the findings in the total UPLIFT population, where no difference in post-bronchodilator FEV1 was detected between the treatment and placebo groups. It should be highlighted that this was a subgroup analysis, and therefore the study was not powered for these secondary analyses.
Pauwels et al. assessed the role of inhaled Budesonide in current smokers with COPD.48 Their group identified subjects 30–65 years old (mean age 52, mean FEV1 77% predicted). This would have included some subjects with early COPD as defined by Martinez et al.21 The authors showed that although inhaled Budesonide was associated with a small one-time improvement in FEV1 (17 ml per year improvement in the Budesonide group and 81 ml per year decline in the placebo group in the first 6 months), it did not affect the long-term FEV1 decline in patients with COPD.
The Detection, Intervention, and Monitoring program of COPD and Asthma (DIMCA) study attempted to identify patients with early disease by recruiting patients with normal spirometry and no prior diagnosis of COPD, but with accelerated decline in lung function.49 Through GP surgeries, the investigators identified adults with an annual FEV1 decline of at least 80 ml per year (mean FEV1% predicted 88.3 in treatment group, 91.2 in placebo group), and randomised participants to receive either Fluticasone propionate 250 mcg twice daily or placebo. The trial was continued for 24 months, followed by a 7-month Fluticasone propionate extension. The authors found no statistically significant difference in the decline in FEV1 between the two treatment arms (primary outcome).
The magnitude of clinical benefit of inhaled therapy in early COPD is limited. Although there is some evidence that bronchodilator therapy offers symptomatic benefit and reduction in exacerbation rates, long-term outcomes such as lung function decline and mortality appear not to be affected on the limited evidence available. In addition, there is no evidence that currently established bronchodilator therapy confers benefit in patients with early COPD as defined previously,21,22 although data is severely limited and these inferences are based on secondary outcomes.
Phosphodiesterase-4 inhibitors (roflumilast)
Roflumilast is a potent and selective inhibitor of the enzyme phosphodiesterase-4 (PDE-4), and targets systemic inflammation associated with COPD. Several clinical trials have reported benefit of Roflumilast over placebo in patients with COPD in terms of FEV1 and exacerbations. It appears to confer benefit in patients with severe and very severe COPD with chronic bronchitis and frequent exacerbations.50,51 Currently, there is no evidence for its use in patients with early disease.
Macrolides and NAC
The main use of anti-inflammatory therapies such as macrolides and N-acetylcysteine (NAC) has been in the reduction of frequency and duration of exacerbations, which are associated with accelerated decline in lung function.52,53
Macrolides have been demonstrated to reduce the exacerbation frequency in patients with COPD. It has been suggested that its anti-inflammatory activity is not related to the anti-bacterial activity, as the anti-inflammatory effect is seen at concentrations lower than the minimal inhibitory concentration for airway bacteria.54 Unfortunately, studies of this group of therapy in patients with early COPD are not currently available.
The 12-month HIACE study,55 which investigated the effects of high-dose NAC (600 mg twice daily) in Chinese patients with COPD, reported a significant improvement in FEF 25–75% and forced oscillometry parameters with NAC compared with placebo. The primary endpoint for this study was the effect on small airways disease, which is now widely accepted as the site of early disease. A meta-analysis of the available evidence for NAC showed that NAC is effective at reducing exacerbations, but the benefit was greater in subjects with chronic bronchitis without a spirometric diagnosis of COPD.56 As chronic bronchitis often precedes development of spirometric diagnosis of COPD, it is plausible that this may provide another therapeutic avenue in patients with early COPD.
Vaccination
Influenza, respiratory syncytial virus and other viral infections are known to be contributing factors for COPD exacerbations and hospital admissions.57 Similarly, pneumococcal colonisation, which occurs more commonly in patients with COPD compared with healthy controls, is known to increase the frequency of exacerbations. Although there are no current licensed vaccines against RSV,58 studies evaluating the effectiveness of influenza and pneumococcal vaccinations have reported a reduction in the number of exacerbations, hospital admissions, incidence of pneumonia, and risk of death, and is useful in elderly subjects even with preserved lung function.59–61 Similarly, a recent study reported the safety of non-typeable Haemophilus influenzae vaccination, demonstrating good immunogenicity in patients with moderate/severe COPD.62 However, there are no studies reporting specifically on the benefit of vaccination in patients with early COPD.
Pulmonary rehabilitation
Physical inactivity is an important determinant of health-related quality of life (HRQoL) in patients with COPD and is a predictor of hospitalisation and mortality.63 Physical deconditioning that follows leads to even more exertional dyspnoea and further deconditioning. Studies have demonstrated reduced skeletal muscle strength even in patients with mild airflow obstruction as well as in smokers without airflow obstruction, suggesting that physical deconditioning is present in early disease.64,65
Traditionally, pulmonary rehabilitation was prescribed for patients with severe disease. However, recent data, including a systematic review of the available data for pulmonary rehabilitation in patients with mild COPD,64 showed evidence of improved exercise capacity and HRQoL , and improvement in 6-min walk test, suggesting their potential benefit even in early disease.
There is currently not enough capacity to deliver conventional pulmonary rehabilitation for large numbers of patients with early disease, and new modes of increasing exercise and fitness levels such as digital interventions will need to be tested in the context of these patient groups.66
Nutrition
There has been much debate on the relationship between obesity and long-term outcomes in COPD.67
Several studies indicate that obesity has a protective effect against mortality in patients with COPD. A meta-analysis of 22 studies involving 22,150 participants concluded that underweight individuals with COPD had a higher mortality while both overweight and obese individuals had a lower mortality.68,69
Data from the Copenhagen City Heart Study showed that there was a U-shaped relationship between patients with mild to moderate COPD and mortality, with the lowest mortality risk in the normal weight and overweight subjects.70 In contrast, in severe COPD, mortality continued to decrease with increasing body mass index (BMI).
This suggests that obesity may exert different effects depending on disease severity. Unfortunately, the pathophysiological basis for this apparent obesity paradox is unknown. Loss of skeletal muscle mass is the main cause of weight loss in patients with COPD, which could then translate into diaphragmatic weakness. It is also possible that obesity itself contributes to a reduction in FEV1 and therefore these patients are classified into a class of COPD that is higher than their true pulmonary impairment, or that the decline in BMI in these patients is a marker of advanced disease and therefore higher mortality.67,68
The role of nutrition in patients with early COPD is a potential area of research, especially in terms of disease progression including risk of exacerbations, exercise tolerance, as well as mortality.
Novel diagnostic approaches and future research areas: role of biomarkers in early detection of COPD
Apart from alpha-1 antitrypsin, deficiency of which is the only known hereditary cause of COPD, no biomarker is currently in routine clinical use to identify at-risk individuals, and therefore subjects with potential early disease. However, there are several biomarkers under investigation that have been implicated in the pathogenesis of COPD, that have the potential to help detect early disease. Most of these biomarkers are involved in inflammatory pathways and oxidative stress that are known to be major pathogenic factors in the development of COPD.
For example, there are established associations between elevated C-reactive protein and fibrinogen levels, and risk of COPD. It has been postulated that regulating factors for these markers, that is, interleukin-1 (IL-1), interleukin-6 (IL-6) and tumour necrosis factor alpha (TNF-alpha) are involved in pathogenesis, rather than these markers themselves. IL-1 and TNF-alpha initiates and maintains inflammation and activates endothelial and epithelial cells, whereas IL-6 has a major role in vascular permeability and cell proliferation. The enzyme epoxide hydrolase-1 (EPHX-1) and superoxide dismutase 3 may inhibit increased oxidative stress in the lungs from cigarette smoke inhalation and are therefore potential markers of interest in COPD research.71
Matrix metalloproteinases (MMPs) are currently under active research as potential biomarkers in the pathogenesis of COPD. These include MMP-8, MMP-9, MMP-12, and TIMP (the main endogenous inhibitor of MMP-8 and MMP-9). In particular, there is evidence that in patients with normal lung function, sputum MMP-8 levels are higher in symptomatic smokers compared with asymptomatic smokers and non-smokers,38,72 and perhaps represents a potential biomarker for diagnosis of early COPD.
Increased levels of cathepsin S and cystatin C have also been reported in symptomatic smokers compared with asymptomatic smokers and non-smokers, and represent another potential area of research to identify patients with early disease.73
The role of imaging in early diagnosis of COPD is currently under active evaluation. Investigators from the COPDGene Study showed that PRM,26 which compares quantification of emphysema on inspiratory scans to that of gas trapping on expiratory scans, has the potential to identify functional small airways disease, which correlates to subsequent FEV1 decline in patients particularly with milder stages of COPD. This association was also evident in GOLD 0 subjects, suggesting that this technique has potential for early detection of disease.
The implementation of biomarkers for diagnosis of early COPD in clinical practice still requires much research and validation, but the studies mentioned previously are promising. It is crucial to identify reliable biomarkers for early disease, a stage at which novel therapies might have the most impact on modifying disease course and preserving lung function.
The concept of early COPD is increasingly being recognised and trials are now underway to study cohorts of young smokers who are potentially at risk of developing COPD. These include the British Lung Foundation (BLF) Early COPD Development Study in the UK [ClinicalTrials.gov identifier: NCT03480347] and the study of Determinants of Onset and Progression of COPD in Young Adults (Early COPD) in Spain [ClinicalTrials.gov identifier: NCT02352220]. The UK BLF Early COPD Development Study is designed to monitor young smokers (30–45 years of age) with at least 10 pack-year smoking history for an initial duration of 3 years, with information collected on serum and sputum biomarkers as well as data on pulmonary function testing and thoracic imaging. One of the ultimate aims of this study will be to identify markers of early disease, which will be a vital step in development of treatment modalities aimed at early COPD.
Conclusion
Early COPD, although not a completely novel concept, remains poorly explored. The diagnosis of COPD as defined by currently established criteria indicate an established disease process which is irreversible. As no current pharmacological treatment is known to halt or reverse the progression of established COPD, it is essential for disease to be diagnosed early and prior to establishment of irreversible pathology, in order to allow timely interventions. Identification of pathological factors involved in the development of early disease will facilitate development of therapies targeting these early changes, and therefore potentially arrest or even reverse the disease process.
Unfortunately, currently available pharmacological therapy for COPD does not appear to impact on early disease as defined by authors.21,22 A universally accepted definition of early COPD is essential in the quest to identify the pathology of early COPD and pave way for design of novel treatments that have the ability to make an impact at this early and potentially reversible stage.
Supplemental Material
Supplemental material, Author_Response for Early COPD: current evidence for diagnosis and management by Aishath Fazleen and Tom Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Early COPD: current evidence for diagnosis and management by Aishath Fazleen and Tom Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Early COPD: current evidence for diagnosis and management by Aishath Fazleen and Tom Wilkinson in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Aishath Fazleen: Conceptualization; Investigation; Project administration; Writing-original draft; Writing-review & editing.
Tom Wilkinson: Conceptualization; Investigation; Supervision; Writing-review & editing.
Conflict of interest statement: Aishath Fazleen has nothing to disclose. Tom Wilkinson reports personal fees from MyMHealth, grants from Innovate UK, grants from GSK, grants and personal fees from AstraZeneca, grants and personal fees from Synairgen, personal fees from BI, outside the submitted work.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aishath Fazleen  https://orcid.org/0000-0002-1286-9374
https://orcid.org/0000-0002-1286-9374
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Aishath Fazleen, University Hospital Southampton NHS Foundation Trust, Tremona Road, Southampton, Hampshire SO16 6YD, UK; Faculty of Medicine, University of Southampton, Hampshire, UK.
Tom Wilkinson, University Hospital Southampton NHS Foundation Trust, Tremona Road, Southampton, Hampshire, UK; Faculty of Medicine, University of Southampton, Hampshire, UK.
References
- 1. Wedzicha JA, Wilkinson T. Impact of chronic obstructive pulmonary disease exacerbations on patients and payers. Proc Am Thorac Soc 2006; 3: 218–221. [DOI] [PubMed] [Google Scholar]
- 2. Mannino DM, Make BJ. Is it time to move beyond the “O” in early COPD? Eur Respir J 2015; 46: 1535–1537. [DOI] [PubMed] [Google Scholar]
- 3. Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax 2010; 65: 837–841. [DOI] [PubMed] [Google Scholar]
- 4. Tavares N, Jarrett N, Hunt K, et al. Palliative and end-of-life care conversations in COPD: a systematic literature review. ERJ Open Res 2017; 3: 00068-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract 2015; 69: 336–349. [DOI] [PubMed] [Google Scholar]
- 6. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet 2007; 370: 741–750. [DOI] [PubMed] [Google Scholar]
- 7. Haughney J, Gruffydd-Jones K, Roberts J, et al. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J 2014; 43: 993–1002. [DOI] [PubMed] [Google Scholar]
- 8. Barrecheguren M, Gonzalez C, Miravitlles M. What have we learned from observational studies and clinical trials of mild to moderate COPD? Respir Res 2018; 19: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agusti A, Celli B. Natural history of COPD: gaps and opportunities. ERJ Open Res 2017; 3: 00117-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016; 374: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh D, D’Urzo AD, Donohue JF, et al. Weighing the evidence for pharmacological treatment interventions in mild COPD; a narrative perspective. Respir Res 2019; 20: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rossi A, Butorac-Petanjek B, Chilosi M, et al. Chronic obstructive pulmonary disease with mild airflow limitation: current knowledge and proposal for future research - a consensus document from six scientific societies. Int J Chron Obstruct Pulmon Dis 2017; 12: 2593–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ray E, Culliford D, Kruk H, et al. Specialist Respiratory outreach: a case-finding initiative for identifying undiagnosed COPD in primary care. Submitted to NPJ Prim Care Respir Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agusti A, Celli B. Avoiding confusion in COPD: from risk factors to phenotypes to measures of disease characterisation. Eur Respir J 2011; 38: 749–751. [DOI] [PubMed] [Google Scholar]
- 15. Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet 2015; 385: 1778–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soriano JB, Polverino F, Cosio BG. What is early COPD and why is it important? Eur Respir J 2018; 52: 1801448. [DOI] [PubMed] [Google Scholar]
- 17. Sapey E, Bafadhel M, Bolton CE, et al. Building toolkits for COPD exacerbations: lessons from the past and present. Thorax 2019; 74: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams NP, Coombs NA, Johnson MJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis 2017; 12: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim VL, Coombs NA, Staples KJ, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J 2017; 50: 1700853. [DOI] [PubMed] [Google Scholar]
- 20. Hurst JR, Wilkinson TMA, Perera WR, et al. Relationships among bacteria, upper airway, lower airway, and systemic inflammation in COPD. Chest 2005; 127: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 21. Martinez FJ, Han MK, Allinson JP, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siafakas N, Bizymi N, Mathioudakis A, et al. EARLY versus MILD chronic obstructive pulmonary disease (COPD). Respir Med 2018; 140: 127–131. [DOI] [PubMed] [Google Scholar]
- 23. McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365: 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hogg JC, Pare PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev 2017; 97: 529–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh D. Small airway disease in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis (Seoul) 2017; 80: 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galban CJ, Han MK, Boes JL, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012; 18: 1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allinson JP, Hardy R, Donaldson GC, et al. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med 2017; 196: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harber P, Tashkin DP, Simmons M, et al. Effect of occupational exposures on decline of lung function in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176: 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J 2017; 49: 1700214. [DOI] [PubMed] [Google Scholar]
- 30. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 2012; 7: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bridevaux PO, Gerbase MW, Probst-Hensch NM, et al. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008; 63: 768–774. [DOI] [PubMed] [Google Scholar]
- 32. Mannino DM, Doherty DE, Sonia Buist A. Global initiative on obstructive lung disease (GOLD) classification of lung disease and mortality: findings from the atherosclerosis risk in communities (ARIC) study. Respir Med 2006; 100: 115–122. [DOI] [PubMed] [Google Scholar]
- 33. Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med 2015; 175: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harvey BG, Strulovici-Barel Y, Kaner RJ, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J 2015; 46: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frantz S, Nihlen U, Dencker M, et al. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med 2012; 106: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 36. Lipworth BJ, Jabbal S. What can we learn about COPD from impulse oscillometry? Respir Med 2018; 139: 106–109. [DOI] [PubMed] [Google Scholar]
- 37. Ostridge K, Wilkinson TM. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur Respir J 2016; 48: 216–228. [DOI] [PubMed] [Google Scholar]
- 38. Ostridge K, Williams N, Kim V, et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax 2016; 71: 126–132. [DOI] [PubMed] [Google Scholar]
- 39. Ostridge K, Williams N, Kim V, et al. Distinct emphysema subtypes defined by quantitative CT analysis are associated with specific pulmonary matrix metalloproteinases. Respir Res 2016; 17: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Csikesz NG, Gartman EJ. New developments in the assessment of COPD: early diagnosis is key. Int J Chron Obstruct Pulmon Dis 2014; 9: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ostridge K, Gove K, Paas KHW, et al. Using novel computed tomography analysis to describe the contribution and distribution of emphysema and small airways disease in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2018; 16: 990–997. [DOI] [PubMed] [Google Scholar]
- 42. Gove K, Wilkinson T, Jack S, et al. Systematic review of evidence for relationships between physiological and CT indices of small airways and clinical outcomes in COPD. Respir Med 2018; 139: 117–125. [DOI] [PubMed] [Google Scholar]
- 43. Bhatt SP, Soler X, Wang X, et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2016; 194: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scanlon PCJ, Waller L. Smoking cessation and lung function in mild to moderate COPD: the lung health study. Am J Respir Crit Care Med 2000; 161: 381–390. [DOI] [PubMed] [Google Scholar]
- 45. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med 2002; 166: 675–679. [DOI] [PubMed] [Google Scholar]
- 46. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking cessation and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the lung health study. JAMA Intern Med 1994; 272: 1497–1505. [PubMed] [Google Scholar]
- 47. Morice AH, Celli B, Kesten S, et al. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir Med 2010; 104: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 48. Pauwels RA, Löfdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med 1999; 340: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 49. van Grunsven P, Schermer T, Akkermans R, et al. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir Med 2003; 97: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 50. Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis 2016; 11:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lipari M, Benipal H, Kale-Pradhan P. Roflumilast in the management of chronic obstructive pulmonary disease. Am J Health Syst Pharm 2013; 70: 2087–2095. [DOI] [PubMed] [Google Scholar]
- 52. Wilkinson T, Wedzicha JA. Strategies for Improving Outcomes of COPD Exacerbations. Int J Chron Obstruct Pulmon Dis 2006: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilkinson TM, Donaldson GC, Hurst JR, et al. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169: 1298–1303. [DOI] [PubMed] [Google Scholar]
- 54. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008; 178: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 55. Tse HN, Raiteri L, Wong KY, et al. High-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest 2013; 144: 106–118. [DOI] [PubMed] [Google Scholar]
- 56. Cazzola M, Calzetta L, Page C, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015; 24: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Warson A, Spalluto CM, McCrae C, et al. Dynamics of IFN-β responses during respiratory viral infection. Insights for therapeutic strategies. Am J Respir Crit Care Med 2020; 201: 83–94. [DOI] [PubMed] [Google Scholar]
- 58. Bagga B, Cehelsky JE, Vaishnaw A, et al. Effect of preexisting serum and mucosal antibody on experimental respiratory syncytial virus (RSV) challenge and infection of adults. J Infect Dis 2015; 212: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 59. Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004; 1: 115–120. [DOI] [PubMed] [Google Scholar]
- 60. Pleguezuelos O, Robinson S, Fernandez A, et al. A synthetic influenza virus vaccine induces a cellular immune response that correlates with reduction in symptomatology and virus shedding in a randomized phase Ib live-virus challenge in humans. Clin Vaccine Immunol 2015; 22: 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sanei F, Wilkinson T. Influenza vaccination for patients with chronic obstructive pulmonary disease: understanding immunogenicity, efficacy and effectiveness. Ther Adv Respir Dis 2016; 10: 349–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilkinson TMA, Schembri S, Brightling C, et al. Non-typeable Haemophilus influenzae protein vaccine in adults with COPD: a phase 2 clinical trial. Vaccine 2019; 37: 6102–6111. [DOI] [PubMed] [Google Scholar]
- 63. Donaldson GC, Wilkinson TM, Hurst JR, et al. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 446–452. [DOI] [PubMed] [Google Scholar]
- 64. Jacome C, Marques A. Pulmonary rehabilitation for mild COPD: a systematic review. Respir Care 2014; 59: 588–594. [DOI] [PubMed] [Google Scholar]
- 65. Montes de, Oca M, Loeb E, Torres SH, et al. Peripheral muscle alterations in non-COPD smokers. Chest 2008; 133: 13–18. [DOI] [PubMed] [Google Scholar]
- 66. Bourne S, DeVos R, North M, et al. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open 2017; 7: e014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hanson C, Rutten EP, Wouters EF, et al. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis 2014; 9: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cao C, Wang R, Wang J, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One 2012; 7: e43892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lavie CJ, Ventura HO, Milani RV. The “obesity paradox”: is smoking/lung disease the explanation? Chest 2008; 134: 896–868. [DOI] [PubMed] [Google Scholar]
- 70. Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 1856–1861. [DOI] [PubMed] [Google Scholar]
- 71. Dahl M, Nordestgaard BG. Markers of early disease and prognosis in COPD. Int J Chron Obstruct Pulmon Dis 2009; 4: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ilumets H, Rytilä P, Demedts I, et al. Matrix metalloproteinases -8, -9 and -12 in smokers and patients with stage 0 COPD. Int J Chron Obstruct Pulmon Dis 2007; 2: 369–379. [PMC free article] [PubMed] [Google Scholar]
- 73. Nakajima T, Nakamura H, Owen CA, et al. Plasma cathepsin s and cathepsin s/cystatin c ratios are potential biomarkers for COPD. Dis Markers 2016; 2016: 4093870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response for Early COPD: current evidence for diagnosis and management by Aishath Fazleen and Tom Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Early COPD: current evidence for diagnosis and management by Aishath Fazleen and Tom Wilkinson in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Early COPD: current evidence for diagnosis and management by Aishath Fazleen and Tom Wilkinson in Therapeutic Advances in Respiratory Disease